Microfiltration Membranes for the Removal of Bisphenol A from Aqueous Solution: Adsorption Behavior and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Experiments
2.2.1. Batch Adsorption
2.2.2. Rapid Filtration Adsorption
2.3. Characterization and Analytical Methods
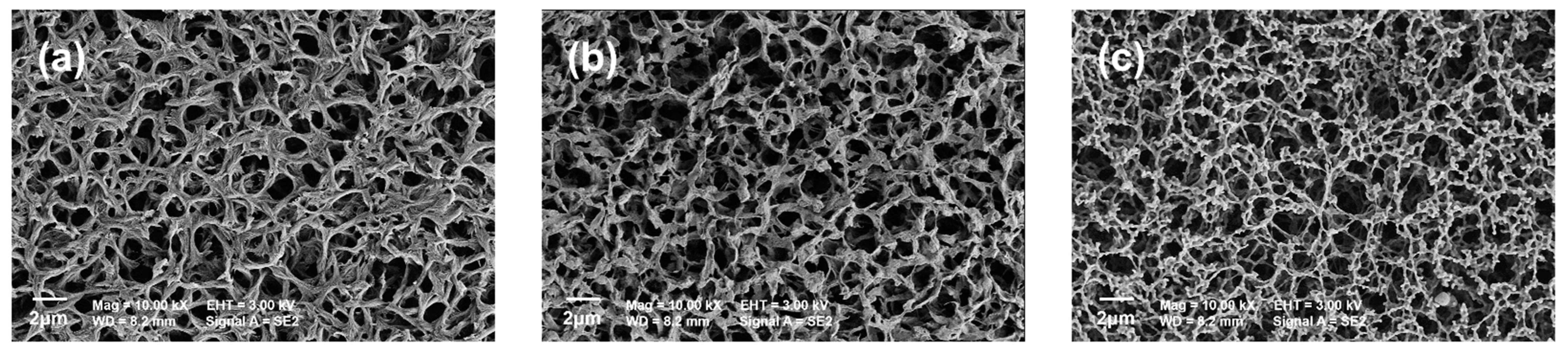
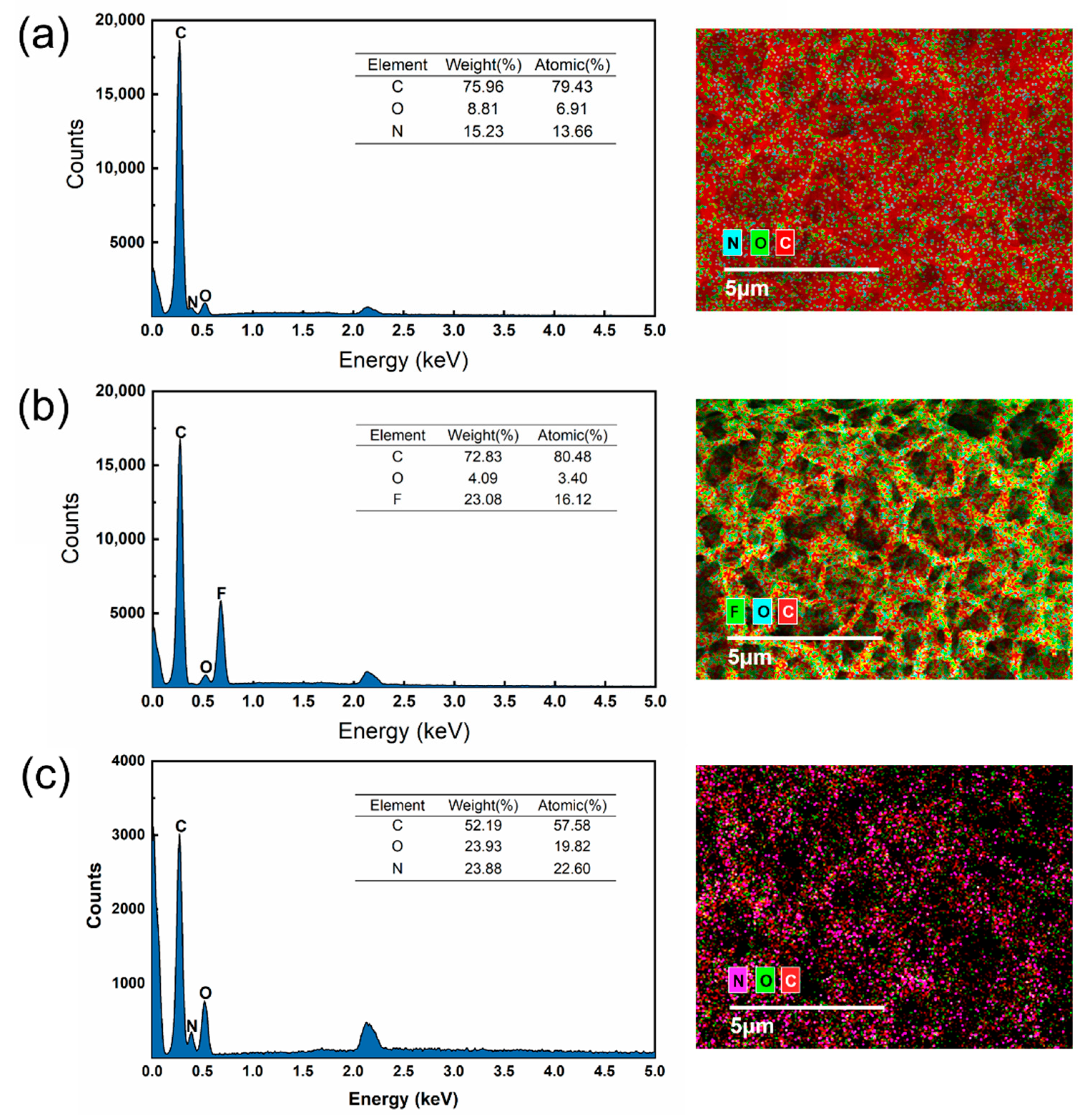
2.3.1. Characterization Methods
2.3.2. Adsorption Kinetics
2.3.3. Adsorption Isotherms
2.3.4. Adsorption Site Energy Distribution Theory Analysis
3. Results and Discussion
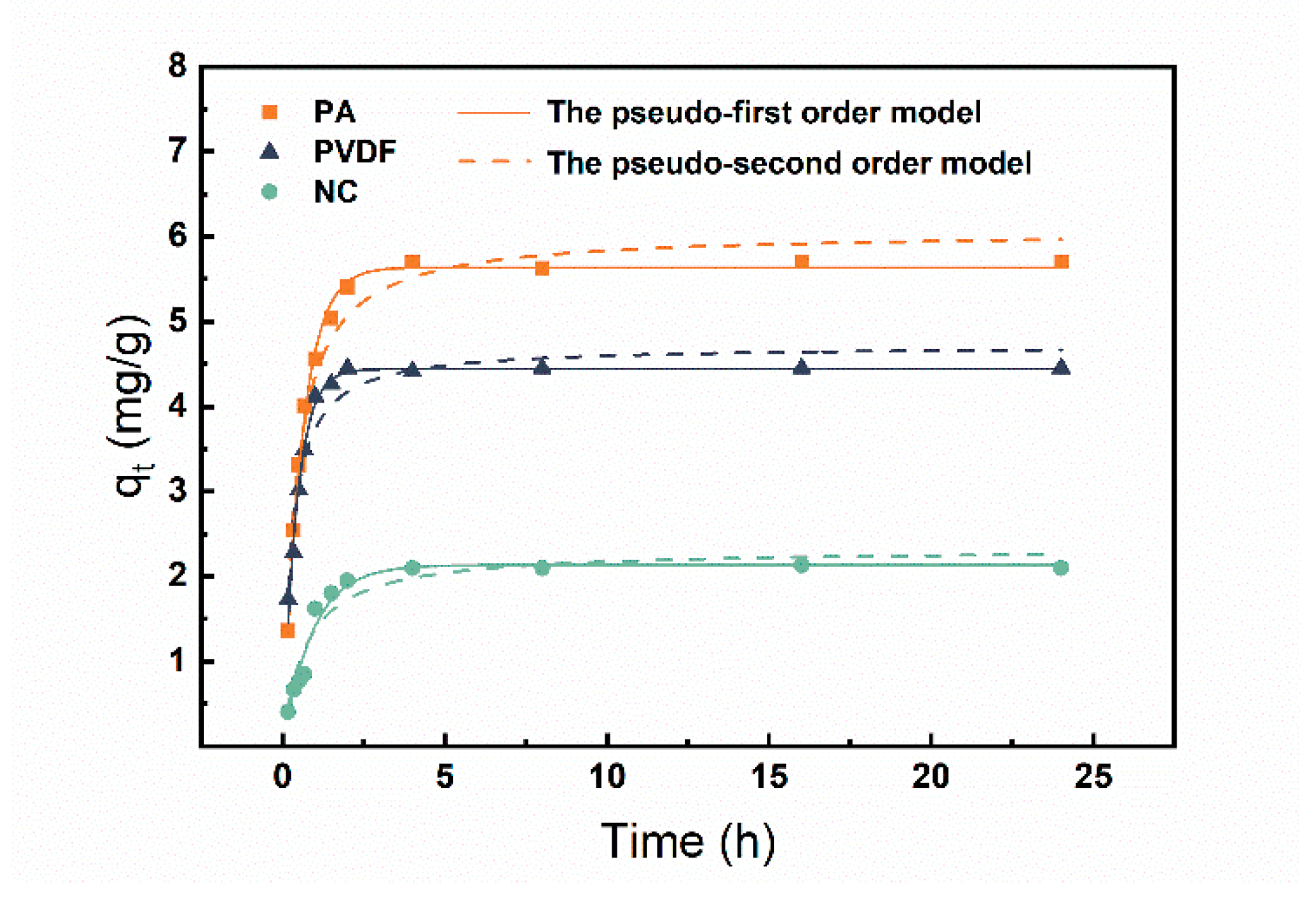
3.1. Adsorption Kinetic
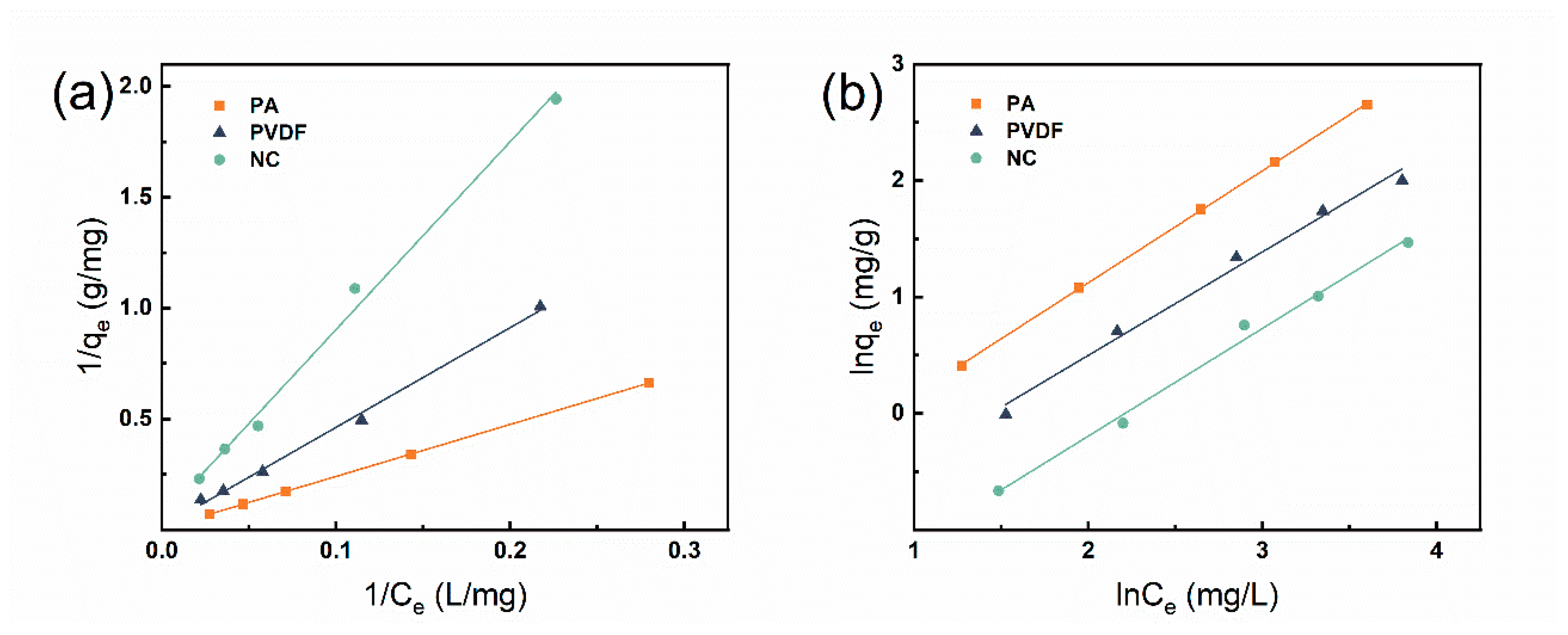
3.2. Adsorption Isothermal
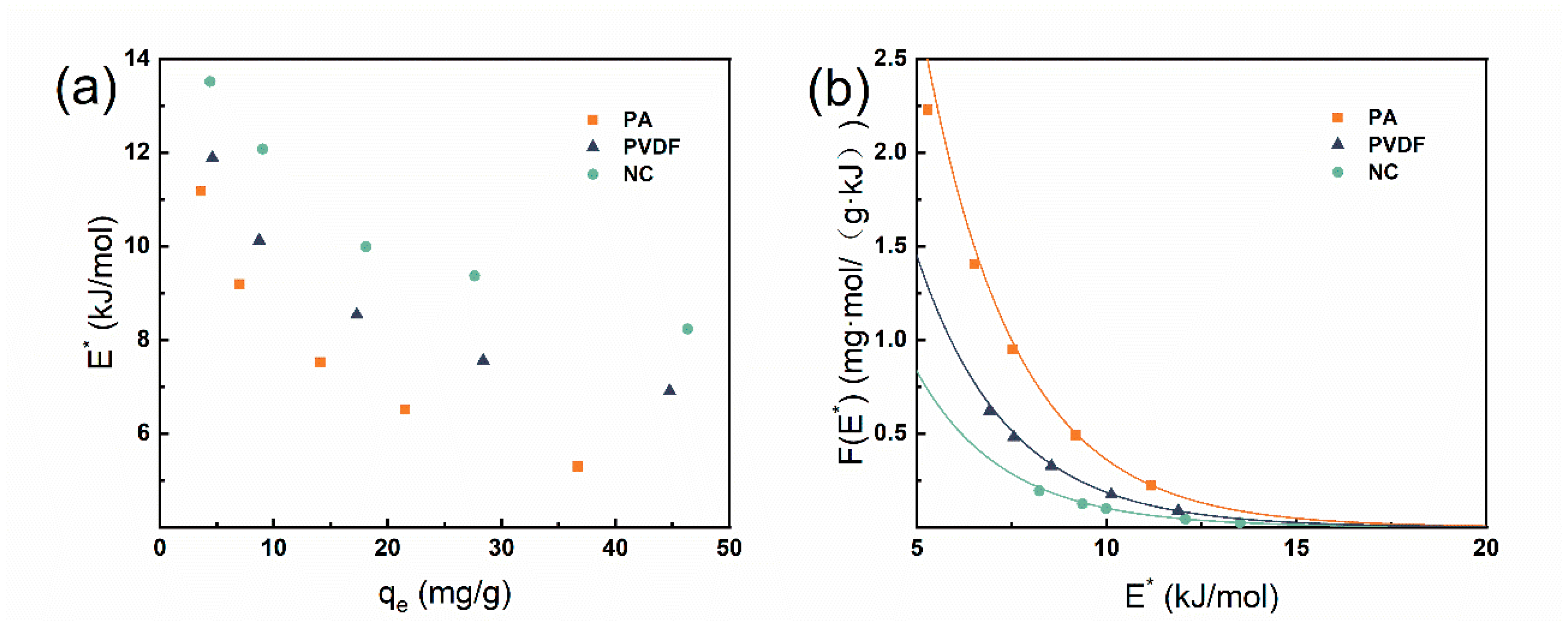
3.3. Site Energy Distribution Analysis
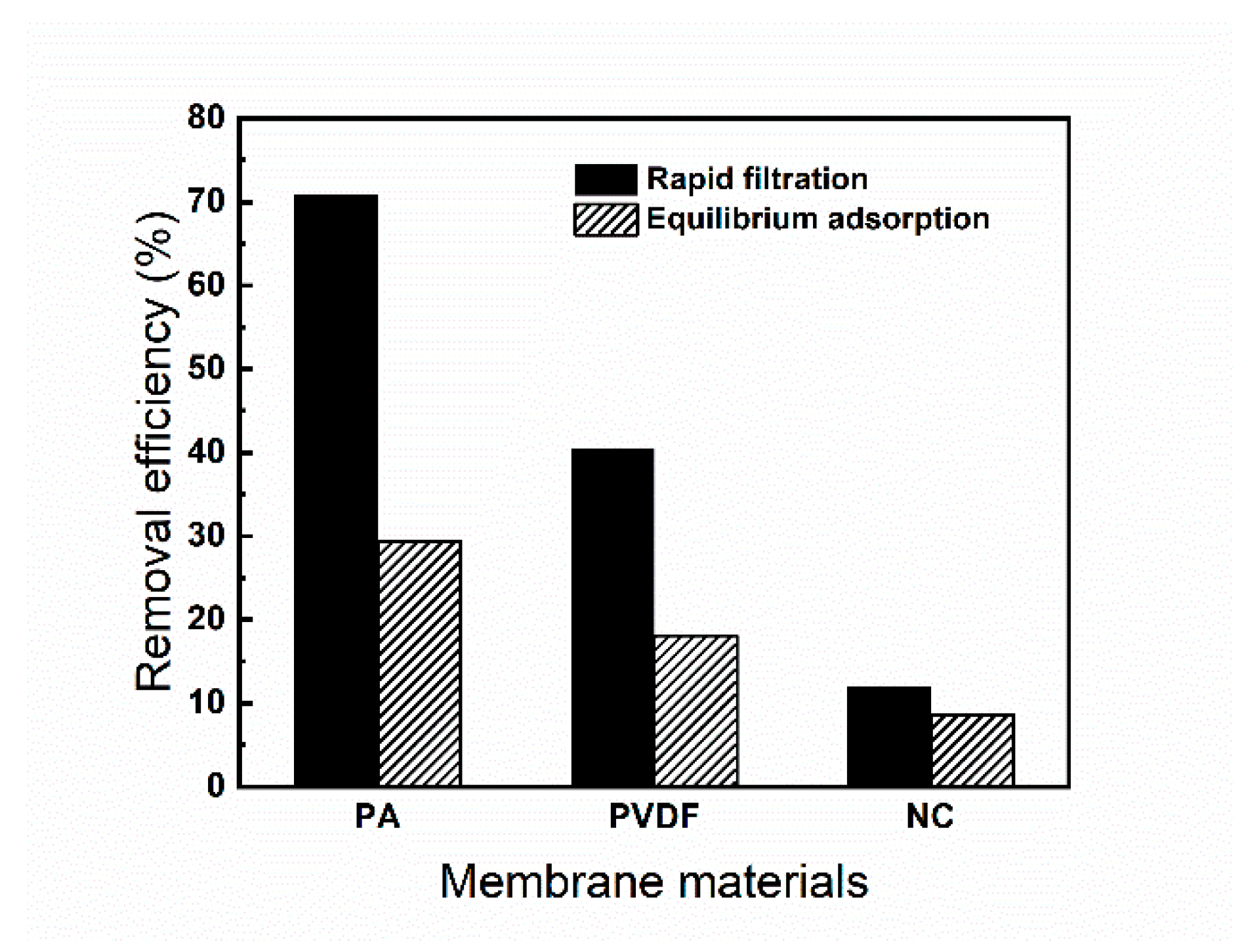
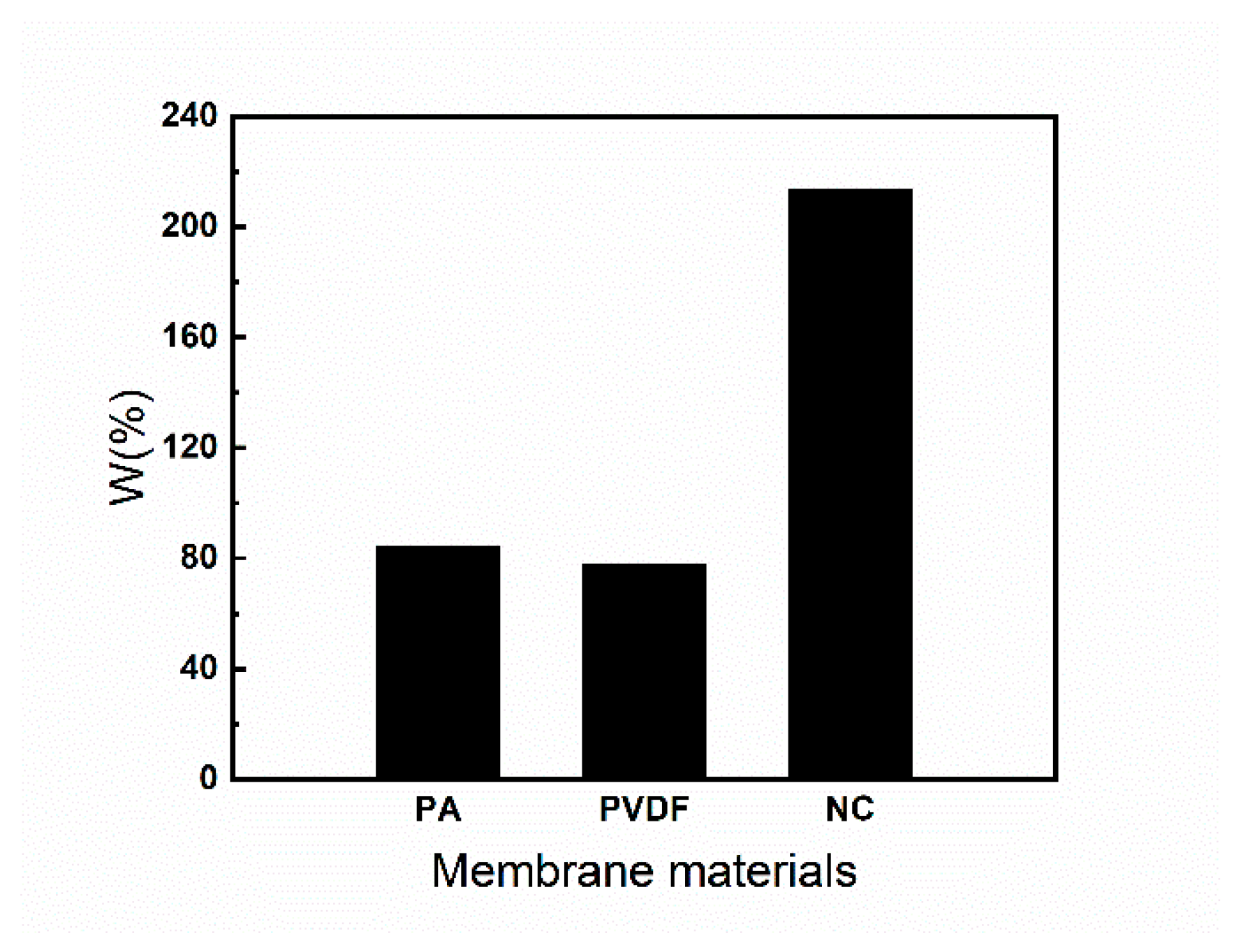
3.4. Rapid Filtration Adsorption and Equilibrium Adsorption
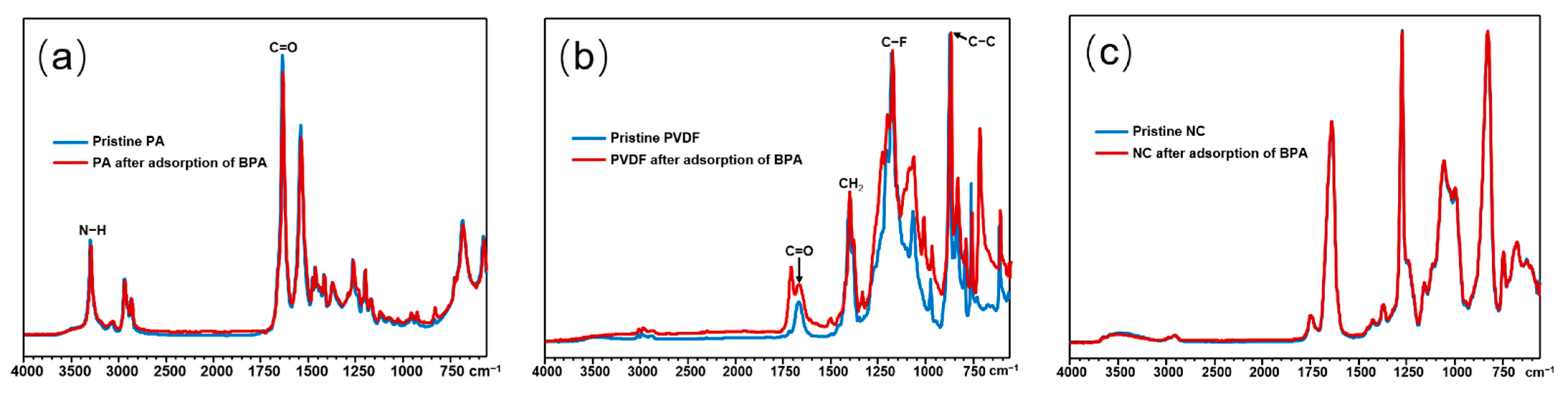
3.5. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wee, S.Y.; Aris, A.Z. Occurrence and Public-Perceived Risk of Endocrine Disrupting Compounds in Drinking Water. NPJ Clean Water 2019, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Corsini, E.; Ruffo, F.; Racchi, M. Steroid Hormones, Endocrine Disrupting Compounds and Immunotoxicology. Curr. Opin. Toxicol. 2018, 10, 69–73. [Google Scholar] [CrossRef]
- Guo, C.; Ren, F.; Jin, J.; Zhang, H.; Wang, L.; Zhang, H.; Chen, J. Internal Exposure of Chinese Children from a Typical Coastal City to Bisphenols and Possible Association with Thyroid Hormone Levels. Environ. Int. 2021, 156, 106759. [Google Scholar] [CrossRef]
- Kawa, I.A.; Masood, A.; Fatima, Q.; Mir, S.A.; Jeelani, H.; Manzoor, S.; Rashid, F. Endocrine Disrupting Chemical Bisphenol A and Its Potential Effects on Female Health. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.G.B.; Liu, S.; Ying, G.; Zheng, G.J.S.; Lee, J.H.W.; Leung, K.M.Y. The Occurrence and Ecological Risks of Endocrine Disrupting Chemicals in Sewage Effluents from Three Different Sewage Treatment Plants, and in Natural Seawater from a Marine Reserve of Hong Kong. Mar. Pollut. Bull. 2014, 85, 352–362. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, M.; Onanong, S.; Snow, D.D.; Ray, C. Occurrence and Removal of Pharmaceutical Compounds and Steroids at Four Wastewater Treatment Plants in Hawai’i and Their Environmental Fate. Sci. Total Environ. 2018, 631–632, 1360–1370. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Benteke Momba, M.N. Endocrine-Disruptive Chemicals as Contaminants of Emerging Concern in Wastewater and Surface Water: A Review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Čelić, M.; Škrbić, B.D.; Insa, S.; Živančev, J.; Gros, M.; Petrović, M. Occurrence and Assessment of Environmental Risks of Endocrine Disrupting Compounds in Drinking, Surface and Wastewaters in Serbia. Environ. Pollut. 2020, 262, 114344. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Guo, M.; Xu, H.; Zhang, S.; Shi, L.; Yao, C. Occurrence, Distribution, and Risk Assessment of Alkylphenols, Bisphenol A, and Tetrabromobisphenol A in Surface Water, Suspended Particulate Matter, and Sediment in Taihu Lake and Its Tributaries. Mar. Pollut. Bull. 2016, 112, 142–150. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Song, N.; Guo, R.; Chen, M.; Mai, D.; Yan, Z.; Han, Z.; Chen, J. Occurrence, Distribution and Sources of Bisphenol Analogues in a Shallow Chinese Freshwater Lake (Taihu Lake): Implications for Ecological and Human Health Risk. Sci. Total Environ. 2017, 599–600, 1090–1098. [Google Scholar] [CrossRef]
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.-H.; Svoboda, D.; Auerbach, S.S.; et al. A Scoping Review of the Health and Toxicological Activity of Bisphenol A (BPA) Structural Analogues and Functional Alternatives. Toxicology 2019, 424, 152235. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and Its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef]
- Naveira, C.; Rodrigues, N.; Santos, F.S.; Santos, L.N.; Neves, R.A.F. Acute Toxicity of Bisphenol A (BPA) to Tropical Marine and Estuarine Species from Different Trophic Groups. Environ. Pollut. 2021, 268, 115911. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of Endocrine Disrupting Chemicals in Pigs. Environ. Pollut. 2020, 263, 114505. [Google Scholar] [CrossRef] [PubMed]
- Azizi, D.; Arif, A.; Blair, D.; Dionne, J.; Filion, Y.; Ouarda, Y.; Pazmino, A.G.; Pulicharla, R.; Rilstone, V.; Tiwari, B.; et al. A Comprehensive Review on Current Technologies for Removal of Endocrine Disrupting Chemicals from Wastewaters. Environ. Res. 2021, 207, 112196. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Latest Advanced Oxidative Processes Applied for the Removal of Endocrine Disruptors from Aqueous Media—A Critical Report. J. Environ. Chem. Eng. 2021, 9, 105748. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable Polymers and Their Nano-Composites for the Removal of Endocrine-Disrupting Chemicals (EDCs) from Wastewater: A Review. Environ. Res. 2021, 202, 111694. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the Simulation of Interlayer Structure. Appl. Clay Sci. 2019, 169, 40–47. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. In-Depth Study of Heavy Metal Removal by an Etidronic Acid-Functionalized Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2022, 14, 7450–7463. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.-N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of Adsorption–Membrane Hybrid Systems for Water and Wastewater Treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Hao, S.; Jia, Z.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Progress in Adsorptive Membranes for Separation—A Review. Sep. Purif. Technol. 2021, 255, 117772. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Zhang, B.; Fang, J.; Zhu, L. Interfacially Crosslinked β-Cyclodextrin Polymer Composite Porous Membranes for Fast Removal of Organic Micropollutants from Water by Flow-through Adsorption. J. Hazard. Mater. 2020, 384, 121187. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Utomo, D.P. Removal of Organic Pollutants from Rubber Wastewater Using Hydrophilic Nanocomposite RGO-ZnO/PES Hybrid Membranes. J. Environ. Chem. Eng. 2021, 9, 106421. [Google Scholar] [CrossRef]
- Qu, M.; He, D.; Luo, Z.; Wang, R.; Shi, F.; Pang, Y.; Sun, W.; Peng, L.; He, J. Facile Preparation of a Multifunctional Superhydrophilic PVDF Membrane for Highly Efficient Organic Dyes and Heavy Metal Ions Adsorption and Oil/Water Emulsions Separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128231. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Fang, C.; Liu, Z.; Fang, J.; Zhu, L. Macroporous Membranes Doped with Micro-Mesoporous β-Cyclodextrin Polymers for Ultrafast Removal of Organic Micropollutants from Water. Carbohydr. Polym. 2019, 222, 114970. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Y.; Min, G.; Zhang, W.; Lv, L.; Zhang, W. A Mini Review of Multifunctional Ultrafiltration Membranes for Wastewater Decontamination: Additional Functions of Adsorption and Catalytic Oxidation. Sci. Total Environ. 2021, 762, 143083. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur, R.; Turken, T.; Guclu, S.; Pasaoglu, M.E. Advances in Water Treatment by Microfiltration, Ultrafiltration, and Nanofiltration. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 83–128. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of Bisphenol A (BPA) from Water by Various Nanofiltration (NF) and Reverse Osmosis (RO) Membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Zeng, H.; Yang, H.; Shen, J.; Darvishmanesh, S.; Luis, P.; Sotto, A.; Van der Bruggen, B. Fractionation of Direct Dyes and Salts in Aqueous Solution Using Loose Nanofiltration Membranes. J. Membr. Sci. 2015, 477, 183–193. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration Membrane Processes: A Review of Research Trends over the Past Decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Qiu, W.; Yang, H.; Wan, L.; Xu, Z. Co-Deposition of Catechol/Polyethyleneimine on Porous Membranes for Efficient Decolorization of Dye Water. J. Mater. Chem. A 2015, 3, 14438–14444. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, L.; Zhao, Y.; Zhu, L. Interfacially Crosslinked Composite Porous Membranes for Ultrafast Removal of Anionic Dyes from Water through Permeating Adsorption. J. Hazard. Mater. 2017, 337, 217–225. [Google Scholar] [CrossRef]
- Niavarani, Z.; Breite, D.; Prager, A.; Abel, B.; Schulze, A. Estradiol Removal by Adsorptive Coating of a Microfiltration Membrane. Membranes 2021, 11, 99. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Hu, J.; Gao, W. Chemisorption of Estrone in Nylon Microfiltration Membranes: Adsorption Mechanism and Potential Use for Estrone Removal from Water. Water Res. 2012, 46, 873–881. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Orbell, J.; Gray, S. Molecular Simulations of Polyamide Membrane Materials Used in Desalination and Water Reuse Applications: Recent Developments and Future Prospects. J. Membr. Sci. 2017, 524, 436–448. [Google Scholar] [CrossRef]
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.R.M.; Li, K. Poly(Vinylidene Fluoride) (PVDF) Membranes for Fluid Separation. React. Funct. Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Wu, L.; Jin, X.; Zhao, T.; Wang, H.; Dai, Z. Impact Factors of the Degradation of Bisphenol A by Nitrocellulose Membrane under Illumination. J. Environ. Sci. 2021, 100, 193–202. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Xu, Z.; Liu, Y.; Cheng, F. Simultaneous Anti-Fouling and Flux-Enhanced Membrane Distillation via Incorporating Graphene Oxide on PTFE Membrane for Coking Wastewater Treatment. Appl. Surf. Sci. 2020, 531, 147349. [Google Scholar] [CrossRef]
- Dai, R.; Li, J.; Wang, Z. Constructing Interlayer to Tailor Structure and Performance of Thin-Film Composite Polyamide Membranes: A Review. Adv. Colloid Interface Sci. 2020, 282, 102204. [Google Scholar] [CrossRef]
