Distribution of Microplastics in Beach Sand on the Can Gio Coast, Ho Chi Minh City, Vietnam
Abstract
:1. Introduction
2. Materials and Methods
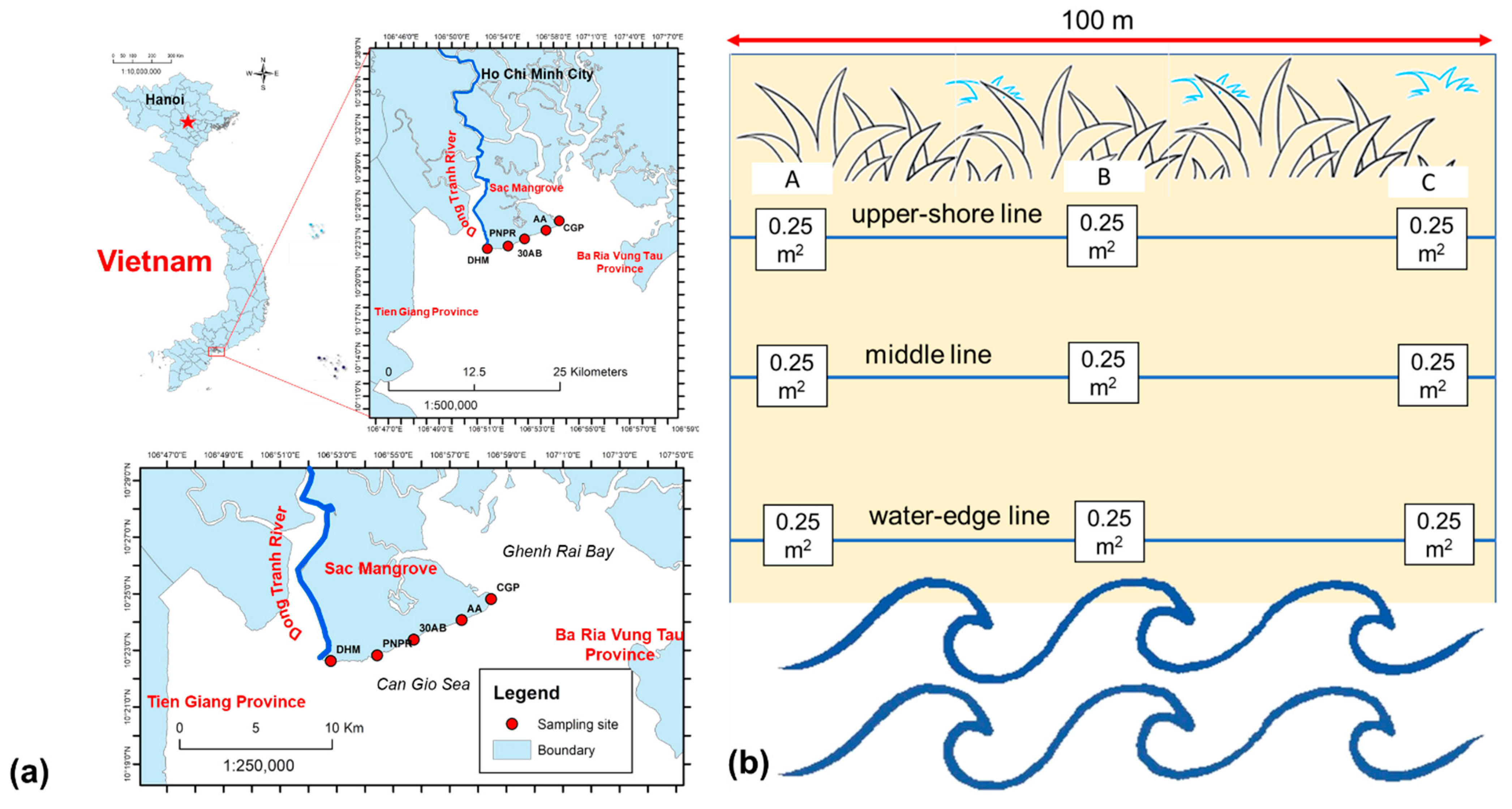
2.1. Study Area
2.2. Sampling Method
2.3. Microplastic Analysis
2.4. Quality Assurance and Quality Control
3. Results
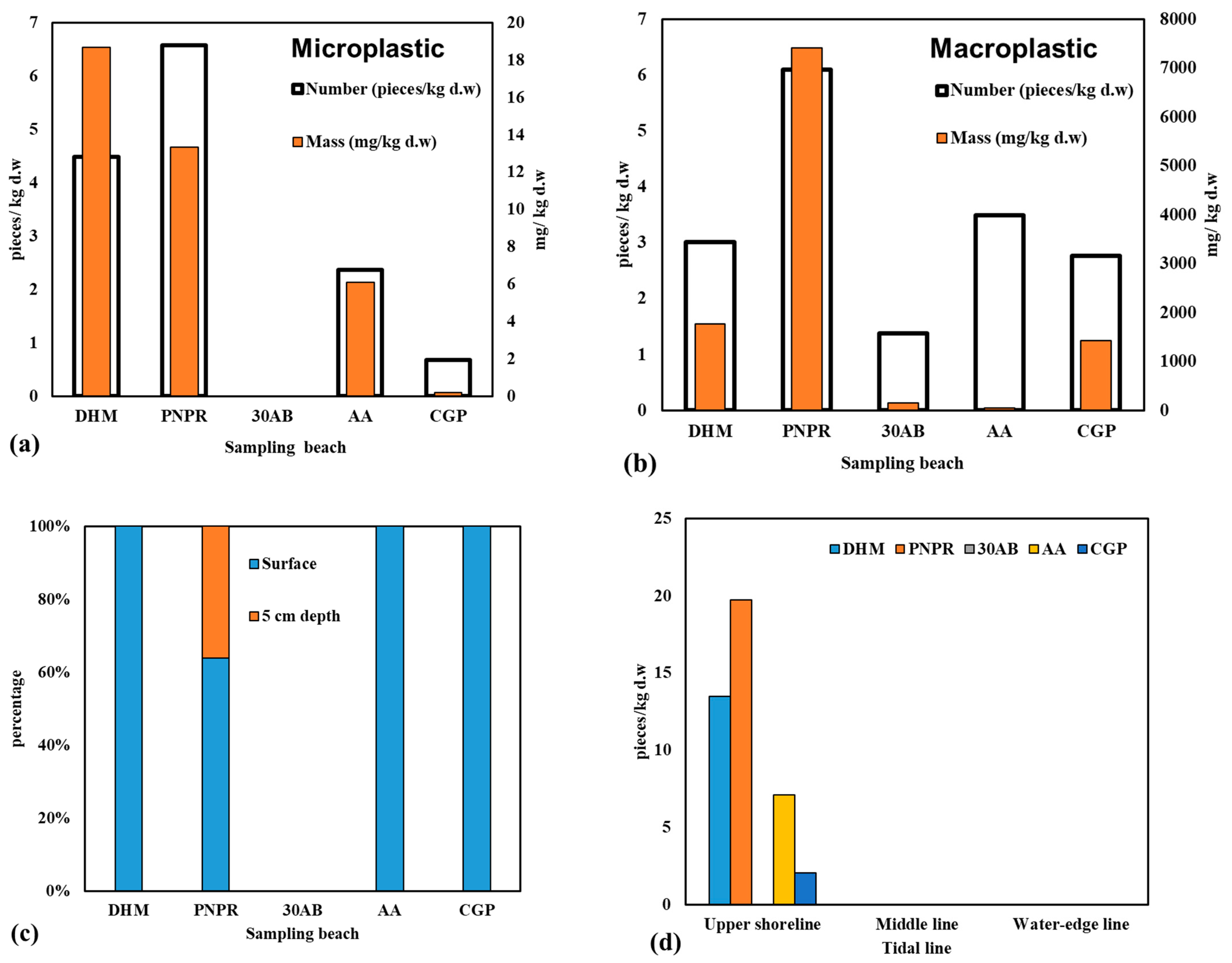
3.1. Abundance of Microplastics
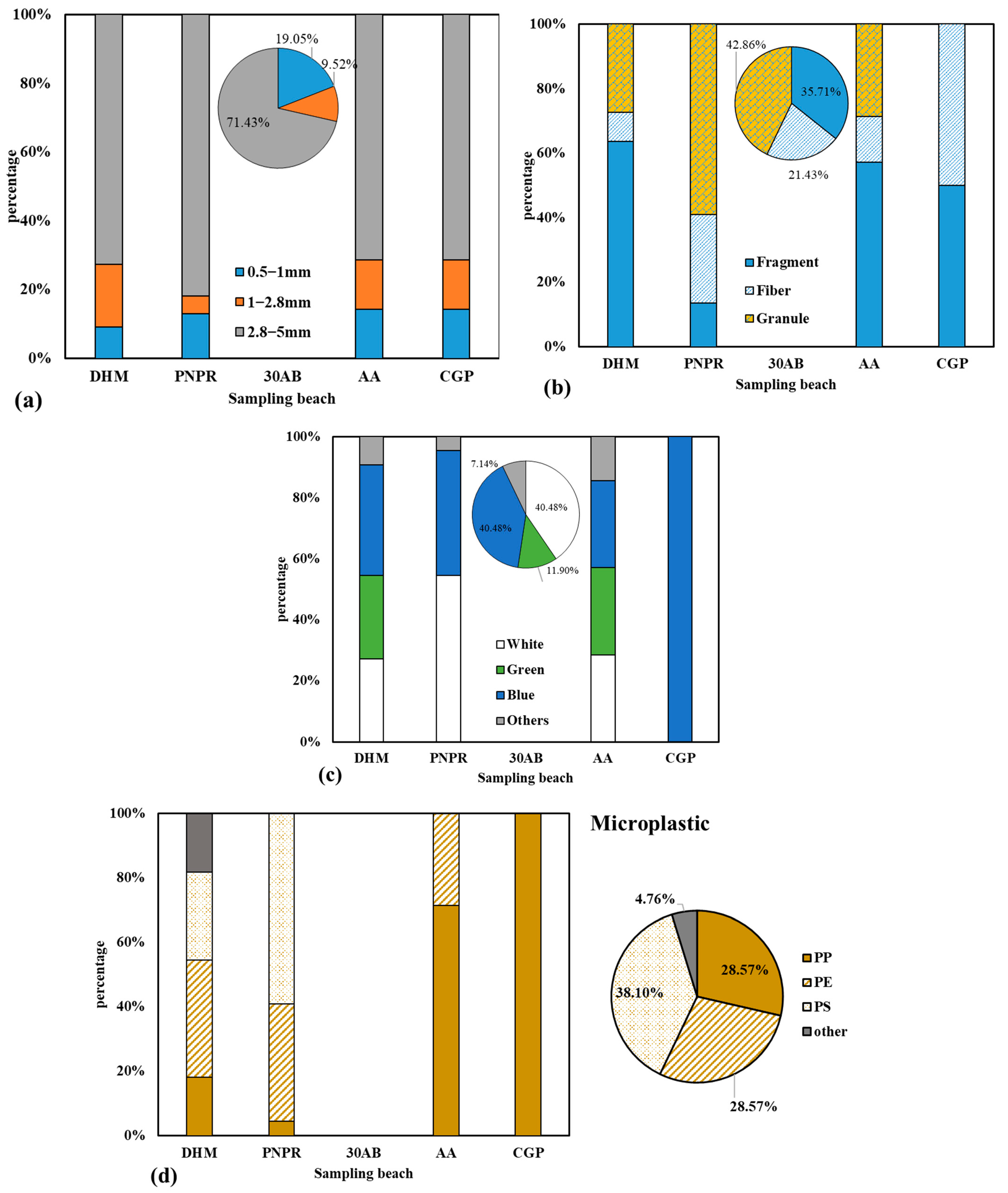
3.2. Physical Characteristics of Microplastics
