Synergistic Effects of Calcium Peroxide and Fe3O4@BC Composites on AVS Removal, Phosphorus and Chromium Release in Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Material Preparation
2.2.1. Preparation of Biochar
2.2.2. Preparation of Fe3O4@BC
2.2.3. Characterization of the Biochar and Fe3O4@BC
2.3. Experiment Design
2.4. Measuring Method
2.5. Statistical Analysis
3. Results
3.1. Characterization of Fe3O4@BC
3.1.1. SEM and EDS
3.1.2. XRD and FT-IR
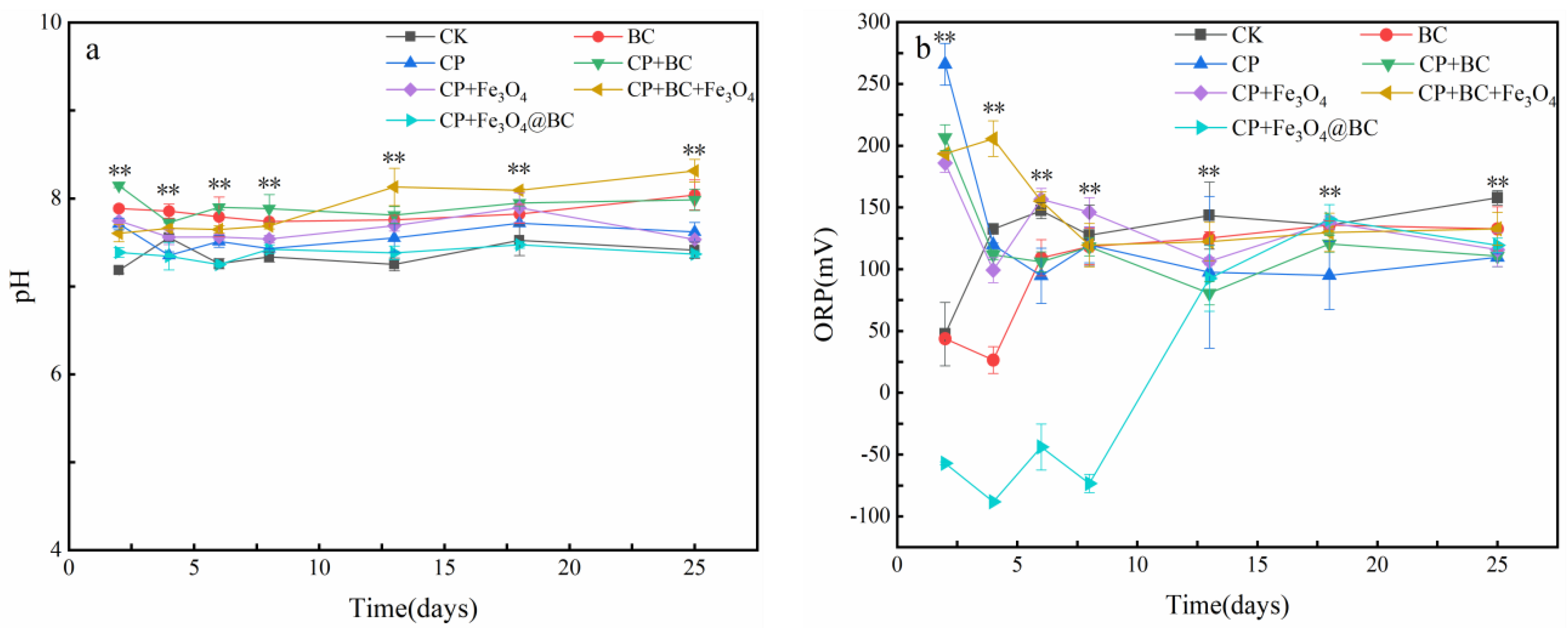
3.2. pH and OPR
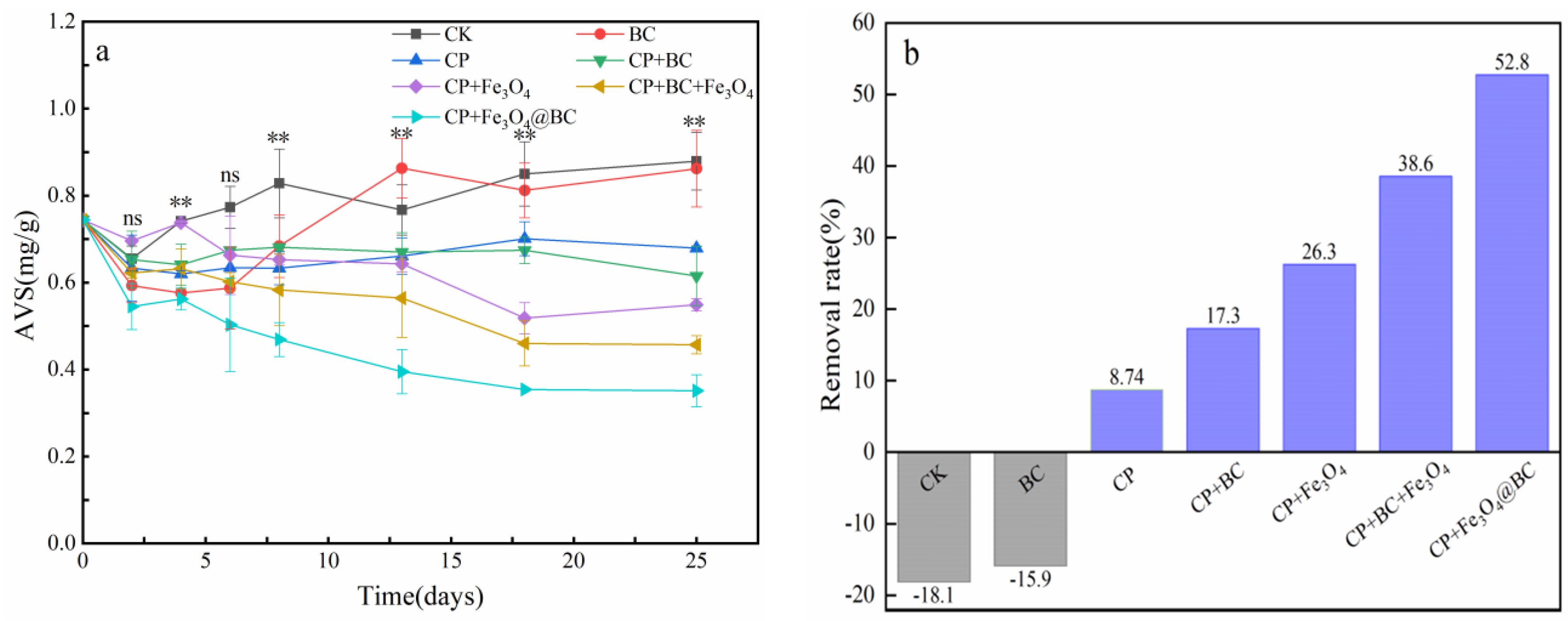
3.3. Removal of AVS in the Sediment
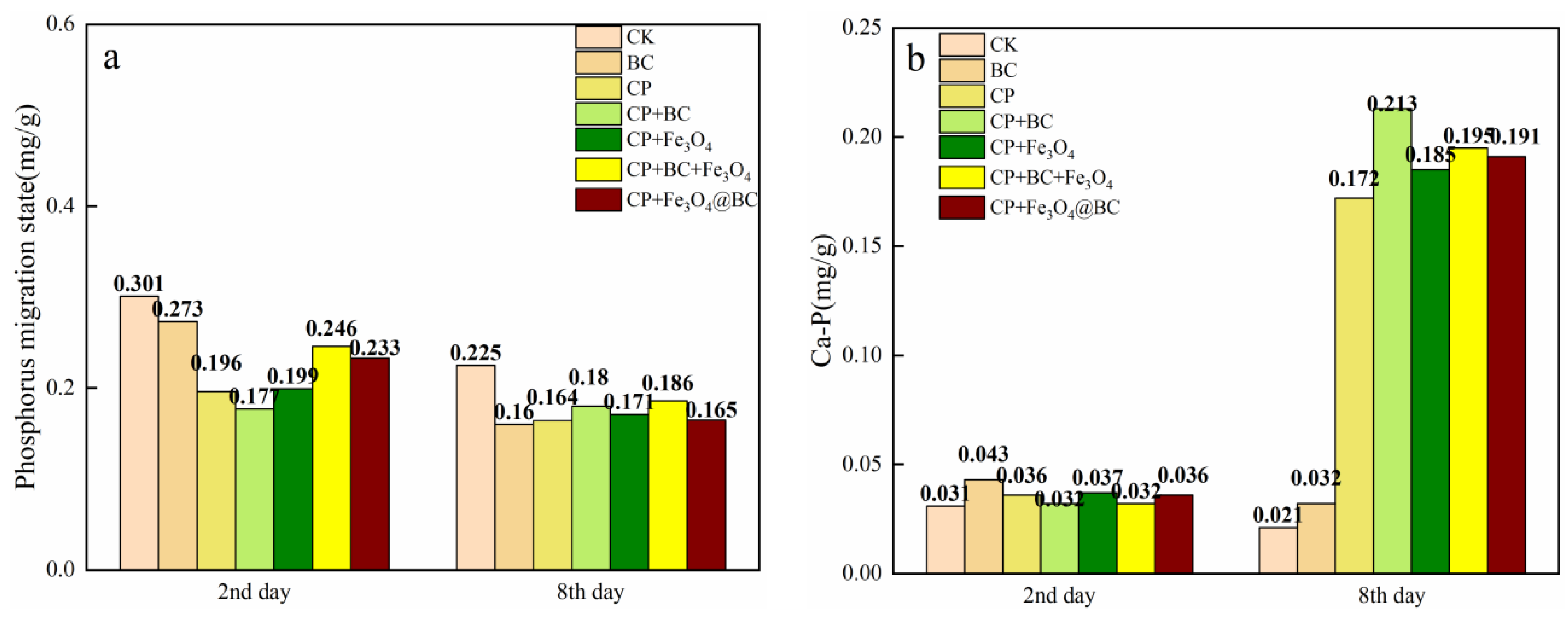
3.4. Changes of TP Content in the Overlying Water and the Content of Different Fractions of Phosphorus in the Sediments
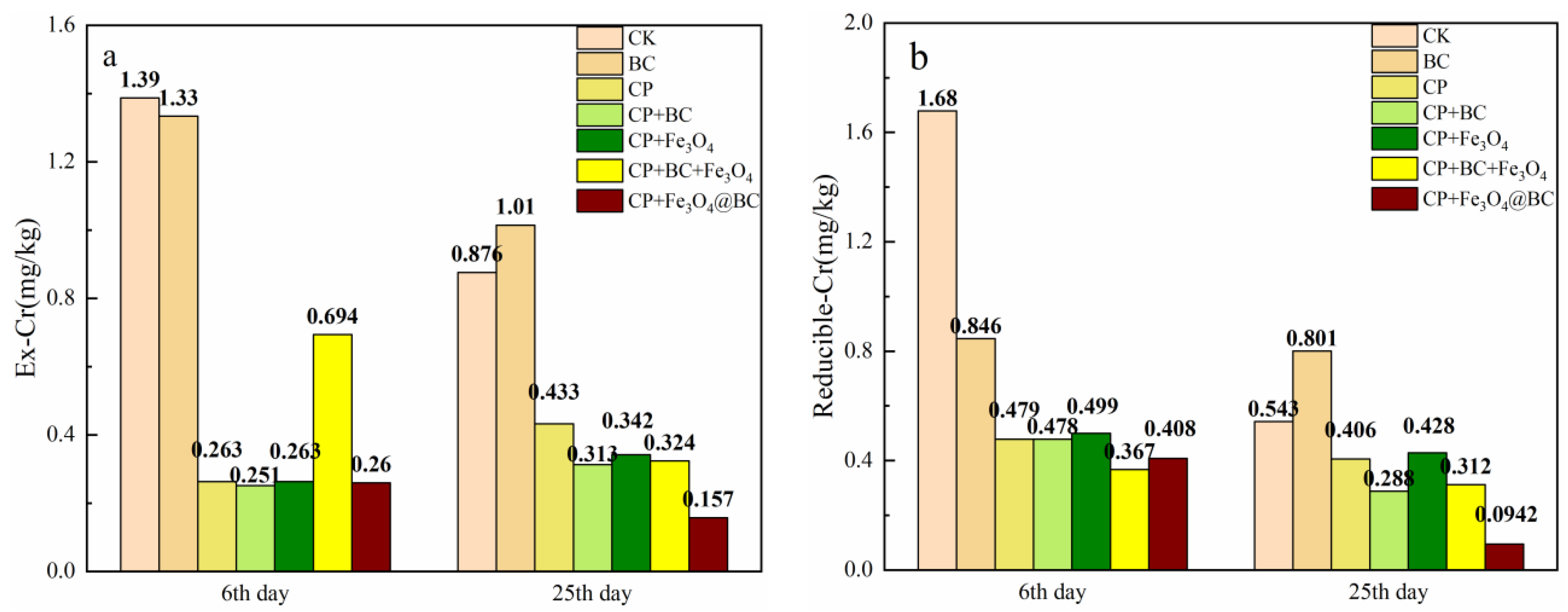
3.5. Changes of Cr Content of Different Fractions in the Sediments
3.6. Changes in Nitrogen Form
3.6.1. Variation of NH4+-N, NO3−-N and NO2−-N in the Sediment
3.6.2. Variation of NH4+-N, NO3−-N and NO2−-N in the Overlying Water
3.7. Analysis of Microbial Changes in Sediments
3.7.1. Microbial Diversity
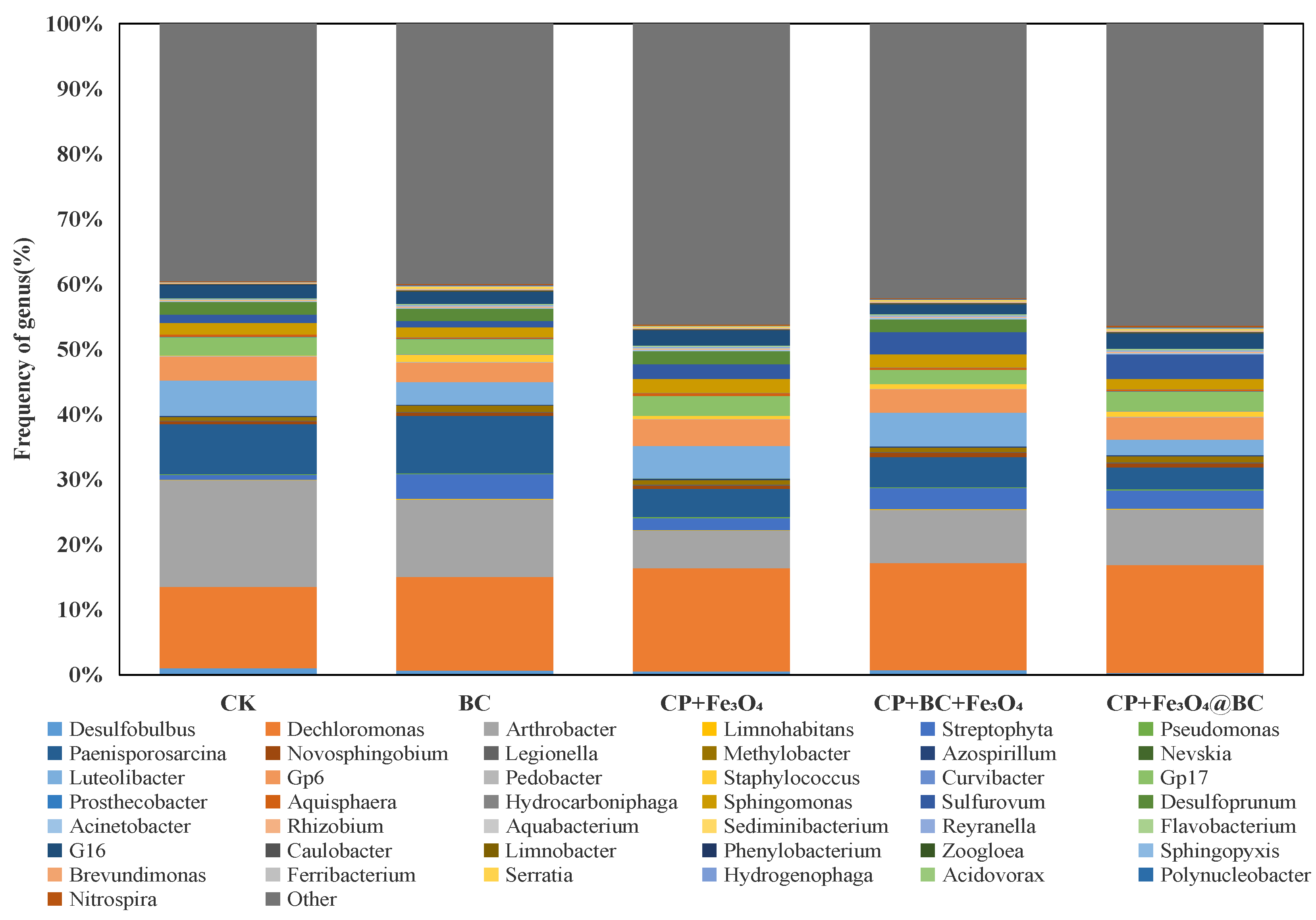
3.7.2. Microorganisms in Sediments
4. Discussions
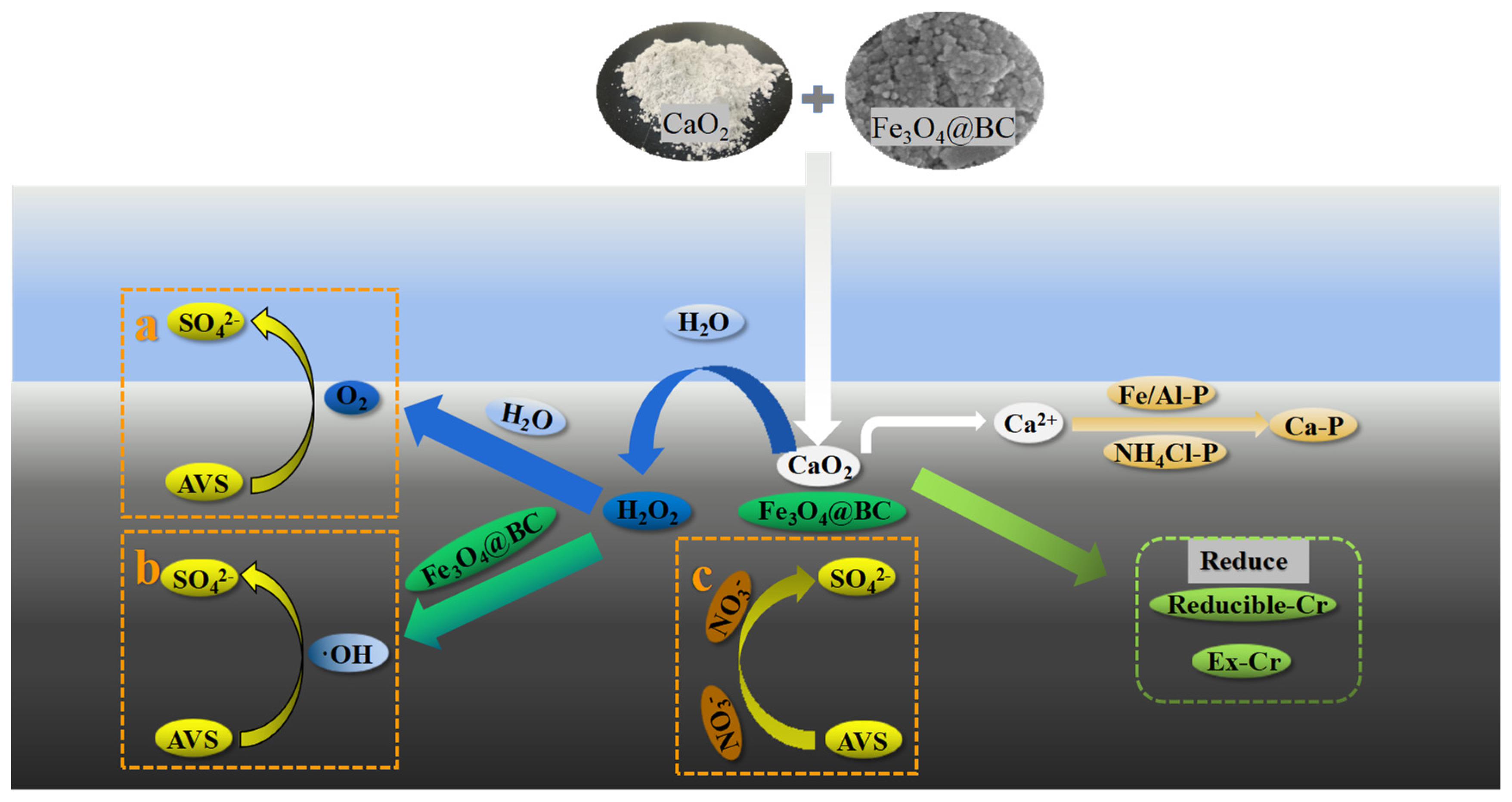
4.1. AVS Removal Mechanism
4.2. Mechanism of Phosphorus and Chromium Change in Different Fractions in the Sediment
4.2.1. Phosphorus
4.2.2. Chromium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Tang, Z.; Ji, J.; Song, Y. Discussion on Urban Black Odor Water Body Treatment and Long-term Management and Maintenance. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 428, p. 12010. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Zhang, C.; Duan, Z. Pollutants’ Release, Redistribution and Remediation of Black Smelly River Sediment Based on Re-Suspension and Deep Aeration of Sediment. Int. J. Environ. Res. Public Health 2017, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- DeFu, H.; RuiRui, C.; EnHui, Z.; Na, C.; Bo, Y.; HuaHong, S.; MinSheng, H. Toxicity bioassays for water from black-odor rivers in Wenzhou, China. Environ. Sci. Pollut. Res. 2014, 22, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Liang, Y.; Cheng, M.; He, Z.; Yu, G. Coupling oxidation of acid volatile sulfide, ferrous iron, and ammonia nitrogen from black-odorous sediment via autotrophic denitrification-anammox by nitrate addition. Sci. Total Environ. 2021, 790, 147972. [Google Scholar] [CrossRef] [PubMed]
