Nitrogen Removal of Water and Sediment in Grass Carp Aquaculture Ponds by Mixed Nitrifying and Denitrifying Bacteria and Its Effects on Bacterial Community
Abstract
:1. Introduction
2. Materials and Methods
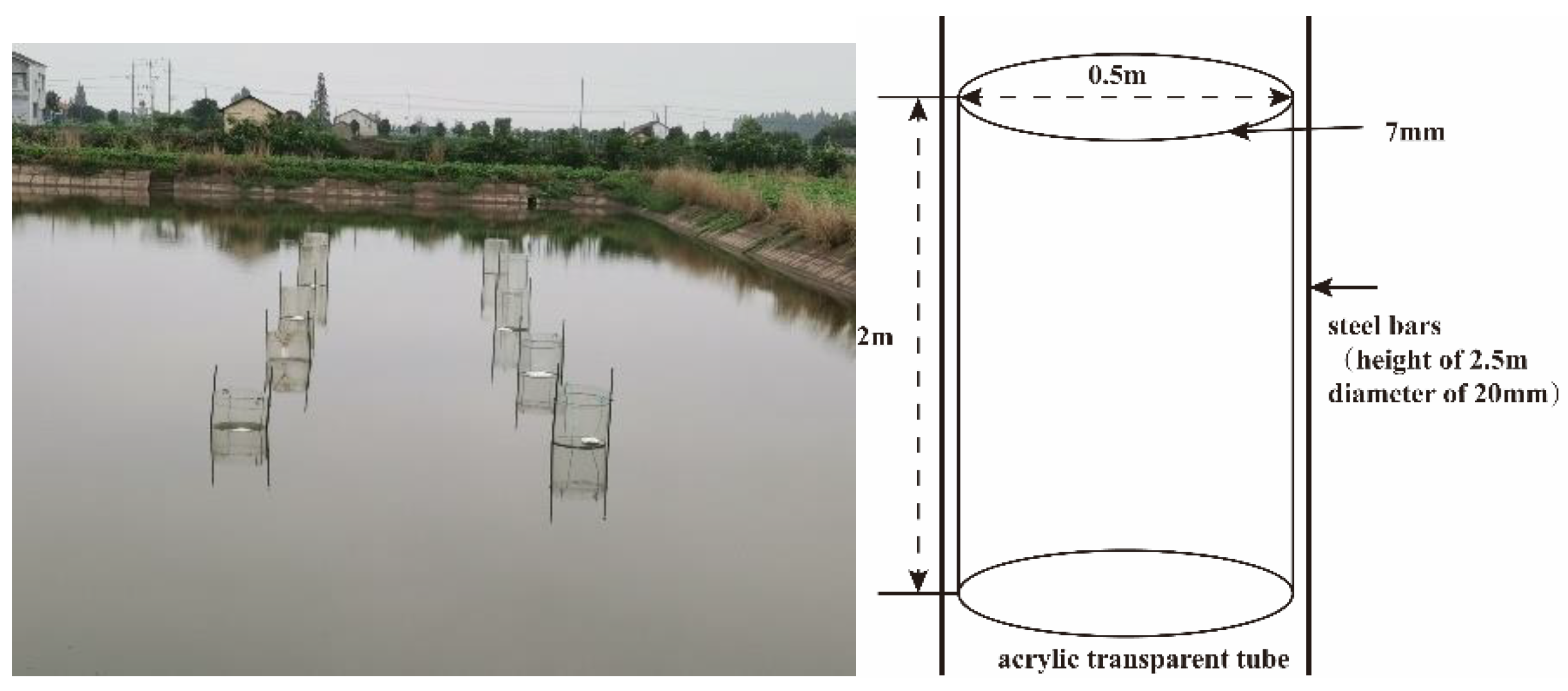
2.1. Pond Environment
2.2. Sample Collection
2.3. Determination of Carbon and Nitrogen
2.4. DNA Extraction
2.5. High-Throughput Sequence Analysis
Quality filtering of the raw tags was performed under specific filtering conditions with QIIME (Version 1.9.0, http://qiime.org/index.html, accessed on 1 March 2021) in order to obtain high-quality clean tags. The tags were compared with the reference database (Gold database; http://drive5.com/uchime/uchime_download.html, accessed on 1 March 2021) using the UCHIME algorithm (UCHIME Algorithm; http://www.drive5.com/usearch/manual/uchime_algo.html, accessed on 1 March 2021) to detect chimeric sequences, which were then removed. The data were de-noised, and the sequences with more than 150 base pairs (bp), less than 8 bp, and ambiguous base pairs and chimeric sequences were eliminated to ensure the high quality of sequence reads. All similar high-quality reads with a similarity of ≥97% were aggregated into an operational classification unit (OTU) [35]. An “OTU table” showing the number of reads from each sample assigned to each OTU was created by using the usearch_global command. α and β diversity were calculated by QIIME based on processed pyrosequencing data (Version 5.1) software. Similarly, QIIME was used to analyze species composition by statistical analysis after the feature table after singleton was removed. The environmental data were combined with the statistics of the feature table after singleton was removed. Environmental and microbial redundancy analyses (RDA) were calculated by QIIME [36].
