Effects of Temperature on the Growth Performance, Biochemical Indexes and Growth and Development-Related Genes Expression of Juvenile Hybrid Sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experimental Design
2.3. Sample Collection
2.4. Determination of SGR and RWG
RWG = 100% × (W2 − W1)/W1
2.5. Determination of Plasma Biochemical Indexes and Hormones
2.6. RNA Extraction and cDNA Synthesis
2.7. Gene Expression
2.8. Statistical Analysis
3. Results
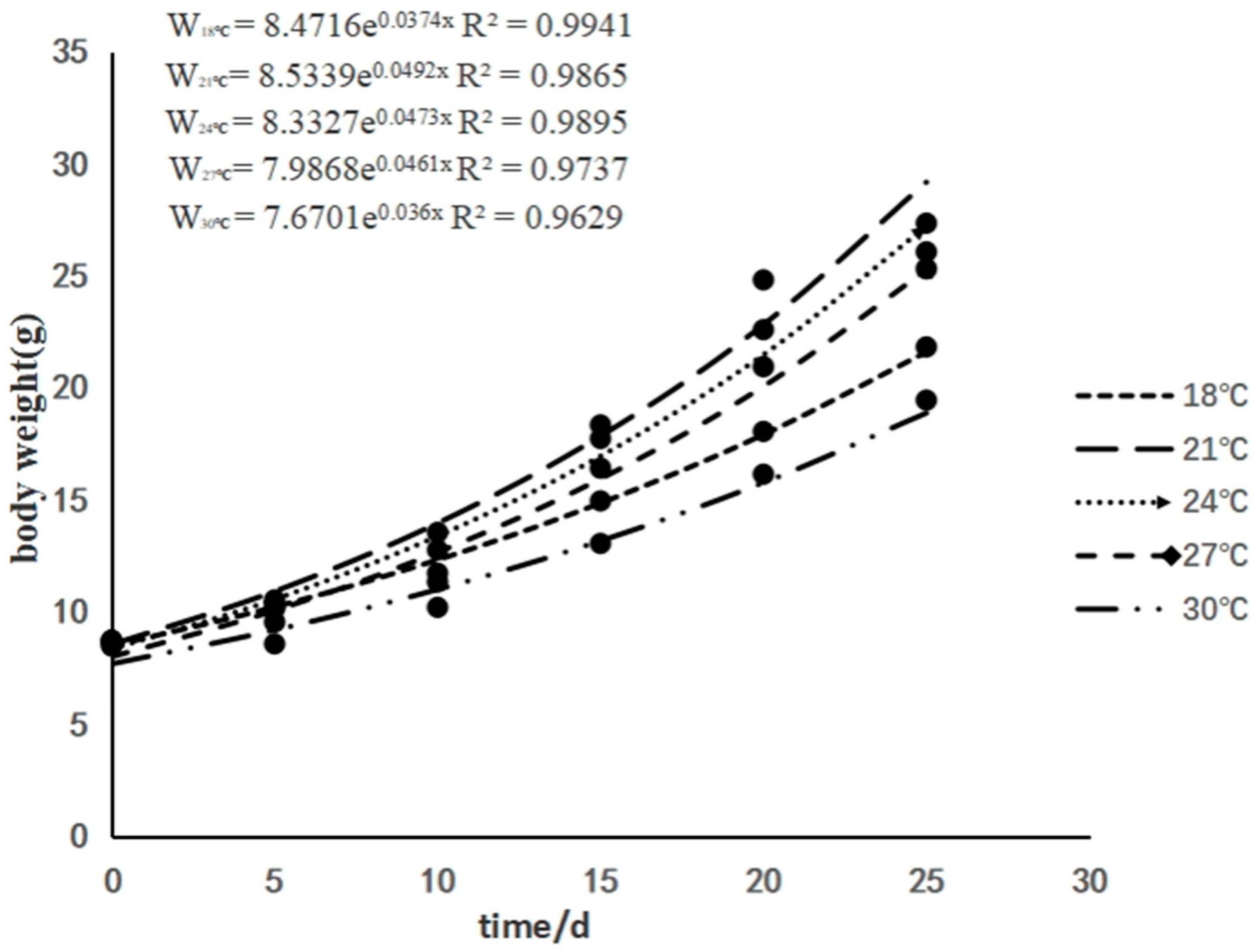
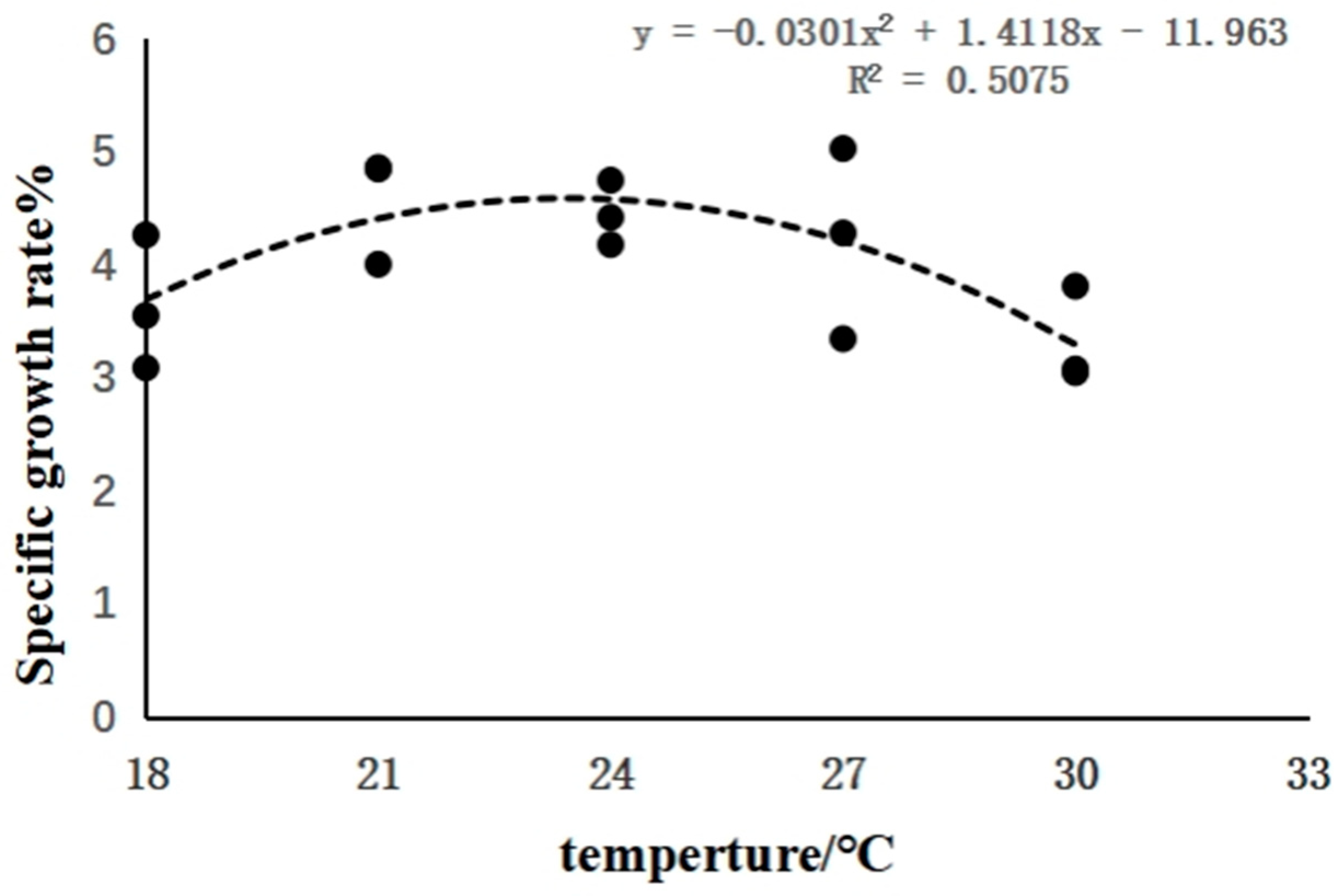
3.1. The Effects of Rearing Temperature on the Growth of Juvenile Sturgeon
3.2. The Effects of Rearing Temperature on Plasma Biochemical Indexes of Juvenile Sturgeon
3.3. The Effects of Rearing Temperature on Antioxidant Capacity of Juvenile Sturgeon
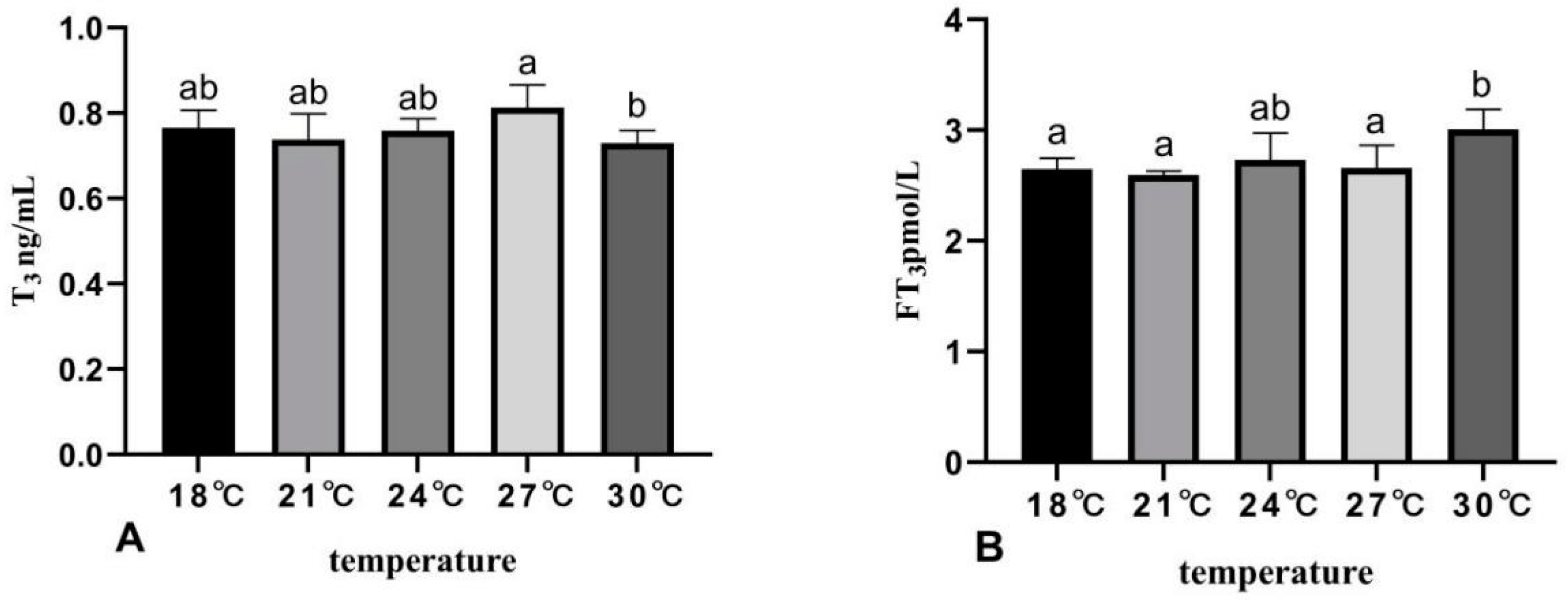
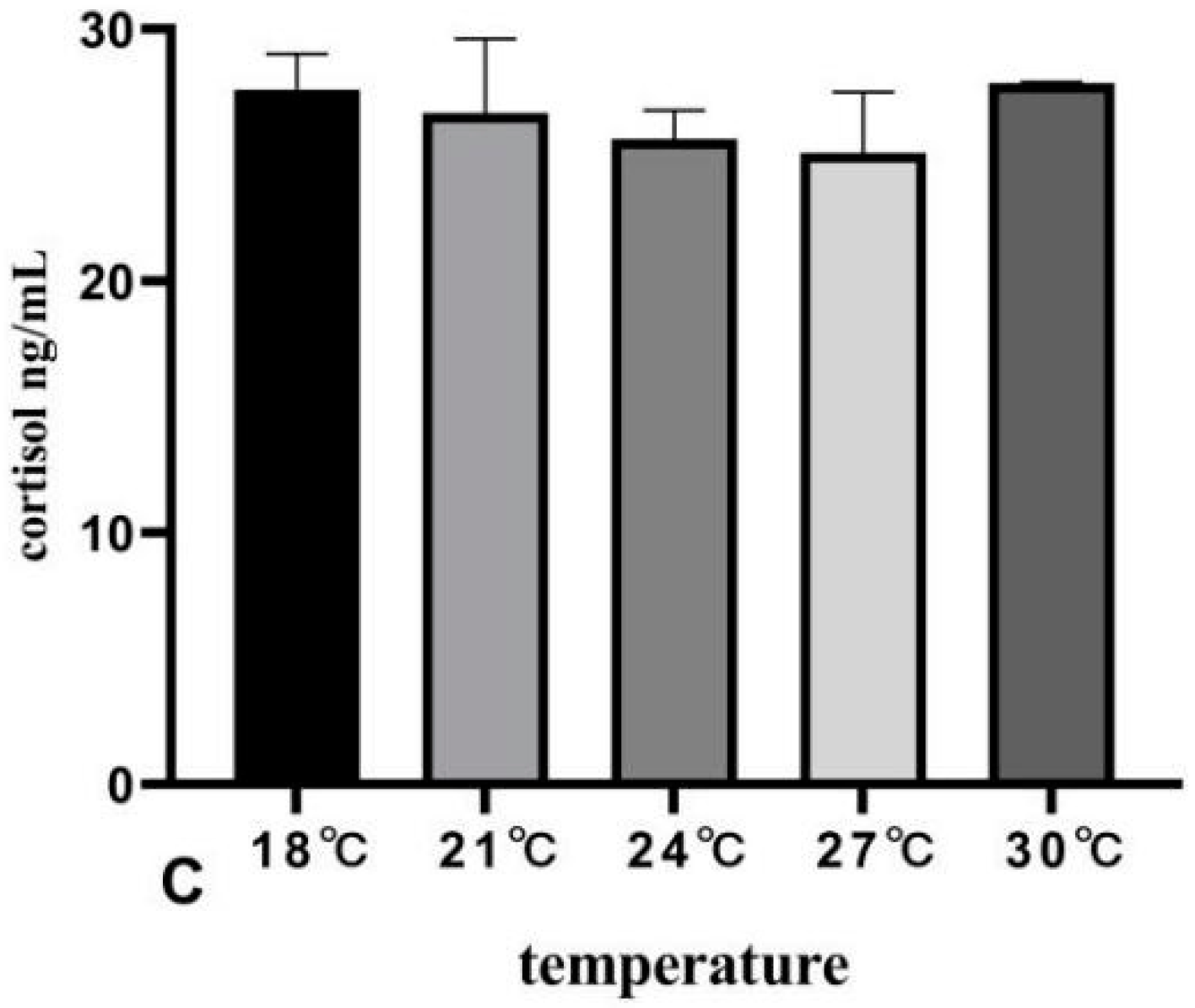
3.4. The Effects of Rearing Temperature on Hormone Levels in Juvenile Sturgeon
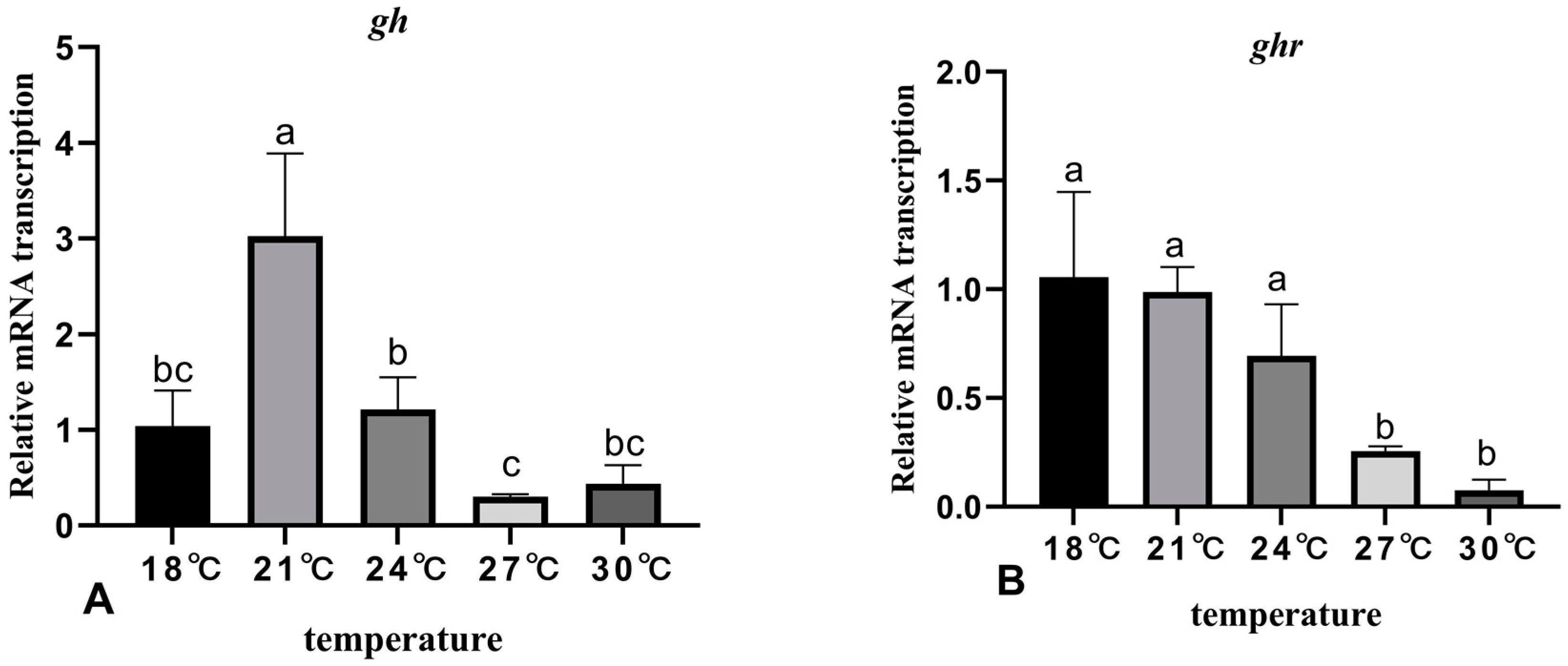
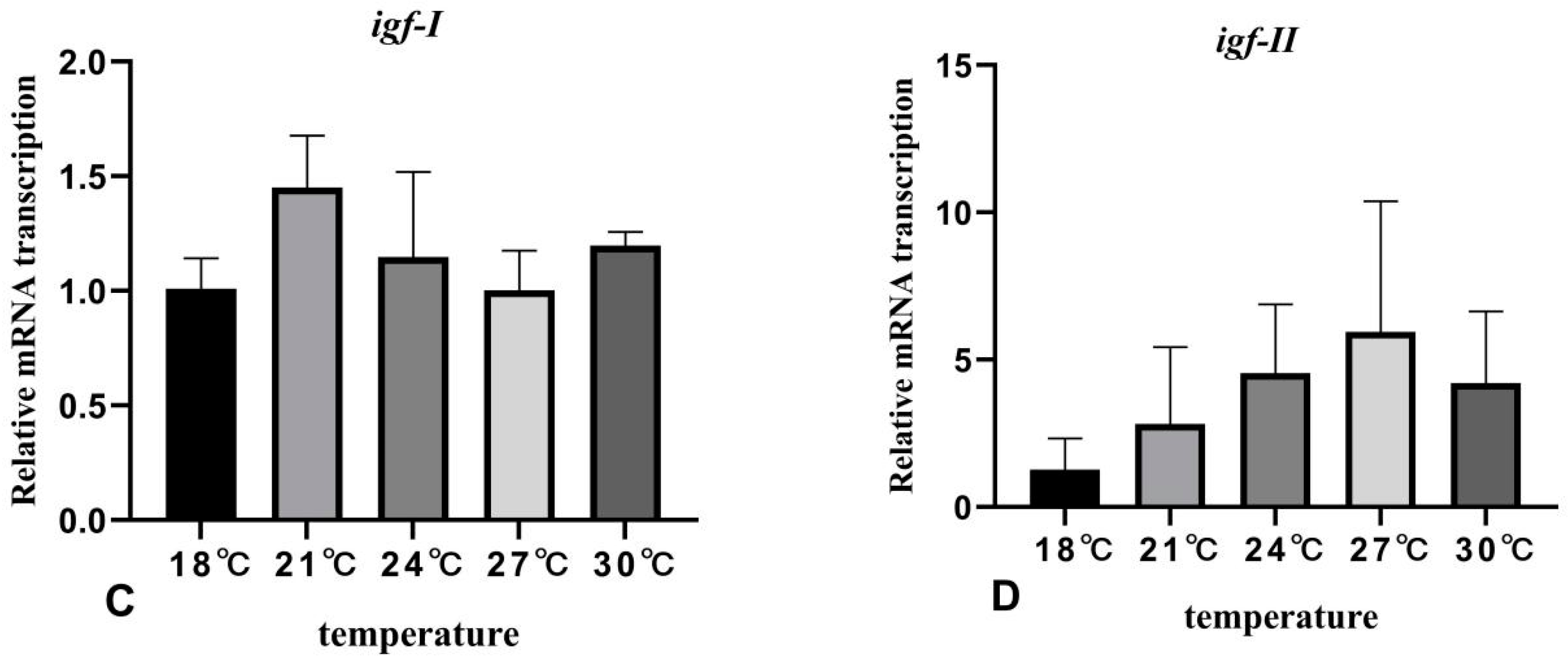
3.5. The Effects of Rearing Temperature on the Expression Levels of Growth-Related Genes in Juvenile Sturgeon
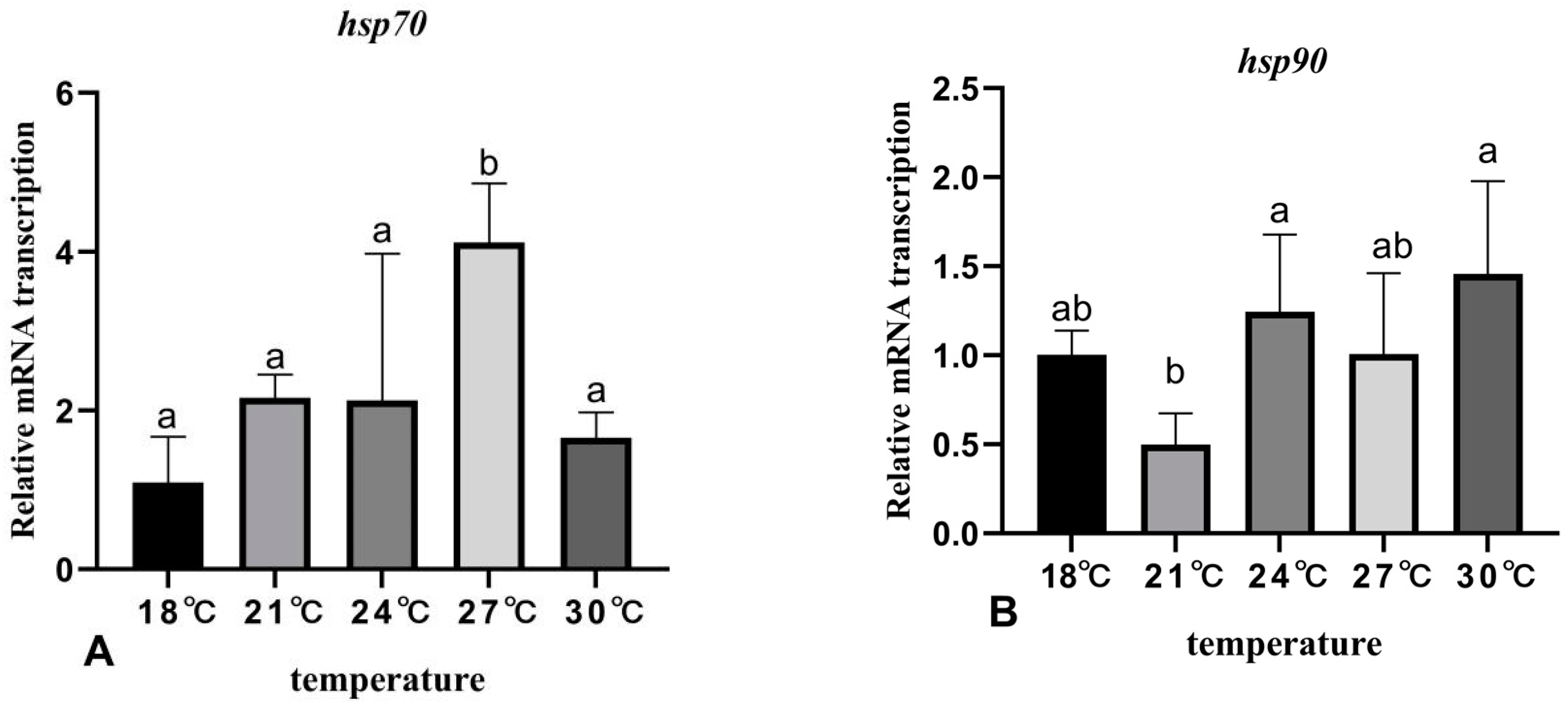
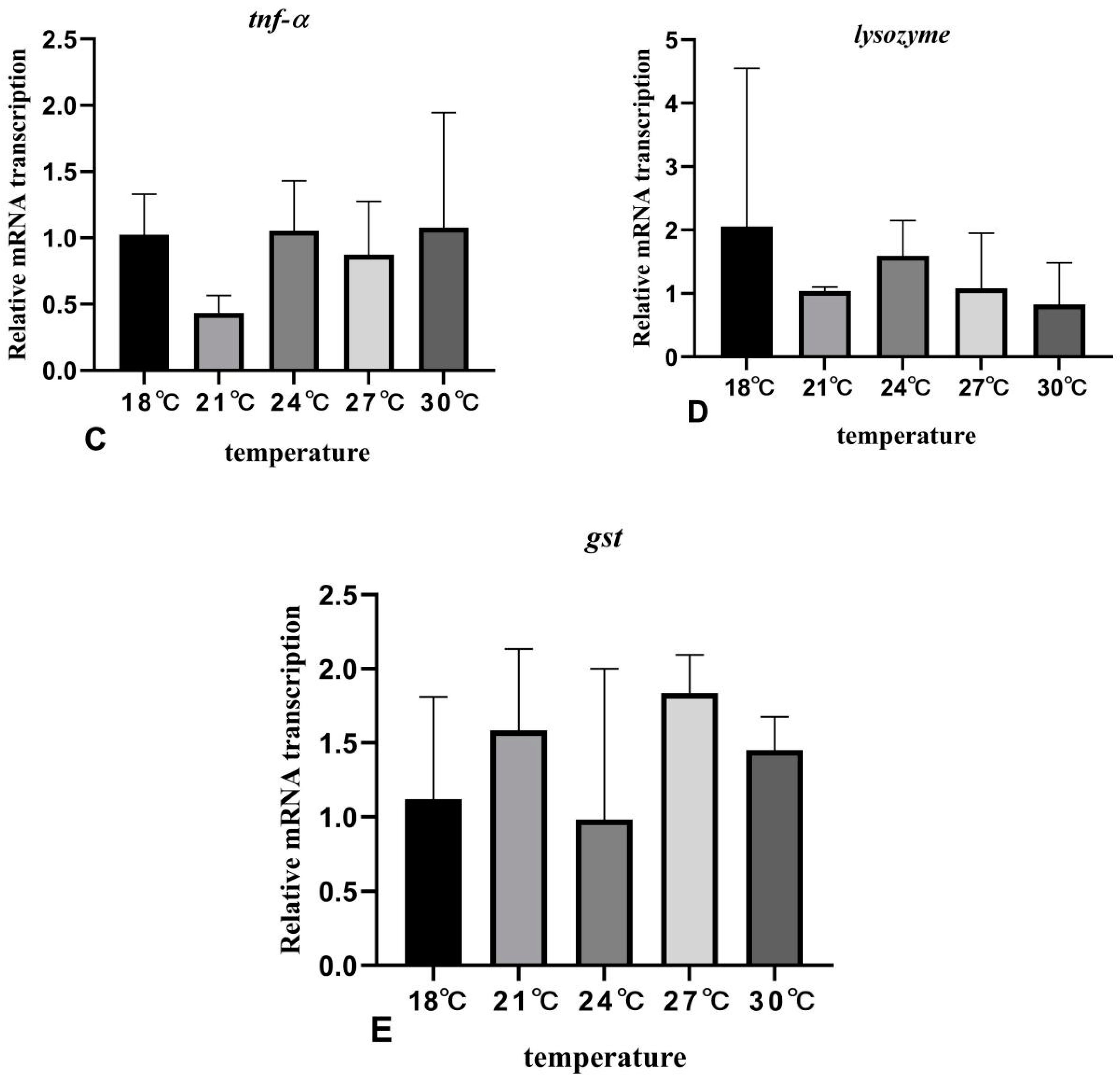
3.6. The Effects of Rearing Temperature on the Expression Levels of Stress- and Immunity-Related Genes in Juvenile Sturgeon
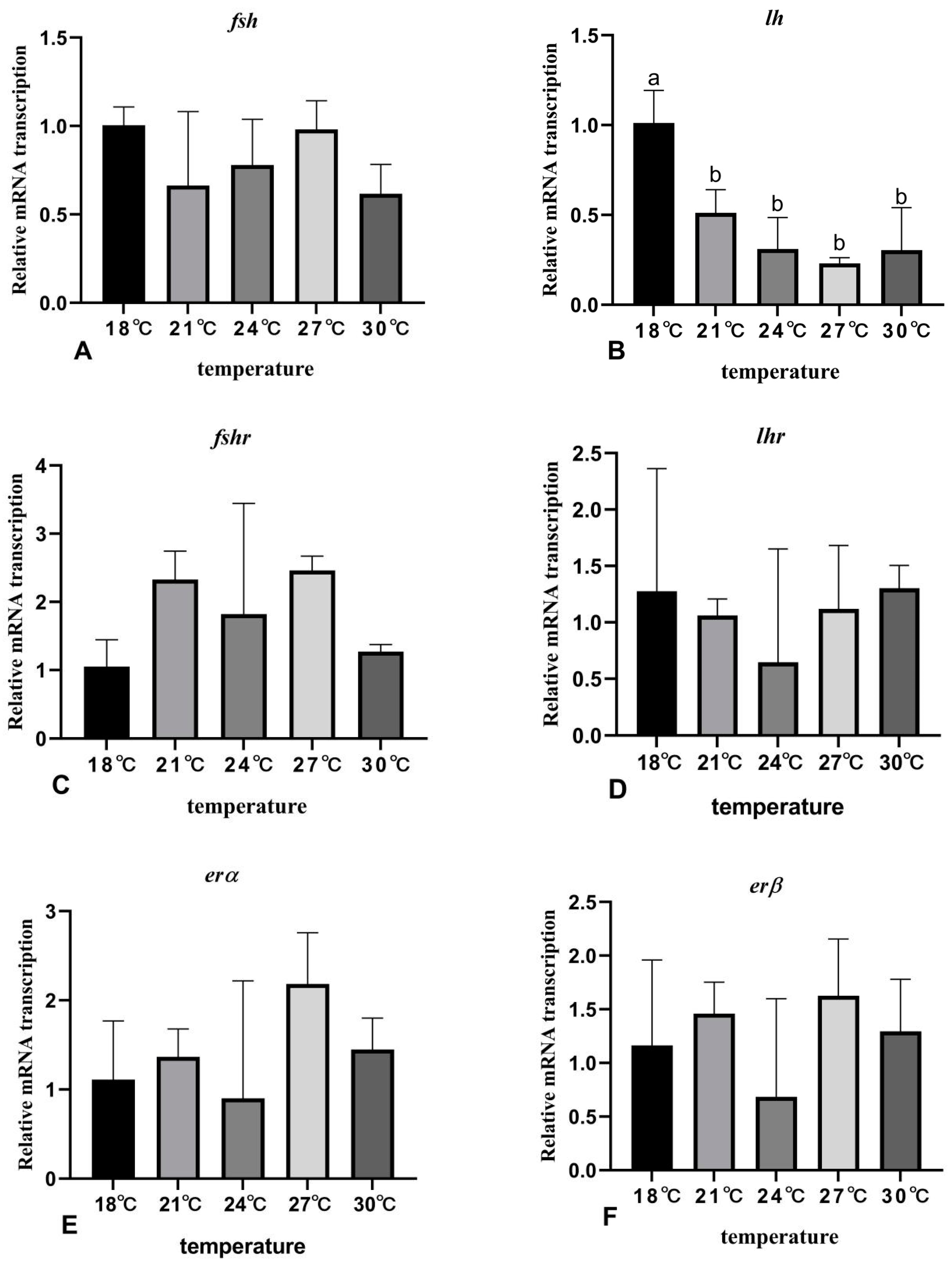
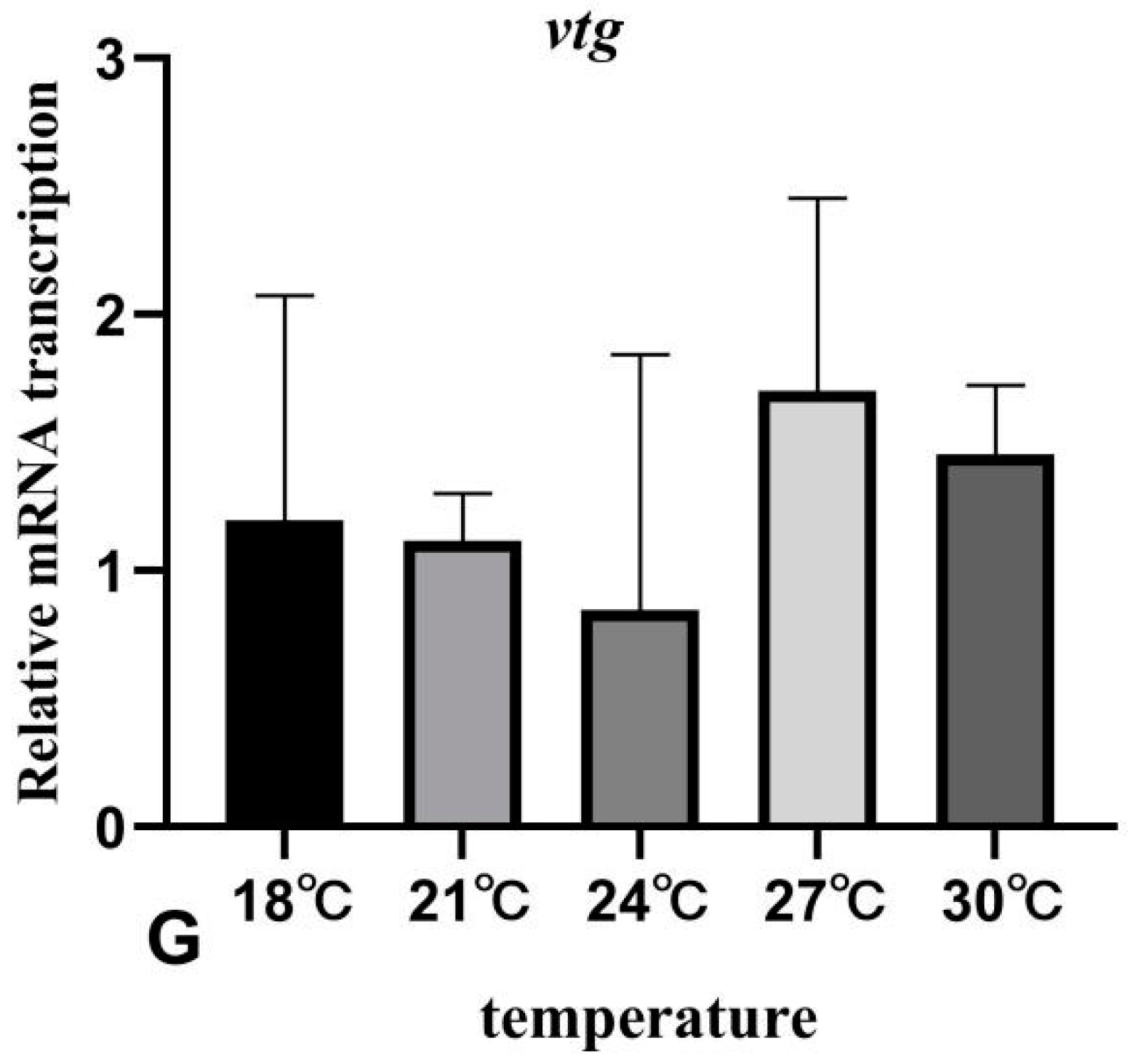
3.7. The Effects of Rearing Temperature on the Expression Levels of Gonad-Development-Related Genes in Juvenile Sturgeon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACW. Temperature in Relation to Fish. Nature 1887, 36, 213–214. [Google Scholar] [CrossRef] [Green Version]
- Mjia, B.; Mjs, B.; Mb, B.; Sz, B.; Ak, A. Extreme ambient temperature effects in European seabass, Dicentrarchus labrax: Growth performance and hemato-biochemical parameters. Aquaculture 2020, 522, 735093. [Google Scholar]
- Hahjahan, M.; Uddin, M.H.; Bain, V.; Haque, M.M. Increased water temperature altered hemato-biochemical parameters and structure of peripheral erythrocytes in striped catfish Pangasianodon hypophthalmus. Fish Physiol. Biochem. 2018, 44, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Cyr, D.G.; Idler, D.R.; Audet, C.; Mcleese, J.M.; Eales, J.G. Effects of long-term temperature acclimation on thyroid hormone deiodinase function, plasma thyroid hormone levels, growth, and reproductive status of male Atlantic cod, Gadus morhua. Gen. Comp. Endocrinol. 1998, 109, 24–36. [Google Scholar] [CrossRef]