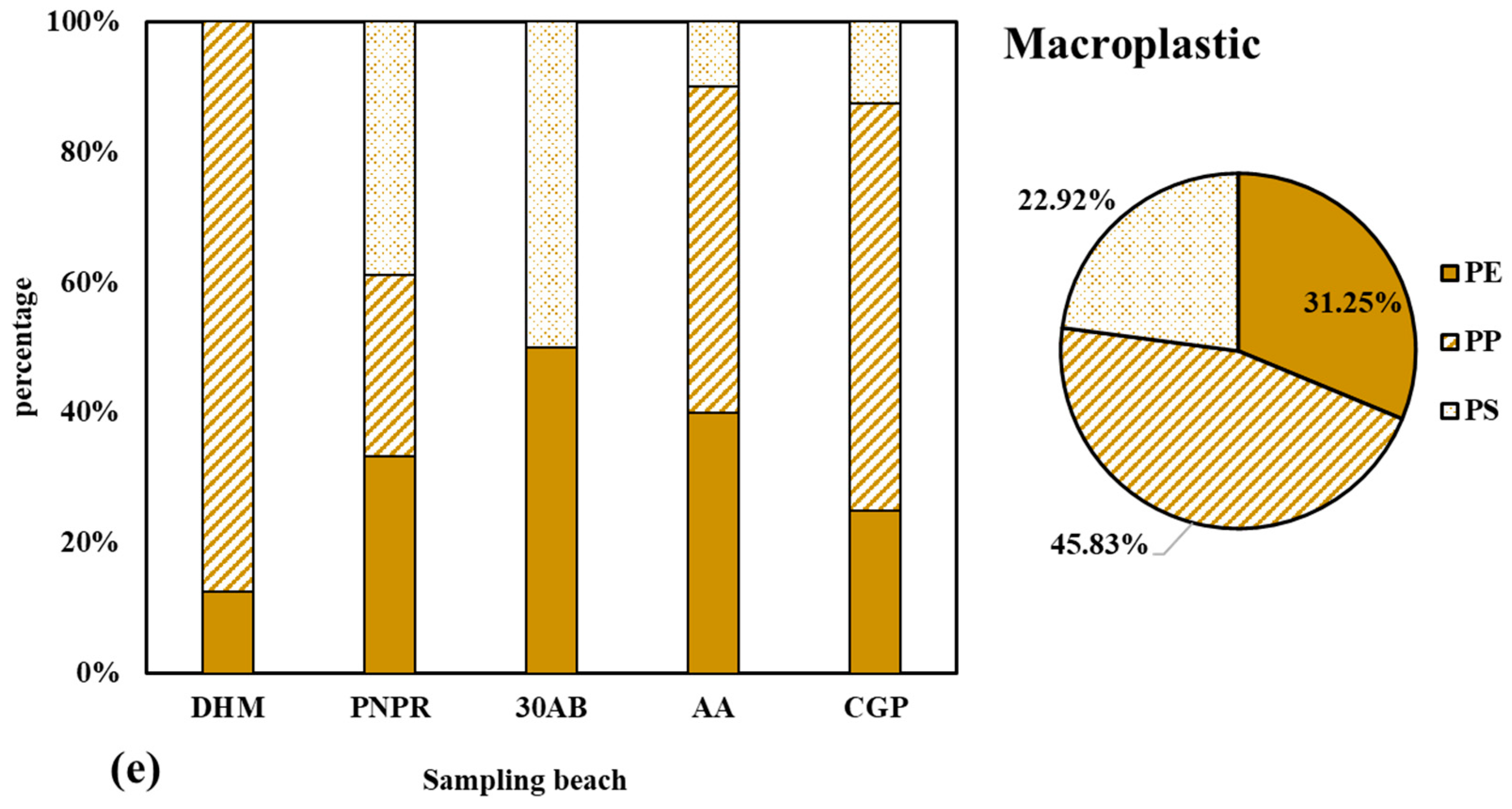
3.3. Composition of Microplastics
4. Discussion
4.1. Abundance of Microplastics in Beach Sand
4.1.1. Comparison with Other Beaches
4.1.2. Distribution of MPs at Different Sampling Areas and Sand Depths
4.2. Physical Characteristics of Microplastics
4.3. Composition of Microplastics
4.4. Prediction of Microplastic Origins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2020, 107, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hannah Ritchie and Max Roser, Plastic Pollution, Our World Data. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 28 May 2021).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; Van Der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants. Elsevier Gezondheidszorg: 2016. Available online: https://research-portal.uws.ac.uk/en/publications/microplastic-pollutants (accessed on 1 September 2022).