2.6. Statistical Analyses
3. Results
3.1. Changes in Water Quality Index
3.2. C/N Ratio in Water and Sediment
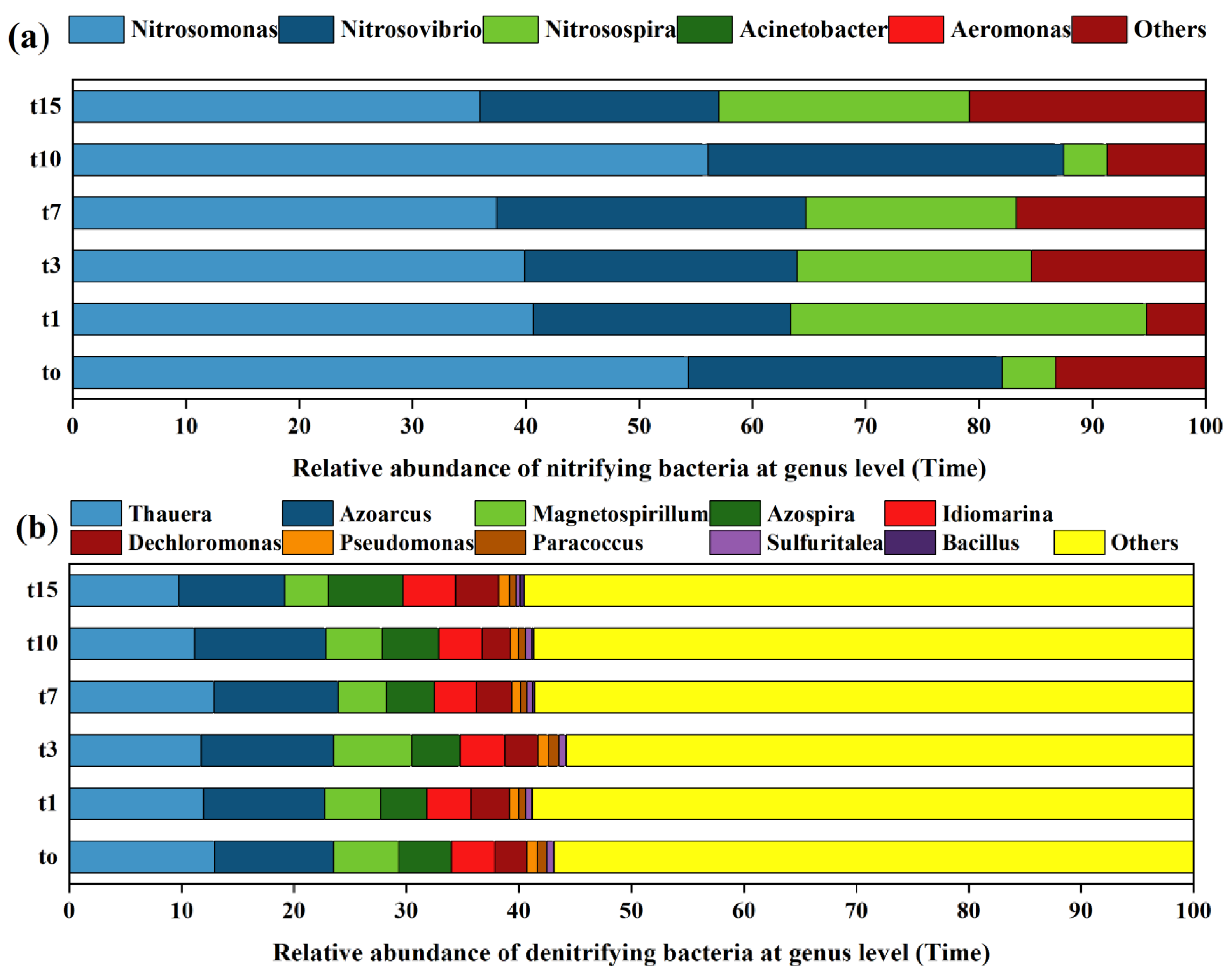
3.3. Species Composition Analysis of Nitrifying and Denitrifying Bacteria
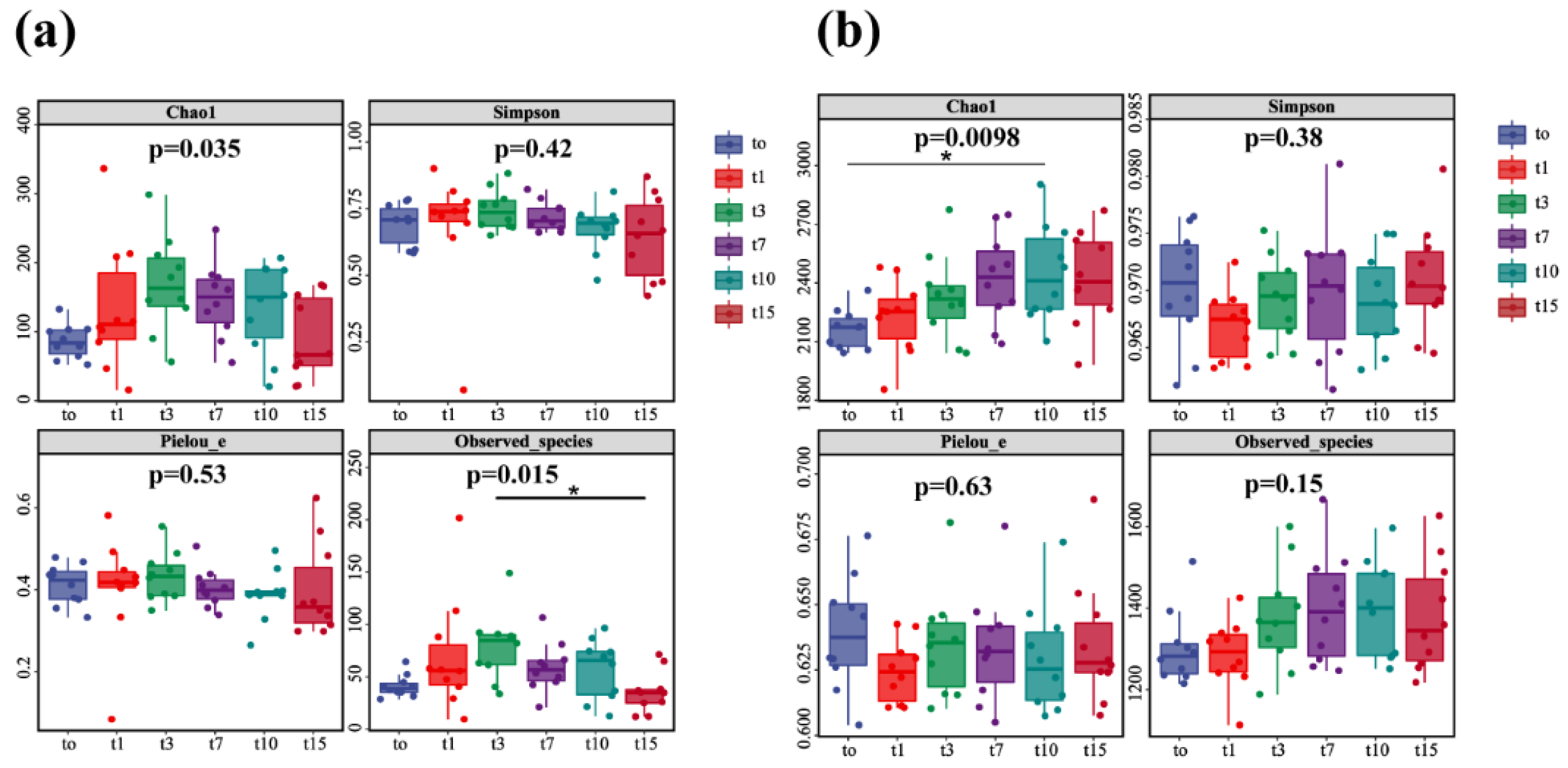
3.4. Diversity Analysis of Nitrifying and Denitrifying Bacteria
3.5. Environmental and Microbial Redundancy Analysis (RDA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nhan, D.K.; Verdegem, M.C.J.; Milstein, A.; Verreth, J.A.V. Water and nutrient budgets of ponds in integrated agriculture-aquaculture systems in the Mekong Delta, Vietnam. Aquac. Res. 2008, 39, 1216–1228. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, S.P.; Wang, X.F.; Huang, L.G.; Liu, J.X.; Xiong, X.Y. Nitrogen and phosphorus budgets of polyculture system of mud crab scylla paramamosain, tiger shrimp penaeus monodon and razor clamsinonovacula constricta. Res. Appl. 2021, 40, 483–491. [Google Scholar]
- Kolath, A.S.; Pedersen, C.S.; Gangelhof, U.L.; Egemose, S. Biodiversity and Sediment Contamination in Wet Stormwater Ponds Depending on Design and Catchment Characteristics. Sustainability 2021, 13, 11809. [Google Scholar] [CrossRef]
- Tulsankar, S.S.; Cole, A.J.; Gagnon, M.M.; Fotedar, R. Effects of seasonal variations and pond age on trace elements and their correlations with plankton productivity in commercial freshwater crayfish (cherax cainii austin, 2002) earthen ponds. Aquac. Res. 2020, 51, 1913–1922. [Google Scholar] [CrossRef]
- Ke, X.; Bao, Q.H.; Qi, Y.F.; Huang, X.Y.; Zhang, H.J. Toxicity assessment of sediments from the Liaohe River Protected Area (China) under the influence of ammonia nitrogen, heavy metals and organic contaminants. Environ. Toxicol. Phar. 2018, 59, 34–42. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hu, H.W.; Huang, X.; Ge, T.D.; Li, Y.F.; Zhu, Z.K.; Liu, X.M.; Tan, W.F.; Jia, Z.J.; Di, H.J.; et al. Canonical ammonia oxidizers, rather than comammox Nitrospira, dominated autotrophic nitrification during the mineralization of organic substances in two paddy soils. Soil Biol. Biochem. 2021, 156, 108192. [Google Scholar] [CrossRef]
- Wang, W.W.; Jiang, X.; Zheng, B.H.; Chen, J.Y.; Zhao, L.; Zhang, B.; Wang, S.H. Composition, mineralization potential and release risk of nitrogen in the sediments of Keluke Lake, a Tibetan Plateau freshwater lake in China. R. Soc. Open Sci. 2018, 5, 180612. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; He, Y.; Zhu, Y.S.; Huang, M.S.; Zhang, Y.P. Biogeochemical sulfur cycling coupling with dissimilatory nitrate reduction processes in freshwater sediments. Environ. Rev. 2018, 26, 121–132. [Google Scholar] [CrossRef]
- Coyne, K.J.; Parker, A.E.; Lee, C.K.; Sohm, J.A.; Kalmbach, A.; Gunderson, T.; Leon-Zayas, R.; Capone, D.G.; Carpenter, E.J.; Cary, S.C. The distribution and relative ecological roles of autotrophic and heterotrophic diazotrophs in the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 2020, 96, fiaa010. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, M.C.M.; Begum, M.; Frerichs, G.N.; Millar, S. The Ingestion of Bacteria in Suspension by the Tilapia Oreochromis-Niloticus. Aquaculture 1989, 81, 373–378. [Google Scholar] [CrossRef]