- Lau, W.; Lai, G.; Li, J.; Gray, S.; Hu, Y.; Misdan, N.; Goh, P.; Matsuura, T.; Azelee, I.W.; Ismail, A.F. Development of Microporous Substrates of Polyamide Thin Film Composite Membranes for Pressure-Driven and Osmotically-Driven Membrane Processes: A Review. J. Ind. Eng. Chem. 2019, 77, 25–59. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Polyvinylidene Fluoride (PVDF) Membrane for Oil Rejection from Oily Wastewater: A Performance Review. J. Water Process Eng. 2016, 14, 41–59. [Google Scholar] [CrossRef]
- Sun, S.; Feng, S.; Ji, C.; Shi, M.; He, X.; Xu, F.; Lu, T.J. Microstructural Effects on Permeability of Nitrocellulose Membranes for Biomedical Applications. J. Membr. Sci. 2020, 595, 117502. [Google Scholar] [CrossRef]
- Tang, Y.; Xing, L.; Wang, P. Preparation of a Hydrophilic Nitrocellulose Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022035. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and Perspectives in PTFE Membrane: Preparation, Modification, and Applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Lagergen, S. Zur Theorie der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Carter, M.C.; Kilduff, J.E.; Weber, W.J. Site Energy Distribution Analysis of Pieloaded Adsorbents. Environ. Sci. Technol. 1995, 29, 1773–1780. [Google Scholar] [CrossRef]
- Cerofolini, G.F. Localized Adsorption on Heterogeneous Surfaces. Thin Solid Film. 1974, 23, 129–152. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical Models of Sorption Kinetics Including a Surface Reaction Mechanism: A Review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption Isotherm Models: A Comprehensive and Systematic Review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Jasni, M.J.F.; Arulkumar, M.; Sathishkumar, P.; Mohd Yusoff, A.R.; Buang, N.A.; Gu, F.L. Electrospun Nylon 6,6 Membrane as a Reusable Nano-Adsorbent for Bisphenol A Removal: Adsorption Performance and Mechanism. J. Colloid Interface Sci. 2017, 508, 591–602. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Liu, K.; Jiang, Y.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Rapid Elimination of Trace Bisphenol Pollutants with Porous β-Cyclodextrin Modified Cellulose Nanofibrous Membrane in Water: Adsorption Behavior and Mechanism. J. Hazard. Mater. 2021, 403, 123666. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, J.; Zhang, H.; Liu, K.; Kong, Z.; Dong, Y.; Jin, G.; Tian, J.; Qin, Z. Fabrication of Composite Membrane with Adsorption Property and Its Application to the Removal of Endocrine Disrupting Compounds during Filtration Process. Chem. Eng. J. 2018, 352, 53–63. [Google Scholar] [CrossRef]