- Chenillat, F.; Huck, T.; Maes, C.; Grima, N.; Blanke, B. Fate of floating plastic debris released along the coasts in a global ocean model. Mar. Pollut. Bull. 2021, 165, 112116. [Google Scholar] [CrossRef]
- Everaert, G.; De Rijcke, M.; Lonneville, B.; Janssen, C.; Backhaus, T.; Mees, J.; van Sebille, E.; Koelmans, A.; Catarino, A.; Vandegehuchte, M. Risks of floating microplastic in the global ocean. Environ. Pollut. 2020, 267, 115499. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Wang, J.; Bassi, A. Current research trends on microplastic pollution from wastewater systems: A critical review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 207–230. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.; Duarte, A.C. Characterization and Analysis of Microplastics; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: A case study in Changzhou, China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Hildebrandt, L.; El Gareb, F.; Zimmermann, T.; Klein, O.; Kerstan, A.; Emeis, K.-C.; Pröfrock, D. Spatial distribution of microplastics in the tropical Indian Ocean based on laser direct infrared imaging and microwave-assisted matrix digestion. Environ. Pollut. 2022, 307, 119547. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, K.; Costa, E.; Morgana, S.; Gambardella, C.; Piazza, V.; Faimali, M.; Minetti, R.; Zeri, C.; Thyssen, M.; Ben Ismail, S.; et al. Microplastics in the Mediterranean: Variability from Observations and Model Analysis. Front. Mar. Sci. 2022, 9, 784937. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef]
- NOAA. What Are Microplastics? 2021. Available online: https://oceanservice.noaa.gov/facts/microplastics.html (accessed on 14 May 2021).
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Axtell, F.; Rothon, R.; Gilbert, M. Additives for Plastics. In Brydson’s Plastics Materials (pp. 127–168). Butterworth-Heinemann. 2017. Available online: https://books.google.com.sg/books?hl=zh-TW&lr=&id=ERWKCgAAQBAJ&oi=fnd&pg=PP1&dq=21.%09Al-Malaika,+S.,+Axtell,+F.,+Rothon,+R.,+%26+Gilbert,+M.+Additives+for+plastics.+In+Brydson%27s+plastics+materials+(pp.+127-168).+But-ter-worth-Heinemann&ots=QQugdY1Srv&sig=cGEnZce6EEtUuYpQc4YvGnkpYWE&redir_esc=y#v=onepage&q&f=false (accessed on 1 September 2022).
- Lambert, S.; Sinclair, C.; Boxall, A. Occurrence, Degradation, and Effect of Polymer-Based Materials in the Environment. Reviews of Environmental Contamination and Toxicology, Volume Springer 227, 1–53. 2014. Available online: https://link.springer.com/chapter/10.1007/978-3-319-01327-5_1 (accessed on 1 September 2022).
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter (p. 447). Springer Nature. 2015. Available online: file:///C:/Users/MDPI/Downloads/1001966.pdf (accessed on 1 September 2022).