- Dilmi, A.; Refes, W.; Meknachi, A. Effects of C/N Ratio on Water Quality, Growth Performance, Digestive Enzyme Activity and Antioxidant Status of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) in Biofloc Based Culture System. Turk. J. Fish. Aquat. Sci. 2022, 22, 19754. [Google Scholar] [CrossRef]
- Gou, J.W.; Hong, C.U.; Deng, M.; Chen, J.Y.; Hou, J.; Li, D.P.; He, X.G. Effect of Carbon to Nitrogen Ratio on Water Quality and Community Structure Evolution in Suspended Growth Bioreactors through Biofloc Technology. Water 2019, 11, 1640. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Lee, J.; Lee, K.; Kim, M. The effects on operation conditions of sludge retention time and carbon/nitrogen ratio in an intermittently aerated membrane bioreactor (IAMBR). Bioresour. Technol. 2008, 99, 5397–5401. [Google Scholar] [CrossRef] [PubMed]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Wang, L.; Ma, L.; Sun, J.; Zhang, Y.; Zhou, Q.H.; Wu, Z.B.; He, F. Effects of different aquaculture methods for introduced bivalves (Hyriopsis cumingii) on seston removal and phosphorus balance at the water-sediment interface. J. Freshwater Ecol. 2018, 33, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part II-mechanical and biological control methods. Harmful Algae 2021, 109, 102119. [Google Scholar] [CrossRef]
- Yin, H.B.; Yang, C.H.; Yang, P.; Kaksonen, A.H.; Douglas, G.B. Contrasting effects and mode of dredging and in situ adsorbent amendment for the control of sediment internal phosphorus loading in eutrophic lakes. Water Res. 2021, 189, 116644. [Google Scholar] [CrossRef]
- Sun, Q.; Ding, S.M.; Chen, M.S.; Gao, S.S.; Lu, G.H.; Wu, Y.X.; Gong, M.D.; Wang, D.; Wang, Y. Long-term effectiveness of sediment dredging on controlling the contamination of arsenic, selenium, and antimony. Environ. Pollut. 2019, 245, 725–734. [Google Scholar] [CrossRef]
- Yang, L.M.; Chen, F.F.; Xiao, Y.; Li, H.K.; Li, J.; Leng, L.J.; Liu, H.; Zhong, Y.M.; Li, K.; Lu, Q.; et al. Microbial community-assisted water quality control and nutrients recovery: Emerging technologies for the sustainable development of aquaponics. J. Chem. Technol. Biot. 2019, 94, 2405–2411. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.W.; Tang, R.; He, X.G.; Li, L.; Takagi, Y.; Li, D.P. Improvement of Muscle Quality of Grass Carp (Ctenopharyngodon idellus) With a Bio-Floating Bed in Culture Ponds. Front. Physiol. 2019, 10, 683. [Google Scholar] [CrossRef]
- Costa, L.F.A.; Lima, J.S.; Siqueira, A.M.A.; da Silva, E.C.C.; Lima, V.C.F.; da Silva, L.A.; da Silva, S.R.; Santos, T.F.; Fugimura, M.M.S.; Vaz, L.J.; et al. Effect of multi-species probiotic administration in Colossoma macropomum juvenile rearing: Supplementation and bioremediation. Aquac. Nutr. 2021, 27, 1721–1729. [Google Scholar] [CrossRef]
