Fe0-Supported Anaerobic Digestion for Organics and Nutrients Removal from Domestic Sewage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Sludge and Wastewater
2.1.2. Fe0 Materials
2.2. Experimental Procedure
2.2.1. Fe0 Reactivity
2.2.2. Contaminants Removal
2.3. Analytical Methods
2.4. Statistical Analysis
2.5. Determination of Pollutants’ Removal Efficiency
3. Results and Discussion
3.1. Fe0 Reactivity
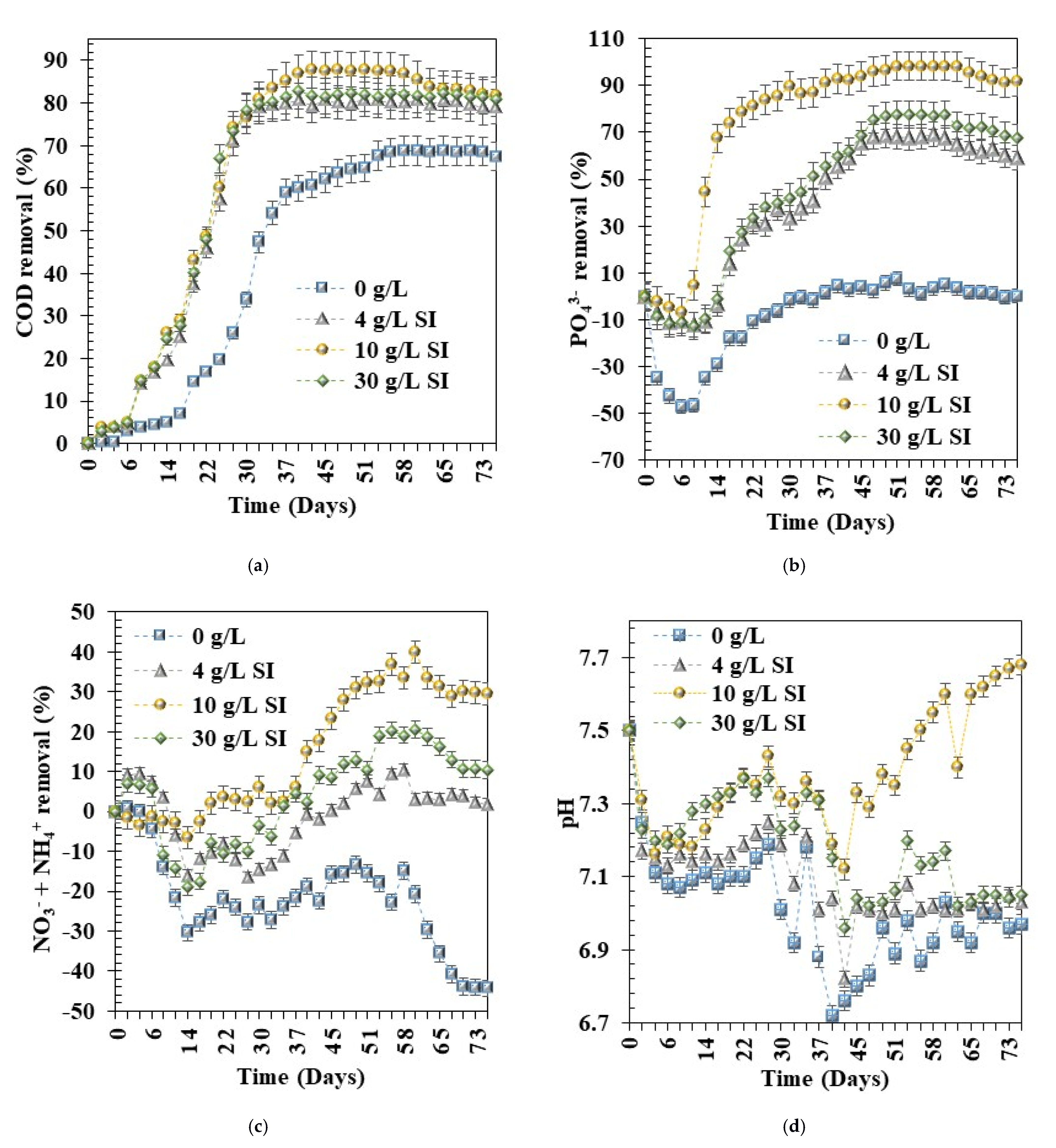
3.2. Effect of Fe0 Materials Dosage on Pollutants Removal
3.2.1. COD
3.2.2. PO43−
3.2.3. NO3− + NH4+
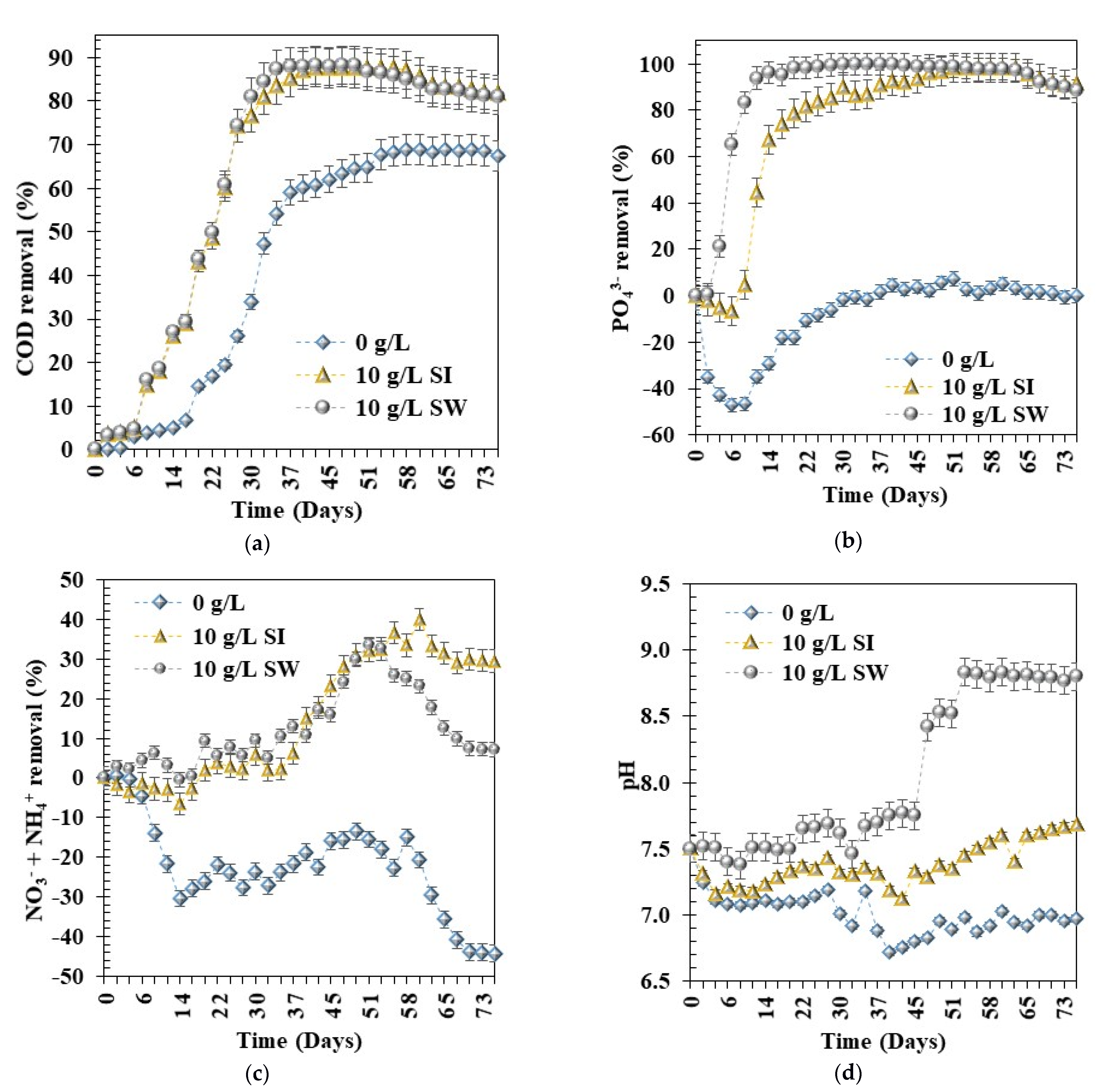
3.3. Effects of Types of Fe0 Materials on Pollutants Removal
3.4. Optimisation of Fe0 Materials Dosage
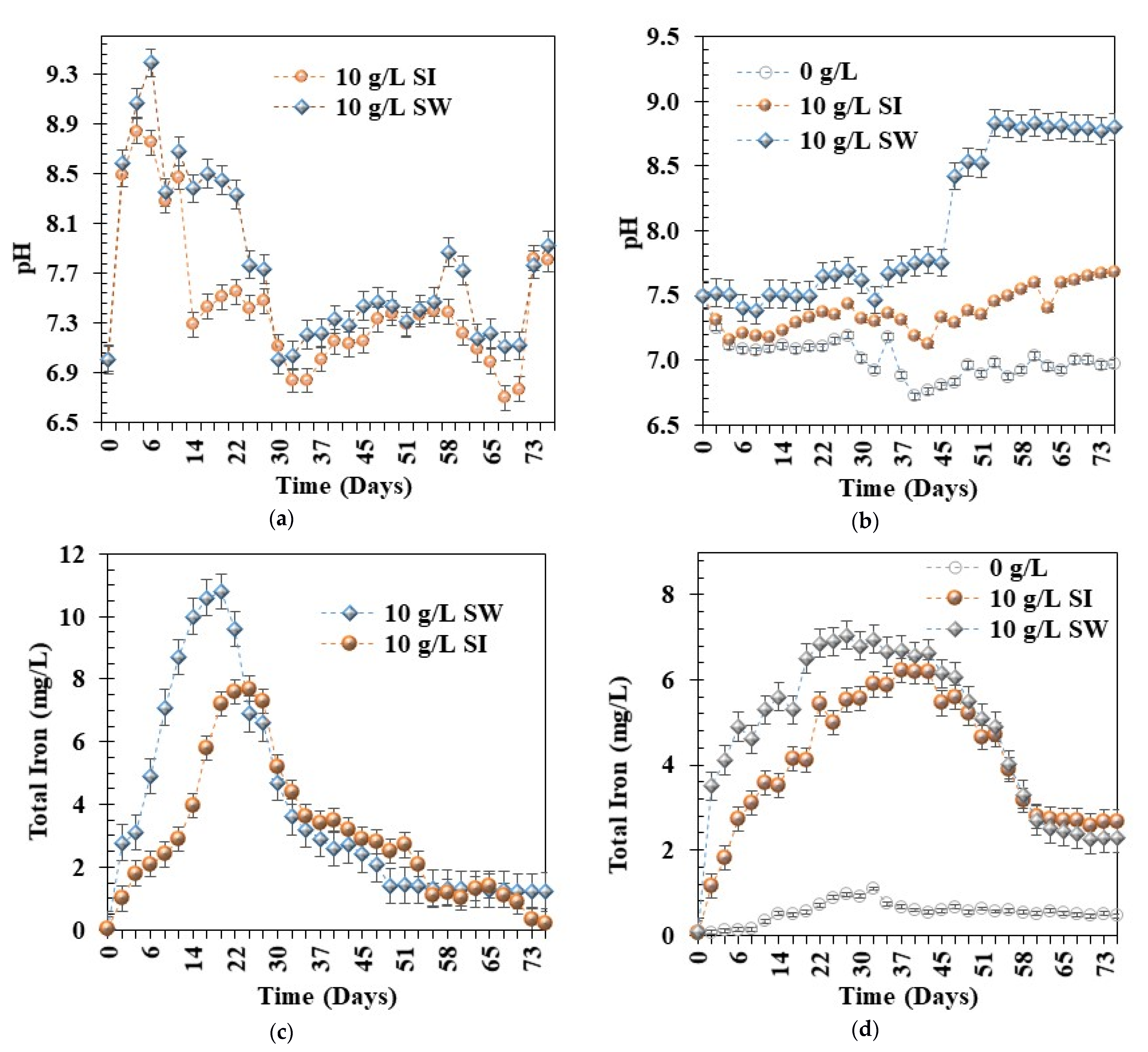
3.5. Behaviour of Fe0 Materials in Domestic Sewage
3.6. Significance of the Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melidis, P.; Vaiopoulou, E.; Athanasoulia, E.; Aivasidis, A. Anaerobic treatment of domestic wastewater using an anaerobic fixed-bed loop reactor. Desalination 2009, 248, 716–722. [Google Scholar] [CrossRef]
- Alexiou, G.; Mara, D. Anaerobic waste stabilization ponds. Appl. Biochem. Biotechnol. 2003, 109, 241–252. [Google Scholar] [CrossRef]
- Moran, S. An Applied Guide to Water and Effluent Treatment Plant Design; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Kujawa-Roeleveld, K.; Zeeman, G. Anaerobic Treatment in Decentralised and Source-Separation-Based Sanitation Concepts. Rev. Environ. Sci. Bio/Technol. 2006, 5, 115–139. [Google Scholar] [CrossRef]
- Riffat, R.; Husnain, T. Fundamentals of Wastewater Treatment and Engineering; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Tchobanoglous, G. Wastewater Engineering: Treatment and Resource Recovery; McGraw-Hill: New York, NY, USA, 2014; Volume 2. [Google Scholar]
- Diatta, J.; Waraczewska, Z.; Grzebisz, W.; Niewiadomska, A.; Tatuśko-Krygier, N. Eutrophication Induction Via N/P and P/N Ratios Under Controlled Conditions—Effects of Temperature and Water Sources. Water Air Soil Pollut. 2020, 231, 149. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Deng, S.; Peng, S.; Xie, B.; Yang, X.; Sun, S.; Yao, H.; Li, D. Influence characteristics and mechanism of organic carbon on denitrification, N2O emission and NO2−Accumulation in the iron [Fe (0)]-oxidizing supported autotrophic denitrification process. Chem. Eng. J. 2020, 393, 124736. [Google Scholar]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Yuan, T.; Bian, S.; Ko, J.H.; Liu, J.; Shi, X.; Xu, Q. Exploring the roles of zero-valent iron in two-stage food waste anaerobic digestion. Waste Manag. 2020, 107, 91–100. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef]
- Domrongpokkaphan, V.; Phalakornkule, C.; Khemkhao, M. In-situ methane enrichment of biogas from anaerobic digestion of palm oil mill effluent by addition of zero valent iron (ZVI). Int. J. Hydrogen Energy 2021, 46, 30976–30987. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Li, Q.; Quan, X. Adding Fe0 powder to enhance the anaerobic conversion of propionate to acetate. Biochem. Eng. J. 2013, 73, 80–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Y.; Quan, X.; Liu, Y.; Onu, P. A built-in zero valent iron anaerobic reactor to enhance treatment of azo dye wastewater. Water Sci. Technol. 2011, 63, 741–746. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Zang, Y.; Yang, Y.; Hu, Y.; Ngo, H.H.; Wang, X.C.; Li, Y.-Y. Zero-valent iron enhanced anaerobic digestion of pre-concentrated domestic wastewater for bioenergy recovery: Characteristics and mechanisms. Bioresour. Technol. 2020, 310, 123441. [Google Scholar] [CrossRef]
- Till, B.A.; Weathers, L.J.; Alvarez, P.J.J. Fe(0)-Supported Autotrophic Denitrification. Environ. Sci. Technol. 1998, 32, 634–639. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J.; Yang, G.; Zhuang, L. Methanogenesis affected by the co-occurrence of iron(III) oxides and humic substances. FEMS Microbiol. Ecol. 2014, 88, 107–120. [Google Scholar] [CrossRef]
- Yamada, C.; Kato, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015, 119, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.; Sivagurunathan, P.; Lee, M.-K.; Yun, Y.-M.; Song, Y.-C.; Kim, D.-H. Enhanced hydrogen fermentation by zero valent iron addition. Int. J. Hydrogen Energy 2019, 44, 3387–3394. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A Novel and Facile Method to Characterize the Suitability of Metallic Iron for Water Treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, J.; Huang, T.; Guan, X. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L.; Lv, B.; Yang, Y.; Zhang, F. Application of zero valent iron coupling with biological process for wastewater treatment: A review. Rev. Environ. Sci. Bio/Technol. 2017, 16, 667–693. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Noubactep, C. Metallic Iron for Water Remediation: Plenty of Room for Collaboration and Convergence to Advance the Science. Water 2022, 14, 1492. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Noubactep, C. Metallic iron (Fe0)-based materials for aqueous phosphate removal: A critical review. J. Environ. Manag. 2022, 315, 115157. [Google Scholar]
- Yu, X.; Amrhein, C.; Deshusses, M.A.; Matsumoto, M. Perchlorate Reduction by Autotrophic Bacteria in the Presence of Zero-Valent Iron. Environ. Sci. Technol. 2006, 40, 1328–1334. [Google Scholar] [CrossRef]
- Yu, X.; Amrhein, C.; Deshusses, M.A.; Matsumoto, M.R. Perchlorate Reduction by Autotrophic Bacteria Attached to Zerovalent Iron in a Flow-Through Reactor. Environ. Sci. Technol. 2007, 41, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, D.; Yang, X.; Xing, W.; Li, J.; Zhang, Q. Biological denitrification process based on the Fe(0)–carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition. Bioresour. Technol. 2016, 219, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Kang, Y.; Quan, H.; Han, Y.; Sun, J.; Feng, Y. Bioremediation of copper-containing wastewater by sulfate reducing bacteria coupled with iron. J. Environ. Manag. 2013, 129, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Boontian, N. Effect of Zero Valent Iron (ZVI) in Wastewater Treatment: A Review. Appl. Mech. Mater. 2015, 775, 180–184. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B.J. Testing the Suitability of Zerovalent Iron Materials for Reactive Walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo, M.; Gwenzi, W.; Noubactep, C. Characterizing the Suitability of Granular Fe0 for the Water Treatment Industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-M.; Li, C.-W.; Chen, S.-S. Fluidized zero valent iron bed reactor for nitrate removal. Chemosphere 2005, 59, 753–759. [Google Scholar] [CrossRef]