- Waajen, G.; van Oosterhout, F.; Douglas, G.; Lürling, M. Geo-engineering experiments in two urban ponds to control eutrophication. Water Res. 2016, 97, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, P.; Kong, M. Effects of nitrate dosing on the migration of reduced sulfur in black odorous river sediment and the influencing factors. Chem. Eng. J. 2019, 371, 516–523. [Google Scholar] [CrossRef]
- Townsend, A.R.; Howarth, R.W.; Bazzaz, F.A.; Booth, M.S.; Cleveland, C.C.; Collinge, S.K.; Dobson, A.P.; Epstein, P.R.; Holland, E.A.; Keeney, D.R.; et al. Human health effects of a changing global nitrogen cycle. Front. Ecol. Environ. 2003, 1, 240–246. [Google Scholar] [CrossRef]
- Ma, S.-N.; Wang, H.-J.; Wang, H.-Z.; Zhang, M.; Li, Y.; Bian, S.-J.; Liang, X.-M.; Søndergaard, M.; Jeppesen, E. Effects of nitrate on phosphorus release from lake sediments. Water Res. 2021, 194, 116894. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Huang, Y.; Li, Y.; Liu, C.; Li, J.; Li, N. Assessment of Calcium Nitrate Addition on the AVS Removal, Phosphorus Locking, and Pb Release in Sediment. Water Air Soil Pollut. 2021, 232, 501. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, Q.-S.; Wei, W.; Sun, J.; Dai, X.; Ni, B.-J. Improving the treatment of waste activated sludge using calcium peroxide. Water Res. 2020, 187, 116440. [Google Scholar] [CrossRef]
- Wang, W.-H.; Wang, Y.; Fan, P.; Chen, L.-F.; Chai, B.-H.; Zhao, J.-C.; Sun, L.-Q. Effect of calcium peroxide on the water quality and bacterium community of sediment in black-odor water. Environ. Pollut. 2018, 248, 18–27. [Google Scholar] [CrossRef]
