Deciphering the Composition of Fulvic Acids from Recirculating Aquaculture Systems by Quadrupole Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
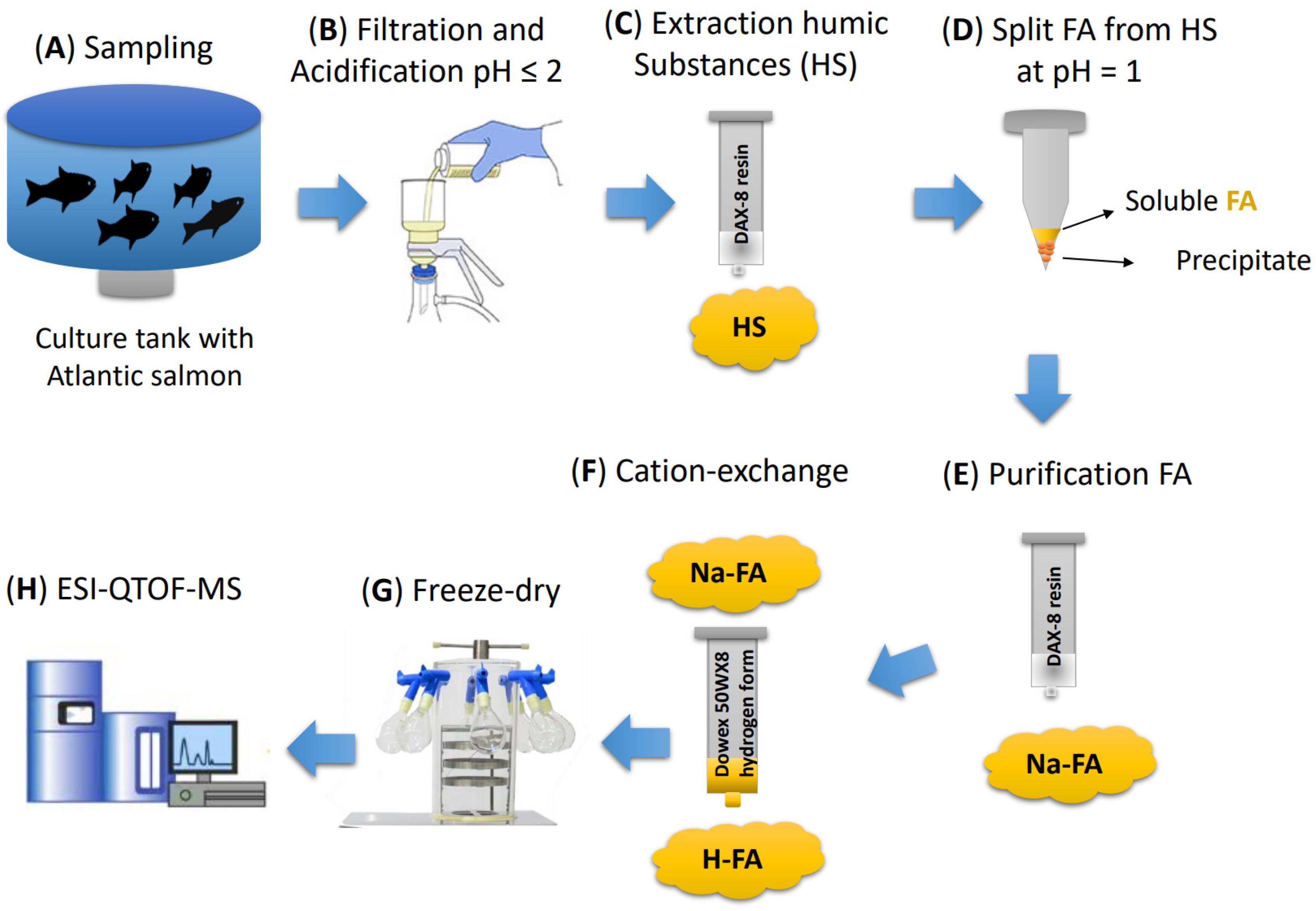
2.1. RAS Water Sample Collection
2.2. Extraction of the Fulvic Acids from RAS and Sample Preparation
2.3. QTOF-MS Analysis
2.4. Data Clean-Up and Visualization
2.5. Accuracy in the Elemental Composition Assignment
3. Results and Discussion
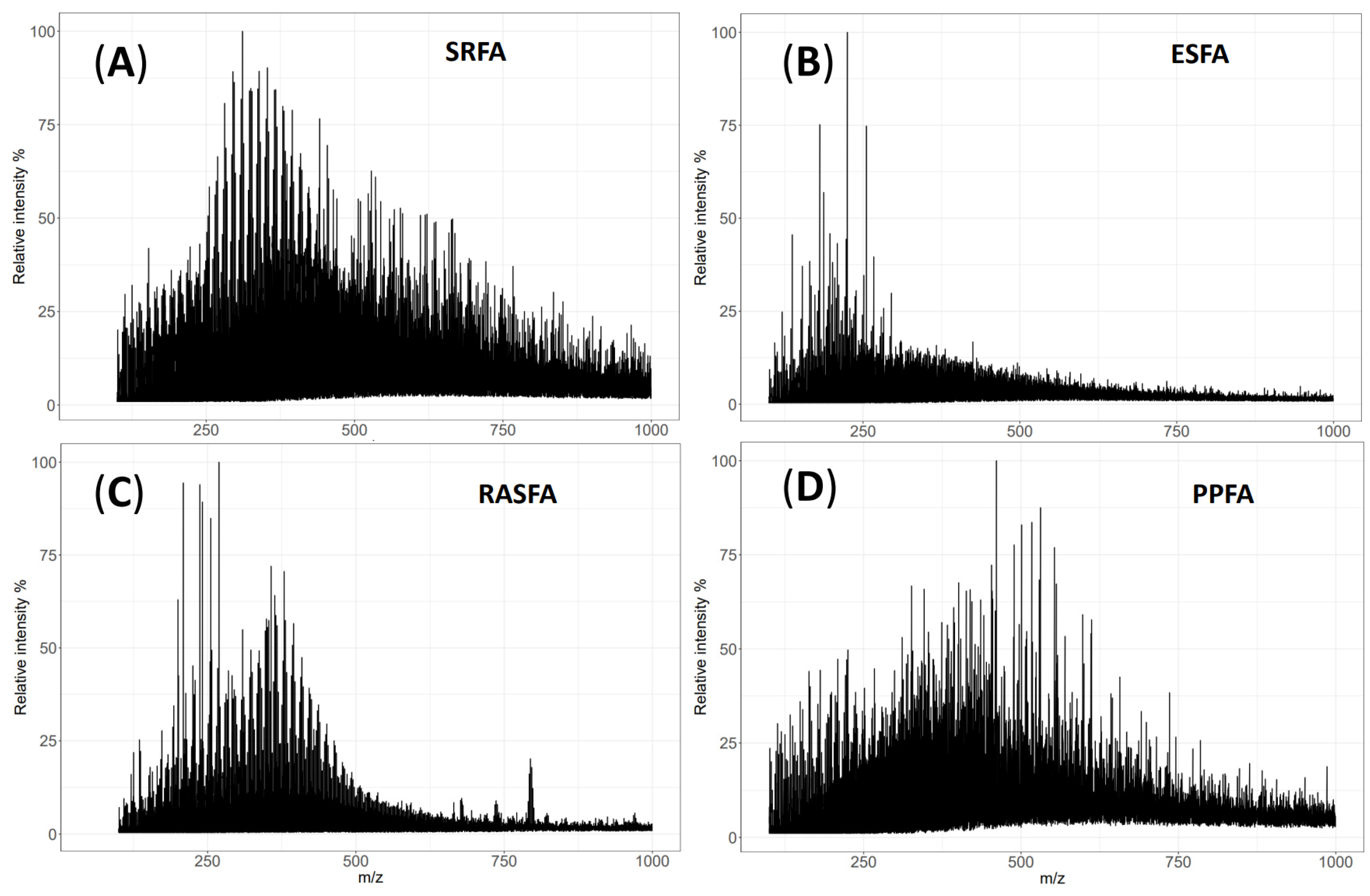
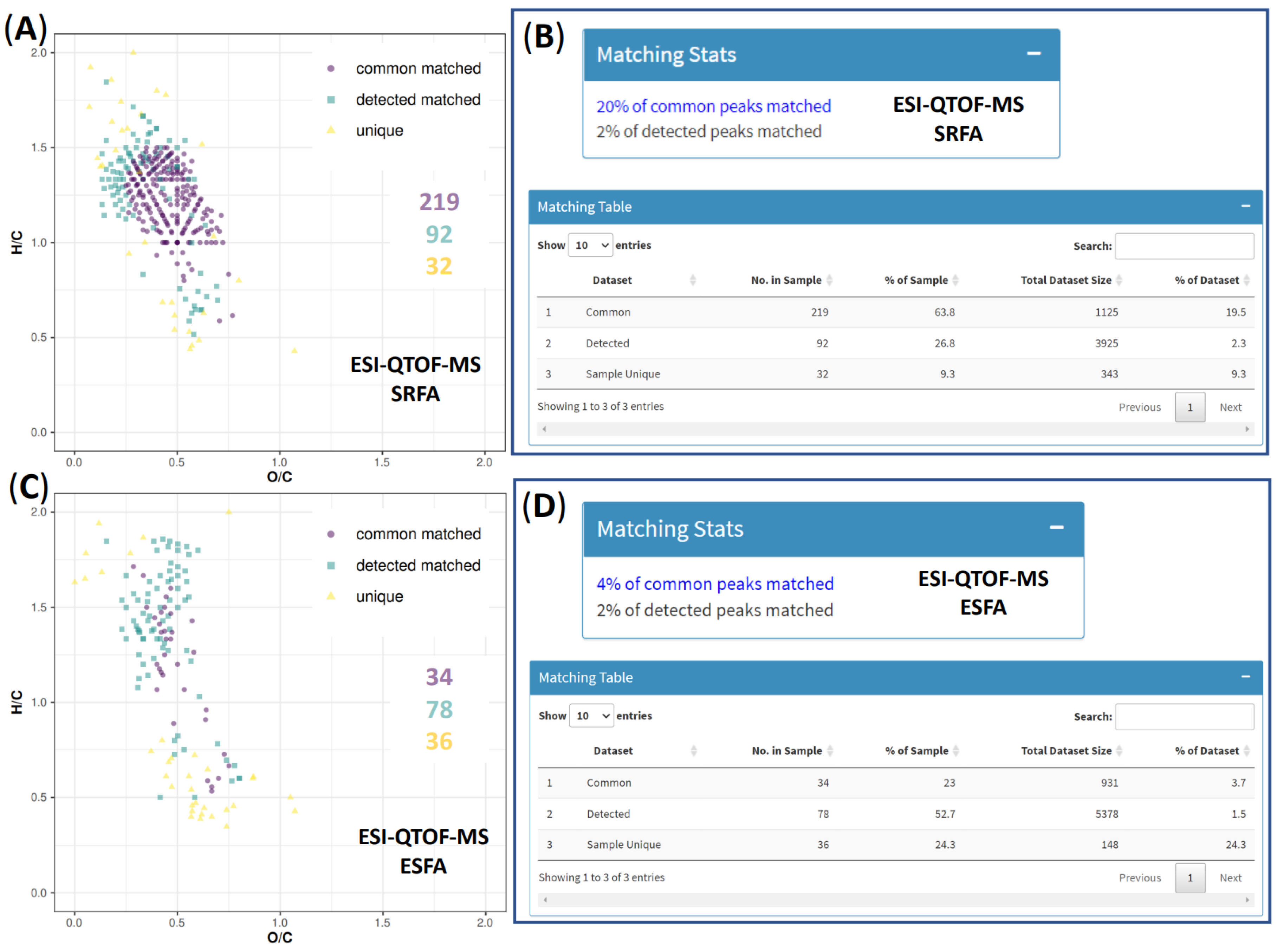
3.1. QTOF-MS of SRFA and ESFA
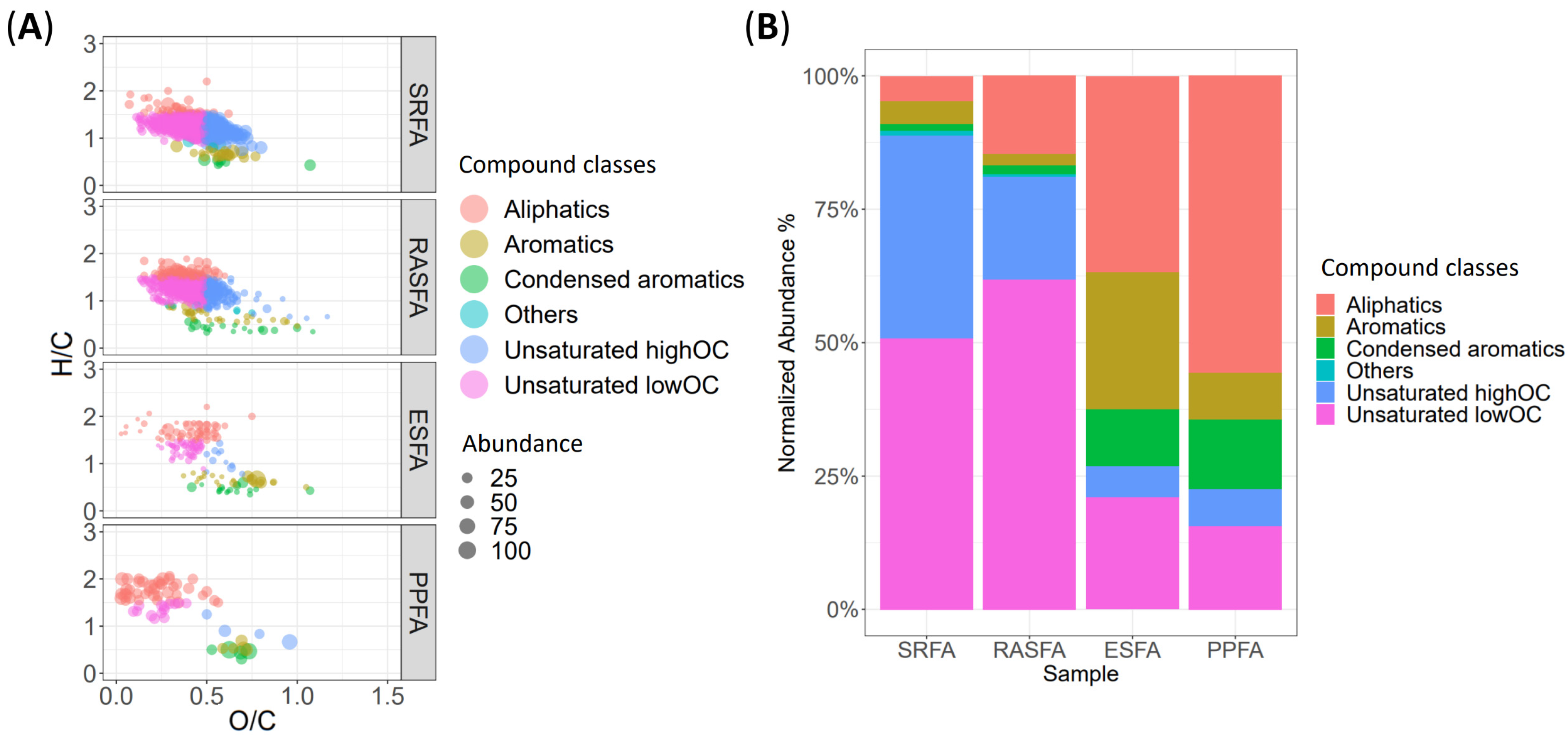
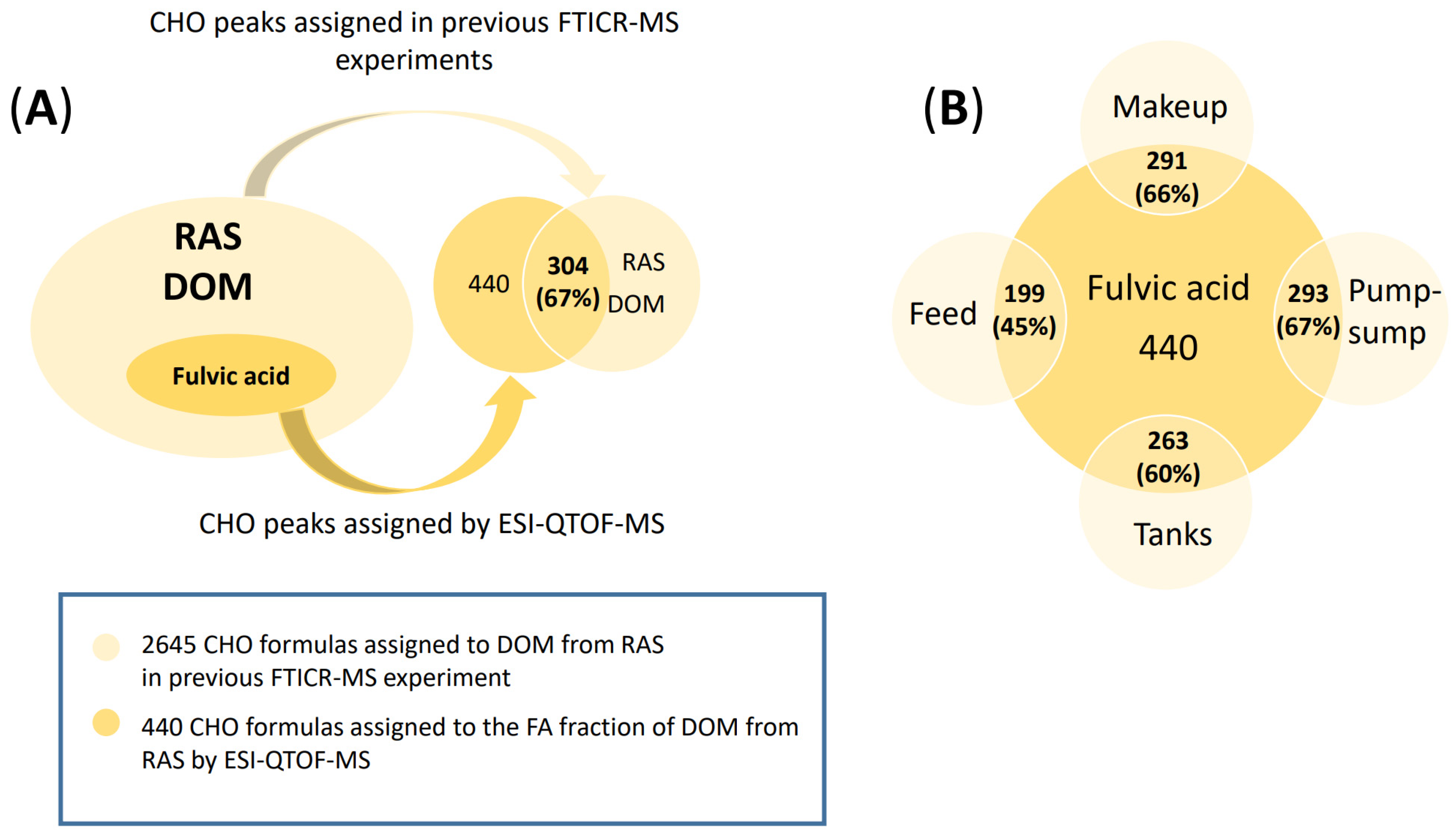
3.2. RASFA by QTOF-MS
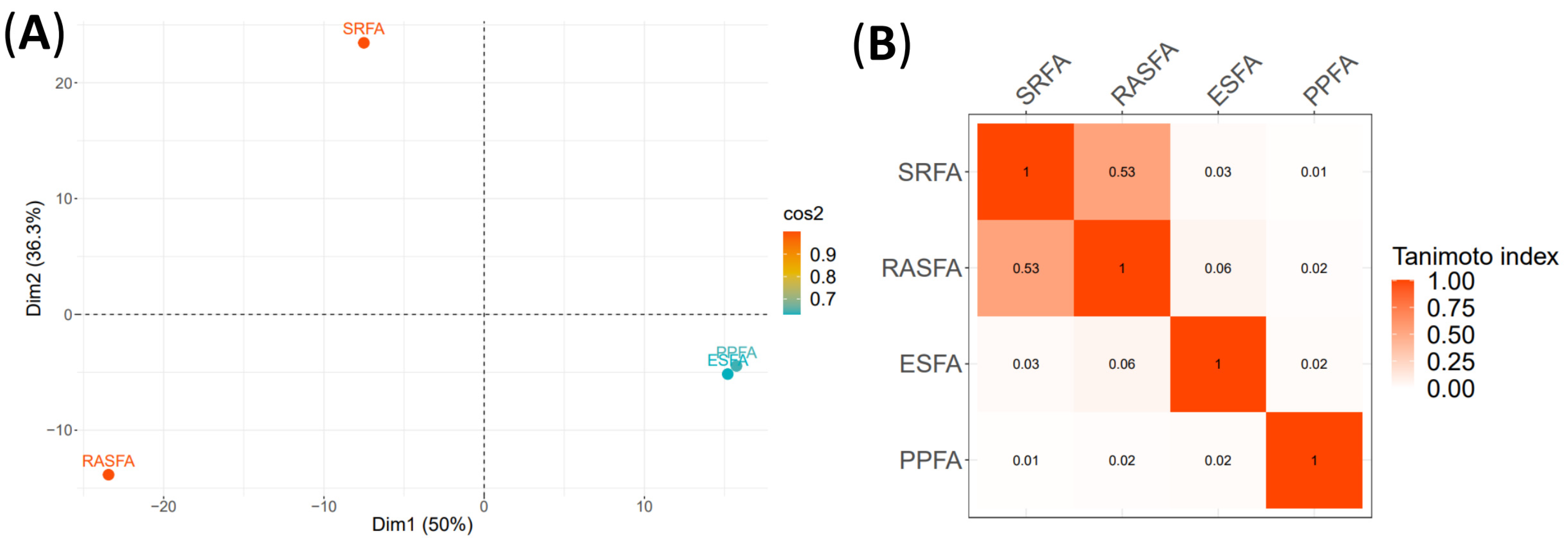
3.3. RASFA and FA Standards from IHSS by QTOF-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tidwell, J.H.; Allan, G.L. Fish as food: Aquaculture’s contribution. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Lem, A.; Bjørndal, T.; Lappo, A. Economic Analysis of Supply and Demand for Food up to 2030. Special Focus on Fish and Fishery Products; FAO: Rome, Italy, 2014; Volume 1089, ISBN 9789251084007. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture Systems. In Aquaculture Production Systems; Cayuga Aqua Ventures: Ithaca, NY, USA, 2007. [Google Scholar]
- Aguilar-Alarcón, P.; Zherebker, A.; Rubekina, A.; Shirshin, E.; Simonsen, M.A.; Kolarevic, J.; Lazado, C.C.; Nikolaev, E.N.; Asimakopoulos, A.G.; Mikkelsen, Ø. Impact of ozone treatment on dissolved organic matter in land-based recirculating aquaculture systems studied by Fourier transform ion cyclotron resonance mass spectrometry. Sci. Total Environ. 2022, 843, 157009. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Alarcón, P.; Gonzalez, S.V.; Simonsen, M.A.; Borrero-santiago, A.R.; Sanchís, J.; Meriac, A.; Kolarevic, J.; Asimakopoulos, A.G.; Mikkelsen, Ø. Characterizing changes of dissolved organic matter composition with the use of distinct feeds in recirculating aquaculture systems via high-resolution mass spectrometry. Sci. Total Environ. 