- Gabillard, J.C. Differential expression of two GH receptor mRNAs following temperature change in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2006, 190, 29–37. [Google Scholar] [CrossRef]
- Gabillard, J.C.; Rescan, P.Y.; Fauconneau, B.; Weil, C.; Bail, P. Effect of temperature on gene expression of the Gh/Igf system during embryonic development in rainbow trout (Oncorhynchus mykiss). J. Exp. Zool. Part A Comp. Exp. Biol. 2003, 298, 134–142. [Google Scholar] [CrossRef]
- Stacey, N.E. Hormones and reproductive behaviour in teleosts. Control. Processes Fish Physiol. 1983, 145, 117–129. [Google Scholar]
- Zanug, S.; Carrillo, M.; Ruiz, F. Delayed gametogenesis and spawning of sea bass (Dicentrarchus labrax) kept under different photoperiod and temperature regimes. Fish Physiol. Biochem. 1986, 2, 53–63. [Google Scholar]
- Chen, H.; Bi, B.; Kong, L.; Rong, H.; Su, Y.; Hu, Q. Seasonal changes in plasma hormones, sex-related genes transcription in brain, liver and ovary during gonadal development in female Rainbow Trout (Oncorhynchus mykiss). Fishes 2021, 6, 62. [Google Scholar] [CrossRef]
- Miranda, L.A.; Strüssmann, C.A.; Somoza, G.M. Effects of light and temperature conditions on the expression of GnRH and GtH genes and levels of plasma steroids in Odontesthes bonariensis females. Fish Physiol. Biochem. 2009, 35, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Swanson, P.; Pankhurst, N.; King, H.; Elizur, A. Effect of thermal challenge on plasma gonadotropin levels and ovarian steroidogenesis in female maiden and repeat spawning Tasmanian Atlantic salmon (Salmo salar). Aquaculture 2012, 334, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Soria, F.N.; Strüssmann, C.A.; Miranda, L.A. High water temperatures impair the reproductive ability of the pejerrey fish Odontesthes bonariensis: Effects on the hypophyseal-gonadal axis. Physiol. Biochem. Zool. 2008, 81, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Brian, J.V.; Harris, C.A.; Runnalls, T.J.; Fantinati, A.; Pojana, G.; Marcomini, A.; Sumpter, J.P. Evidence of temperature-dependent effects on the estrogenic response of fish: Implications with regard to climate change. Sci. Total Environ. 