- Nathanailides, C.; Kolygas, M.; Choremi, K.; Mavraganis, T.; Gouva, E.; Vidalis, K.; Athanassopoulou, F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes 2021, 6, 76. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probioticBacillusspecies in aquaculture with special reference toBacillus subtilis. Rev. Aquac. 2021, 13, 862–906. [Google Scholar] [CrossRef]
- Hahn, M.W. The microbial diversity of inland waters. Curr. Opin. Biotech. 2006, 17, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Liu, Z.; Li, Y.J.; Wang, J.F. Bacterial diversity in saline-alkali ponds rearing common carp (Cyprinus carpio) as revealed by 16S rRNA gene sequences. Biologia 2014, 69, 727–734. [Google Scholar] [CrossRef]
- Tang, X.M.; Li, L.L.; Shao, K.Q.; Wang, B.W.; Cai, X.L.; Zhang, L.; Chao, J.Y.; Gao, G. Pyrosequencing analysis of free-living and attached bacterial communities in Meiliang Bay, Lake Taihu, a large eutrophic shallow lake in China. Can. J. Microbiol. 2015, 61, 22–31. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.L.; Liu, B.; Xuan, Y.M.; Jiang, M.; Pan, Y.S.; Zhang, Y.M.; Gong, Y.P.; Lu, X.P.; Yu, D.S.; et al. Dynamic changes of microbial communities in Litopenaeus vannamei cultures and the effects of environmental factors. Aquaculture 2016, 455, 97–108. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Tang, J.Y.; Dai, Y.X. Bacterial communities in Chinese grass carp (Ctenopharyngodon idellus) farming ponds. Aquac. Res. 2013, 45, 138–149. [Google Scholar] [CrossRef]
- Xia, Z.G.; Wang, Q.; She, Z.L.; Gao, M.C.; Zhao, Y.G.; Guo, L.; Jin, C.J. Nitrogen removal pathway and dynamics of microbial community with the increase of salinity in simultaneous nitrification and denitrification process. Sci. Total Environ. 2019, 697, 134047. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.K.L.Y.; Xu, A.L.; Song, Z.W. Effect of Microecological Preparation on the Establishment of Nitrification Function in Mariculture System. J. Microbiol. 2019, 39, 16–21. [Google Scholar]
- Jia, J.P.J.L.; Wang, Y.J.; Li, Y.; Li, X.H.; Wu, W.B.; Fu, S.S.; Yuan, Q.S. Study on nitrogen removal from low C/N wastewater by denitrification microbial fuel cell. Environ. Pollut. Control. 2021, 43, 937–941. [Google Scholar]
- Yang, K.W.L.Y.; Wu, Z.Y.; Tong, L.; Feng, L. Effects of denitrifying bacteria inoculation on bacterial communities in a lake. Safety Environ. Eng. 2020, 27, 81–87. [Google Scholar]
- Chinese, N.E.P.A. Water and Wastewater Monitoring Methods; Chinese Environmental Science Publishing House: Beijing, China, 2012. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.C.; Dong, H.L.; Yu, B.S.; Lv, G.; Deng, S.C.; Berzins, N.; Dai, M.H. Diversity and Abundance of Ammonia-Oxidizing Archaea and Bacteria in Qinghai Lake, Northwestern China. Geomicrobiol. J 2009, 26, 199–211. [Google Scholar] [CrossRef]