2020, 749, 142326. [Google Scholar] [CrossRef] [PubMed]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Chen, S.; Timmons, M.B.; Aneshansley, D.J.; Bisogni, J.J. Suspended solids characteristics from recirculating aquacultural systems and design implications. Aquaculture 1993, 112, 143–155. [Google Scholar] [CrossRef]
- Cripps, S.J.; Bergheim, A. Solids management and removal for intensive land-based aquaculture production systems. Aquac. Eng. 2000, 22, 33–56. [Google Scholar] [CrossRef]
- Yamin, G.; Borisover, M.; Cohen, E.; van Rijn, J. Accumulation of humic-like and proteinaceous dissolved organic matter in zero-discharge aquaculture systems as revealed by fluorescence EEM spectroscopy. Water Res. 2017, 108, 412–421. [Google Scholar] [CrossRef]
- Hambly, A.C.; Arvin, E.; Pedersen, L.F.; Pedersen, P.B.; Seredyńska-Sobecka, B.; Stedmon, C.A. Characterising organic matter in recirculating aquaculture systems with fluorescence EEM spectroscopy. Water Res. 2015, 83, 112–120. [Google Scholar] [CrossRef]
- Malcolm, R.L. The uniqueness of humic substances in each of soil, stream and marine environments. Anal. Chim. Acta 1990, 232, 19–30. [Google Scholar] [CrossRef]
- Christman, R.F.; Ghassemi, M. Chemical Nature of Organic Color in Water. J.-Am. Water Work. Assoc. 1966, 58, 723–741. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Liu, C.-Q.; Mottaleb, M.A.; Wan, G.; Ogawa, H.; Vione, D.; Yoshioka, T.; Wu, F. Dissolved organic matter in natural waters. In Photobiogeochemistry of Organic Matter; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–137. [Google Scholar] [CrossRef]
- Oliver, B.G.; Thurman, E.M.; Malcolm, R.L. The contribution of humic substances to the acidity of colored natural waters. Geochim. Cosmochim. Acta 1983, 47, 2031–2035. [Google Scholar] [CrossRef]