- Antwi, P.; Li, J.; Boadi, P.O.; Meng, J.; Shi, E.; Chi, X.; Deng, K.; Ayivi, F. Dosing effect of zero valent iron (ZVI) on biomethanation and microbial community distribution as revealed by 16S rRNA high-throughput sequencing. Int. Biodeterior. Biodegrad. 2017, 123, 191–199. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q.; Yin, X. Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem. Eng. J. 2016, 303, 458–466. [Google Scholar] [CrossRef]
- Charalambous, P.; Vyrides, I. In situ biogas upgrading and enhancement of anaerobic digestion of cheese whey by addition of scrap or powder zero-valent iron (ZVI). J. Environ. Manag. 2021, 280, 111651. [Google Scholar] [CrossRef]
- Hu, R.; Ndé-Tchoupé, A.I.; Lufingo, M.; Xiao, M.; Nassi, A.; Noubactep, C.; Njau, K.N. The impact of selected pretreatment procedures on iron dissolution from metallic iron specimens used in water treatment. Sustainability 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Günther, S.; Koch, C.; Hübschmann, T.; Röske, I.; Müller, R.A.; Bley, T.; Harms, H.; Müller, S. Correlation of Community Dynamics and Process Parameters As a Tool for the Prediction of the Stability of Wastewater Treatment. Environ. Sci. Technol. 2012, 46, 84–92. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Benyounis, K.Y.; Messeiry, M.; Hussain, A.; Abadir, E.F. Optimization of Alkaline Treatment Conditions of Flax Fiber Using Box–Behnken Method. J. Nat. Fibers 2012, 9, 256–276. [Google Scholar] [CrossRef]
- Akteke-Ozturk, B.; Koksal, G.; Weber, G.-W. Nonconvex optimization of desirability functions. Qual. Eng. 2018, 30, 293–310. [Google Scholar] [CrossRef]
- Summer, D.; Schöftner, P.; Watzinger, A.; Reichenauer, T.G. Inhibition and stimulation of two perchloroethene degrading bacterial cultures by nano- and micro-scaled zero-valent iron particles. Sci. Total Environ. 2020, 722, 137802. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2019, 148, 70–85. [Google Scholar] [CrossRef]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 10, 1383–1398. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M.S. Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt. J. Pet. 2014, 23, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Alrawashdeh, K.A.B.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, W.-H.; Shih, C.J. Heavy metal removal from wastewater using zero-valent iron nanoparticles. Water Sci. Technol. 2008, 58, 1947–1954. [Google Scholar] [CrossRef]
- Fu, F.; Han, W.; Tang, B.; Hu, M.; Cheng, Z. Insights into environmental remediation of heavy metal and organic pollutants: Simultaneous removal of hexavalent chromium and dye from wastewater by zero-valent iron with ligand-enhanced reactivity. Chem. Eng. J. 2013, 232, 534–540. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. Investigating the Fe0/H2O systems using the methylene blue method: Validity, applications, and future directions. Chemosphere 2022, 291, 132913. [Google Scholar]
- Noubactep, C. On the mechanism of microbe inactivation by metallic iron. J. Hazard. Mater. 2011, 198, 383–386. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and Inactivation of Waterborne Viruses Using Zerovalent Iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.; Batstone, D.J. Low pH anaerobic digestion of waste activated sludge for enhanced phosphorous release. Water Res. 2015, 81, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Mehta, C.M.; Batstone, D. Nutrient solubilization and its availability following anaerobic digestion. Water Sci. Technol. 2013, 67, 756–763. [Google Scholar] [CrossRef]
- Martí, N.; Bouzas, A.; Seco, A.; Ferrer, J. Struvite precipitation assessment in anaerobic digestion processes. Chem. Eng. J. 2008, 141, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Gan, J.; Rajendran, A.; Reis, C.E.R.; Hu, B. Phosphorus Removal and Recovery from Digestate after Biogas Production. In Biofuels-Status and Perspective; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Schievano, A.; D’Imporzano, G.; Salati, S.; Adani, F. On-field study of anaerobic digestion full-scale plants (Part I): An on-field methodology to determine mass, carbon and nutrients balance. Bioresour. Technol. 2011, 102, 7737–7744. [Google Scholar] [CrossRef]