- Nykänen, A.; Kontio, H.; Klutas, O.; Penttinen, O.-P.; Kostia, S.; Mikola, J.; Romantschuk, M. Increasing lake water and sediment oxygen levels using slow release peroxide. Sci. Total Environ. 2012, 429, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Mukwaturi, M.; Lin, C. Mobilization of heavy metals from urban contaminated soils under water inundation conditions. J. Hazard. Mater. 2015, 285, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, J.; Zhang, R.; Tang, W. Performance of physical and chemical methods in the co-reduction of internal phosphorus and nitrogen loading from the sediment of a black odorous river. Sci. Total Environ. 2019, 663, 68–77. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Feng, Y.; Ren, H.; Zhang, Y.; Gu, N.; Lin, X. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J. Soils Sediments 2011, 11, 1408–1417. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Fortification of micronutrients for efficient agronomic production: A review. Agron. Sustain. Dev. 2016, 36, 7. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Xie, S.; Huang, X.; Qiu, X. Ionothermal synthesis of Fe3O4 magnetic nanoparticles as efficient heterogeneous Fenton-like catalysts for degradation of organic pollutants with H2O2. J. Hazard. Mater. 2017, 322, 152–162. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B Environ. 2012, 123–124, 117–126. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Ahmed, A.; Kurian, J.; Raghavan, V. Biochar influences on agricultural soils, crop production, and the environment: A review. Environ. Rev. 2016, 24, 495–502. [Google Scholar] [CrossRef]
- Huang, L.Q.; Fu, C.; Li, T.Z.; Yan, B.; Wu, Y.; Zhang, L.; Ping, W.; Yang, B.R.; Chen, L. Advances in research on effects of biochar on soil nitrogen and phosphorus. IOP Conf. Series: Earth Environ. Sci. 2020, 424, 12015. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, W.; Jin, X.; Shan, B. Using biochar capping to reduce nitrogen release from sediments in eutrophic lakes. Sci. Total Environ. 2018, 646, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Xing, B. Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Wu, J.; Huang, L.; Brookes, P.C.; Rodrigues, J.L.M.; Xu, J.; Liu, X. Effects of magnetic biochar-microbe composite on Cd remediation and microbial responses in paddy soil. J. Hazard. Mater. 2021, 414, 125494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohyd. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, H.; Xu, P.; Hou, B.; Jia, S.; Wang, D.; Li, K. Biodegradation of quinoline by Streptomyces sp. N01 immobilized on bamboo carbon supported Fe3O4 nanoparticles. Biochem. Eng. J. 2015, 99, 44–47. [Google Scholar] [CrossRef]