2008, 397, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.; King, H.; Pankhurst, N.; Ruff, N.; Pankhurst, P.; Elizur, A. Effect of elevated temperature on estrogenic induction of vitellogenesis and zonagenesis in juvenile Atlantic salmon (Salmo salar). Mar. Freshw. Behav. Phy. 2012, 45, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Din, S.Y.; Hurvitz, A.; Goldberg, D.; Jackson, K.; Levavi-Sivan, B.; Degani, G. Cloning of Russian sturgeon (Acipenser gueldenstaedtii) growth hormone and insulin-like growth factor I and their expression in male and female fish during the first period of growth. J. Endocrinol. Investig. 2008, 31, 201–210. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, K.; Yang, J.; Liu, X.; Wang, B.; Zeng, Q.; Du, H. Effects of feeding frequency on juvenile Chinese sturgeon Acipenser sinensis. Sci. Rep. 2020, 10, 17399. [Google Scholar] [CrossRef]
- Long, L.; Zhang, H.; Ni, Q.; Liu, H.; Wu, F.; Wang, X. Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 25–34. [Google Scholar] [CrossRef]
- Fajkowska, M.; Głowacka, D.K.; Adamek-Urbańska, D.; Ostaszewska, T.; Krajnik, K.A.; Rzepkowska, M. Sex-related gene expression profiles in various tissues of juvenile Russian sturgeon (Acipenser gueldenstaedtii). Aquaculture 2019, 500, 532–539. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.; Tian, Z.; Sun, A.; Dong, Y.; Dong, T.; Hu, H. Effects of 11-Ketotestosterone on development of the previtellogenic ovary in the sterlet, Acipenser ruthenus. Front. Endocrinol. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Atre, I.; Mizrahi, N.; Yebra-Pimentel, E.S.; Hausken, K.; Yom-Din, S.; Hurvitz, A.; Levavi-Sivan, B. Molecular characterization of two Russian sturgeon gonadotropin receptors: Cloning, expression analysis, and functional activity. Gen. Comp. Endocrinol. 2020, 298, 113557. [Google Scholar] [CrossRef]
- Jackson, K.; Hurvitz, A.; Din, S.Y.; Goldberg, D.; Pearlson, O.; Degani, G.; Levavi-Sivan, B. Anatomical, hormonal and histological descriptions of captive Russian sturgeon (Acipenser gueldenstaedtii) with intersex gonads. Gen. Comp Endocrinol. 2006, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Nguen, T.H.P. Effects of temperatures on growth performance, hematological parameters and plasma IGF-1 level of Tra catfish (Pangasianodon hypophthalmus). Hydrobiol. J. 2022, 58, 87–101. [Google Scholar] [CrossRef]
