Coupling of Advanced Oxidation Technologies and Biochar for the Removal of Dyes in Water
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Removal of Dyes by Biochar
4.1.1. Production of Biochar
4.1.2. Dye Removal by Adsorption Using Biochar
| Biomass | Thermal Decomposition Method | Dye | Optimal Operation Conditions | Removal Efficiency | Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Chrysanthemum morifolium Ramat straw | Hydrothermal carbonization 220 °C | Basic red 46 | pH = 10; [Biochar] = 0.03 g; t = 120 min | 53.19 mg/g | Electrostatic attraction and H-bonding-π-π interaction | [54] |
| Chromolaena odorata | Slow pyrolysis 800 °C for 3 h | Indigo carmine | [Biochar] = 30 mg T = 30 °C; t = 2 h | 98.8 mg/g | Physical adsorption and electrostatic attraction | [55] |
| Pinus patula wood | Gasification 700 °C, atmospheric air as gasification agent | Malachite green | pH =10; [Biochar] = 9.80 g/L; Biochar particle size =150–300 µm; t = 60 min; [dye] = 50 mg/L | >99.70% | Not specified | [56] |
| Date palm petioles | Slow pyrolysis 700 °C, 3 h of retention time | Crystal violet | pH = 7; T = 30 °C | 209 mg/g | Electrostatic attraction, pore-filling, H-bonding and π-π interaction | [13] |
| Groundnut shell | Slow pyrolysis 350 °C during 120 min | Basic red 09 | pH = 8; [Biochar] = 1 g/L; T = 35 °C | 46.3 mg/g | Ion exchange | [57] |
4.1.3. Biochar Regeneration and Final Disposal
4.2. Dye removal by Advanced Oxidation Processes
4.2.1. Fenton Process
Heterogeneous Fenton Process
Photo-Fenton Process
Electro-Fenton Process
Sono-Fenton Process
4.2.2. UV/H2O2 System
4.2.3. Photocatalysis and Sono-photocatalysis
4.2.4. Persulfate-Based AOPs
| Advanced Oxidation Process | Dye | Operation Conditions | Results | Ref. |
|---|---|---|---|---|
| Sono-photocatalysis with ZnO microparticles | Rhodamine B | λ = 554 nm; pH = 5.8; frequency = 59 kHz, [catalyst] = 0.5 g/L; [dye] = 2.5 mg/L |
| [78] |
| Heterogeneous sono-Fenton with magnetite (Fe3O4) nanoparticles | Basic violet 10 | pH = 3; [catalyst] = 1.5 g/L; [H2O2] = 36 mM; ultrasonic power = 450 W/L; [dye] = 30 mg/L |
| [115] |
| Heterogeneous sono-Fenton like with Fe3O4 nanoparticles | Reactive orange 107 (RO107) and real textile wastewater | pH = 5 (simulated water), 8.1 (real textile wastewater); [catalyst] = 0.8 g/L; [H2O2] = 10 mM; frequency = 24 kHz |
| [116] |
| Photo-Fenton | Congo red | pH = 3; λ = 507 nm; [Fe2+] = 10 mg/L; [H2O2] = 50 mg/L |
| [117] |
| Electro-Fenton | Reactive red 195 | pH = 3; [dye] = 50 mg/L; superficial oxygen velocity = 0.012 cm/s; t = 60 min; current density = 2 mA/cm2 |
| [118] |
| Sono-Fenton | Acid violet 7 | pH = 3; [Fe2+] = 10 mg/L; [H2O2] = 50 mg/L; [dye] = 20 mg/L; frequency = 40 kHz |
| [119] |
| Persulfate sono-catalysis with magnetic CaFe2O4 nanoparticles | Brilliant green (BG) | [Dye] = 50 mg/L; [catalyst] = 0.5 g/L; [persulfate] = 200 mg/L; frequency = 23 kHz; pH = 8.1 |
| [120] |
4.3. Coupling Biochar with AOPs for the Elimination of Dyes in Water
4.4. Biochar Modification
4.5. Biochar Reusability and Final Disposal
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of chitin and chitosan based biomaterials for the adsorptive removal of textile dyes from water-A comprehensive review. Carbohydr. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, E.; Butter, B.; Santander, P.; Sánchez, J. Polyelectrolytes applied to remove methylene blue and methyl orange dyes from water via polymer-enhanced ultrafiltration. J. Environ. Chem. Eng. 2021, 9, 106297. [Google Scholar] [CrossRef]
- Lei, S.; Wang, S.; Gao, B.; Zhan, Y.; Zhao, Q.; Jin, S.; Song, G.; Lyu, X.; Zhang, Y.; Tang, Y. Ultrathin dodecyl-sulfate-intercalated Mg-Al layered double hydroxide nanosheets with high adsorption capability for dye pollution. J. Colloid Interface Sci. 2020, 577, 181–190. [Google Scholar] [CrossRef]