- Lots, F.A.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Shaifuddin, S.N.M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.T.; Olsen, Y.S.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; Mcgonigle, D.F.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar]
- Korez, Š.; Gutow, L.; Saborowski, R. Microplastics at the strandlines of Slovenian beaches. Mar. Pollut. Bull. 2019, 145, 334–342. [Google Scholar] [CrossRef]
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Zhao, J.; Ran, W.; Teng, J.; Liu, Y.; Liu, H.; Yin, X.; Cao, R.; Wang, Q. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018, 640–641, 637–645. [Google Scholar] [CrossRef]
- Auta, H.; Emenike, C.; Fauziah, S. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Tse, H.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.; Song, Y.K.; Hong, S.H.; Jang, Y.C.; Jang, M.; Heo, N.W.; Han, G.M.; Lee, M.J.; Kang, D.; et al. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.; Hong, S.H.; Song, Y.K.; Lee, J.; Lee, J.; Shim, W.J. Abundance, composition, and distribution of microplastics larger than 20 μm in sand beaches of South Korea. Environ. Pollut. 2018, 238, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Castillo-Olaya, V.A.; Granados-Briceño, A.F.; García, L.M.B.; Díaz, L.F.E. Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar. Pollut. Bull. 2019, 145, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.A.; Kunz, A.; Hu, C.-S. Type and quantity of coastal debris pollution in Taiwan: A 12-year nationwide assessment using citizen science data. Mar. Pollut. Bull. 2018, 135, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Hien, T.T.; Nhon, N.T.T.; Thu, V.T.M.; Quyen, D.T.T.; Nguyen, N.T. The Distribution of Microplastics in Beach Sand in Tien Giang Province and Vung Tau City, Vietnam. J. Eng. Technol. Sci. 2020, 52, 208–221. [Google Scholar] [CrossRef]
- Yu, X.; Peng, J.; Wang, J.; Wang, K.; Bao, S. Occurrence of microplastics in the beach sand of the Chinese inner sea: The Bohai Sea. Environ. Pollut. 2016, 214, 722–730. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Lahens, L.; Strady, E.; Kieu-Le, T.-C.; Dris, R.; Boukerma, K.; Rinnert, E.; Gasperi, J.; Tassin, B. Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ. Pollut. 2018, 236, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Mỹ, T.T.Á.; Dũng, P.T. Analytical conditions for determination of microplastics in fish. Hue Univ. J. Sci. Nat. Sci. 2020, 129, 85–92. [Google Scholar]
- Nguyen, Q.A.T.; Nguyen, H.N.Y.; Strady, E.; Nguyen, Q.T.; Trinh-Dang, M.; Vo, V.M. Characteristics of microplastics in shoreline sediments from a tropical and urbanized beach (Da Nang, Vietnam). Mar. Pollut. Bull. 2020, 161, 111768. [Google Scholar] [CrossRef] [PubMed]
- Yukioka, S.; Tanaka, S.; Nabetani, Y.; Suzuki, Y.; Ushijima, T.; Fujii, S.; Takada, H.; Van Tran, Q.; Singh, S. Occurrence and characteristics of microplastics in surface road dust in Kusatsu (Japan), Da Nang (Vietnam), and Kathmandu (Nepal). Environ. Pollut. 2019, 256, 113447. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Cohen, M. Studies in Can Gio Mangrove Biosphere Reserve, Ho Chi Minh City, Vietnam; ISME Mangrove Ecosystems Technical Reports; International Society for Mangrove Ecosystems (ISME): Okinawa, Japan, 2014; Volume 6, p. 75. [Google Scholar]
- Le Nguyen, H.T.; Luong, H.P.V. Erosion and deposition processes from field experiments of hydrodynamics in the coastal mangrove area of Can Gio, Vietnam. Oceanologia 2018, 61, 252–264. [Google Scholar] [CrossRef]
- Kim, I.-S.; Chae, D.-H.; Kim, S.-K.; Choi, S.; Woo, S.-B. Factors Influencing the Spatial Variation of Microplastics on High-Tidal Coastal Beaches in Korea. Arch. Environ. Contam. Toxicol. 2015, 69, 299–309. [Google Scholar] [CrossRef]
- Hanvey, J.S.; Lewis, P.; Lavers, J.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2016, 9, 1369–1383. [Google Scholar] [CrossRef]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; de Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582. [Google Scholar] [CrossRef]
- Nel, H.A.; Froneman, P.W. A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar. Pollut. Bull. 2015, 101, 274–279. [Google Scholar] [CrossRef]
- Carson, H.S.; Colbert, S.L.; Kaylor, M.J.; McDermid, K.J. Small plastic debris changes water movement and heat transfer through beach sediments. Mar. Pollut. Bull. 2011, 62, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Turra, A.; Manzano, A.B.; Dias, R.J.S.; Mahiques, M.; Barbosa, L.; Balthazar-Silva, D.; Moreira, F.T. Three-dimensional distribution of plastic pellets in sandy beaches: Shifting paradigms. Sci. Rep. 2014, 4, 4435. [Google Scholar] [CrossRef]
- Frias, J.; Pagter, E.; Nash, R.; O’Connor, I.; Carretero, O.; Filgueiras, A.; Gerdts, G. Standardised Protocol for Monitoring Microplastics in Sediments; Deliverable 4.2. 2018. Available online: https://repository.oceanbestpractices.org/handle/11329/1206 (accessed on 1 September 2022).
- Saliu, F.; Montano, S.; Garavaglia, M.G.; Lasagni, M.; Seveso, D.; Galli, P. Microplastic and charred microplastic in the Faafu Atoll, Maldives. Mar. Pollut. Bull. 2018, 136, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zheng, H.; Wang, J.; Chen, C. Microplastics in surface water and sediments of Chongming Island in the Yangtze Estuary, China. Environ. Sci. Eur. 2020, 32, 15. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. 2015. Available online: https://scholar.google.com.hk/scholar?hl=zh-TW&as_sdt=0%2C5&q=61.%09Masura%2C+J.%2C+Baker%2C+J.%2C+Foster%2C+G.%2C+%26+Arthur%2C+C.+%282015%29.+Laboratory+Methods+for+the+Analysis+of+Microplas-tics+in+the+Marine+En-vironment%3A+Recommendations+for+quantifying+synthetic+particles+in+waters+and+sediments&btnG= (accessed on 1 September 2022).