- Dahlhoff, E.P. Biochemical indicators of stress and metabolism: Applications for marine ecological studies. Annu. Rev. Physiol. 2004, 66, 183–207. [Google Scholar] [CrossRef] [Green Version]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Almroth, B.C.; Asker, N.; Wassmur, B.; Rosengren, M.; Jutfelt, F.; Gräns, A.; Sturve, J. Warmer water temperature results in oxidative damage in an Antarctic fish, the bald notothen. J. Exp. Mar. Biol. Ecol. 2015, 468, 130–137. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Preville, X.; Salvemini, F.; Giraud, S.; Chaufour, S.; Paul, C.; Stepien, G.; Ursini, M.V.; Arrigo, A.P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 1999, 247, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, Y.; Li, X.; Cao, H.; Xu, M.; Dai, J. The identification of heat shock protein genes in goldfish (Carassius auratus) and their expression in a complex environment in Gaobeidian Lake, Beijing, China. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Hermesz, E.; Ábrahám, M.; Nemcsók, J. Tissue-specific expression of two metallothionein genes in common carp during cadmium exposure and temperature shock. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 128, 457–465. [Google Scholar] [CrossRef]
- Wu, C.X.; Zhao, F.Y.; Zhang, Y.; Zhu, Y.J.; Ma, M.S.; Mao, H.L.; Hu, C.Y. Overexpression of Hsp90 from grass carp (Ctenopharyngodon idella) increases thermal protection against heat stress. Fish Shellfish Immunol. 2012, 33, 42–47. [Google Scholar] [CrossRef]
- Sharma, J.G.; Singh, S.P.; Chakrabarti, R. Effect of temperature on digestive physiology, immune-modulatory parameters, and expression level of Hsp and LDH genes in Catla catla (Hamilton, 1822). Aquaculture 2017, 479, 134–141. [Google Scholar] [CrossRef]
- Pérez-Casanova, J.C.; Rise, M.L.; Dixon, B.; Afonso, L.O.B.; Hall, J.R.; Johnson, S.C.; Gamperl, A.K. The immune and stress responses of Atlantic cod to long-term increases in water temperature. Fish Shellfish Immunol. 2008, 24, 600–609. [Google Scholar] [CrossRef]
- Ndong, D.; Chen, Y.Y.; Lin, Y.H.; Vaseeharan, B.; Chen, J.C. The immune response of Tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694. [Google Scholar] [CrossRef]