- Akunna, J.C. Anaerobic Waste-Wastewater Treatment and Biogas Plants: A Practical Handbook; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Schädler, S.; Burkhardt, C.; Hegler, F.; Straub, K.L.; Miot, J.; Benzerara, K.; Kappler, A. Formation of Cell-Iron-Mineral Aggregates by Phototrophic and Nitrate-Reducing Anaerobic Fe(II)-Oxidizing Bacteria. Geomicrobiol. J. 2009, 26, 93–103. [Google Scholar] [CrossRef]
- Yin, W.; Wu, J.; Li, P.; Wang, X.; Zhu, N.; Wu, P.; Yang, B. Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: The effects of pH, iron dosage, oxygen and common dissolved anions. Chem. Eng. J. 2012, 184, 198–204. [Google Scholar] [CrossRef]
- Sleiman, N.; Deluchat, V.; Wazne, M.; Mallet, M.; Courtin-Nomade, A.; Kazpard, V.; Baudu, M. Phosphate removal from aqueous solutions using zero valent iron (ZVI): Influence of solution composition and ZVI aging. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Liao, Z.; Yao, D.; Yang, Z.; Wu, Y.; Tang, C. Phosphorus immobilization in water and sediment using iron-based materials: A review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Sheng, G.-P.; Mu, Y.; Yu, H.-Q. A modeling approach to describe ZVI-based anaerobic system. Water Res. 2013, 47, 6007–6013. [Google Scholar] [CrossRef]
- Kassab, G.; Khater, D.; Odeh, F.; Shatanawi, K.; Halalsheh, M.; Arafah, M.; Van Lier, J.B. Impact of Nanoscale Magnetite and Zero Valent Iron on the Batch-Wise Anaerobic Co-Digestion of Food Waste and Waste-Activated Sludge. Water 2020, 12, 1283. [Google Scholar] [CrossRef]
- Kong, X.; Niu, J.; Zhang, W.; Liu, J.; Yuan, J.; Li, H.; Yue, X. Mini art review for zero valent iron application in anaerobic digestion and technical bottlenecks. Sci. Total Environ. 2021, 791, 148415. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Lu, J.; Ye, J.; Zhou, Y. The effects and mechanisms of zero-valent iron on anaerobic digestion of solid waste: A mini-review. J. Clean. Prod. 2021, 278, 123567. [Google Scholar] [CrossRef]
