Characteristics and Causes of Changing Groundwater Quality in the Boundary Line of the Middle and Lower Yellow River (Right Bank)
Abstract
:1. Introduction
2. Date and Materials
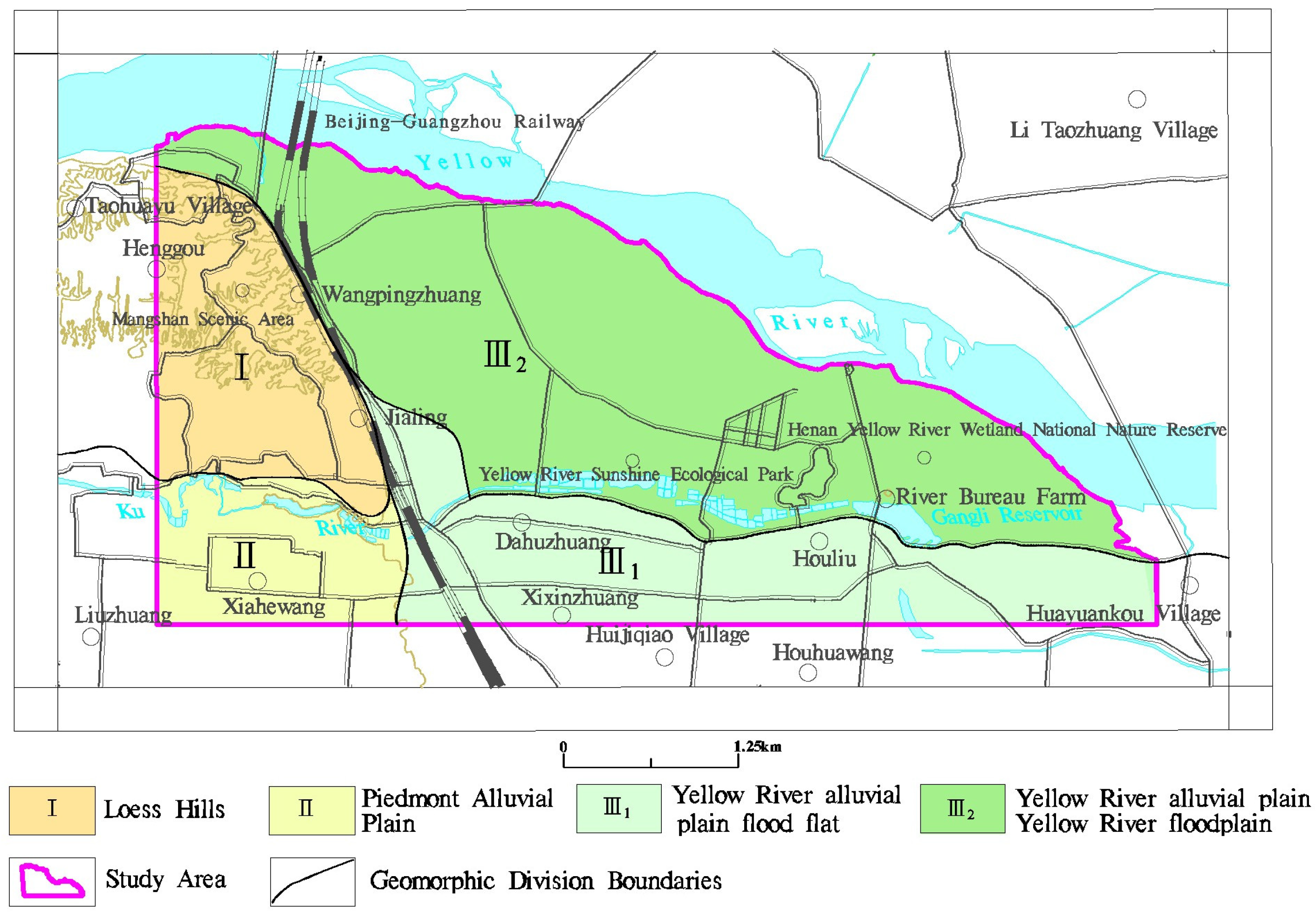
2.1. Study Area
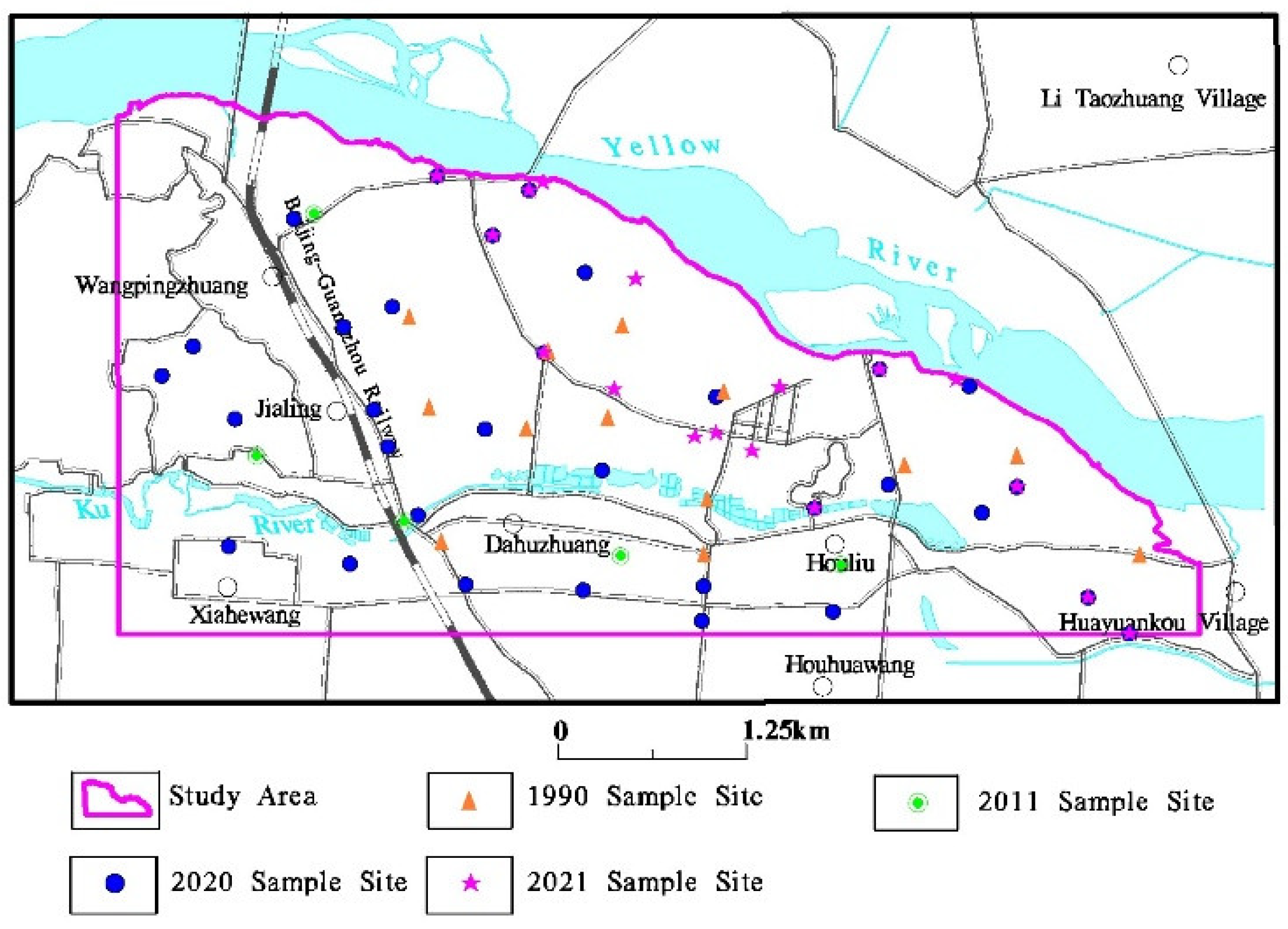
2.2. Sample Collection