- Garg, A.; Chopra, L. Dye Waste: A significant environmental hazard. Mater. Today Proc. 2021, 48, 1310–1315. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Danish, M.; Singh, R.S.; Rafatullah, M.; HPS, A.K. Exploiting microbial biomass in treating azo dyes contaminated wastewater: Mechanism of degradation and factors affecting microbial efficiency. J. Water Process Eng. 2021, 43, 102255. [Google Scholar] [CrossRef]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J. Water Process Eng. 2020, 37, 101455. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Ikhlaq, A.; Zahid, M.; Alazmi, A. Subcritical and supercritical water oxidation for dye decomposition. J. Environ. Manag. 2021, 290, 112605. [Google Scholar] [CrossRef]
- Çelekli, A.; Al-Nuaimi, A.I.; Bozkurt, H. Adsorption kinetic and isotherms of Reactive Red 120 on Moringa oleifera seed as an eco-friendly process. J. Mol. Struct. 2019, 1195, 168–178. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Binh, Q.A.; Nguyen, X.C.; Nguyen, T.T.H.; Vo, Q.N.; Nguyen, T.D.; Tran, T.C.P.; Nguyen, T.A.H.; Kim, S.Y.; Nguyen, T.P.; et al. Metal salt-modified biochars derived from agro-waste for effective congo red dye removal. Environ. Res. 2021, 200, 111492. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioesour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef]
- Chahinez, H.O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Serrano-Martínez, A.; Mercader-Ros, M.T.; Martínez-Alcalá, I.; Lucas-Abellán, C.; Gabaldón, J.A.; Gómez-López, V.M. Degradation and toxicity evaluation of azo dye Direct red 83:1 by an advanced oxidation process driven by pulsed light. J. Water Process Eng. 2020, 37, 101530. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.; Han, X.; Zhao, Q.; Wang, D.; Fu, F.; Liang, Y. Highly efficient visible-light-driven photo-Fenton catalytic performance over FeOOH/Bi2WO6 composite for organic pollutant degradation. J. Alloy. Compd. 2020, 816, 152560. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X.; et al. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, S.; Nguyen, T.T.; Gao, X.; Luo, S.; Guo, M. Biochar loaded on MnFe2O4 as Fenton catalyst for Rhodamine B removal: Characterizations, catalytic performance, process optimization and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127651. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Akre, A.P.; Sreedharan, V.; Kumar, M.S. Potential role of biochar in advanced oxidation processes: A sustainable approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Wang, J.; Cai, J.; Wang, S.; Zhou, X.; Ding, X.; Ali, J.; Zheng, L.; Wang, S.; Yang, L.; Xi, S.; et al. Biochar-based activation of peroxide: Multivariate-controlled performance, modulatory surface reactive sites and tunable oxidative species. Chem. Eng. J. 2021, 428, 131233. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Chon, K. Fenton oxidation of synthetic food dyes by Fe-embedded coffee biochar catalysts prepared at different pyrolysis temperatures: A mechanism study. Chem. Eng. J. 2021, 421, 129943. [Google Scholar] [CrossRef]
- Leichtweis, J.; Silvestri, S.; Carissimi, E. New composite of pecan nutshells biochar-ZnO for sequential removal of acid red 97 by adsorption and photocatalysis. Biomass Bioenergy 2020, 140, 105648. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Wang, T.; Mei, M.; Chen, S.; Li, J. Peroxydisulfate activation by digestate-derived biochar for azo dye degradation: Mechanism and performance. Sep. Purif. Technol. 2021, 279, 119687. [Google Scholar] [CrossRef]
- Urán-Duque, L.; Saldarriaga-Molina, J.C.; Rubio-Clemente, A. Advanced oxidation processes based on sulfate radicals for wastewater treatment: Research trends. Water 2021, 13, 2445. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Wang, X.; He, Y.; Zhang, C.; Xue, G.; Liu, Z.; Lao, H.; Song, H.; Chen, W.; et al. Dyeing and finishing wastewater treatment in China: State of the art and perspective. J. Clean. Prod. 2021, 326, 129353. [Google Scholar] [CrossRef]
- Diaz-Elsayed, N.; Rezaei, N.; Ndiaye, A.; Zhang, Q. Trends in the environmental and economic sustainability of wastewater-based resource recovery: A review. J. Clean. Prod. 2020, 265, 121598. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Murad, H.A.; Ahmad, M.; Bundschuh, J.; Hashimoto, Y.; Zhang, M.; Sarkar, B.; Ok, Y.S. A remediation approach to chromium-contaminated water and soil using engineered biochar derived from peanut shell. Environ. Res. 2021, 204, 112125. [Google Scholar] [CrossRef]