- Hassan, A.M.; El Nahas, A.F.; Mahmoud, S.; Barakat, M.E.; Ammar, A.Y. Thermal stress of ambient temperature modulate expression of stress and immune-related genes and DNA fragmentation in Nile tilapia (Oreochromis niloticus (Linnaeus, 1758)). Appl. Ecol. Environ. Res. 2017, 15, 1343–1354. [Google Scholar] [CrossRef]
- Bagnyukova, T.V.; Lushchak, O.V.; Storey, K.B.; Lushchak, V.I. Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23 °C. J. Therm. Biol. 2007, 32, 227–234. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef] [Green Version]
- Blanton, M.L.; Specker, J.L. The Hypothalamic-Pituitary-Thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit. Rev. Toxicol. 2007, 37, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Rabah, S.A.; Gowan, I.L.; Pagnin, M.; Osman, N.; Richardson, S.J. Thyroid hormone distributor proteins during development in vertebrates. Front. Endocrinol. 2019, 10, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loter, T.C.; MacKenzie, D.S.; McLeese, J.; Eales, J. G Seasonal changes in channel catfish thyroid hormones reflect increased magnitude of daily thyroid hormone cycles. Aquaculture 2007, 262, 451–460. [Google Scholar] [CrossRef]
- Peyghan, R.; Enayati, A.; Sabzevarizadeh, M. Effect of salinity level on TSH and thyroid hormones of grass carp, Ctenophayngodon idella. Vet. Res. Forum 2013, 4, 175–178. [Google Scholar] [PubMed]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish. Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Strange, R.J.; Schreck, C.B.; Golden, J.T. Corticoid stress responses to handling and temperature in salmonids. Trans. Am. Fish. Soc. 1977, 106, 213–218. [Google Scholar] [CrossRef]
- Cho, H.C.; Kim, J.E.; Kim, H.B.; Baek, H.J. Effects of water temperature change on the hematological responses and plasma cortisol levels in growing of red spotted grouper, Epinephelus akaara. Dev. Reprod. 2015, 19, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.R.; Sun, L.T.; Lee, Y.H.; Chang, C.F. Characteristics of blood in common carp, Cyprinus carpio, exposed to low temperatures. J. Appl. Aquac. 1995, 5, 21–31. [Google Scholar] [CrossRef]