- Yu, M.J.; Kim, Y.H.; Han, I.; Kim, H.C. Characterization of Humic Substances Isolated from Han River Water and Change in the Structural and Chemical Characteristics by Ozonation. Environ. Technol. 2010, 26, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Abbt-Braun, G.; Lankes, U.; Frimmel, F.H. Structural characterization of aquatic humic substances—The need for a multiple method approach. Aquat. Sci. 2004, 66, 151–170. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V. Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules 2021, 26, 2706. [Google Scholar] [CrossRef] [PubMed]
- Thurman, E.M. Organic Geochemistry of Natural Waters, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9789401087520. [Google Scholar]
- Rodríguez-Vidal, F.J.; Núñez, L.A. Characterization of aquatic humic substances. Water Environ. J. 2011, 25, 163–170. [Google Scholar] [CrossRef]
- Koparal, A.; Yildiz, Y.; Keskinler, B.; Demircioglu, N. Effect of initial pH on the removal of humic substances from wastewater by electrocoagulation. Sep. Purif. Technol. 2008, 59, 175–182. [Google Scholar] [CrossRef]
- Réveillé, V.; Mansuy, L.; Émilie Jardé, A.; Garnier-Sillam, É. Characterisation of sewage sludge-derived organic matter: Lipids and humic acids. Org. Geochem. 2003, 34, 615–627. [Google Scholar] [CrossRef]
- Yang, X.; De Buyck, P.-J.; Zhang, R.; Manhaeghe, D.; Wang, H.; Chen, L.; Zhao, Y.; Demeestere, K.; Van Hulle, S.W. Van Enhanced removal of refractory humic- and fulvic-like organics from biotreated landfill leachate by ozonation in packed bubble columns. Sci. Total Environ. 2022, 807, 150762. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Simpson, A.J. Humic Substances in Soils: Are They Really Chemically Distinct? Environ. Sci. Technol. 2006, 40, 4605–4611. [Google Scholar] [CrossRef]
- Graber, E.R.; Rudich, Y.; Dagan, B. Atmospheric HULIS: How humic-like are they? A comprehensive and critical review. Atmos. Chem. Phys. 2006, 6, 729–753. [Google Scholar] [CrossRef]