- Mosier, A.C.; Francis, C.A. Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ. Microbiol. 2008, 10, 3002–3016. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Hou, L.J.; Liu, M.; Lu, M.; Zhao, H.; Yin, G.Y.; Zhou, J.L. Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl. Microbiol. Biot. 2013, 97, 8351–8363. [Google Scholar] [CrossRef]
- Bock, E.; Wagner, M. Oxidation of Inorganic Nitrogen Compounds as an Energy Source. In Prokaryotes: A Handbook on the Biology of Bacteria, 3rd ed.; 2006; Volume 2, pp. 457–495. [Google Scholar] [CrossRef]
- Castignetti, D.; Gunner, H.B. Sequential Nitrification by an Alcaligenes Sp and Nitrobacter-Agilis. Can. J. Microbiol. 1980, 26, 1114–1119. [Google Scholar] [CrossRef]
- Srithep, P.; Pornkulwat, P.; Limpiyakorn, T. Contribution of ammonia-oxidizing archaea and ammonia-oxidizing bacteria to ammonia oxidation in two nitrifying reactors. Environ. Sci. Pollut. R. 2018, 25, 8676–8687. [Google Scholar] [CrossRef]
- Wang, J.L.; Gong, B.Z.; Wang, Y.M.; Wen, Y.H.; Zhou, J.; He, Q. The potential multiple mechanisms and microbial communities in simultaneous nitrification and denitrification process treating high carbon and nitrogen concentration saline wastewater. Bioresour. Technol. 2017, 243, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Head, I.M.; Hiorns, W.D.; Embley, T.M.; Mccarthy, A.J.; Saunders, J.R. The Phylogeny of Autotrophic Ammonia-Oxidizing Bacteria as Determined by Analysis of 16s Ribosomal-Rna Gene-Sequences. J. Gen. Microbiol. 1993, 139, 1147–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purkhold, U.; Wagner, M.; Timmermann, G.; Pommerening-Roser, A.; Koops, H.P. 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: Extension of the dataset and proposal of a new lineage within the nitrosomonads. Int. J. Syst. Evol. Microbiol. 2003, 53, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.B.; O’Mullan, G.D. Worldwide distribution of Nitrosococcus oceani, a marine ammonia-oxidizing gamma-proteobacterium, detected by PCR and sequencing of 16S rRNA and amoA genes. Appl. Environ. Microbiol. 2002, 68, 4153–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, D.D.; Drahos, D.D.; Marsh, L.; Flick, G.J. Evaluation of nitrifying bacteria product to improve nitrification efficacy in recirculating aquaculture systems. Aquac. Eng. 2010, 43, 78–82. [Google Scholar] [CrossRef]
- Benakova, A.; Wanner, J. Application of fluorescence in situ hybridization for the study and characterization of nitrifying bacteria in nitrifying/denitrifying wastewater treatment plants. Environ. Technol. 2013, 34, 2415–2422. [Google Scholar] [CrossRef]
- Toor, U.A.; Han, D.W.; Kim, D.J. N2O emission during wastewater nitrification with enriched nitrifying bacteria. Desalin Water Treat. 2016, 57, 661–669. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, S.; Yuan, H.; Fu, F.; Zhang, Y. Effects of chloramphenicol on the bacterial community structure and simultaneous nitrification and denitrification performance in a sequencing biofilm batch reactor. J. Water Process Eng. 2021, 42, 102095. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Liu, Y.; Gao, X.Y.; Ai, G.M.; Miao, L.L.; Liu, Z.P. Characterization of a marine origin aerobic nitrifying-denitrifying bacterium. J. Biosci. Bioeng. 2012, 114, 33–37. [Google Scholar] [CrossRef]