- Wu, X.; Zhong, C.; Wang, T.; Zou, X.; Zang, Z.; Li, Q.; Chenglong, W. Occurrence and distribution of microplastics on recreational beaches of Haichow Bay, China. Environ. Sci. Pollut. Res. 2020, 28, 6132–6145. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Guaita, L.; Behrens, P. Microplastic pollution on Caribbean beaches in the Lesser Antilles. Mar. Pollut. Bull. 2018, 133, 442–447. [Google Scholar] [CrossRef]
- Wessel, C.C.; Lockridge, G.R.; Battiste, D.; Cebrian, J. Abundance and characteristics of microplastics in beach sediments: Insights into microplastic accumulation in northern Gulf of Mexico estuaries. Mar. Pollut. Bull. 2016, 109, 178–183. [Google Scholar] [CrossRef]
- Kunz, A.; Walther, B.A.; Löwemark, L.; Lee, Y.-C. Distribution and quantity of microplastic on sandy beaches along the northern coast of Taiwan. Mar. Pollut. Bull. 2016, 111, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Nuri, M.; Amiri, P.; Niyogi, S. Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar. Pollut. Bull. 2019, 146, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tu, C.; Fu, C.; Li, Y.; Zhang, H.; Xiong, K.; Zhao, X.; Li, L.; Waniek, J.J.; Luo, Y. Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci. Total Environ. 2019, 703, 134807. [Google Scholar] [CrossRef] [PubMed]
- Piñon-Colin, T.D.J.; Rodriguez-Jimenez, R.; Pastrana-Corral, M.A.; Rogel-Hernandez, E.; Wakida, F.T. Microplastics on sandy beaches of the Baja California Peninsula, Mexico. Mar. Pollut. Bull. 2018, 131, 63–71. [Google Scholar] [CrossRef]
- Young, A.M.; Elliott, J.A. Characterization of microplastic and mesoplastic debris in sediments from Kamilo Beach and Kahuku Beach, Hawai’i. Mar. Pollut. Bull. 2016, 113, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Besley, A.; Vijver, M.G.; Behrens, P.; Bosker, T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 2017, 114, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
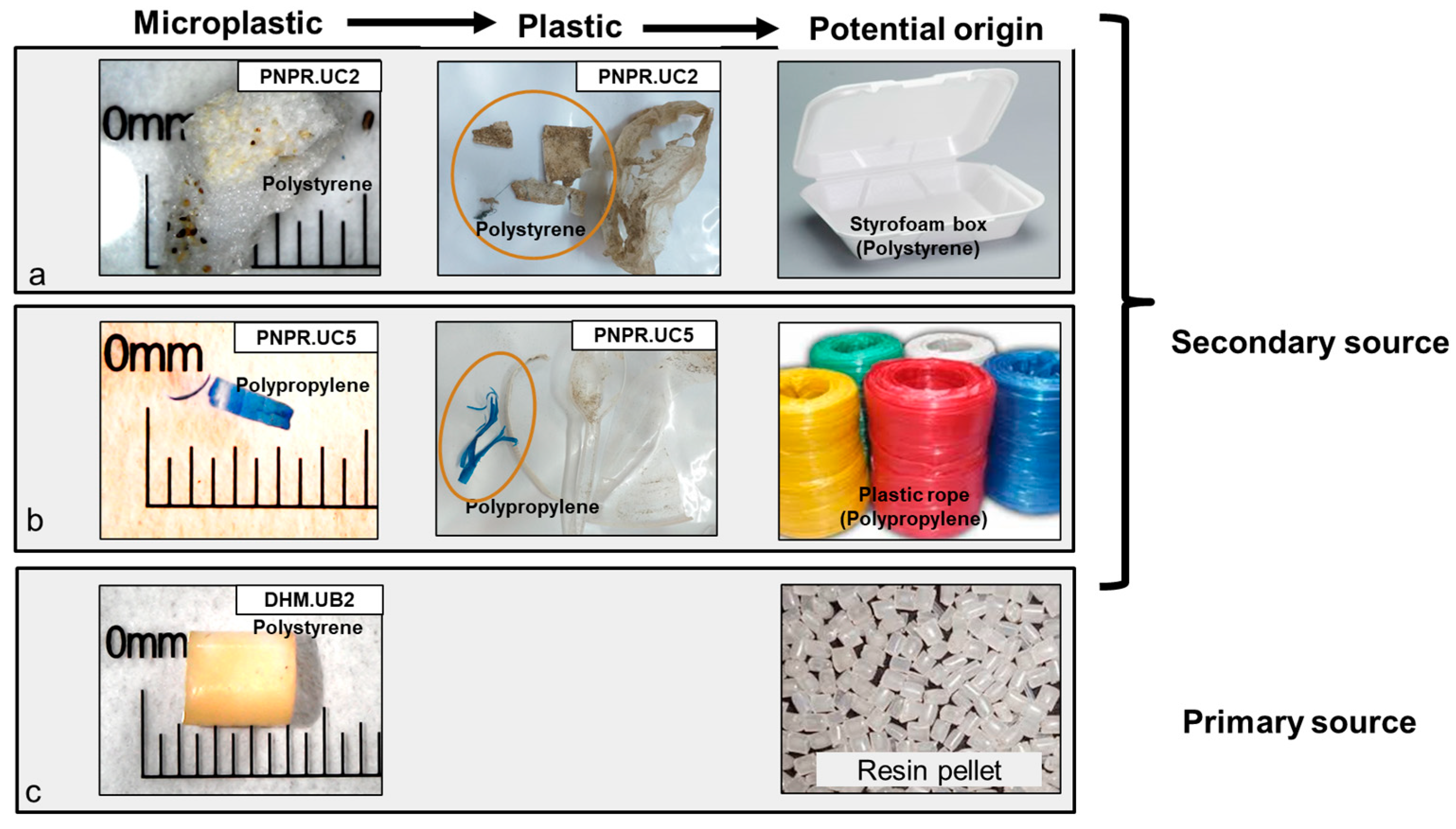
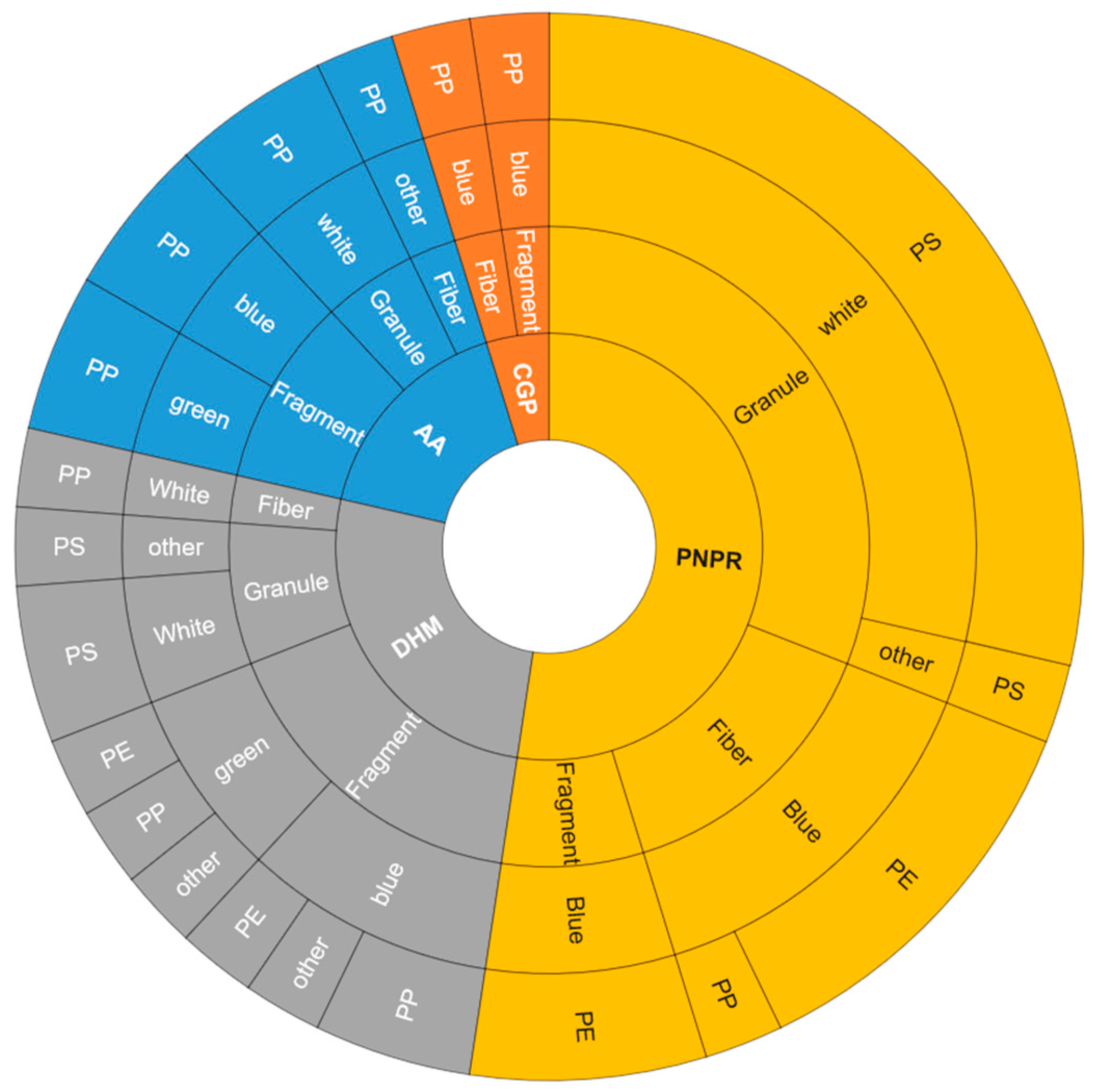