- Liu, M.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.; Zeng, G.; Zhu, Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2021, 806, 150531. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil–bottlenecks and scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Phytotoxic condensed organic compounds are common in fast but not slow pyrolysis biochars. Bioresour. Technol. Rep. 2021, 13, 100613. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application, and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Raja, J.D.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Gul, E.; Alrawashdeh, K.A.B.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Zaichenko, V. Properties of biochar obtained by hydrothermal carbonization and torrefaction of peat. Fuel 2019, 256, 115929. [Google Scholar] [CrossRef]
- Guo, F.; Jia, X.; Liang, S.; Zhou, N.; Chen, P.; Ruan, R. Development of biochar-based nanocatalysts for tar cracking/reforming during biomass pyrolysis and gasification. Bioresour. Technol. 2020, 298, 122263. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Mishra, R.; Kumar, S. Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci. Total Environ. 2021, 806, 150444. [Google Scholar] [CrossRef]
- Low, Y.W.; Yee, K.F. A review on lignocellulosic biomass waste into biochar-derived catalyst: Current conversion techniques, sustainable applications and challenges. Biomass Bioenergy 2021, 154, 106245. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, Z.; Diao, R.; Zhu, X. Combining torrefaction pretreatment and co-pyrolysis to upgrade biochar derived from bio-oil distillation residue and walnut shell. Energy Convers. Manag. 2019, 199, 111970. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Ong, H.C.; Show, P.L.; Ling, T.C.; Chen, W.H.; Yu, K.L.; Abdullah, R. Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent. Energy Convers. Manag. 2018, 165, 152–162. [Google Scholar] [CrossRef]
- Sbizzaro, M.; Sampaio, S.C.; dos Reis, R.R.; de Assis Beraldi, F.; Rosa, D.M.; de Freitas Maia, C.M.B.; de Carvalho, C.; do Nascimento, C.T.; da Silva, E.A.; Borba, C.E. Effect of production temperature in biochar properties from bamboo culm and its influences on atrazine adsorption from aqueous systems. J. Mol. Liq. 2021, 343, 117667. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Huang, H.; Xiao, R.; Li, R.; Zhang, Z. Influence of temperature and residence time on characteristics of biochars derived from agricultural residues: A comprehensive evaluation. Process Saf. Environ. Prot. 2020, 139, 218–229. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning oxygenated functional groups on biochar for water pollution control: A critical review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advanced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Song, G.; Qin, F.; Yu, J.; Tang, L.; Pang, Y.; Zhang, C.; Wang, J.; Deng, L. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2021, 424, 127663. [Google Scholar] [CrossRef] [PubMed]
- Barquilha, C.E.; Braga, M.C. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Gnansounou, E.; Chaturvedi, P. Biochar and environmental sustainability: Emerging trends and techno-economic perspectives. Bioresour. Technol. 2021, 332, 125102. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Song, Y.; Zhuang, H.; Tang, H. Removal of cationic dye BR46 by biochar prepared from Chrysanthemum morifolium Ramat straw: A study on adsorption equilibrium, kinetics and isotherm. J. Mol. Liq. 2021, 340, 116617. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Isotherm, kinetics, thermodynamics studies and effects of carbonization temperature on adsorption of Indigo Carmine (IC) dye using C. odorata biochar. Chem. Data Collect. 2021, 33, 100673. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Gutiérrez, J.; Henao, H.; Melo, A.M.; Pérez, J.F.; Chica, E. Adsorption capacity of the biochar obtained from Pinus patula wood micro-gasification for the treatment of polluted water containing malachite green dye. J. King Saud Univ. Eng. Sci. 2021. In press. [Google Scholar] [CrossRef]