- Preena, P.G.; Kumar, V.J.R.; Singh, I.S.B. Nitrification and denitrification in recirculating aquaculture systems: The processes and players. Rev. Aquac. 2021, 13, 2053–2075. [Google Scholar] [CrossRef]
- Li, X.M.; Xue, S.X.; Zhao, Y.C. Control and effect of nitrifying bacteria in factory culture. Tianjin Aquat. 2004, 2, 15–20. [Google Scholar]
- Pan, J.M. Utilization of commercial nitrobacteria addtive in the inoculation of a new recirculating aquaculture system. J. Fish. 2004, 28, 419–424. [Google Scholar]
- Ju, Z.; Zhou, C.L.; Du, J. Studies on the culture and Applicayion of a nitrite-oxidizing bacterium with high nitrification activity. Microbiol. China 2005, 5, 56–61. [Google Scholar]
- Koops, H.P.; Pommerening-Roser, A. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol. Ecol. 2001, 37, 1–9. [Google Scholar] [CrossRef]
- Lin, Y.; He, Y.L.; Kong, H.N.; Liu, B.B.; Li, Y.; Inamon, Y. Isolation and characterization of heterotrophic nitrifying bacteria in MBR. J. Environ. Sci. 2005, 17, 589–592. [Google Scholar]
- Fujitani, H.; Nomachi, M.; Takahashi, Y.; Hasebe, Y.; Eguchi, M.; Tsuneda, S. Successful enrichment of low-abundant comammox Nitrospira from nitrifying granules under ammonia-limited conditions. FEMS Microbiol. Lett. 2020, 367, fnaa025. [Google Scholar] [CrossRef]
- Foesel, B.U.; Gieseke, A.; Schwermer, C.; Stief, P.; Koch, L.; Cytryn, E.; de la Torre, J.R.; van Rijn, J.; Minz, D.; Drake, H.L.; et al. Nitrosomonas Nm143-like ammonia oxidizers and Nitrospira marina-like nitrite oxidizers dominate the nitrifier community in a marine aquaculture biofilm. FEMS Microbiol. Ecol. 2008, 63, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.-Y.; Qu, Y.; Yu, D.-S.; Guo, S.-S.; Yang, R.-X. Comparison of Heterotrophic Nitrification and Aerobic Denitrification System by Strain qy37 and Its Accelerating Removal Characteristic of NH4+-N. Huan Jing Ke Xue Huanjing Kexue 2010, 31, 1819–1826. [Google Scholar]
- Foss, S.; Harder, J. Thauera linaloolentis sp. nov. and Thauera terpenica sp. nov., isolated on oxygen-containing monoterpenes (linalool, menthol, and eucalyptol) and nitrate. Syst. Appl. Microbiol. 1998, 21, 365–373. [Google Scholar] [CrossRef]
- Ma, W.W.; Han, Y.X.; Ma, W.C.; Han, H.J.; Zhu, H.; Xu, C.Y.; Li, K.; Wang, D.X. Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Bioresour. Technol. 2017, 244, 84–91. [Google Scholar] [CrossRef]