2.3. Methods
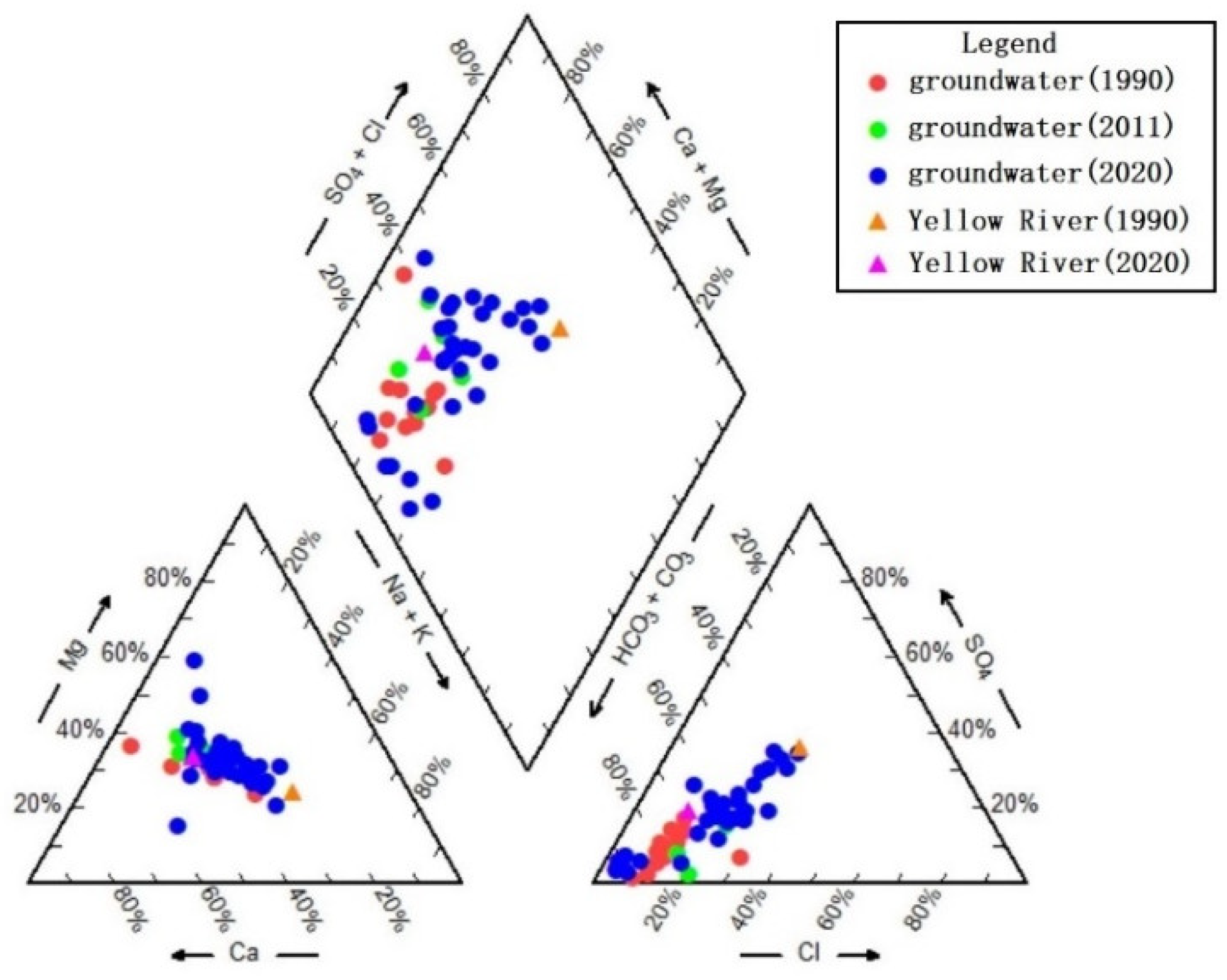
2.3.1. Piper Diagram
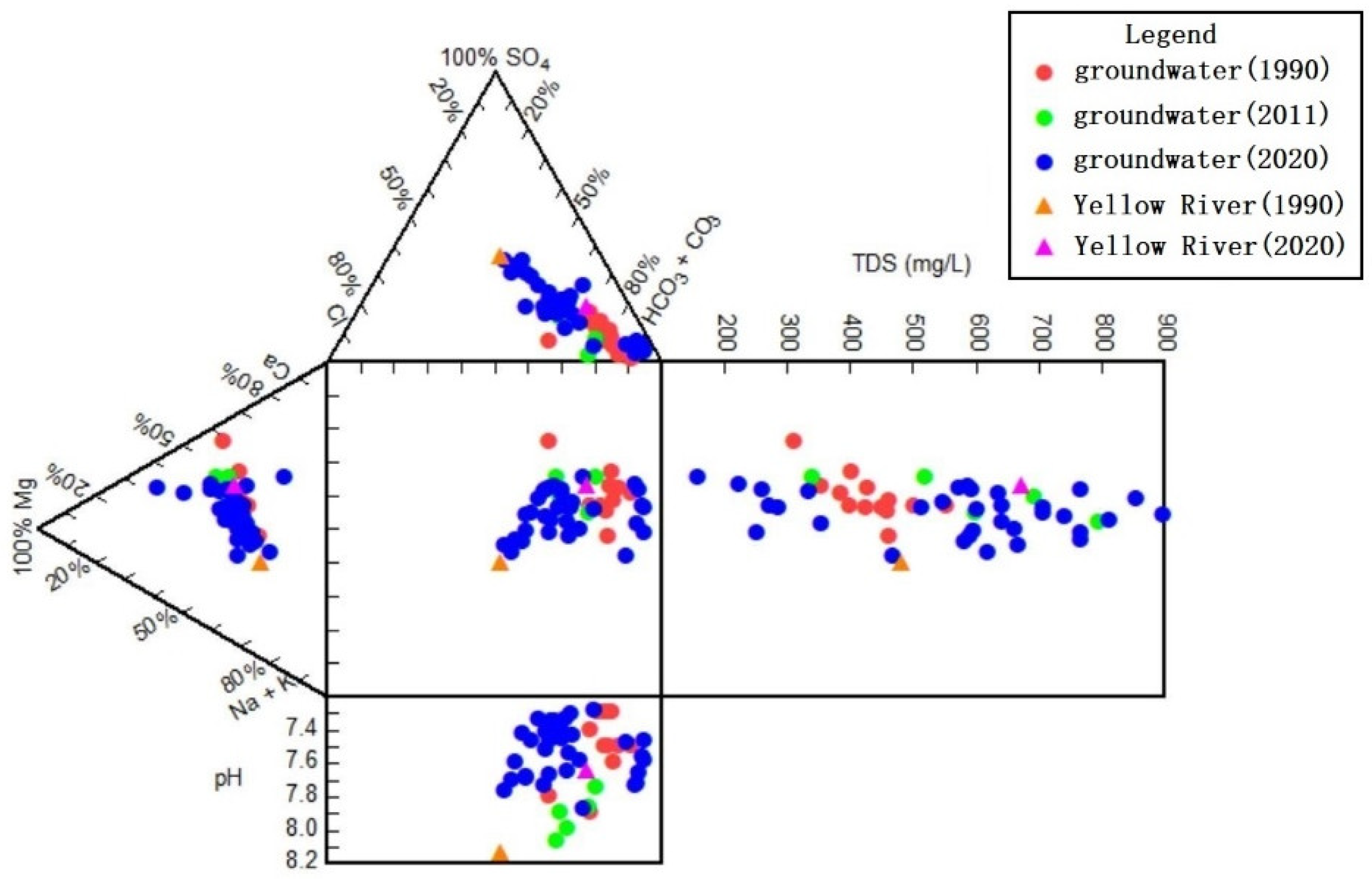
2.3.2. Durov Diagram
2.3.3. Spatial Analysis
3. Results
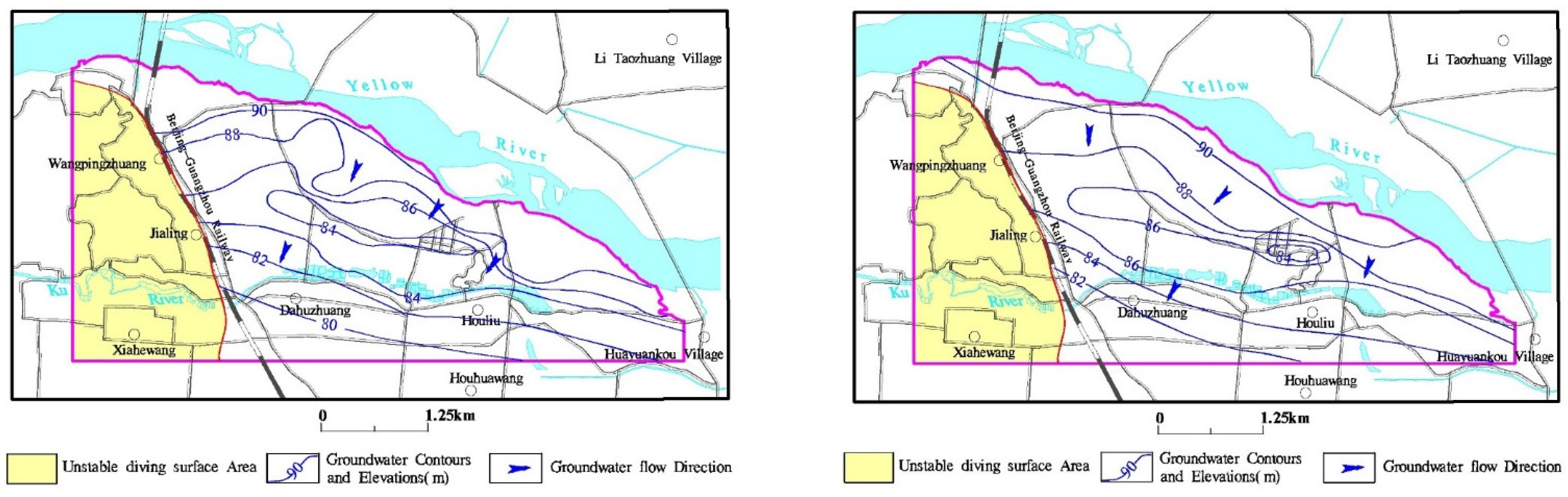
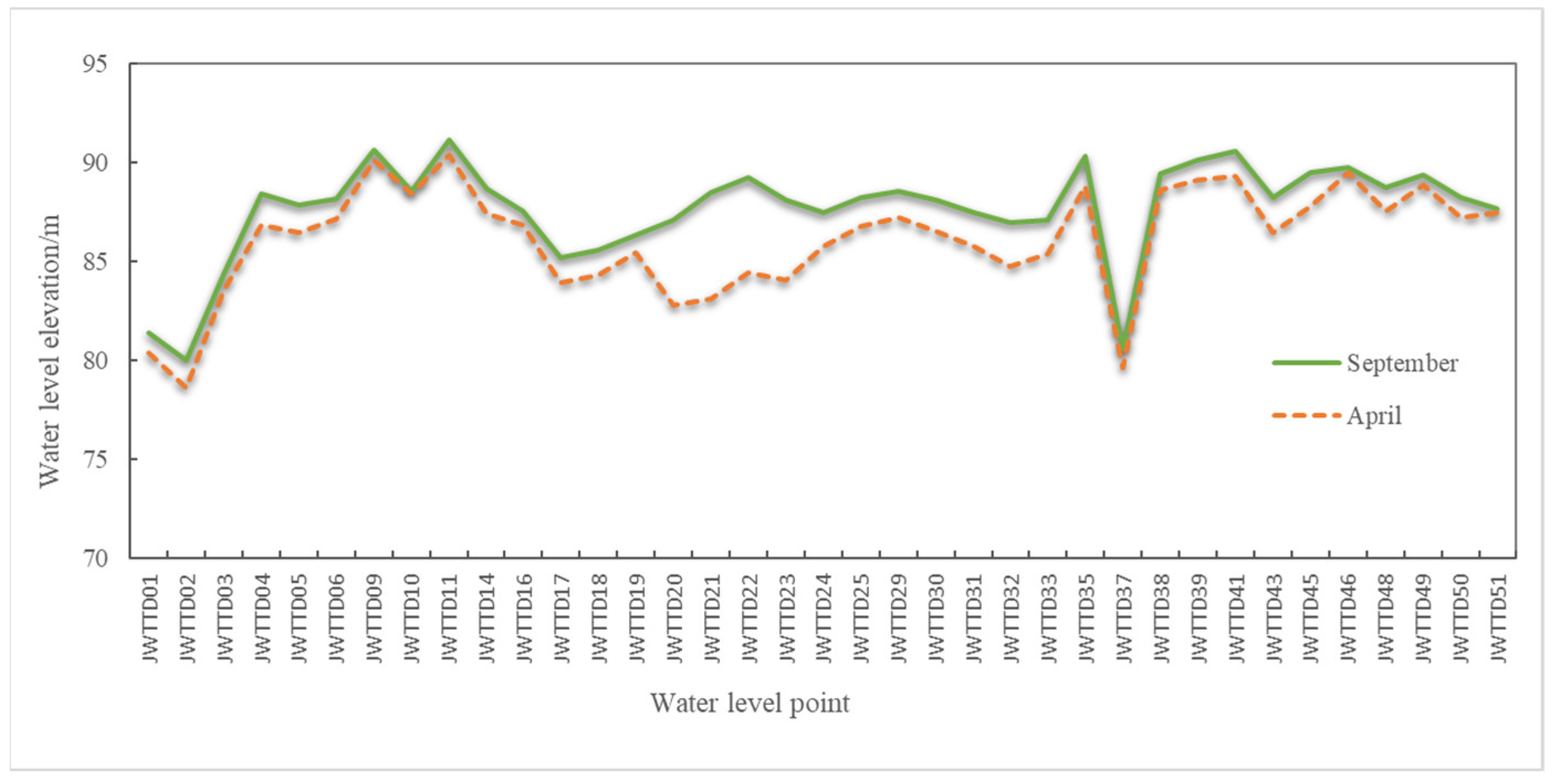
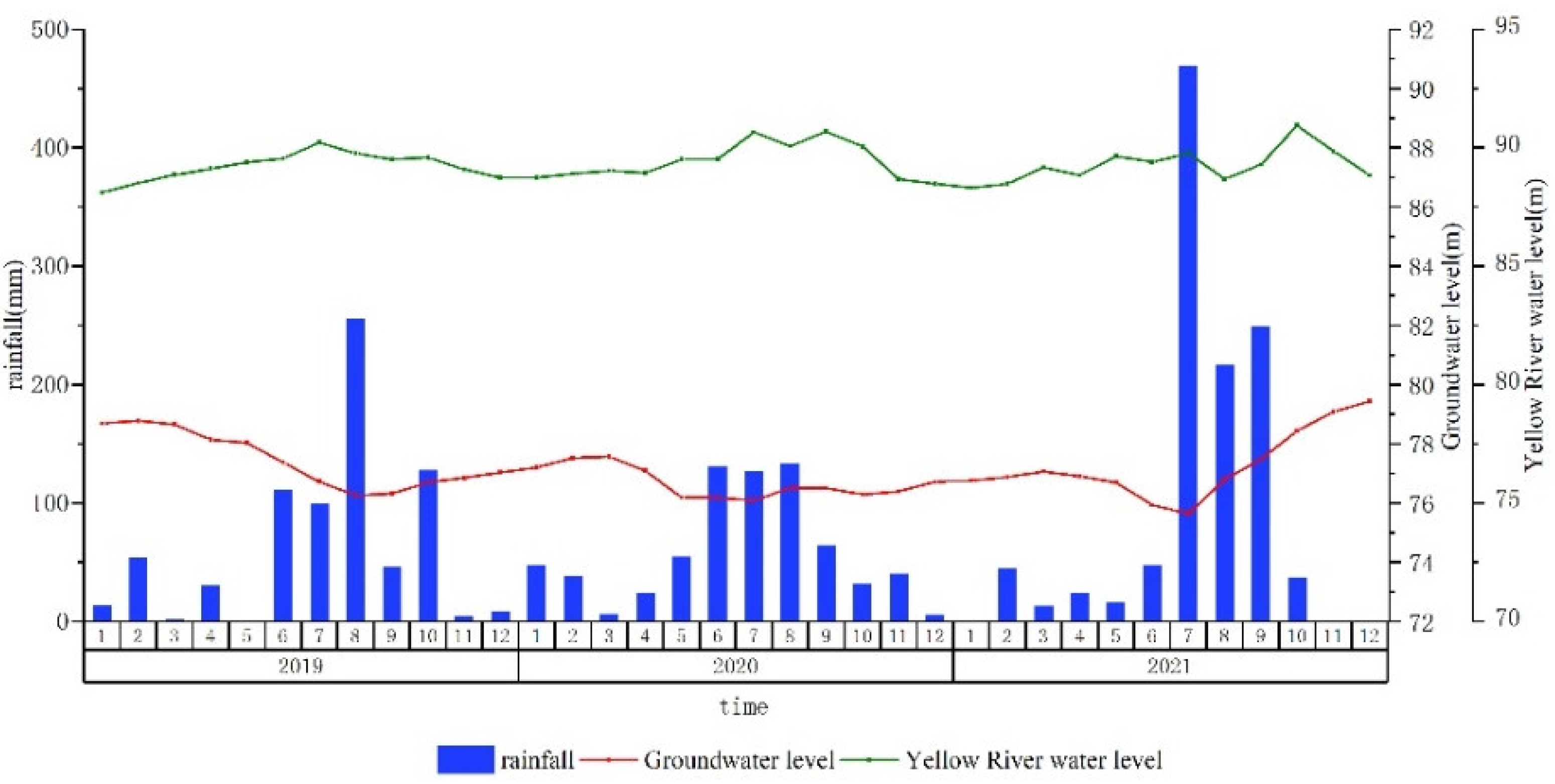