- Gabillard, J.C.; Weil, C.; Rescan, P.Y.; Navarro, I.; Gutiérrez, J.; Le Bail, P.Y. Effects of environmental temperature on IGF1, IGF2, and IGF type I receptor expression in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2003, 133, 233–242. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N. Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: A review. Rev. Fish Biol. Fish. 2009, 19, 97–120. [Google Scholar] [CrossRef]
- Shahjahan, M.; Zahangir, M.M.; Islam, S.M.; Ashaf-Ud-Doulah, M.; Ando, H. Higher acclimation temperature affects growth of rohu (Labeo rohita) through suppression of GH and IGFs genes expression actuating stress response. J. Therm. Biol. 2021, 100, 103032. [Google Scholar] [CrossRef]
- Hevrøy, E.M.; Hunskår, C.; de Gelder, S.; Shimizu, M.; Waagbø, R.; Breck, O.; Hansen, T. GH–IGF system regulation of attenuated muscle growth and lipolysis in Atlantic salmon reared at elevated sea temperatures. J. Comp. Physiol. B 2013, 183, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Maruska, K.P.; Fernald, R.D. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 2011, 26, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Pankhurst, N.; King, H.; Elizur, A. Effect of thermal challenge on the expression of genes involved in ovarian steroidogenesis in Tasmanian Atlantic salmon (Salmo salar). Aquaculture 2017, 479, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Shahjahan, M.D.; Kitahashi, T.; Ando, H. Temperature affects sexual maturation through the control of kisspeptin, kisspeptin receptor, GnRH and GTH subunit gene expression in the grass puffer during the spawning season. Gen. Comp. Endocrinol. 2017, 243, 138–145. [Google Scholar] [CrossRef]
- Bock, S.L.; Chow, M.I.; Forsgren, K.L.; Lema, S.C. Widespread alterations to hypothalamic-pituitary-gonadal (HPG) axis signaling underlie high temperature reproductive inhibition in the eurythermal sheepshead minnow (Cyprinodon variegatus). Mol. Cell. Endocrinol. 2021, 537, 111447. [Google Scholar] [CrossRef]
- Tan, N.S.; Frecer, V.; Lam, T.J.; Ding, J.L. Temperature dependence of estrogen binding: Importance of a subzone in the ligand binding domain of a novel piscine estrogen receptor. Biochim. Biophys. Acta (BBA)-Mol. Cell. Res. 1999, 1452, 103–120. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, S.; Islinger, M.; Völkl, A.; Braunbeck, T. Temperature-dependent vitellogenin-mRNA expression in primary cultures of rainbow trout (Oncorhynchus mykiss) hepatocytes at 14 °C and 18 °C. Toxicol. In Vitro 2000, 14, 531–540. [Google Scholar] [CrossRef]