- Mechichi, T.; Stackebrandt, E.; Gad’on, N.; Fuchs, G. Phylogenetic and metabolic diversity of bacteria degrading aromatic compounds under denitrifying conditions, and description of Thauera phenylacetica sp nov., Thauera aminoaromatica sp. nov., and Azoarcus buckelii sp nov. Arch. Microbiol. 2002, 178, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Scholten, E.; Lukow, T.; Auling, G.; Kroppenstedt, R.M.; Rainey, F.A.; Diekmann, H. Thauera mechernichensis sp nov., an aerobic denitrifier from a leachate treatment plant. Int. J. Syst. Bacteriol. 1999, 49, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Song, B.K.; Palleroni, N.J.; Kerkhof, L.J.; Haggblom, M.M. Characterization of halobenzoate-degrading. denitrifying Azoarcus and Thauera isolates and description of Thauera chlorobenzoica sp. nov. Int. J. Syst. Evol. Microbiol. 2001, 51, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reino, C.; Suarez-Ojeda, M.E.; Perez, J.; Carrera, J. Kinetic and microbiological characterization of aerobic granules performing partial nitritation of a low-strength wastewater at 10 degrees C. Water Res. 2016, 101, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Hira, D.; Aiko, N.; Yabuki, Y.; Fujii, T. Impact of aerobic acclimation on the nitrification performance and microbial community of landfill leachate sludge. J. Environ. Manag. 2018, 209, 188–194. [Google Scholar] [CrossRef]
- Khardenavis, A.A.; Kapley, A.; Purohit, H.J. Simultaneous nitrification and denitrification by diverse Diaphorobacter sp. Appl. Microbiol. Biot. 2007, 77, 403–409. [Google Scholar] [CrossRef]
- Liu, C.; Hou, L.J.; Liu, M.; Zheng, Y.L.; Yin, G.Y.; Han, P.; Dong, H.P.; Gao, J.; Gao, D.Z.; Chang, Y.K.; et al. Coupling of denitrification and anaerobic ammonium oxidation with nitrification in sediments of the Yangtze Estuary: Importance and controlling factors. Estuar. Coast. Shelf Sci. 2019, 220, 64–72. [Google Scholar] [CrossRef]
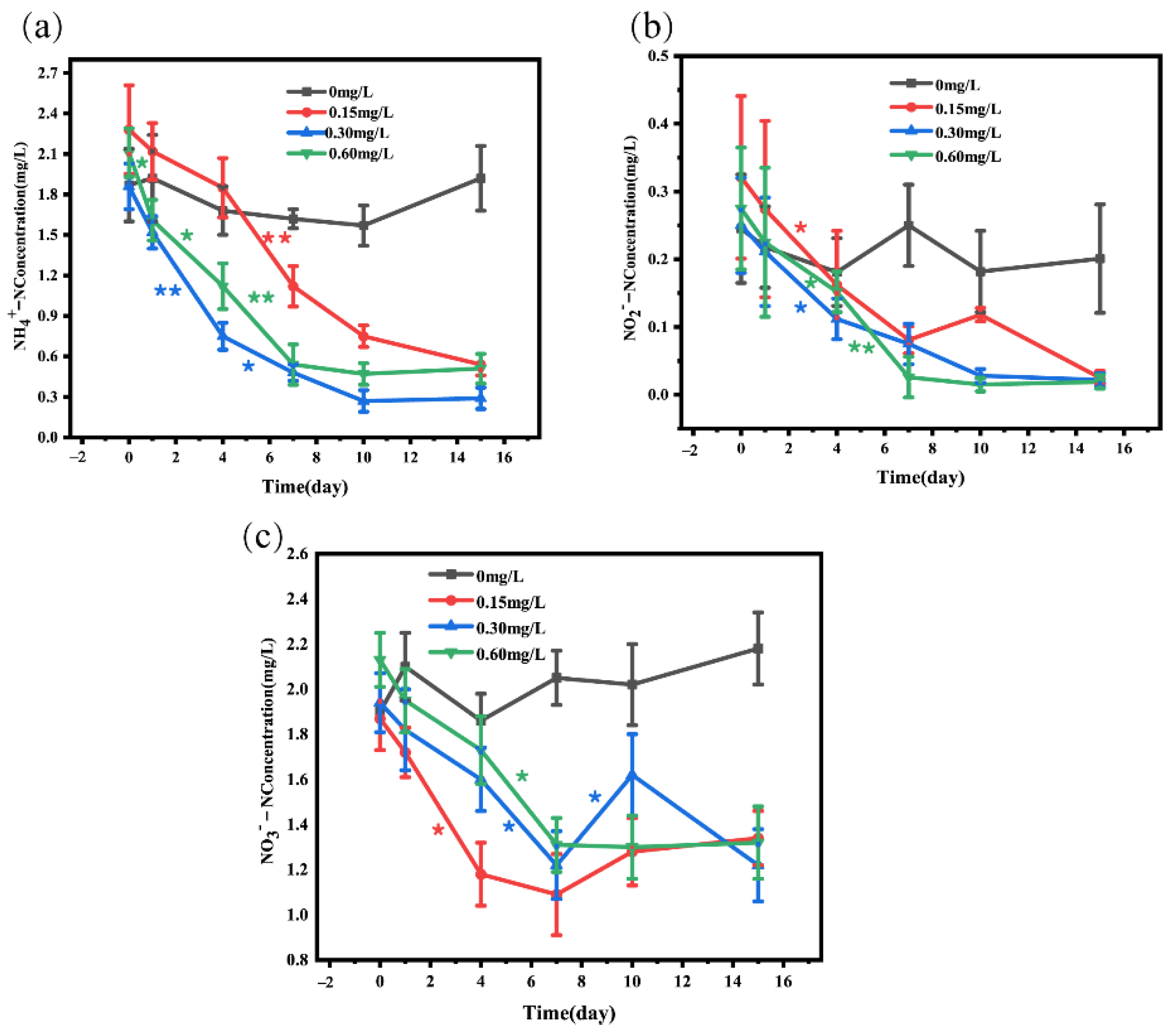
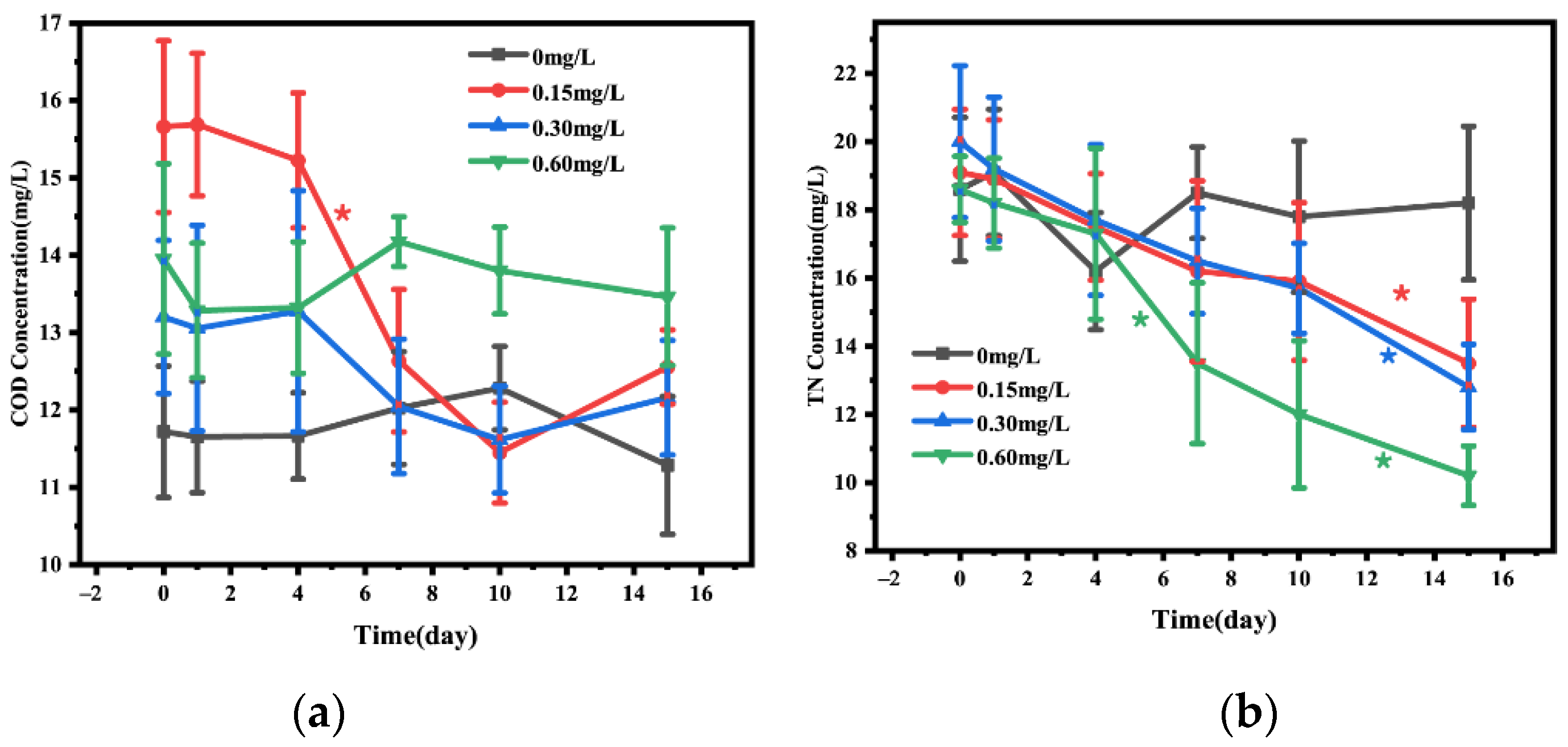

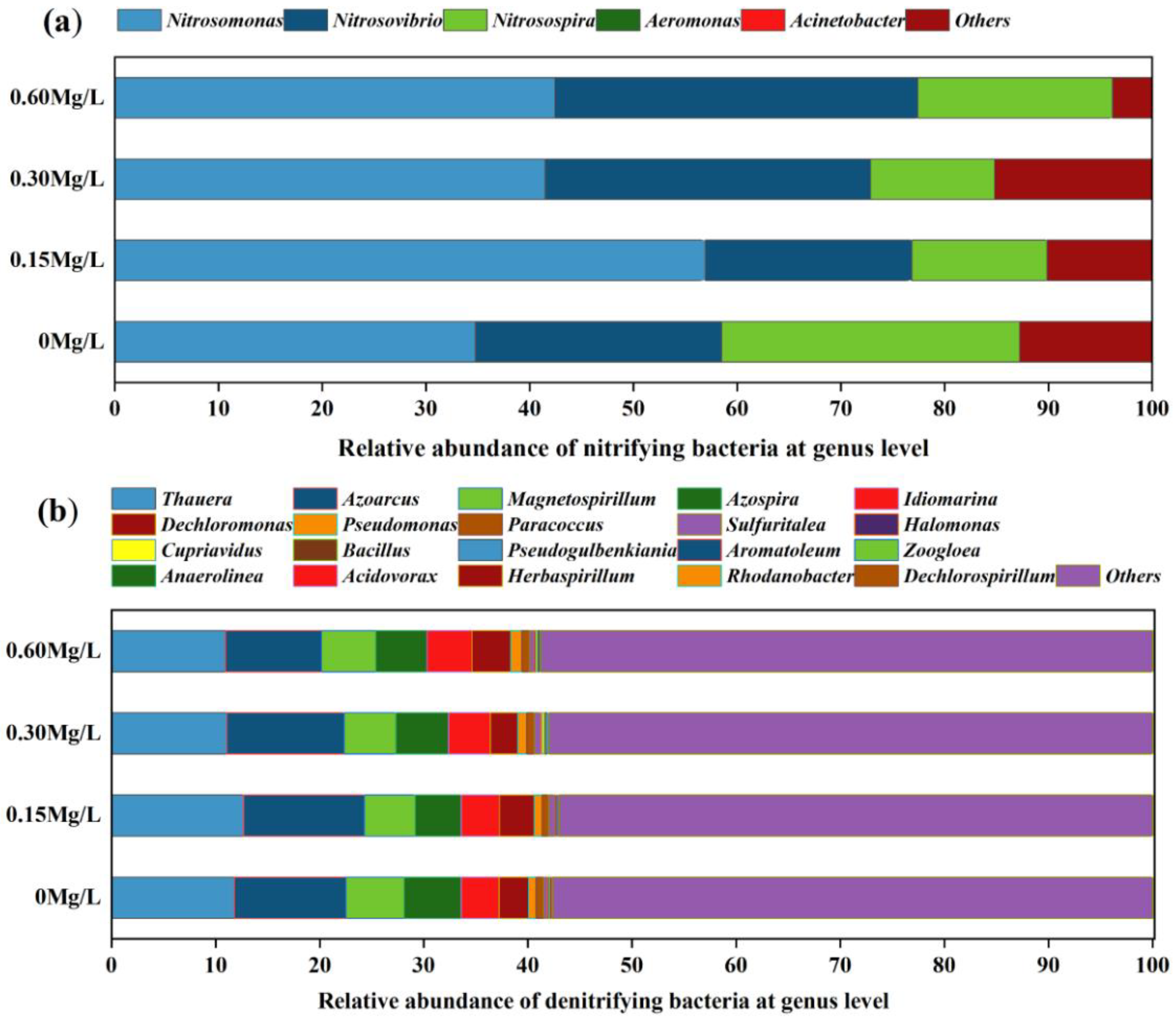


| Group | Time (Day) | Water | TM | DO (mg/L) | pH | ||||
|---|---|---|---|---|---|---|---|---|---|
| NH4+-N (mg/L) | NO2−-N (mg/L) | NO3−-N (mg/L) | TN (mg/L) | COD (mg/L) | |||||
| 0 m/L | 0 | 1.87 ± 0.27 | 0.25 ± 0.08 | 1.90 ± 0.37 | 18.6 ± 2.11 | 11.72 ± 0.85 | 10.0 ± 0.02 | 9.73 ± 0.56 | 8.62 ± 0.41 |
| 1 | 1.92 ± 0.32 | 0.22 ± 0.06 | 2.10 ± 0.35 | 19.1 ± 1.85 | 11.65 ± 0.72 | 8.5 ± 0.01 | 8.95 ± 0.37 | 8.92 ± 0.15 | |
| 4 | 1.68 ± 0.18 | 0.18 ± 0.05 | 1.86 ± 0.32 | 16.2 ± 1.72 | 11.66 ± 0.56 | 9.5 ± 0.01 | 10.50 ± 0.34 | 8.67 ± 0.47 | |
| 7 | 1.62 ± 0.07 | 0.25 ± 0.06 | 2.05 ± 0.42 | 18.5 ± 1.34 | 12.03 ± 0.73 | 6.7 ± 0.02 * | 10.32 ± 0.42 | 8.70 ± 0.17 | |
| 10 | 1.57 ± 0.15 | 0.18 ± 0.06 | 2.02 ± 0.28 | 17.8 ± 2.22 | 12.28 ± 054 | 8.6 ± 0.01 | 9.97 ± 0.55 | 8.72 ± 0.26 | |
| 15 | 1.92 ± 0.24 | 0.20 ± 0.08 | 2.18 ± 0.26 | 18.2 ± 2.25 | 11.28 ± 0.89 | 8.2 ± 0.01 | 10.89 ± 0.87 | 8.69 ± 0.28 | |
| 0.15 mg/L | 0 | 2.28 ± 0.33 | 0.32 ± 0.12 | 1.87 ± 0.34 | 19.1 ± 1.85 | 15.66 ± 1.52 | 10.1 ± 0.01 | 10.02 ± 0.35 | 8.57 ± 0.45 |
| 1 | 2.12 ± 0.21 | 0.27 ± 0.13 | 1.72 ± 0.31 | 18.9 ± 1.74 | 15.69 ± 1.33 | 8.4 ± 0.02 | 9.52 ± 0.38 | 9.10 ± 0.47 | |
| 4 | 1.85 ± 0.22 | 0.16 ± 0.08 * | 1.18 ± 0.24 * | 17.5 ± 1.56 | 15.23 ± 1.87 | 9.5 ± 0.02 | 11.12 ± 0.77 | 9.34 ± 0.37 | |