- Lijklema, A.H.M.H. Fractionation of Inorganic Phosphates in Calcareous Sediments. J. Environ. Qual. 1980, 9, 405–407. [Google Scholar] [CrossRef]
- Wang, L.; Long, X.; Chong, Y.; Yu, G. Potential risk assessment of heavy metals in sediments during the denitrification process enhanced by calcium nitrate addition: Effect of AVS residual. Ecol. Eng. 2016, 87, 333–339. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Yang, L.; Liu, C.; Li, J.; Li, N. Combined impact of organic matter, phosphorus, nitrate, and ammonia nitrogen on the process of blackwater. Environ. Sci. Pollut. Res. 2021, 28, 32831–32843. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.; Chen, Q. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model. J. Clean. Prod. 2019, 237, 117770. [Google Scholar] [CrossRef]
- Li, L.; Zhong, D.; Xu, Y.; Zhong, N. A novel superparamagnetic micro-nano-bio-adsorbent PDA/Fe3O4/BC for removal of hexavalent chromium ions from simulated and electroplating wastewater. Environ. Sci. Pollut. Res. 2019, 26, 23981–23993. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ji, D.; Lin, M.; Chen, Y.; Sun, Y.; Huo, S.; Zhu, J.; Xi, B. Developing surface water quality standards in China. Resour. Conserv. Recycl. 2017, 117, 294–303. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, J.M.; Yang, H.; Chen, J. Phosphorus fractions in sediment profiles and their potential contributions to eutrophication in Dianchi Lake. Environ. Earth Sci. 2005, 48, 835–844. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, N.; Meng, F.; Li, F.; Xie, Y.; Zhang, H. Speciation and Release Kinetics Simulation of Zn and Cd from River Sediment Contaminated by Gold Mining. Water Ai Soil Pollut. 2021, 232, 21. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Sun, Q.-L.; Zhang, J.; Wang, M.-X.; Cao, L.; Du, Z.-F.; Sun, Y.-Y.; Liu, S.-Q.; Li, C.-L.; Sun, L. High-Throughput Sequencing Reveals a Potentially Novel Sulfurovum Species Dominating the Microbial Communities of the Seawater–Sediment Interface of a Deep-Sea Cold Seep in South China Sea. Microorganisms 2020, 8, 687. [Google Scholar] [CrossRef]