- Praveen, S.; Gokulan, R.; Pushpa, T.B.; Jegan, J. Techno-economic feasibility of biochar as biosorbent for basic dye sequestration. J. Indian Chem. Soc. 2021, 98, 100107. [Google Scholar] [CrossRef]
- Gokulan, R.; Avinash, A.; Prabhu, G.G.; Jegan, J. Remediation of remazol dyes by biochar derived from Caulerpa scalpelliformis—An eco-friendly approach. J. Environ. Chem. Eng. 2019, 7, 103297. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Highly efficient adsorbent for removal of crystal violet dye from aqueous solution by CaAl/LDH supported on biochar. Appl. Clay Sci. 2021, 214, 106297. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; da Gama, B.M.V.; da Silva Gonçalves, A.H.; Medeiros, J.A.; de Souza Abud, A.K. Basic-dye adsorption in albedo residue: Effect of pH, contact time, temperature, dye concentration, biomass dosage, rotation and ionic strength. J. King Saud Univ. Eng. Sci. 2020, 32, 351–359. [Google Scholar]
- Hanumanthu, J.R.; Ravindiran, G.; Subramanian, R.; Saravanan, P. Optimization of process conditions using RSM and ANFIS for the removal of remazol brilliant orange 3R in a packed bed column. J. Indian Chem. Soc. 2021, 98, 100086. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.; Luo, X. Enhanced adsorption of rhodamine B from water by Fe-N co-modified biochar: Preparation, performance, mechanism and reusability. Bioresour. Technol. 2021, 343, 126103. [Google Scholar] [CrossRef]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, J.; Feng, P.; Huang, G.; Xu, C.; Lin, B. High-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar. Chemosphere 2020, 246, 125734. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, F.; Chen, H.; Wang, H.; Li, G. Fe2O3/TiO2 functionalized biochar as a heterogeneous catalyst for dyes degradation in water under Fenton processes. J. Environ. Chem. Eng. 2020, 8, 103905. [Google Scholar] [CrossRef]
- Zazycki, M.A.; Borba, P.A.; Silva, R.N.; Peres, E.C.; Perondi, D.; Collazzo, G.C.; Dotto, G.L. Chitin derived biochar as an alternative adsorbent to treat colored effluents containing methyl violet dye. Adv. Powder Technol. 2019, 30, 1494–1503. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Choi, Y.K.; Kim, H.J.; Song, H.S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Koh, J.; et al. Application of macroalgal biomass derived biochar and bioelectrochemical system with Shewanella for the adsorptive removal and biodegradation of toxic azo dye. Chemosphere 2021, 264, 128539. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Gautam, R.K.; Goswami, M.; Mishra, R.K.; Chaturvedi, P.; Awashthi, M.K.; Singh, R.S.; Giri, B.S.; Pandey, A. Biochar for remediation of agrochemicals and synthetic organic dyes from environmental samples: A review. Chemosphere 2021, 272, 129917. [Google Scholar] [CrossRef] [PubMed]
- Ramrakhiani, L.; Halder, A.; Majumder, A.; Mandal, A.K.; Majumdar, S.; Ghosh, S. Industrial waste derived biosorbent for toxic metal remediation: Mechanism studies and spent biosorbent management. Chem. Eng. J. 2017, 308, 1048–1064. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment-unexpected nitration side reactions-a serious environmental issue: A review. Chem. Eng. J. 2021, 430, 133002. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction A. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Ramos, R.O.; Albuquerque, M.V.; Lopes, W.S.; Sousa, J.T.; Leite, V.D. Degradation of indigo carmine by photo-Fenton, Fenton, H2O2/UV-C and direct UV-C: Comparison of pathways, products and kinetics. J. Water Process Eng. 2020, 37, 101535. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnadez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro-and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Anushree, C.; Krishna, D.N.G.; Philip, J. Efficient dye degradation via catalytic persulfate activation using iron oxide-manganese oxide core-shell particle doped with transition metal ions. J. Mol. Liq. 2021, 337, 116429. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Santana, C.S.; Velloso, C.C.V.; da Silva, A.H.M.; Magalhães, F.; Aguiar, A. A review on the treatment of textile industry effluents through Fenton processes. Process Saf. Environ. Prot. 2021, 155, 366–386. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Rial, J.B.; Ferreira, M.L. Challenges of dye removal treatments based on IONzymes: Beyond heterogeneous Fenton. J. Water Process Eng. 2021, 41, 102065. [Google Scholar] [CrossRef]