| Site Symbol | Location | Coordinate | Microplastic Abundance | Macroplastic Abundance | ||
|---|---|---|---|---|---|---|
| Pieces/kg d.w. | mg/kg d.w. | Pieces/kg d.w. | mg/kg d.w. | |||
| DHM | Dong Hoa Market | 10.376830, 106.879996 | 4.49 | 18.66 | 3.01 | 1766.24 |
| PNPR | Phuong Nam Pearl Resort | 10.377025, 106.894204 | 6.58 | 13.33 | 6.10 | 7403.80 |
| 30AB | 30 April Beach | 10.389663, 106.928722 | ND | ND | 1.37 | 157.84 |
| AA | Aquaculture area | 10.400064, 106.954130 | 2.37 | 6.09 | 3.49 | 52.28 |
| CGP | Can Gio Park | 10.413471, 106.974338 | 0.68 | 0.20 | 2.76 | 1423.70 |
| Sampling Beach | n = 18 (Pieces/kg d.w.) | Surface Layer | Upper Shorline | Surface Layer of the Upper Shoreline | Surface Layer of the Upper Shoreline |
|---|---|---|---|---|---|
| n = 9 (Pieces/kg d.w.) | n = 6 (Pieces/kg d.w.) | n = 3 (Pieces/kg d.w.) | n = 3 (Pieces/m2) | ||
| DHM | 4.49 | 8.34 | 13.47 | 26.94 | 107.76 |
| PNPR | 6.58 | 9.55 | 19.74 | 25.33 | 101.32 |
| 30AB | ND | ND | ND | ND | ND |
| AA | 2.37 | 4.93 | 7.11 | 14.22 | 56.88 |
| CGP | 0.68 | 1.40 | 2.05 | 4.1 | 16.4 |
| Min-Max | 0–6.58 | 0–9.55 | 0–19.74 | 0–26.94 | 0–107.76 |
| Median | 2.37 | 4.93 | 7.11 | 14.22 | 56.88 |
| Average | 2.82 | 4.84 | 8.47 | 14.12 | 56.47 |
| Reference | Research Area | Sampling | Sampling Area’s Characteristics | Microplastics Abundance |
|---|---|---|---|---|
| [36] | South Korea | 3 tidal lines 100 m stretch 0.5 × 0.5 m2 quadrat Upper 2.5 cm of sand | beaches with different features:
| 1400–62,800 pieces/m2 (small microplastics) 0–2088 pieces/m2 (large microplastics) |
| [62] | China | 3 tidal lines 100 m stretch 0.5 × 0.5 m2 quadrat Upper 5 cm of sand | Recreational beaches with different levels of impact. | 106.50 ± 34.41 items/kg |
| [63] | Netherland | Strandline 0.5 × 0.5 m2 quadrat Upper 5 cm of sand | highly exposed area to seasonal extreme events (tropical hurricanes). | 68 ± 19–620 ± 96 pieces/kg d.w. (261 ± 6 pieces/kg d.w.) |
| [64] | USA | Wrack line 0.25 × 0.25 quadrat Upper 3–6 cm of sand |