- Fan, J.; Luo, J.; Zhang, X.; Zhen, B.; Dong, C.; Li, Y.; Shen, J.; Cheng, Y.; Chen, H. A Novel Electrospun β-CD/CS/PVA Nanofiber Membrane for Simultaneous and Rapid Removal of Organic Micropollutants and Heavy Metal Ions from Water. Chem. Eng. J. 2019, 378, 122232. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Hu, J.; Cai, Q.; Li, X.; Wang, W.; Faraj, Y.; Ju, X.; Xie, R.; Chu, L. β-Cyclodextrin-Modified Graphene Oxide Membranes with Large Adsorption Capacity and High Flux for Efficient Removal of Bisphenol A from Water. J. Membr. Sci. 2020, 595, 117510. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, F.; Zhang, X.; Zhu, R.; Shen, X.; Sun, B.; Wang, B. Design and Synthesis of Organic Rectorite-Based Composite Nanofiber Membrane with Enhanced Adsorption Performance for Bisphenol A. Environ. Sci. Pollut. Res. 2019, 26, 28860–28870. [Google Scholar] [CrossRef]
- Shen, X.; Guo, X.; Zhang, M.; Tao, S.; Wang, X. Sorption Mechanisms of Organic Compounds by Carbonaceous Materials: Site Energy Distribution Consideration. Environ. Sci. Technol. 2015, 49, 4894–4902. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Zheng, Z.; Mu, B. Synthesis of an Absorption Material Based on Oil Shale Semi-Coke: Discussion to Adsorption Mechanism and Corresponding Site Energy Distribution Analysis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128251. [Google Scholar] [CrossRef]
- Kozak, J.; Weber, J.B.; Sheets, T.J. Adsorption of prometryn and metolachlor by selected soil organic matter fractions. Soil Sci. 1983, 136, 94–101. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K. Site Energy Distribution Analysis and Influence of Fe3O4 Nanoparticles on Sulfamethoxazole Sorption in Aqueous Solution by Magnetic Pine Sawdust Biochar. Environ. Pollut. 2018, 233, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tisserat, B.H. Coating Applications to Natural Fiber Composites to Improve Their Physical, Surface and Water Absorption Characters. Ind. Crops Prod. 2018, 112, 196–199. [Google Scholar] [CrossRef]
- Yuan, S.; Tan, Z. Effect and Mechanism of Changes in Physical Structure and Chemical Composition of New Biochar on Cu(II) Adsorption in an Aqueous Solution. Soil Ecol. Lett. 2022, 4, 237–253. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Rana, D.; Matsuura, T.; Tabe-Mohammadi, A. Negatively Charged Polyethersulfone Hollow Fiber Nanofiltration Membrane for the Removal of Bisphenol A from Wastewater. Sep. Purif. Technol. 2010, 73, 92–99. [Google Scholar] [CrossRef]