| 7 | 1.12 ± 0.15 ** | 0.08 ± 0.02 | 1.09 ± 0.18 | 16.2 ± 2.65 | 12.64 ± 0.92 * | 6.8 ± 0.01 * | 11.34 ± 0.67 | 9.13 ± 0.29 | |
| 10 | 0.75 ± 0.08 | 0.12 ± 0.01 | 1.28 ± 0.15 | 15.9 ± 2.31 | 11.45 ± 0.65 | 8.6 ± 0.02 | 10.18 ± 0.65 | 9.24 ± 0.26 | |
| 15 | 0.54 ± 0.08 | 0.03 ± 0.01 | 1.34 ± 0.12 | 13.5 ± 1.88 * | 12.56 ± 0.48 | 8.3 ± 0.01 | 11.52 ± 0.59 | 9.16 ± 0.25 | |
| 0.30 mg/L | 0 | 1.86 ± 0.17 | 0.25 ± 0.07 | 1.94 ± 0.33 | 20.0 ± 2.23 | 13.20 ± 0.99 | 10.2 ± 0.01 | 9.85 ± 0.27 | 8.72 ± 0.37 |
| 1 | 1.52 ± 0.12 | 0.21 ± 0.08 | 1.82 ± 0.18 | 19.2 ± 2.11 | 13.06 ± 1.33 | 8.4 ± 0.02 | 8.88 ± 0.85 | 8.42 ± 0.31 | |
| 4 | 0.75 ± 0.10 ** | 0.11 ± 0.03 * | 1.60 ± 0.24 | 17.7 ± 2.21 | 13.28 ± 1.56 | 9.6 ± 0.01 | 9.87 ± 0.86 | 8.55 ± 0.23 | |
| 7 | 0.48 ± 0.06 * | 0.08 ± 0.03 | 1.22 ± 0.15 * | 16.5 ± 1.54 | 12.05 ± 0.87 | 6.8 ± 0.01 * | 9.23 ± 0.36 | 8.79 ± 0.28 | |
| 10 | 0.27 ± 0.08 | 0.03 ± 0.01 | 1.62 ± 0.18 * | 15.7 ± 1.32 | 11.62 ± 0.69 | 8.5 ± 0.01 | 9.17 ± 0.28 | 8.62 ± 0.44 | |
| 15 | 0.29 ± 0.08 | 0.02 ± 0.01 | 1.22 ± 0.16 | 12.8 ± 1.25 * | 12.16 ± 0.74 | 8.2 ± 0.02 | 10.03 ± 0.27 | 8.34 ± 0.28 | |
| 0.60 mg/L | 0 | 2.11 ± 0.18 | 0.28 ± 0.09 | 2.13 ± 0.32 | 18.6 ± 0.97 | 13.95 ± 1.23 | 10.0 ± 0.02 | 9.77 ± 0.55 | 8.65 ± 0.37 |
| 1 | 1.61 ± 0.15 * | 0.23 ± 0.11 | 1.95 ± 0.24 | 18.2 ± 1.32 | 13.29 ± 0.87 | 8.5 ± 0.01 | 9.03 ± 0.58 | 8.55 ± 0.35 | |
| 4 | 1.12 ± 0.17 * | 0.15 ± 0.03 * | 1.73 ± 0.15 | 17.3 ± 2.51 | 13.32 ± 0.85 | 9.5 ± 0.02 | 9.92 ± 0.64 | 8.69 ± 0.42 | |
| 7 | 0.54 ± 0.15 ** | 0.03 ± 0.01 ** | 1.31 ± 0.22* | 13.5 ± 2.36 * | 14.18 ± 032 | 6.9 ± 0.01 * | 9.55 ± 0.36 | 8.72 ± 0.39 | |
| 10 | 0.47 ± 0.08 | 0.02 ± 0.01 | 1.30 ± 0.24 | 12.0 ± 2.16 | 13.80 ± 0.56 | 8.6 ± 0.01 | 9.26 ± 0.38 | 8.24 ± 0.41 | |
| 15 | 0.51 ± 0.11 | 0.02 ± 0.01 | 1.32 ± 0.26 | 10.2 ± 0.87 * | 13.46 ± 0.89 | 8.2 ± 0.01 | 10.13 ± 0.95 | 8.13 ± 0.32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Hu, Z.; Li, D.; Tang, R. Nitrogen Removal of Water and Sediment in Grass Carp Aquaculture Ponds by Mixed Nitrifying and Denitrifying Bacteria and Its Effects on Bacterial Community. Water 2022, 14, 1855. https://doi.org/10.3390/w14121855
Han D, Hu Z, Li D, Tang R. Nitrogen Removal of Water and Sediment in Grass Carp Aquaculture Ponds by Mixed Nitrifying and Denitrifying Bacteria and Its Effects on Bacterial Community. Water. 2022; 14(12):1855. https://doi.org/10.3390/w14121855
Chicago/Turabian StyleHan, Dengfeng, Zhenyi Hu, Dapeng Li, and Rong Tang. 2022. "Nitrogen Removal of Water and Sediment in Grass Carp Aquaculture Ponds by Mixed Nitrifying and Denitrifying Bacteria and Its Effects on Bacterial Community" Water 14, no. 12: 1855. https://doi.org/10.3390/w14121855
APA StyleHan, D., Hu, Z., Li, D., & Tang, R. (2022). Nitrogen Removal of Water and Sediment in Grass Carp Aquaculture Ponds by Mixed Nitrifying and Denitrifying Bacteria and Its Effects on Bacterial Community. Water, 14(12), 1855. https://doi.org/10.3390/w14121855