- Purdy, K.J.; Nedwell, D.B.; Embley, T.M.; Takii, S. Use of 16S rRNA-targeted oligonucleotide probes to investigate the distribution of sulphate-reducing bacteria in estuarine sediments. FEMS Microbiol. Ecol. 2001, 36, 165–168. [Google Scholar] [CrossRef]
- Yan, Y.U.; Yueyue, W.A.N.G.; Duxian, F.A.N.G.; Jie, R.E.N.; Ying, W.A.N.G. Bacterial diversity in surface sediments of Baiyangdian lake and its influencing factors. Chin. J. Environ. Eng. 2021, 15, 1121–1130. [Google Scholar]
- Duffner, C.; Holzapfel, S.; Wunderlich, A.; Einsiedl, F.; Schloter, M.; Schulz, S. Dechloromonas and close relatives prevail during hydrogenotrophic denitrification in stimulated microcosms with oxic aquifer material. FEMS Microbiol. Ecol. 2021, 97, fiab004. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.Z.; Yang, X.N.; Wu, F.P.; Zhao, W.; Zhang, H.W.; Zhang, X. Microbial Community Structure and Diversity in Cellar Water by 16S rRNA High-throughput Sequencing. Environ. Sci. 2017, 38, 1704–1716. [Google Scholar] [CrossRef]
- Nakamura, Y.; Satoh, H.; Kindaichi, T.; Okabe, S. Community Structure, Abundance, and in Situ Activity of Nitrifying Bacteria in River Sediments as Determined by the Combined Use of Molecular Techniques and Microelectrodes. Environ. Sci. Technol. 2006, 40, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fang, A.; Yu, X.; Zhang, K.; He, Z.; Wang, C.; Peng, Y.; Xiao, F.; Yang, T.; Zhang, W.; et al. Microbially-driven sulfur cycling microbial communities in different mangrove sediments. Chemosphere 2020, 273, 128597. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Q.; Zhao, D.; Xu, M.; Shen, Q.; Wang, D.; Wang, Y.; Ding, S. A critical review of the appearance of black-odorous waterbodies in China and treatment methods. J. Hazard. Mater. 2020, 385, 121511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Shi, J.; Dong, H. Sulfur-based mixotrophic bio-reduction for efficient removal of chromium (VI) in groundwater. Geochim. Cosmochim. Acta 2019, 268, 296–309. [Google Scholar] [CrossRef]