3.1. Change of Groundwater Levels
3.2. Characteristics of Groundwater Quality
3.2.1. Results of Statistical Analysis
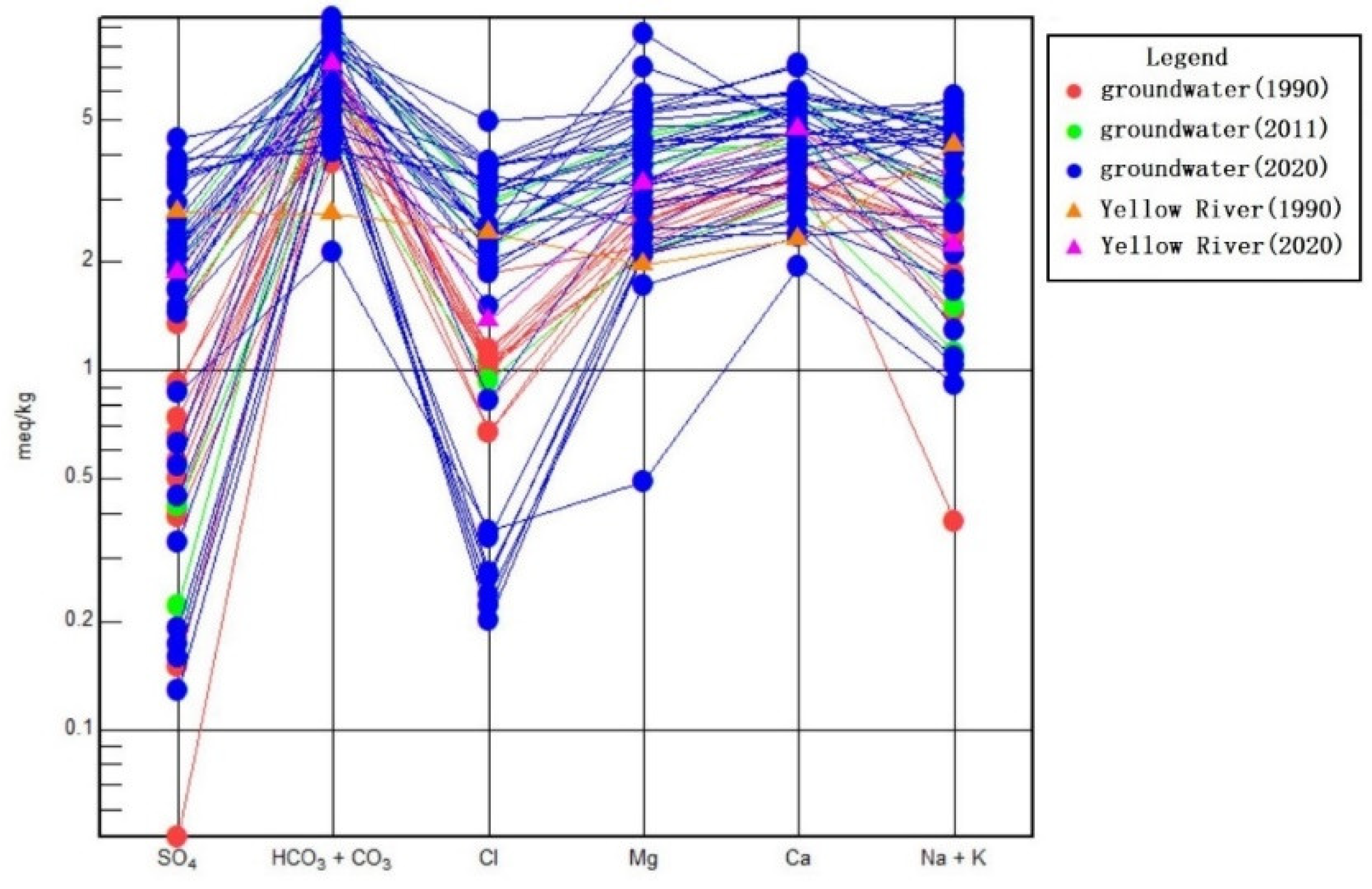
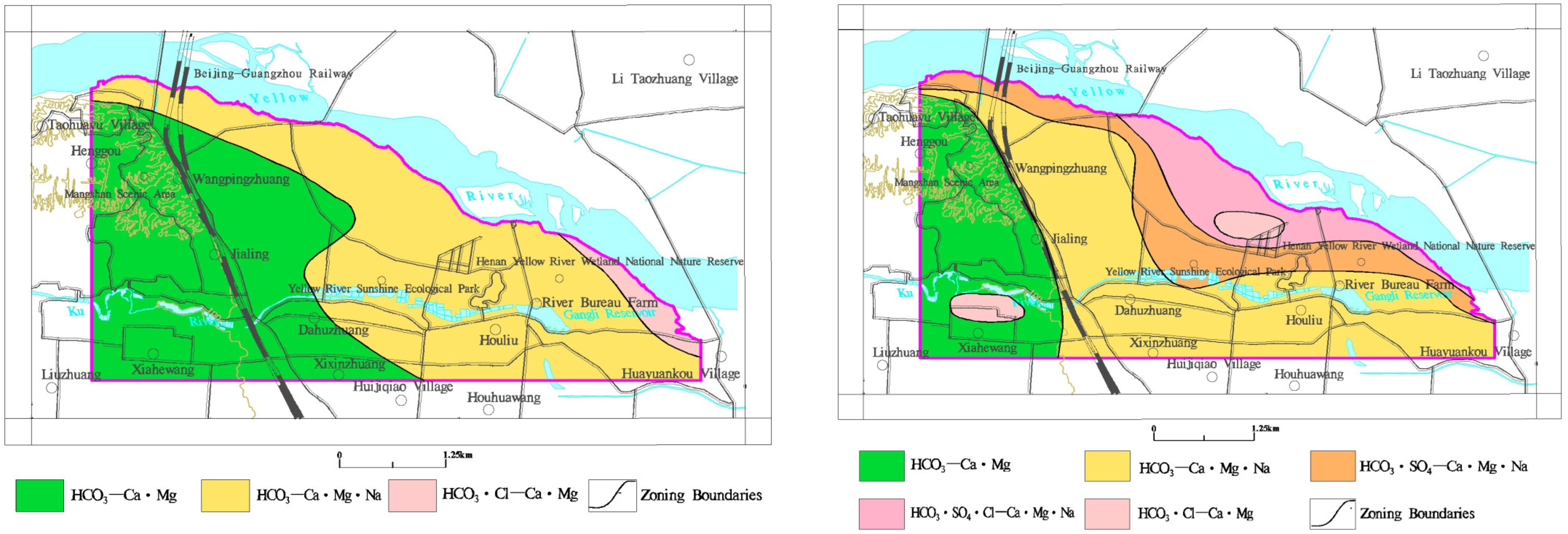
3.2.2. Characteristics of Groundwater Chemical Types
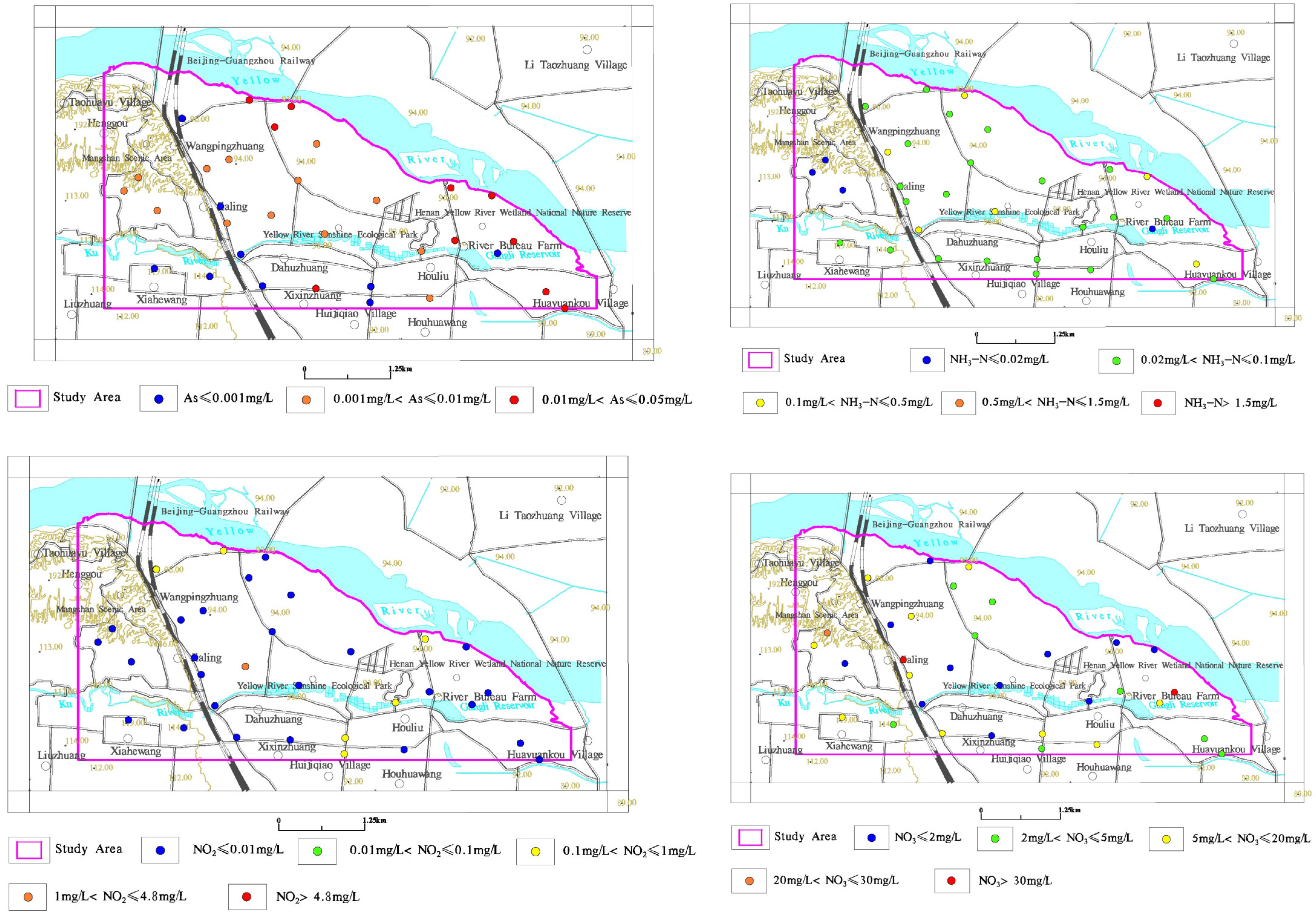