- Han, J.; Meng, S.; Dong, Y.; Hu, J.; Gao, W. Capturing Hormones and Bisphenol A from Water via Sustained Hydrogen Bond Driven Sorption in Polyamide Microfiltration Membranes. Water Res. 2013, 47, 197–208. [Google Scholar] [CrossRef]
- Tizaoui, C.; Fredj, S.B.; Monser, L. Polyamide-6 for the Removal and Recovery of the Estrogenic Endocrine Disruptors Estrone, 17β-Estradiol, 17α-Ethinylestradiol and the Oxidation Product 2-Hydroxyestradiol in Water. Chem. Eng. J. 2017, 328, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, F.; Wang, D.; Pei, X.F.; Zhang, W.; Jin, J. A Novel Zwitterionic Polyelectrolyte Grafted PVDF Membrane for Thoroughly Separating Oil from Water with Ultrahigh Efficiency. J. Mater. Chem. A 2013, 1, 5758. [Google Scholar] [CrossRef]
- Fridley, G.E.; Holstein, C.A.; Oza, S.B.; Yager, P. The Evolution of Nitrocellulose as a Material for Bioassays. MRS Bull. 2013, 38, 326–330. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, J.; Liu, K.; Liu, N.; Zhou, B. Adsorption of Bisphenol A Based on Synergy between Hydrogen Bonding and Hydrophobic Interaction. Langmuir 2014, 30, 13861–13868. [Google Scholar] [CrossRef] [PubMed]
- Palacio, L.; Calvo, J.I.; Prádanos, P.; Hernández, A.; Väisänen, P.; Nyström, M. Contact Angles and External Protein Adsorption onto UF Membranes. J. Membr. Sci. 1999, 152, 189–201. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Meng, S.; Gao, W. Removal of Ethinylestradiol (EE2) from Water via Adsorption on Aliphatic Polyamides. Water Res. 2012, 46, 5715–5724. [Google Scholar] [CrossRef]
- Huang, J.; Huang, K.; Liu, S.; Luo, Q.; Xu, M. Adsorption Properties of Tea Polyphenols onto Three Polymeric Adsorbents with Amide Group. J. Colloid Interface Sci. 2007, 315, 407–414. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I. Adsorption and Transport of Trace Contaminant Estrone in NF/RO Membranes. Environ. Eng. Sci. 2002, 19, 441–451. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Cao, Z.; Hu, J.; Gao, W. Adsorption of Ethinylestradiol (EE2) on Polyamide 612: Molecular Modeling and Effects of Water Chemistry. Water Res. 2013, 47, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
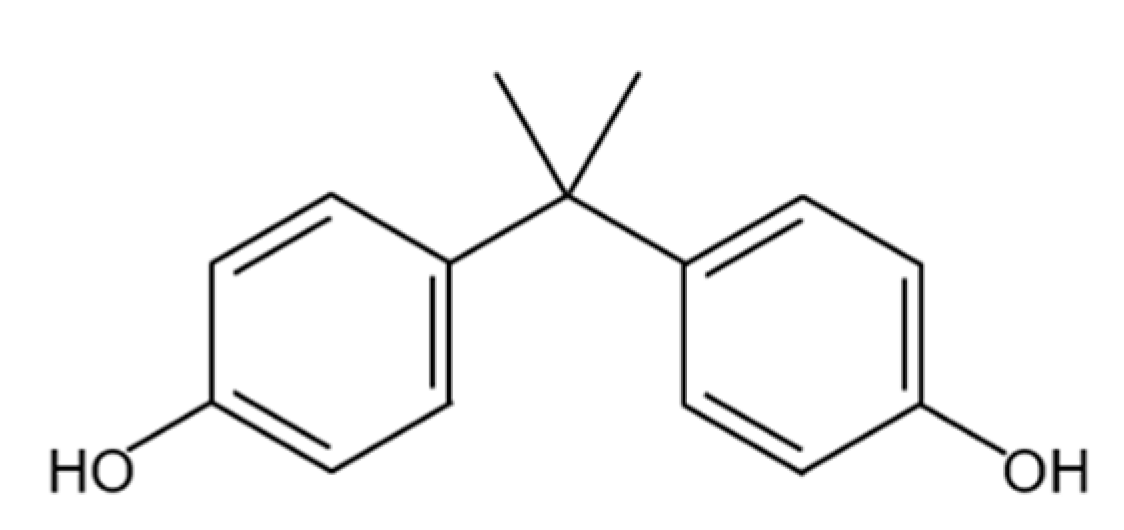

| Membrane Material | Molecular Structure | pH Tolerance | Temperature Tolerance | Performance |
|---|---|---|---|---|
| PA | 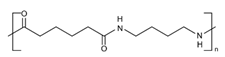 | 3–10 | 100 °C | Excellent mechanical intensity, high separation number, chemical resistance, high thermal stability [39,40] |
| PVDF | 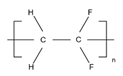 | 3–10 | 100 °C | Low tensile strength, brittle membrane, resistant to dilute acid and weak base, flammable, and produces toxic oxides of nitrogen during combustion [41] |