- Teuchies, J.; Bervoets, L.; Cox, T.J.S.; Meire, P.; de Deckere, E. The effect of waste water treatment on river metal concentrations: Removal or enrichment? J. Soil Sediment 2011, 11, 364–372. [Google Scholar] [CrossRef] [Green Version]
- De Lange, H.; Van Griethuysen, C.; Koelmans, A. Sampling method, storage and pretreatment of sediment affect AVS concentrations with consequences for bioassay responses. Environ. Pollut. 2008, 151, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, L.; Huang, W.; Zhou, H.; Li, J.; Liu, C.; Lai, B.; Li, N. Effective Peroxymonosulfate Activation by Natural Molybdenite for Enhanced Atrazine Degradation: Role of Sulfur Vacancy, Degradation Pathways and Mechanism. J. Hazard. Mater. 2022, 435, 128899. [Google Scholar] [CrossRef]
- Yang, X.; Zou, R.; Tang, K.; Andersen, H.R.; Angelidaki, I.; Zhang, Y. Degradation of metoprolol from wastewater in a bio-electro-Fenton system. Sci. Total Environ. 2021, 771, 145385. [Google Scholar] [CrossRef]
- Jian, H.; Yang, F.; Gao, Y.; Zhen, K.; Tang, X.; Zhang, P.; Wang, Y.; Wang, C.; Sun, H. Efficient removal of pyrene by biochar supported iron oxide in heterogeneous Fenton-like reaction via radicals and high-valent iron-oxo species. Sep. Purif. Technol. 2021, 265, 118518. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Zhang, L.; Li, X.; Wang, F.; Li, G.; Li, J.; Li, W. In-situ remediation of sediment by calcium nitrate combined with composite microorganisms under low-DO regulation. Sci. Total Environ. 2019, 697, 134109. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.-Y.; Guo, J.-H.; Qiu, Y.Y.; Guo, J.H.; Zhang, L.; Chen, G.-H.; Jiang, F. A high-rate sulfidogenic process based on elemental sulfur reduction: Cost-effectiveness evaluation and microbial community analysis. Biochem. Eng. J. 2017, 128, 26–32. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, C.; Huang, W.; Ding, S. Microorganisms and typical organic matter responsible for lacustrine “black bloom”. Sci. Total Environ. 