- Ahile, U.J.; Wuana, R.A.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total Environ. 2020, 710, 134872. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M. Advanced oxidation processes and nanomaterials-a review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. RECENT trends and developments in Fenton processes for industrial wastewater treatment–A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Scaria, J.; Gopinath, A.; Nidheesh, P.V. A versatile strategy to eliminate emerging contaminants from the aqueous environment: Heterogeneous Fenton process. J. Clean. Prod. 2021, 278, 124014. [Google Scholar] [CrossRef]
- Lai, C.; Shi, X.; Li, L.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; Yi, H.; Qin, L.; Zhang, M.; et al. Enhancing iron redox cycling for promoting heterogeneous Fenton performance: A review. Sci. Total Environ. 2021, 775, 145850. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H. Chemical kinetic modeling of organic pollutant degradation in Fenton and solar photo-Fenton processes. J. Taiwan Inst. Chem. Eng. 2021, 123, 175–184. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S. Photo-Fenton applied to the removal of pharmaceutical and other pollutants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar] [CrossRef]
- Ismail, S.A.; Ang, W.L.; Mohammad, A.W. Electro-Fenton technology for wastewater treatment: A bibliometric analysis of current research trends, future perspectives and energy consumption analysis. J. Water Process Eng. 2021, 40, 101952. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Xiao, F.; Postole, G.; Zhao, H.; Zhao, G. Recent advances and trends of heterogeneous electro-Fenton process for wastewater treatment-review. Chin. Chem. Lett. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- Divyapriya, G.; Scaria, J.; Singh, T.A.; Nidheesh, P.V.; Babu, D.S.; Kumar, M.S. Advanced treatment of real wastewater effluents by an electrochemical approach. In Water Pollution and Remediation: Heavy Metals; Springer: Cham, Switzerland, 2021; pp. 85–122. [Google Scholar]
- Casado, J. Towards industrial implementation of Electro-Fenton and derived technologies for wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 102823. [Google Scholar] [CrossRef]
- de Andrade, F.V.; Augusti, R.; de Lima, G.M. Ultrasound for the remediation of contaminated waters with persistent organic pollutants: A short review. Ultrason. Sonochem. 2021, 78, 105719. [Google Scholar] [CrossRef]
- Camargo-Perea, A.L.; Rubio-Clemente, A.; Peñuela, G.A. Use of ultrasound as an advanced oxidation process for the degradation of emerging pollutants in water. Water 2020, 12, 1068. [Google Scholar] [CrossRef]
- Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.Q.; Ho, S.H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2020, 409, 128207. [Google Scholar]
- Prakash, L.V.; Gopinath, A.; Gandhimathi, R.; Velmathi, S.; Ramesh, S.T.; Nidheesh, P.V. Ultrasound aided heterogeneous Fenton degradation of Acid Blue 15 over green synthesized magnetite nanoparticles. Sep. Purif. Technol. 2021, 266, 118230. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Mousavi-Tashar, A.; Masoumi, Z.; Azarian, G. Integrated advanced oxidation process, sono-Fenton treatment, for mineralization and volume reduction of activated sludge. Ecotoxicol. Environ. Saf. 2019, 168, 120–126. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Korpe, S.; Rao, P.V. Application of advanced oxidation processes and cavitation techniques for treatment of tannery wastewater-A review. J. Environ. Chem. Eng. 2021, 9, 105234. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Recent advancements in the sonophotocatalysis (SPC) and doped-sonophotocatalysis (DSPC) for the treatment of recalcitrant hazardous organic water pollutants. Ultrason. Sonochem. 2017, 36, 481–496. [Google Scholar] [CrossRef]