3.2.3. Spatial Distribution Characteristics of Typical Indicators
3.3. Study of the Chemical Causes of Groundwater
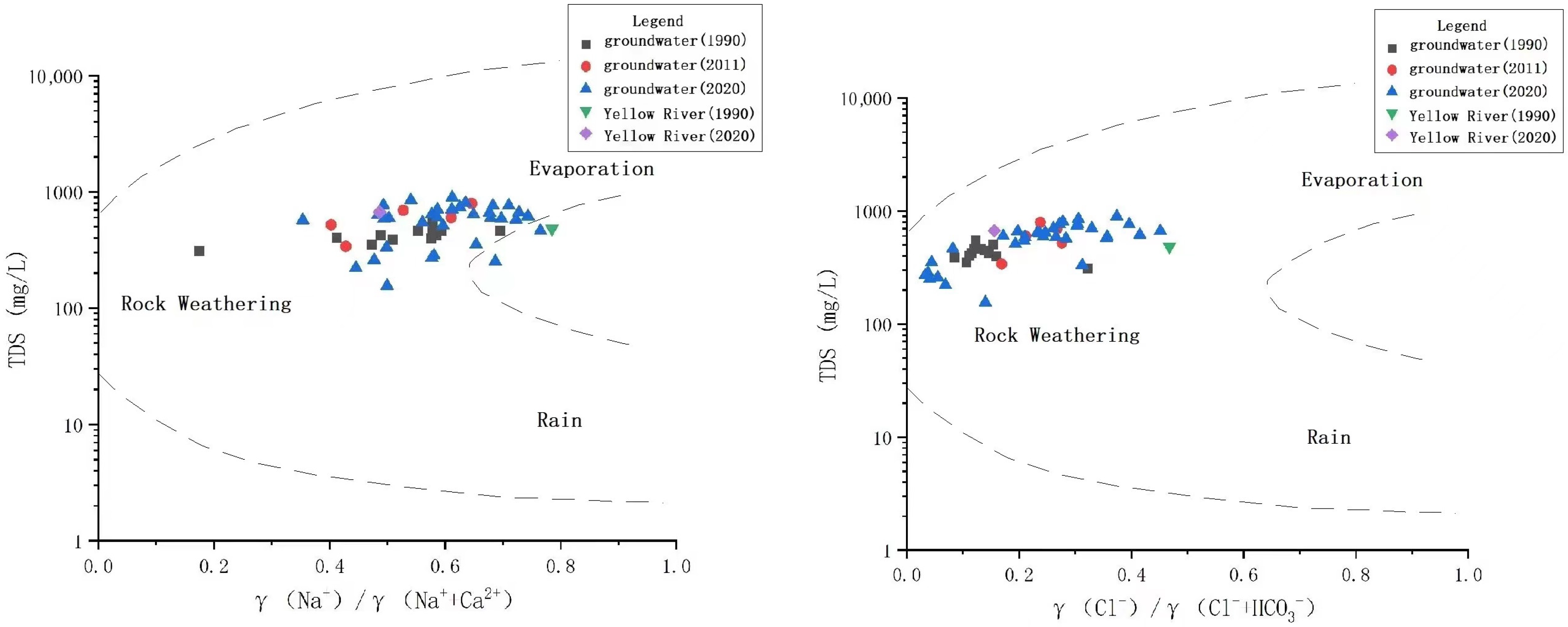
3.3.1. Analysis Based on Gibbs Diagram
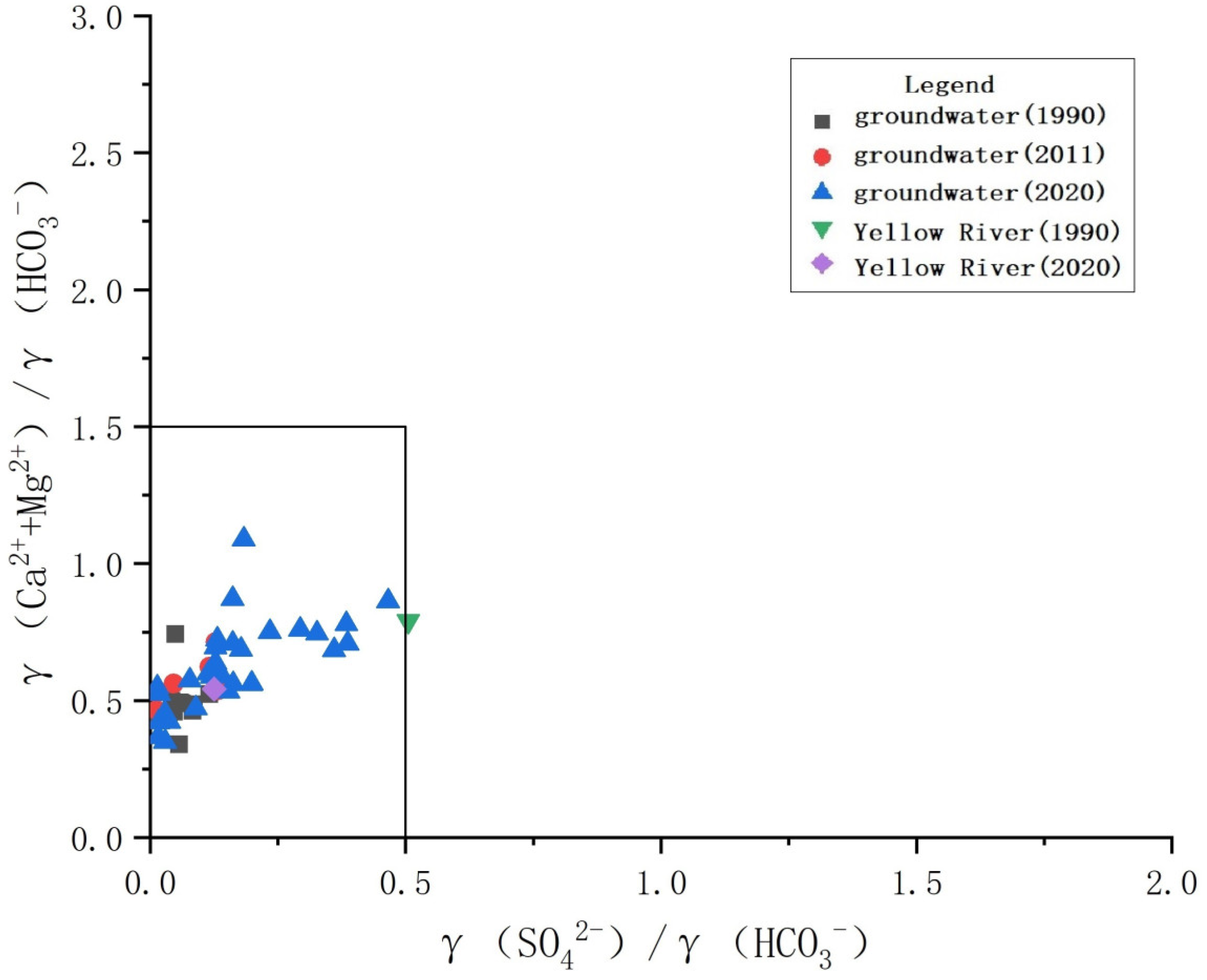
3.3.2. Analysis Based on the Ion Proportion Coefficient
3.3.3. Analysis of Excessive Arsenic in Groundwater
3.3.4. Analysis of Excessive Three Nitrogen in Groundwater
4. Conclusions
- (1)
- The shallow groundwater in the study area is weakly alkaline, and the TDS is low. The groundwater chemical types are mainly HCO3−Na·Ca·Mg and HCO3−Na·Ca. In the past 10 years, the hydrochemical types have become more complex, and the contents of SO42− and Cl− have increased locally. The closer HCO3− and Mg2+ are to the Yellow River, the lower the contents are, and the closer SO42−, Cl−, and Na+ are to the Yellow River, the higher the contents are.