2014, 470–471, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, X.; Zhou, B.; Ren, G.; Yuan, D.; Liu, H.; Wei, Z.; Gu, X.; Zhao, B.; Hu, Y.; et al. An integrated migration and transformation model to evaluate the occurrence characteristics and environmental risks of Nitrogen and phosphorus in constructed wetland. Chemosphere 2021, 277, 130219. [Google Scholar] [CrossRef]
- Rydin, E. Potentially mobile phosphorus in Lake Erken sediment. Water Res. 2000, 34, 2037–2042. [Google Scholar] [CrossRef]
- Ribeiro, D.; Martins, G.; Nogueira, R.; Cruz, J.; Brito, A. Phosphorus fractionation in volcanic lake sediments (Azores–Portugal). Chemosphere 2007, 70, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Kaiserli, A.; Voutsa, D.; Samara, C. Phosphorus fractionation in lake sediments—Lakes Volvi and Koronia, N. Greece. Chemosphere 2002, 46, 1147–1155. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, C.J.; Blowes, D.W.; Gould, W.D. Control of mercury and methylmercury in contaminated sediments using biochars: A long-term microcosm study. Appl. Geochem. 2018, 92, 30–44. [Google Scholar] [CrossRef]
- Wang, L.; Ye, M.; Li, Q.; Zou, H.; Zhou, Y. Phosphorus speciation in wetland sediments of Zhujiang (Pearl) River Estuary, China. Chin. Geogr. Sci. 2013, 23, 574–583. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Ding, S.; Yao, L.; Shen, Q.; Zhu, C.; Wang, Y.; Xu, D. Dynamics of phosphorus–iron–sulfur at the sediment–water interface influenced by algae blooms decomposition. J. Hazard. Mater. 2015, 300, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, Y.; Zhu, Y.; Huang, M.; Zhang, Y. Biogeochemical sulfur cycling coupling with dissimilatory nitrate reduction processes in freshwater sediments. Environ. Rev. 2018, 26, 121–132. [Google Scholar] [CrossRef]
- Xiao, H.; Griffiths, B.; Chen, X.; Liu, M.; Jiao, J.; Hu, F.; Li, H. Influence of bacterial-feeding nematodes on nitrification and the ammonia-oxidizing bacteria (AOB) community composition. Appl. Soil Ecol. 2010, 45, 131–137. [Google Scholar] [CrossRef]
- Lal, S.; Singhal, A.; Kumari, P. Exploring carbonaceous nanomaterials for arsenic and chromium removal from wastewater. J. Water Process Eng. 2020, 36, 101276. [Google Scholar] [CrossRef]