- Anandan, S.; Ponnusamy, V.K.; Ashokkumar, M. A review on hybrid techniques for the degradation of organic pollutants in aqueous environment. Ultrason. Sonochem. 2020, 67, 105130. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, N. A systematic review of the sonophotocatalytic process for the decolorization of dyes in aqueous solution: Synergistic mechanisms, degradation pathways, and process optimization. J. Water Process Eng. 2021, 44, 102314. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A comprehensive review on sonocatalytic, photocatalytic, and sonophotocatalytic processes for the degradation of antibiotics in water: Synergistic mechanism and degradation pathway. Chem. Eng. J. 2021, 413, 127412. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Abramova, A.V.; Cravotto, G. Sonochemical processes for the degradation of antibiotics in aqueous solutions: A review. Ultrason. Sonochem. 2021, 74, 105566. [Google Scholar] [CrossRef]
- Leite, C.; Coppola, F.; Monteiro, R.; Russo, T.; Polese, G.; Silva, M.R.; Lourenco, M.; Ferreira, P.; Soares, A.; Pereira, E.; et al. Toxic impacts of rutile titanium dioxide in Mytilus galloprovincialis exposed to warming conditions. Chemosphere 2020, 252, 126563. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Ge, L.; Shao, B.; Liang, Q.; Huang, D.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; et al. Layered double hydroxide based materials applied in persulfate based advanced oxidation processes: Property, mechanism, application and perspectives. J. Hazard. Mater. 2021, 424, 127612. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Hassani, A.; Karaca, C.; Karaca, S.; Khataee, A.; Açışlı, Ö.; Yılmaz, B. Enhanced removal of basic violet 10 by heterogeneous sono-Fenton process using magnetite nanoparticles. Ultrason. Sonochem. 2018, 42, 390–402. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Takdastan, A.; Jorfi, S.; Ghanbari, F.; Ahmadi, M.; Barzegar, G. The performance study on ultrasonic/Fe3O4/H2O2 for degradation of azo dye and real textile wastewater treatment. J. Mol. Liq. 2018, 256, 462–470. [Google Scholar] [CrossRef]
- Masalvad, S.K.S.; Sakare, P.K. Application of photo Fenton process for treatment of textile Congo-red dye solution. Mater. Today Proc. 2021, 46, 5291–5297. [Google Scholar] [CrossRef]
- Elbatea, A.A.; Nosier, S.A.; Zatout, A.A.; Hassan, I.; Sedahmed, G.H.; Abdel-Aziz, M.H.; El-Naggar, M.A. Removal of reactive red 195 from dyeing wastewater using electro-Fenton process in a cell with oxygen sparged fixed bed electrodes. J. Water Process Eng. 2021, 41, 102042. [Google Scholar] [CrossRef]
- Kodavatiganti, S.; Bhat, A.P.; Gogate, P.R. Intensified degradation of Acid Violet 7 dye using ultrasound combined with hydrogen peroxide, Fenton, and persulfate. Sep. Purif. Technol. 2021, 279, 119673. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Tripathy, B.K.; Debnath, A.; Kumar, M. Enhanced persulfate activated sono-catalytic degradation of brilliant green dye by magnetic CaFe2O4 nanoparticles: Degradation pathway study, assessment of bio-toxicity and cost analysis. Surf. Interfaces 2021, 26, 101412. [Google Scholar] [CrossRef]
- Luo, K.; Pang, Y.; Wang, D.; Li, X.; Wang, L.; Lei, M.; Huang, Q.; Yang, Q. A critical review on the application of biochar in environmental pollution remediation: Role of persistent free radicals (PFRs). J. Environ. Sci. 2021, 108, 201–216. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Yi, Y.; Luo, J.; Fang, Z. Magnetic biochar derived from Eichhornia crassipes for highly efficient Fenton-like degradation of antibiotics: Mechanism and contributions. J. Environ. Chem. Eng. 2021, 9, 106258. [Google Scholar] [CrossRef]
- da Silva Medeiros, D.C.C.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; El-Din, M.G. Pristine and engineered biochar for the removal of contaminants co-existing in several types of industrial wastewaters: A critical review. Sci. Total Environ. 2021, 809, 151120. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.P.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Gasim, M.F.M.; Lim, J.W.; Low, S.C.; Lin, K.Y.A.; Oh, W.D. Can biochar and hydrochar be used as sustainable catalyst for persulfate activation? Chemosphere 2021, 287, 132458. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Wang, P.; Liu, T.; Ahmad, M.; Zhang, T.; Guo, J.; Xiao, H.; Song, J. Highly-efficient nitrogen self-doped biochar for versatile dyes’ removal prepared from soybean cake via a simple dual-templating approach and associated thermodynamics. J. Clean. Prod. 2022, 332, 130069. [Google Scholar] [CrossRef]