- (2)
- The shallow groundwater in the study area is jointly affected by water–rock interactions and evaporation concentrations. The closer to the Yellow River the groundwater is, the shallower its buried depth, the greater the effect of evaporative concentration, and the stronger the cation exchange. The water–rock interaction is manifested in the dissolution of calcite, dolomite, and other carbonate minerals in the study area. At the same time, the carbonate in groundwater is also widely dispersed.
- (3)
- The arsenic content of shallow groundwater in the study area exceeds the local standard. The exceeding points are mainly distributed in the Yellow River alluvial plain, and there is no class V (>0.05 mg/L) point. The class IV (≤0.05 mg/L) points are mainly distributed in the Yellow River Wetland Park in Huiji District, Houliubei, and other places. The possibility of the artificial pollution of excessive arsenic is very small. The primary reductive sedimentary environment of sediment interbedded structures, such as the Yellow River alluvial plain, intensifies the dissolution of arsenic-rich ferromanganese oxides or hydroxides in the strata. At the same time, the strong evaporation and strong cation exchange in the shallow groundwater depth region aggravate the enrichment of arsenic.
- (4)
- There are sporadic local excesses of “three nitrogen” in shallow groundwater in the study area, which are attributable to previous fisheries and human domestic sewage discharge. There are many fish ponds in the study area. The nitrogen element in the feed that is put into the fish pond every day is the original source of ammonia nitrogen and nitrite in the water body. Usually, the nitrogen element in the feed that is not absorbed and utilized by the fish body is in the process of various microorganisms. Under the action, it is converted into ammonia nitrogen and nitrite in water. At present, domestic pond aquaculture basically discharges wastewater without treatment. Nitrite, ammonia nitrogen, and organic nitrogen in domestic sewage, domestic garbage and other discharges enter the groundwater through discharge, leaching, and other channels, resulting in groundwater “three nitrogen” exceeding the standard.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Gao, Z.; Wang, Z.; Xu, X.; Su, Q.; Wang, S.; Xing, T. Hydrogeochemical processes and suitability assessment of groundwater in the Jiaodong Peninsula, China. Environ. Monit. Assess. 2020, 192, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, X.W.; Wan, L.; Han, G.; Guo, H. Hydrogeochemical characterization of groundwater flow systems in the discharge area of a river basin. J. Hydrol. 2015, 527, 433–441. [Google Scholar] [CrossRef]
- Meybeck, M. Global occurrence of major elements in rivers. Treatise Geochem. 2003, 5, 207–223. [Google Scholar]
- Grasby, S.E. Chemical dynamics and weathering rates of a carbonate basin Bow River, southern Alberta. Appl. Geochem. 2003, 15, 67–77. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupre, B.; Allègre, C.J. The global control of silicate weathering rates and the coupling with physical erosion: New insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 2002, 196, 83–98. [Google Scholar] [CrossRef]
- Jia, H.; Qian, H.; Zheng, L.; Feng, W.; Wang, H.; Gao, Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 2020, 717, 137170. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhu, C.; Xue, X.; Qian, K.; Xie, X.; Wang, Y. Hydrogeochemical processes controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain. Sci. Total Environ. 2020, 730, 138877. [Google Scholar] [CrossRef]
- Aher, S.; Deshmukh, K.; Gawali, P.; Zolekar, R.; Deshmukh, P. Hydrogeochemical characteristics and groundwater quality investigation along the basinal cross-section of Pravara River, Maharashtra, India. J. Asian Earth Sci. X 2022, 7, 100082. [Google Scholar] [CrossRef]
- Xu, L.S. Application of GIS in Jiaxing Water Quality Monitoring. Manag. Technol. Small Medium Enterp. 2014, 26, 304–305. [Google Scholar]
- Zhao, L.S.; Li, H.G. Comparative study on the application effect of GIS spatial interpolation method in lake water quality evaluation. Mod. Agric. Sci. Technol. 2014, 5, 245–246. [Google Scholar]
- Gan, Y.; Zhao, K.; Deng, Y.; Liang, X.; Ma, T.; Wang, Y.X. Groundwater flow and hydrogeochemical evolution in the Jianghan Plain, central China. Hydrogeol. J. 2018, 26, 1609–1623. [Google Scholar] [CrossRef]
- Peng, H.; Zou, P.; Ma, C.; Xiong, S.; Lu, T. Elements in potable groundwater in Rugao longevity area, China: Hydrogeochemical characteristics, enrichment patterns and health assessments. Ecotoxicol. Environ. Saf. 2021, 218, 112279. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Han, D.; Song, X.; Liu, S. Environmental isotopes (δ18O, δ2H, 222Rn) and hydrochemical evidence for understanding rainfall-surface water-groundwater transformations in a polluted karst area. J. Hydrol. 2021, 592, 125748. [Google Scholar] [CrossRef]
- Sun, D.M. Analysis of chemical characteristics and formation mechanism of groundwater in Dalian area. Water Resour. Dev. Manag. 2022, 8, 54–58. [Google Scholar]
- Wang, Y.S.; Cheng, X.X.; Zhang, M.N.; Qi, X.F. Hydrochemical characteristics and formation mechanism of Malian River in the Yellow River Basin during dry season. Environ. Chem. 2018, 37, 164–172. [Google Scholar]
- Liu, X.; Xiang, W.; Si, B.C. Hydrochemical characteristics and stable isotope characteristics of hydrogen and oxygen in shallow groundwater in the Fenhe River Basin. Environ. Sci. 2021, 42, 1739–1749. [Google Scholar]
- Ming-Hui, H.; Stallard, R.F.; Edmond, J.M. Major ion chemistry of some large Chinese rivers. Nature 1982, 298, 550–553. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.W.; Letolle, R.; Jusserand, C. Major element chemistry of the Huanghe (Yellow River), China-weathering processes and chemical fluxes. J. Hydrol. 1995, 168, 173–203. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, J. Chemical weathering processes and atmospheric CO2 consumption of Huanghe River and Changjiang River basins. Chin. Geogr. Sci. 2003, 15, 16–21. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wen, Z.C. Discussion on silicate weathering in the Huanghe drainage basin. Period. Ocean Univ. China 2009, 39, 988–994. [Google Scholar]