- van Nes, S.; Andersen, Ø. Temperature effects on sex determination and ontogenetic gene expression of the aromatases cyp19a and cyp19b, and the estrogen receptors esr1 and esr2 in Atlantic halibut (Hippoglossus hippoglossus). Mol. Reprod. Deve. 2006, 73, 1481–1490. [Google Scholar] [CrossRef]

| Gene | Primer Name | Sequence (5′–3′) | TM | GenBank No. or Article Source |
|---|---|---|---|---|
| igf-I | igf-I-F | GAACGAGTGCTGCTTCCAGAG | 57 °C | [18] |
| igf-I-R | AGGCTTTGGCTGGCTTAACA | |||
| igf-II | igf-II-F | ATCGCCCTCACAGTCTACAT | 57 °C | [19] |
| igf-II-R | GTGGCTTGCTGAAATAAAA | |||
| gh | gh-F | ATGGCATCAGGTCTGCTTCT | 57 °C | [19] |
| gh-R | ACGCTGCTCATCTGGAACATAG | |||
| ghr | ghr-F | CATAGAAATCCAGGTTTACCCAACTC | 57 °C | [19] |
| ghr-R | CTGAACATCAAGGACGACGACTC | |||
| hsp70 | hsp70-F | ACAGCCATGTTGTATACTGAGTCC | 53 °C | [20] |
| hsp70-R | TGCACACCTTCTCCAGTTCTT | |||
| hsp90 | hsp90-F | GCCAACCAATTTGATCAGAGC | 53 °C | [20] |
| hsp90-R | TGGACACTGTGACCTGGAAAG | |||
| gst | gst-F | TTGATAGGGCGGCTCTTGT | 57 °C | [20] |
| gst-R | CACCTGGATGTGTCGACTTGT | |||
| lysozyme | lysozyme-F | GAGGGACCCAAATGGAATG | 57 °C | [20] |
| lysozyme-R | CCCACCCAGTTATTTTATGCT | |||
| tnf-α | tnf-α-F | TGTGTCTGTAGAGCACTCCGAT | 57 °C | [20] |
| tnf-α-R | CATGGCCAGCAAGTCGAT | |||
| erα | erα-F | CAGGCCAAGTATGGAAGGCA | 57 °C | [21] |
| erα-R | CACCGCACAGAACCTCATCT | |||
| erβ | erβ-F | ATTGCTGCTGGAGATGCTG | 57 °C | [22] |
| erβ-R | TTCTGGCTTTGAACAGGTGA | |||
| vtg | vtg-F | CAAGTCAGCTAACCCAGCCA | 57 °C | [21] |
| vtg-R | GCATGTTCAGGATCCCCCTC | |||
| lhr | lhr-F | TTCAATCCCTGCGAAGACAT | 57 °C | [23] |
| lhr-R | GCAGAACAAGCAGTACAGCAAA | |||
| fshr | fshr-F | GAGGCTGAGATTGCGAAGAAGGAG | 57 °C | [24] |
| fshr-R | CGCCGTCTGTGTCTGCTATGTAAG | |||
| fsh | fsh-F | GTCTGTCAACACCACCTCCT | 57 °C | EU523732.1 |
| fsh-R | CTTAGGGTGCCACAGTCAGT | |||
| lh | lh-F | TCCTCCTCCTCTTCTCTGCT | 57 °C | EU523733.1 |
| lh-R | CACCGTCACAAAGCGAAGAT | |||
| 18sRNA | 18SRNA-F | CCATAAACGATGCCGACTGG | 57 °C | [21] |
| 18SRNA-R | TGAGGTTCCCCGTGTTGAGT |
| Parameter | 18 °C | 21 °C | 24 °C | 27 °C | 30 °C |
|---|---|---|---|---|---|
| Initial weight (g) | 8.74 ± 0.05 | 8.69 ± 0.19 | 8.55 ± 0.12 | 8.71 ± 0.22 | 8.47 ± 0.14 |
| Final weight (g) | 21.83 ±3.16 ab | 27.35 ± 2.75 a | 26.07 ± 2.24 a | 25.31 ± 4.71 ab | 19.45 ± 1.93 b |