- Shentu, J.; Li, X.; Han, R.; Chen, Q.; Shen, D.; Qi, S. Effect of site hydrological conditions and soil aggregate sizes on the stabilization of heavy metals (Cu, Ni, Pb, Zn) by biochar. Sci. Total Environ. 2021, 802, 149949. [Google Scholar] [CrossRef]
- Arain, M.B.; Kazi, T.G.; Jamali, M.K.; Afridi, H.I.; Jalbani, N.; Sarfraz, R.A.; Baig, J.A.; Kandhro, G.A.; Memon, M.A. Time saving modified BCR sequential extraction procedure for the fraction of Cd, Cr, Cu, Ni, Pb and Zn in sediment samples of polluted lake. J. Hazard. Mater. 2008, 160, 235–239. [Google Scholar] [CrossRef]





| Groups | Sediment (g) | Water (mL) | BC (g) | CaO2 (g) | Fe3O4 (g) | Fe3O4@BC (g) |
|---|---|---|---|---|---|---|
| CK | 75 | 150 | ||||
| BC | 75 | 150 | 4 | |||
| CP | 75 | 150 | 12 | |||
| CP+BC | 75 | 150 | 4 | 12 | ||
| CP+Fe3O4 | 75 | 150 | 12 | 2 | ||
| CP+BC+Fe3O4 | 75 | 150 | 4 | 12 | 2 | |
| CP+Fe3O4@BC | 75 | 150 | 12 | 6 |
| Sample | Shannon | Chao | Simpson | Shannoneven | Coverage | |
|---|---|---|---|---|---|---|
| 13th days | CK | 5.49 | 2977.78 | 0.03 | 0.70 | 0.99 |
| BC | 5.63 | 3207.53 | 0.02 | 0.71 | 0.99 | |
| CP+Fe3O4 | 5.73 | 3030.68 | 0.02 | 0.74 | 0.99 | |
| CP+BC+Fe3O4 | 5.62 | 3068.40 | 0.03 | 0.72 | 0.99 | |
| CP+Fe3O4@BC | 5.97 | 3144.75 | 0.01 | 0.76 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, Y.; Wang, X.; Gou, G.; Liu, C.; Li, J.; He, Y.; Li, N. Synergistic Effects of Calcium Peroxide and Fe3O4@BC Composites on AVS Removal, Phosphorus and Chromium Release in Sediments. Water 2022, 14, 1626. https://doi.org/10.3390/w14101626
Li Y, Huang Y, Wang X, Gou G, Liu C, Li J, He Y, Li N. Synergistic Effects of Calcium Peroxide and Fe3O4@BC Composites on AVS Removal, Phosphorus and Chromium Release in Sediments. Water. 2022; 14(10):1626. https://doi.org/10.3390/w14101626
Chicago/Turabian StyleLi, Yintian, Yanchun Huang, Xueying Wang, Ge Gou, Chao Liu, Jun Li, Yuxin He, and Naiwen Li. 2022. "Synergistic Effects of Calcium Peroxide and Fe3O4@BC Composites on AVS Removal, Phosphorus and Chromium Release in Sediments" Water 14, no. 10: 1626. https://doi.org/10.3390/w14101626