- Maccarthy, P. The principles of humic substances: An introduction to the first principle. Spec. Publ.-R. Soc. Chem. 2001, 273, 19–30. [Google Scholar] [CrossRef]
- Christensen, J.M.; Rusch, K.A.; Malone, R.F. Development of a model for describing accumulation of color and subsequent destruction by ozone in a freshwater recirculating aquaculture system. J. World Aquac. Soc. 2000, 31, 167–174. [Google Scholar] [CrossRef]
- Averett, R.C.; Leenheer, J.A.; McKnight, D.M.; Thorn, K.A. Humic Substances in the Suwannee River, Georgia: Interactions, Properties, and Proposed Structures; US Government Printing Office: Washington, DC, USA, 1994.
- Hirayama, K.; Mizuma, H.; Mizue, Y. The accumulation of dissolved organic substances in closed recirculation culture systems. Aquac. Eng. 1988, 7, 73–87. [Google Scholar] [CrossRef]
- Mladenov, N.; Huntsman-mapila, P.; Wolski, P.; Masamba, W.R.L.; Mcknight, D.M. Dissolved organic matter accumulation, reactivity, and redox state in ground water of a recharge wetland. Wetlands 2008, 28, 747–759. [Google Scholar] [CrossRef]
- Porcal, P.; Koprivnjak, J.; Molot, L.A.; Dillon, P.J. Humic substances—part 7: The biogeochemistry of dissolved organic carbon and its interactions with climate change. Environ. Sci. Pollut. Res. 2009, 16, 714–726. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Foreman, C.M.; Marshall, A.G.; McKnight, D.M. Characterization of IHSS Pony Lake fulvic acid dissolved organic matter by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and fluorescence spectroscopy. Org. Geochem. 2013, 65, 19–28. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Rosario-ortiz, F.L.; Baik, S.; Vanderford, B.J.; Snyder, S.A. Characterization of fulvic acids by liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 1319–1324. [Google Scholar] [CrossRef]