| Specific growth rate (%/d) | 3.64 ± 0.59 ab | 4.57 ± 0.49 a | 4.45 ± 0.29 a | 4.22 ± 0.84 ab | 3.31 ± 0.43 b |
| Relative weight gain rate (%) | 149.97 ± 37.44 ab | 215.07 ± 37.17 a | 204.67 ± 21.84 ab | 191.34 ± 60.35 ab | 129.80 ± 25.58 b |
| Parameter | 18 °C | 21 °C | 24 °C | 27 °C | 30 °C |
|---|---|---|---|---|---|
| Blood Glucose (µg/mL) | 598.83 ± 127.45 ab | 647.47 ± 63.64 bc | 550.37 ± 41.59 ab | 481.70 ± 20.24 a | 739.90 ± 36.13 c |
| Triglyceride (mg/dL) | 1392.49 ± 903.49 | 1126.59 ± 381.39 | 924.86 ± 290.10 | 793.64 ± 128.80 | 877.46 ± 351.57 |
| Total Cholesterol (µmol/dL) | 117.78 ± 36.11 | 121.06 ± 40.44 | 102.13 ± 10.96 | 110.05 ± 0.89 | 123.09 ± 1.75 |
| Total protein (µg/mL) | 16.56 ± 1.65 | 15.45 ± 0.66 | 14.94 ± 3.13 | 14.06 ± 0.32 | 14.69 ± 0.28 |
| Na+ (mmol/L) | 171.66 ± 20.19 | 149.82 ± 9.78 | 139.76 ± 29.19 | 136.52 ± 17.72 | 204.61 ± 78.04 |
| Cl− (mmol/L) | 107.01 ± 8.52 | 113.72 ± 13.22 | 123.33 ± 18.32 | 115.46 ± 3.15 | 121.36 ± 11.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Hu, Q.; Kong, L.; Rong, H.; Bi, B. Effects of Temperature on the Growth Performance, Biochemical Indexes and Growth and Development-Related Genes Expression of Juvenile Hybrid Sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂). Water 2022, 14, 2368. https://doi.org/10.3390/w14152368
Chen H, Hu Q, Kong L, Rong H, Bi B. Effects of Temperature on the Growth Performance, Biochemical Indexes and Growth and Development-Related Genes Expression of Juvenile Hybrid Sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂). Water. 2022; 14(15):2368. https://doi.org/10.3390/w14152368
Chicago/Turabian StyleChen, Huiqin, Qing Hu, Lingfu Kong, Hua Rong, and Baoliang Bi. 2022. "Effects of Temperature on the Growth Performance, Biochemical Indexes and Growth and Development-Related Genes Expression of Juvenile Hybrid Sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂)" Water 14, no. 15: 2368. https://doi.org/10.3390/w14152368
APA StyleChen, H., Hu, Q., Kong, L., Rong, H., & Bi, B. (2022). Effects of Temperature on the Growth Performance, Biochemical Indexes and Growth and Development-Related Genes Expression of Juvenile Hybrid Sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂). Water, 14(15), 2368. https://doi.org/10.3390/w14152368