| NC | 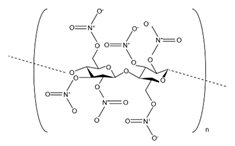 | 3–10 | 75 °C | Preeminent chemical resistance, mechanical stability, strong negative electrostatic, good flexibility [42,43] |
| PTFE | 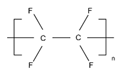 | 3–10 | 100 °C | High water resistance, excellent electrical insulation properties, and a wide range of high and low-temperature use [44] |
| Membrane Material | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 (1/h) | qe (mg/g) | R2 | K2 g/(mg·h) | qe (mg/g) | R2 | |
| PA | 1.739 | 5.637 | 0.996 | 0.625 | 6.063 | 0.965 |
| PVDF | 2.373 | 4.442 | 0.986 | 0.810 | 4.717 | 0.938 |
| NC | 1.086 | 2.134 | 0.967 | 0.419 | 2.320 | 0.916 |
| Membrane Material | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | qmax (mg/g) | R2 | KF (mg/g)/ (mg/L)n | n | R2 | |
| PA | 0.0026 | 161.29 | 1.000 | 0.448 | 1.038 | 0.999 |
| PVDF | 0.0027 | 80.000 | 0.996 | 0.278 | 1.125 | 0.989 |
| NC | 0.0065 | 18.018 | 0.994 | 0.129 | 1.080 | 0.993 |
| Membrane Material | Membrane Type | qm (mg/g) | Ref |
|---|---|---|---|
| PA | MF | 161.29 | This study |
| PVDF | MF | 80.00 | This study |
| NC | MF | 18.02 | This study |
| PTFE | MF | 1.56 | This study |
| NNM | NF | 91.30 | [55] |
| CA-P-CDP | NF | 50.37 | [56] |
| PP-g-SA-HEA-PVDF | Composite membrane | 26.67 | [57] |
| β-CD/CS/PVA | NF | 352.17 | [58] |
| CDGO | Composite membrane | 25.50 | [59] |
| SRt-PAN | NF | 17.50 | [60] |
| Membrane Material | The Energy Range | The Average Site Energy | The Site Energy Heterogeneity |
|---|---|---|---|
| E (kJ/mol) | μ (E*) (kJ/mol) | (kJ/mol) | |
| PA | 5.298–11.173 | 7.940 | 2.059 |
| PVDF | 6.609–11.886 | 9.004 | 1.802 |
| NC | 8.230–13.514 | 10.635 | 1.907 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Jiang, X.; Zhou, Y.; Fan, J.; Zeng, G. Microfiltration Membranes for the Removal of Bisphenol A from Aqueous Solution: Adsorption Behavior and Mechanism. Water 2022, 14, 2306. https://doi.org/10.3390/w14152306
Sun J, Jiang X, Zhou Y, Fan J, Zeng G. Microfiltration Membranes for the Removal of Bisphenol A from Aqueous Solution: Adsorption Behavior and Mechanism. Water. 2022; 14(15):2306. https://doi.org/10.3390/w14152306
Chicago/Turabian StyleSun, Jiaoxia, Xueting Jiang, Yao Zhou, Jianxin Fan, and Guoming Zeng. 2022. "Microfiltration Membranes for the Removal of Bisphenol A from Aqueous Solution: Adsorption Behavior and Mechanism" Water 14, no. 15: 2306. https://doi.org/10.3390/w14152306