- Plancque, G.; Toulhoat, P.; Amekraz, B.; Moulin, C. Molecular structure of fulvic acids by electrospray with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 827–835. [Google Scholar] [CrossRef]
- Reemtsma, T.; These, A. Comparative Investigation of Low-Molecular-Weight Fulvic Acids of Different Origin by SEC-Q-TOF-MS: New Insights into Structure and Formation. Environ. Sci. Technol. 2005, 39, 3507–3512. [Google Scholar] [CrossRef]
- These, A.; Winkler, M.; Thomas, C.; Reemtsma, T. Determination of molecular formulas and structural regularities of low molecular weight fulvic acids by size-exclusion chromatography with electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- These, A.; Reemtsma, T. Structure-Dependent Reactivity of Low Molecular Weight Fulvic Acid Molecules during Ozonation. Environ. Sci. Technol. 2005, 39, 8382–8387. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, C.; Bergquist, J.; Sjöberg, P.J.R.; Tranvik, L.; Hawkes, J.A. Online HPLC-ESI-HRMS Method for the Analysis and Comparison of Different Dissolved Organic Matter Samples. Environ. Sci. Technol. 2018, 52, 2091–2099. [Google Scholar] [CrossRef]
- Patriarca, C.; Balderrama, A.; Može, M.; Sjöberg, P.J.R.; Bergquist, J.; Tranvik, L.J.; Hawkes, J.A. Investigating the Ionization of Dissolved Organic Matter by Electrospray. Anal. Chem. 2020, 92, 14210–14218. [Google Scholar] [CrossRef]
- Patriarca, C.; Hawkes, J.A. High Molecular Weight Spectral Interferences in Mass Spectra of Dissolved Organic Matter. J. Am. Soc. Mass Spectrom. 2021, 32, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Capley, E.N.; Tipton, J.D.; Marshall, A.G.; Stenson, A.C. Chromatographic reduction of isobaric and isomeric complexity of fulvic acids to enable multistage tandem mass spectral characterization. Anal. Chem. 2010, 82, 8194–8202. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Al-Othman, Z.A.; Nagae, N.; Gaitonde, V.D.; Dutta, K.K. Recent trends in ultra-fast HPLC: New generation superficially porous silica columns. J. Sep. Sci. 2012, 35, 3235–3249. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Al-Warthan, A.; Ali, I. Advances in enantiomeric resolution on monolithic chiral stationary phases in liquid chromatography and electrochromatography. J. Sep. Sci. 2014, 37, 1033–1057. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Ali, I. Comparison of the chiral resolution of econazole, miconazole, and sulconazole by HPLC using normal-phase amylose CSPs. Anal. Bioanal. Chem. 2001, 370, 951–955. [Google Scholar] [CrossRef]
- Qin, S.; Xu, C.; Guo, F.; Qi, J.; Xu, L.; Xu, Y.; Song, F. Molecular Signatures of Three Fulvic Acid Standard Samples as Revealed by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Technol. Environ. Sci. Mol. 2019, 4, 13940–13946. [Google Scholar] [CrossRef]
- Hawkes, J.A.; Andrilli, J.D.; Agar, J.N.; Barrow, M.P.; Berg, S.M.; Catalán, N.; Chen, H.; Chu, R.K.; Cole, R.B.; Dittmar, T.; et al. An international laboratory comparison of dissolved organic matter composition by high resolution mass spectrometry: Are we getting the same answer? Limnol. Oceanogr. Methods 2020, 18, 235–258. [Google Scholar] [CrossRef]
- Zherebker, A.; Kim, S.; Schmitt-Kopplin, P.; Spencer, R.G.M.; Lechtenfeld, O.; Podgorski, D.C.; Hertkorn, N.; Harir, M.; Nurfajin, N.; Koch, B.; et al. Interlaboratory comparison of humic substances compositional space as measured by Fourier transform ion cyclotron resonance mass spectrometry (IUPAC Technical Report). Pure Appl. Chem. 2020, 92, 1447–1467. [Google Scholar] [CrossRef]
- Thurman, E.M.; Malcolm, R.L. Preparative Isolation of Aquatic Humic Substances. Environ. Sci. Technol. 1981, 15, 463–466. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical Method for Analysis of Ultrahigh-Resolution Broadband Mass Spectra of Natural Organic Matter, the Van Krevelen Diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- Stenson, A.C.; Marshall, A.G.; Cooper, W.T. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal. Chem. 2003, 75, 1275–1284. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, F.; Xing, B.; Meng, W.; Shi, G.; Giesy, J.P. Isolation and Characterization of Chinese Standard Fulvic Acid Sub-fractions Separated from Forest Soil by Stepwise Elution with Pyrophosphate. Sci. Rep. 2015, 5, 8723. [Google Scholar] [CrossRef]
- Reemtsma, T.; These, A.; Springer, A.; Linscheid, M. Fulvic Acids as Transition State of Organic Matter: Indications from High Resolution Mass Spectrometry. Environ. Sci. Technol. 2006, 40, 5839–5845. [Google Scholar] [CrossRef]
- Morris, E.; Cawley, K.M.; Mcknight, D.M.; Miller, P.; Cory, R. Characterization of fulvic acid fractions of dissolved organic matter during ice-out in a hyper-eutrophic, coastal pond in Antarctica. Environ. Res. Lett. 2013, 8, 045015. [Google Scholar] [CrossRef]
- Terjesen, B.F.; Summerfelt, S.T.; Nerland, S.; Ulgenes, Y.; Fjæra, S.O.; Megård Reiten, B.K.; Selset, R.; Kolarevic, J.; Brunsvik, P.; Bæverfjord, G.; et al. Design, dimensioning, and performance of a research facility for studies on the requirements of fish in RAS environments. Aquac. Eng. 2013, 54, 49–63. [Google Scholar] [CrossRef]
- Rho, H.; Chon, K.; Park, J.; Cho, J. Rapid and Effective Isolation of Dissolved Organic Matter Using Solid-Phase Extraction Cartridges Packed with Amberlite XAD 8/4 Resins. Water 2019, 11, 67. [Google Scholar] [CrossRef]
- Corilo, Y.E.; Rodgers, R.P.; Marshall, A.G. PetroOrg Software; Florida State University: Tallahassee, FL, USA, 2014. [Google Scholar]
- Waters Corporation. Classifying Petroleum Samples with Waters SYNAPT HDMS and Omics LLC PetroOrg Petroleomics Software; Waters Corporation: Milford, MA, USA, 2014; Available online: https://www.waters.com/webassets/cms/library/docs/720005061en.pdf (accessed on 1 April 2022).