- Liu, F.; Zhao, Z.; Yang, L.; Ma, Y.; Xu, Y.; Gong, L.; Liu, H. Geochemical characterization of shallow groundwater using multivariate statistical analysis and geochemical modeling in an irrigated region along the upper Yellow River, Northwestern China. J. Geochem. Explor. 2020, 215, 106565. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Yan, Z.P.; Jiao, H.J. Exploration, Development and Protection of Reserve Groundwater Sources in Yanhuang City, Henan Province; Geological Press: Beijing, China, 2013; p. 51. [Google Scholar]
- Tong, C.S.; Wu, J.C.; Miao, J.X.; Liu, J.C.; Yu, S.H. Development and utilization of groundwater and evolution of water quality in the North Henan Plain. Hydrogeol. Eng. Geol. 2005, 5, 13–16. [Google Scholar]
- Zhai, Q.M.; Ning, Y.X.; Liu, S. Analysis of sediment changes and their impact in the middle and lower reaches of the Yellow River. Henan Sci. Technol. 2020, 16, 78–80. [Google Scholar]
- Zhang, J.L.; Luo, Q.S.; Chen, C.X.; An, Q.H. Joint dynamic regulation of reservoir group-channel flow and sediment in the middle and lower reaches of the Yellow River. Adv. Water Sci. 2021, 32, 649–658. [Google Scholar]
- Han, S.B.; Li, F.C.; Wang, S.; Li, H.X.; Yuan, L.; Liu, J.T.; Shen, H.Y.; Zhang, X.Q.; Li, C.Q.; Wu, X.; et al. The situation of groundwater resources and its ecological environment in the Yellow River Basin. China Geol. 2021, 48, 1001–1019. [Google Scholar]
- Fan, Y.L.; Hu, N. Temporal and spatial dynamic changes of groundwater resources in the middle and lower reaches of the Yellow River. People’s Yellow River 2020, 42, 69–71. [Google Scholar]
- Ying, Y.M.; Qing, S.; Li, H.H.; Wang, W. A preliminary study on the spatial and temporal distribution characteristics of arsenic pollutants in the middle and lower reaches of the Yellow River. China Water Transp. (Second. Half Mon.) 2018, 18, 94–95. [Google Scholar]
- Tong, C.S.; Cheng, S.P.; Wang, X.K.; Guo, D.X.; Zhan, Y.H. Research on groundwater quality and pollution causes in the Huaihe River Basin (Henan Section). Eng. Investig. 2005, 3, 24–26. [Google Scholar]
- Sun, L.; Wang, L.L.; Cao, W.G. Study on the evolution law of hydrochemistry in the influence belt of the lower Yellow River (Henan section). People’s Yellow River 2021, 43, 91–99. [Google Scholar]
- Zhang, Q.Q.; Jin, Z.D.; Zhang, F. Seasonal Variation in river water chemistry of the middle reaches of the Yellow River and its controlling factors. J. Geochem. Explor. 2015, 156, 101–113. [Google Scholar] [CrossRef]
- Ren, Y.; Cao, W.G.; Pan, D.; Wang, S.; Li, Z.Y.; Li, J.C. Evolution characteristics and change mechanism of arsenic and fluorine in shallow groundwater in typical irrigation areas of Henan in the lower reaches of the Yellow River from 2010 to 2020. Rock Miner. Test. 2021, 40, 846–859. [Google Scholar]
- Wang, Y.R.; Shi, L.Q.; Qiu, M. Analysis of chemical characteristics of mine water based on Piper three-line diagram. Shandong Coal Sci. Technol. 2019, 4, 145–147, 150. [Google Scholar]
- Ren, X.Z.; Liu, M.; Zhang, Y.Z.; He, Z.M.; Zhu, B.Q. The realization of Durov’s three-line diagram based on Matlab. Geogr. Arid Reg. 2018, 41, 744–750. [Google Scholar]
- Jiang, Y.L.; Guan, Z.Q.; Zheng, C.X. Application of GIS spatial analysis in water pollution monitoring. Geospat. Inf. 2004, 3, 32–33. [Google Scholar]
- Shen, Z.L. Basis of Hydrology and Geochemistry; Geological Press: Beijing, China, 1999; p. 40. [Google Scholar]
- Liu, B.W.; Dong, S.G.; Tang, Z.H.; Xia, M.H. The seasonal variation characteristics of groundwater chemistry in the Tumochuan Plain, Inner Mongolia. Eng. Investig. 2020, 48, 34–40. [Google Scholar]
- Hu, C.H.; Zhou, W.B.; Xia, S.Q. Characteristics and source analysis of major ions in hydrochemistry in the Poyang Lake Basin. Environ. Chem. 2011, 30, 1620–1626. [Google Scholar]



| HCO3− | SO42− | CI− | K+ + Na+ | Ca2+ | Mg2+ | TDS | pH | NO3− | NH4+ | NO2− | As | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yellow River | 1990 | 166.6 | 132.6 | 85.1 | 97.3 | 46.5 | 23.9 | 483 | 8.15 | 6 | 0.1 | / | / | |
| 2020 | 452 | 89.3 | 48.6 | 51.2 | 94.2 | 40.1 | 672 | 7.66 | 0.85 | / | 0.16 | 0.003 | ||
| Shallow groundwater | 1990 | Min | 238.60 | 2.40 | 23.80 | 8.70 | 62.30 | 25.00 | 311.10 | 7.30 | 0.000 | 0.000 | / | / |
| Max | 497.30 | 69.60 | 65.90 | 83.30 | 92.00 | 35.40 | 553.50 | 7.90 | 10.000 | 0.360 | / | / | ||
| Mean | 400.05 | 32.13 | 36.96 | 49.52 | 72.46 | 30.62 | 430.10 | 7.48 | 2.800 | 0.187 | / | / | ||
| 2011 | Min | 279.65 | 10.57 | 33.15 | 25.84 | 60.14 | 25.79 | 339.99 | 7.75 | 0.46 | / | 0.003 | / | |
| Max | 564.31 | 118.68 | 105.04 | 114.00 | 109.86 | 55.47 | 795.83 | 8.07 | 17.00 | / | 0.119 | / | ||
| Mean | 448.46 | 62.18 | 81.08 | 64.43 | 90.90 | 45.98 | 589.60 | 7.91 | 8.25 | / | 0.031 | / | ||
| 2020 | Min | 133.00 | 6.15 | 7.15 | 22.33 | 39.00 | 5.96 | 156.00 | 7.29 | 0.52 | 0.000 | 0.000 | 0.000478 | |
| Max | 648.00 | 211.00 | 175.00 | 135.04 | 142.00 | 105.00 | 897.00 | 7.87 | 36.00 | 0.153 | 1.840 | 0.0492 | ||
| Mean | 414.79 | 95.87 | 80.08 | 81.48 | 88.63 | 46.71 | 565.42 | 7.54 | 7.25 | 0.061 | 0.133 | 0.00819 | ||
| 2021 | Min | / | 39.20 | 9.40 | 68.70 | / | / | 466.00 | 6.90 | 0.01 | 0.028 | 0.000 | 0.0007 | |
| Max | / | 361.00 | 172.00 | 220.00 | / | / | 1250.00 | 7.90 | 2.48 | 1.130 | 0.283 | 0.0138 | ||
| Mean | / | 135.70 | 86.94 | 102.54 | / | / | 712.76 | 7.48 | 0.73 | 0.334 | 0.052 | 0.003982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Tang, H.; Gan, R.; Li, Z.; He, X.; Gu, S. Characteristics and Causes of Changing Groundwater Quality in the Boundary Line of the Middle and Lower Yellow River (Right Bank). Water 2022, 14, 1846. https://doi.org/10.3390/w14121846
Tong X, Tang H, Gan R, Li Z, He X, Gu S. Characteristics and Causes of Changing Groundwater Quality in the Boundary Line of the Middle and Lower Yellow River (Right Bank). Water. 2022; 14(12):1846. https://doi.org/10.3390/w14121846
Chicago/Turabian StyleTong, Xiaoxia, Hui Tang, Rong Gan, Zitao Li, Xinlin He, and Shuqian Gu. 2022. "Characteristics and Causes of Changing Groundwater Quality in the Boundary Line of the Middle and Lower Yellow River (Right Bank)" Water 14, no. 12: 1846. https://doi.org/10.3390/w14121846
APA StyleTong, X., Tang, H., Gan, R., Li, Z., He, X., & Gu, S. (2022). Characteristics and Causes of Changing Groundwater Quality in the Boundary Line of the Middle and Lower Yellow River (Right Bank). Water, 14(12), 1846. https://doi.org/10.3390/w14121846