- Binda, G.; Faccini, D.; Zava, M.; Pozzi, A.; Dossi, C.; Monticelli, D.; Spanu, D. Exploring the adsorption of Pb on microalgae-derived biochar: A versatile material for environmental remediation and electroanalytical applications. Chemosensors 2022, 10, 168. [Google Scholar] [CrossRef]
- Ling, L.L.; Liu, W.J.; Zhang, S.; Jiang, H. Magnesium oxide embedded nitrogen self-doped biochar composites: Fast and high-efficiency adsorption of heavy metals in an aqueous solution. Environ. Sci. Technol. 2017, 51, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, R.; Huang, R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021, 297, 126681. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, H.J.; Kim, H.W.; Lim, H. Iron-impregnated spent coffee ground biochar for enhanced degradation of methylene blue during cold plasma application. J. Ind. Eng. Chem. 2021, 98, 383–388. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Q.; Wang, Z.; Gao, B.; Fan, Z.; Li, M.; Hao, H.; Wei, X.; Zhong, M. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study. Sci. Total Environ. 2021, 758, 143584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L.; et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe–Mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef]
- Sutar, S.; Otari, S.; Jadhav, J. Biochar based photocatalyst for degradation of organic aqueous waste: A review. Chemosphere 2021, 287, 132200. [Google Scholar] [CrossRef]
- Zhao, Y.; Qamar, S.A.; Qamar, M.; Bilal, M.; Iqbal, H.M. Sustainable remediation of hazardous environmental pollutants using biochar-based nanohybrid materials. J. Environ. Manag. 2021, 300, 113762. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Rehman, M.S.U.; Faisal, A.; Rehman, F. Integrating adsorption and photocatalysis: A cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X. Treatment of wastewater containing Reactive Brilliant Blue KN-R using TiO2/BC composite as heterogeneous photocatalyst and adsorbent. Chemosphere 2018, 206, 777–783. [Google Scholar] [CrossRef]
- Pinna, M.; Binda, G.; Altomare, M.; Marelli, M.; Dossi, C.; Monticelli, D.; Spanu, D.; Recchia, S. Biochar nanoparticles over TiO2 nanotube arrays: A green co-catalyst to boost the photocatalytic degradation of organic pollutants. Catalysts 2021, 11, 1048. [Google Scholar] [CrossRef]
- Longfei, Z.; Siqun, W.; Zhilin, C.; Yamei, W. Preparation of wood functional coatings with carbon dots grafted TiO2 for its photocatalytic degradation of formaldehyde gas. J. Beiging For. Univ. 2022, 44, 113–122. [Google Scholar]
- Chen, Q.; Lin, G.; Meng, L.; Zhou, L.; Hu, L.; Nong, J.; Li, Y.; Wang, J.; Yu, Q. Enhanced photoelectric performance of TiO2 nanotubes sensitized with carbon dots derived from bagasse. Chem. Phys. Lett. 2020, 749, 137428. [Google Scholar] [CrossRef]
- Gonçalves, M.G.; da Silva Veiga, P.A.; Fornari, M.R.; Peralta-Zamora, P.; Mangrich, A.S.; Silvestri, S. Relationship of the physicochemical properties of novel ZnO/biochar composites to their efficiencies in the degradation of sulfamethoxazole and methyl orange. Sci. Total Environ. 2020, 748, 141381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef] [PubMed]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar–based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process. Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, Q.; Qiu, Y.; Meng, L.; Wei, X.; Sang, W.; Tao, J. Insight into the degradation of Orange G by persulfate activated with biochar modified by iron and manganese oxides: Synergism between Fe and Mn. J. Water Process Eng. 2020, 37, 101470. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, S.; Li, X.; Zhang, S.; Nguyen, T.T.; Guo, M.; Gao, X. Ultrasound-assisted heterogeneous Fenton-like process for methylene blue removal using magnetic MnFe2O4/biochar nanocomposite. Appl. Surf. Sci. 2021, 566, 150654. [Google Scholar] [CrossRef]