| 5–117 pieces/m2 |
| [59] | Maldives | Drift line 1 × 1 m2 grid Upper 1 cm of sand | 2 beaches with different characteristics:
| 22.8 ± 10.5 pieces/m2 |
| [27] | Slovenia | Strandline Upper 4 cm of sand | Slovenian beaches | 0.5 ± 0.5 pieces/kg d.w. to 1.0 ± 0.8 pieces/kg d.w. |
| [65] | Northern Taiwan | Middle of tidal zone 0.5 × 0.5 m2 quadrat 2 layers of sand (0–5 cm, 5–10 cm) |
| 0.23–30.4 pieces/kg d.w. |
| [48] | Danang, Vietnam | Transect from water-edge to vegetation zone 2 layers of sand (0–5 cm, 5–10 cm) |
| 9238 ± 2097 items/kg d.w. synthetic fibers |
| [37] | Colombia Caribbean | Random sites 1 m2 | Ciénaga Grande de Santa Marta mangrove | 31–2863 pieces/kg d.w. |
| [38] | Singapore | Low tide Upper 3–4 cm of sand 1.5 × 1.5 m2 quadrat | Mangrove Ecosystems | 60.7 ± 27.2 pieces/kg d.w. |
| [66] | Iran | Random sites Upper 5 cm of sand | The Iranian mangrove forest is located between the Persian Gulf and the Oman Sea. | 19.5 ± 6.36 to 34.5 ± 0.71 pieces/kg d.w. |
| [67] | China | Random sites 0.3 × 0.3 m2 quadrat Upper 2 cm of sand | China’s Southeast mangroves | 8.3–5738.3 pieces/kg d.w. |
| This study, 2019 | Vietnam | 3 tidal lines 100 m stretch 0.5 × 0.5 m2 quadrat 2 layers of 2 cm (surface and 5 cm depth) |
| 0 to 6.58 pieces/kg d.w. (0–26.32 pieces/m2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhon, N.T.T.; Nguyen, N.T.; Hai, H.T.N.; Hien, T.T. Distribution of Microplastics in Beach Sand on the Can Gio Coast, Ho Chi Minh City, Vietnam. Water 2022, 14, 2779. https://doi.org/10.3390/w14182779
Nhon NTT, Nguyen NT, Hai HTN, Hien TT. Distribution of Microplastics in Beach Sand on the Can Gio Coast, Ho Chi Minh City, Vietnam. Water. 2022; 14(18):2779. https://doi.org/10.3390/w14182779
Chicago/Turabian StyleNhon, Nguyen Thi Thanh, Nguyen Thao Nguyen, Ho Truong Nam Hai, and To Thi Hien. 2022. "Distribution of Microplastics in Beach Sand on the Can Gio Coast, Ho Chi Minh City, Vietnam" Water 14, no. 18: 2779. https://doi.org/10.3390/w14182779