- Patriarca, C.; Sedano-Núñez, V.T.; Garcia, S.L.; Bergquist, J.; Bertilsson, S.; Sjöberg, P.J.R.; Tranvik, L.J.; Hawkes, J.A. Character and environmental lability of cyanobacteria-derived dissolved organic matter. Limnol. Oceanogr. 2021, 66, 496–509. [Google Scholar] [CrossRef]
- Koch, B.P.; Dittmar, T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20, 926–932. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Dittmar, T.; Kothawala, D.N.; Tranvik, L.J. Chemodiversity of dissolved organic matter in lakes driven by climate and hydrology. Nat. Commun. 2014, 5, 3804. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Elvert, M.; Koch, B.P.; Witt, M.; Hinrichs, K.U. Molecular characterization of dissolved organic matter in pore water of continental shelf sediments. Geochim. Cosmochim. Acta 2009, 73, 3337–3358. [Google Scholar] [CrossRef]
- Zherebker, A.Y.; Rukhovich, G.D.; Kharybin, O.N.; Fedoros, E.I.; Perminova, I.V.; Nikolaev, E.N. Fourier transform ion cyclotron resonance mass spectrometry for the analysis of molecular composition and batch-to-batch consistency of plant-derived polyphenolic ligands developed for biomedical application. Rapid Commun. Mass Spectrom. 2020, 34, e8850. [Google Scholar] [CrossRef] [PubMed]
- Mangal, V.; Stock, N.L.; Guéguen, C. Molecular characterization of phytoplankton dissolved organic matter (DOM) and sulfur components using high resolution Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 1891–1900. [Google Scholar] [CrossRef]
- Khreptugova, A.N.; Mikhnevich, T.A.; Molodykh, A.A.; Melnikova, S.V.; Konstantinov, A.I.; Rukhovich, G.D.; Volikov, A.B.; Perminova, I.V. Comparative Studies on Sorption Recovery and Molecular Selectivity of Bondesil PPL versus Bond Elut PPL Sorbents with Regard to Fulvic Acids. Water 2021, 13, 3553. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Dong, Y.; Li, W. Selectivity of solid phase extraction for dissolved organic matter in the hypersaline da Qaidam Lake, China. Environ. Sci. Process. Impacts 2017, 19, 1374–1386. [Google Scholar] [CrossRef]
- Peuravuori, J.; Lehtonen, T.; Pihlaja, K. Sorption of aquatic humic matter by DAX-8 and XAD-8 resins Comparative study using pyrolysis gas chromatography. Anal. Chim. Acta 2002, 471, 219–226. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Bloom, P.R.; Sleighter, R.L.; Cory, R.M.; Khwaja, A.R.; Hatcher, P.G. Chemical differences of aquatic humic substances extracted by XAD-8 and DEAE-cellulose. J. Environ. Chem. Eng. 2015, 3, 2982–2990. [Google Scholar] [CrossRef]
- Li, Y.; Harir, M.; Lucio, M.; Kanawati, B.; Smirnov, K.; Flerus, R.; Koch, B.P.; Schmitt-Kopplin, P.; Hertkorn, N. Proposed Guidelines for Solid Phase Extraction of Suwannee River Dissolved Organic Matter. Anal. Chem. 2016, 88, 6680–6688. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.A.; Dittmar, T.; Patriarca, C.; Tranvik, L.; Bergquist, J. Evaluation of the Orbitrap Mass Spectrometer for the Molecular Fingerprinting Analysis of Natural Dissolved Organic Matter. Anal. Chem. 2016, 88, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Roth, V.N.; Dittmar, T.; Gleixner, G. Molecular Signals of Heterogeneous Terrestrial Environments Identified in Dissolved Organic Matter: A Comparative Analysis of Orbitrap and Ion Cyclotron Resonance Mass Spectrometers. Front. Earth Sci. 2018, 6, 138. [Google Scholar] [CrossRef]
- Green, N.W.; Mcinnis, D.; Hertkorn, N.; Maurice, P.A.; Perdue, E.M. Suwannee River Natural Organic Matter: Isolation of the 2R101N Reference Sample by Reverse Osmosis. Environ. Eng. Sci. 2015, 32, 38–44. [Google Scholar] [CrossRef]
- Raeke, J.; Lechtenfeld, O.J.; Wagner, M.; Reemtsma, T. Selectivity of solid phase extraction of freshwater dissolved organic matter and its effect on ultrahigh resolution mass spectra. Environ. Sci. Process. Impacts 2016, 18, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, U.J.; Geuer, J.K.; Lechtenfeld, O.J.; Koch, B.P.; Murphy, K.R.; Stedmon, C.A. Quantifying the impact of solid-phase extraction on chromophoric dissolved organic matter composition. Mar. Chem. 2018, 207, 33–41. [Google Scholar] [CrossRef]


| Samples | Identified Formulas | H/Cwa | O/Cwa | m/zwa | AIwa |
|---|---|---|---|---|---|
| SRFA | 343 | 1.21 | 0.46 | 374.3 | 0.29 |
| ESFA | 148 | 1.18 | 0.53 | 320.7 | 0.32 |
| PPFA | 69 | 1.37 | 0.35 | 582.6 | 0.24 |
| RASFA | 440 | 1.30 | 0.42 | 387.3 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Alarcón, P.; Gonzalez, S.V.; Asimakopoulos, A.G.; Mikkelsen, Ø. Deciphering the Composition of Fulvic Acids from Recirculating Aquaculture Systems by Quadrupole Time-of-Flight Mass Spectrometry. Water 2022, 14, 2549. https://doi.org/10.3390/w14162549
Aguilar-Alarcón P, Gonzalez SV, Asimakopoulos AG, Mikkelsen Ø. Deciphering the Composition of Fulvic Acids from Recirculating Aquaculture Systems by Quadrupole Time-of-Flight Mass Spectrometry. Water. 2022; 14(16):2549. https://doi.org/10.3390/w14162549
Chicago/Turabian StyleAguilar-Alarcón, Patricia, Susana V. Gonzalez, Alexandros G. Asimakopoulos, and Øyvind Mikkelsen. 2022. "Deciphering the Composition of Fulvic Acids from Recirculating Aquaculture Systems by Quadrupole Time-of-Flight Mass Spectrometry" Water 14, no. 16: 2549. https://doi.org/10.3390/w14162549
APA StyleAguilar-Alarcón, P., Gonzalez, S. V., Asimakopoulos, A. G., & Mikkelsen, Ø. (2022). Deciphering the Composition of Fulvic Acids from Recirculating Aquaculture Systems by Quadrupole Time-of-Flight Mass Spectrometry. Water, 14(16), 2549. https://doi.org/10.3390/w14162549