- Silvestri, S.; Goncalves, M.G.; da Silva Veiga, P.A.; da Silva Matos, T.T.; Peralta-Zamora, P.; Mangrich, A.S. TiO2 supported on Salvinia molesta biochar for heterogeneous photocatalytic degradation of Acid Orange 7 dye. J. Environ. Chem. Eng. 2019, 7, 102879. [Google Scholar] [CrossRef]
- Rubeena, K.K.; Reddy, P.H.P.; Laiju, A.R.; Nidheesh, P.V. Iron impregnated biochars as heterogeneous Fenton catalyst for the degradation of acid red 1 dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef] [PubMed]
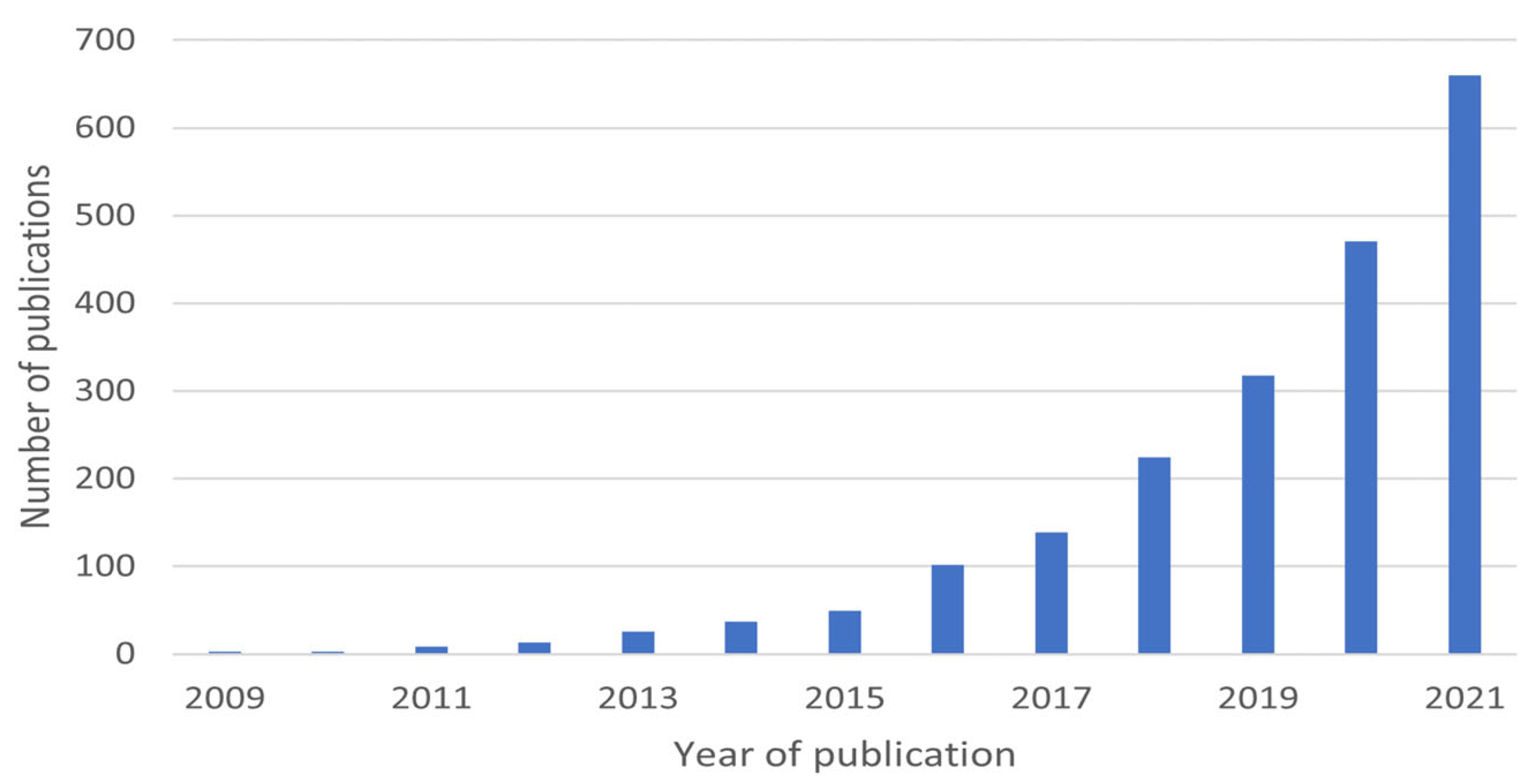

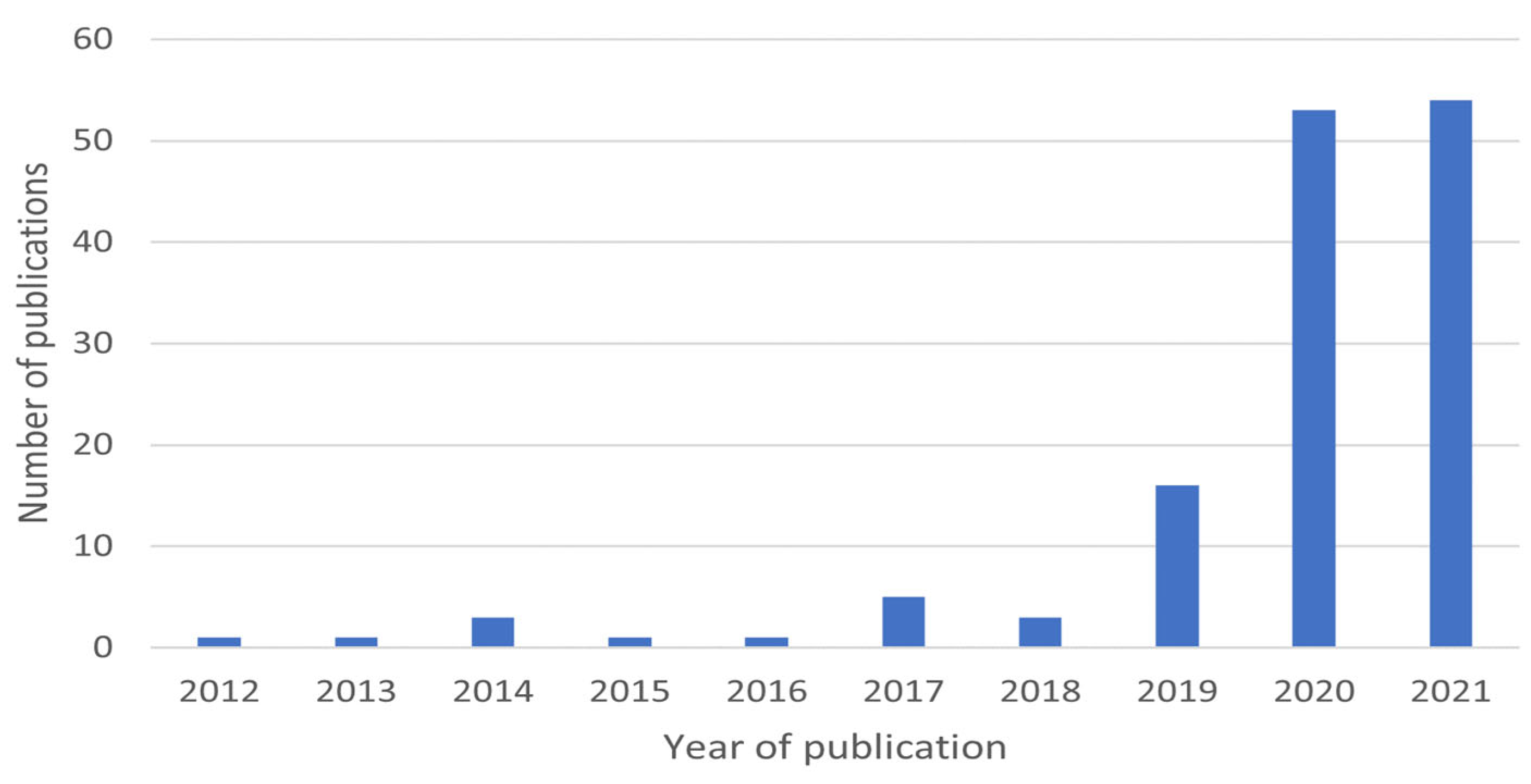
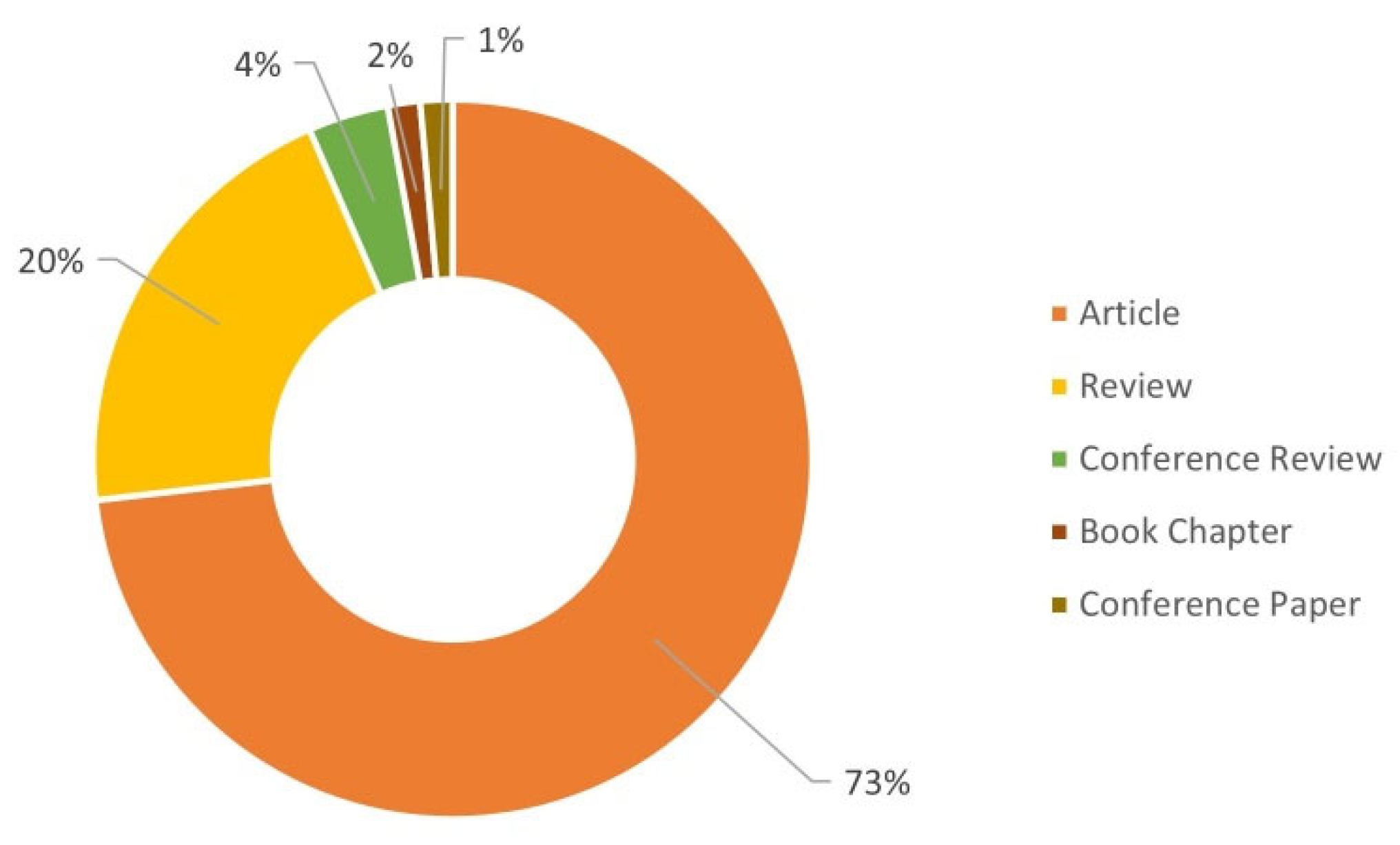
| Process | Dye | Operation Conditions | Results | Reference |
|---|---|---|---|---|
| Fe-biochar, heterogeneous Fenton process | Amaranth (AM) Sunset yellow (SY) | Biochar: slow pyrolysis, waste coffee grounds, 700 °C, biomass treated with Fe(III) chloride (FeCl3) pH = 3.0; [catalyst] = 0.4 g/L; [H2O2] = 5.0 mM; T = 25 °C; t = 60 min |
| [21] |
| Photocatalyst with ZnO-biochar | Reactive red 97 | Biochar preparation: Fast pyrolysis, pecan nutshell, 800 °C, biomass treated with ZnO before pyrolysis pH = 7; t = 67 min |
| [22] |
| Persulfate-AOP with biochar | Reactive brilliant red X-3B | Biochar preparation: pyrolysis, food waste digestate, 800 °C pH = 3.78; [biochar] = 0.5 g/L; [PDS/PS] = 1.5 mM; T = 25 °C; t = 30 min |
| [23] |
| Fe-biochar heterogeneous Fenton-like process | Rhodamine B | Biochar preparation: slow pyrolysis, sawdust, 600 °C, biochar treated with a Fe3+ solution and pyrolyzed at 900 °C pH = 6.5; [biochar] = 2.0 g/L; [H2O2] = 4.0 mM; [dye] = 10 mg/L; T = 30 °C |
| [133] |
| Photocatalyst with ZnO-biochar | Methylene blue | Biochar preparation: slow pyrolysis, bamboo stakes, 600 °C, ball milling for ZnO-biochar composite pH = 6.0; [biochar] = 1.0 g/L; [dye] = 160 mg/L; λ = 665 nm; t = 225 min |
| [16] |
| MnFe2O4-biochar, heterogeneous Fenton process | Rhodamine B | Biochar preparation: slow pyrolysis, poplar wood flour, 600 °C, biochar treated with a FeCl3, manganese sulfate (MnSO4) and sodium hydroxide (NaOH) solution, and dried to obtain Fe/Mn-biochar pH = 4.8; [biochar] = 0.6 g/L; [H2O2] = 115 mM |
| [17] |
| Persulfate-AOP with Mn/Fe-biochar | Orange G | Biochar preparation: slow pyrolysis, sludge, 600 °C, biochar treated with a solution of ferric chloride hexahydrate (FeCl3·6H2O)/Mg(II) chloride (MnCl2·4H2O), pyrolyzed again and treated with ball milling pH = 9; [biochar] = 0.4 g/L; [PMS] = 6 mM; [dye] = 1500 mg/L; T = 25 °C; t = 24 h |
| [148] |
| MnFe2O4-biochar, heterogeneous sono-Fenton-like | Methylene blue | Biochar preparation: slow pyrolysis, poplar wood powder, 250 °C, biochar treated with a FeCl3, MnSO4 and NaOH solution, followed by heating pH = 5; [biochar] = 0.7 g/L; [H2O2] = 15 mmol/L; [dye] = 20 mg/L; frequency = 20 kHz; T = 25 °C; t = 20 min |
| [149] |
| Photocatalyst with TiO2-biochar | Acid orange 7 | Biochar preparation: slow pyrolysis, Salvinia molesta, 350 °C, biomass pretreated with titanyl sulfate TiOSO4 and titanium isopropoxide (C12H28O4Ti) [Biochar] = 20 mg/L; λ = 380–480 nm |
| [150] |
| Fe-biochar, heterogeneous Fenton | Acid red 1 | Biochar preparation: slow pyrolysis, coir pith, 700 °C, biochar treated with a solution of Fe(III) nitrate nonahydrate (Fe (NO3)3·9H2O) and heated again pH = 3; [biochar] = 4 g/L; [H2O2] = 16 mM; [dye] = 50 mg/L |
| [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Coupling of Advanced Oxidation Technologies and Biochar for the Removal of Dyes in Water. Water 2022, 14, 2531. https://doi.org/10.3390/w14162531
Gallego-Ramírez C, Chica E, Rubio-Clemente A. Coupling of Advanced Oxidation Technologies and Biochar for the Removal of Dyes in Water. Water. 2022; 14(16):2531. https://doi.org/10.3390/w14162531
Chicago/Turabian StyleGallego-Ramírez, Carolina, Edwin Chica, and Ainhoa Rubio-Clemente. 2022. "Coupling of Advanced Oxidation Technologies and Biochar for the Removal of Dyes in Water" Water 14, no. 16: 2531. https://doi.org/10.3390/w14162531