- Pyae, H.A.; Dararatana, S. Optimizing bio-methanation by differential zero valent iron (ZVI) particle size in cassava pulp feed CSTRs. Acad. J. Biotechnol. 2019, 6. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Guo, J.; Zhang, C.; Hu, Z. Hydrogen production from the dissolution of nano zero valent iron and its effect on anaerobic digestion. Water Res. 2016, 88, 475–480. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Zhou, H.; Cao, Z.; Ying, Z.; Liu, J.; Hu, T.; Zhang, M.; Zhang, J. Effects of zero-valent iron and enzymes on the anaerobic co-digestion of sewage sludge and corn silage. Environ. Prot. Eng. 2020, 46, 41–56. [Google Scholar] [CrossRef]
- Hu, R.; Ndé-Tchoupé, A.I.; Cao, V.; Gwenzi, W.; Noubactep, C. Metallic iron for environmental remediation: The fallacy of the electron efficiency concept. Front. Environ. Chem. 2021, 2, 677813. [Google Scholar]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef] [Green Version]
- Tsinde, R.T. Designing and Piloting a Household Filter for the Peri-Urban Population of Douala (Cameroon). Freib. Online Geosci. 2021, 61, 1–90. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive Dehalogenation of Chlorinated Methanes by Iron Metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Sarr, D. Zero-Valent-Iron Permeable Reactive Barriers—How Long Will They Last? Remediation 2001, 11, 1–18. [Google Scholar]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Sustaining the efficiency of the Fe (0)/H2O system for Cr (VI) removal by MnO2 amendment. Chemosphere 2019, 214, 389–398. [Google Scholar]
- Antia, D. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz [mH2O], and n-Fex [Cation] nOyHz [Anion] m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 66–71. [Google Scholar]
- Kumar, A.; Ahammed, M.M.; Shaikh, I.N. Zero-valent iron-modified sand filters for greywater treatment. Int. J. Environ. Sci. Technol. 2022, 19, 1–14. [Google Scholar] [CrossRef]
- Tao, R.; Yang, H.; Cui, X.; Xiao, M.; Gatcha-Bandjun, N.; Kenmogne-Tchidjo, J.F.; Lufingo, M.; Konadu Amoah, B.; Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; et al. The Suitability of Hybrid Fe0/Aggregate Filtration Systems for Water Treatment. Water 2022, 14, 260. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ruppert, H.; Noubactep, C. Modeling porosity loss in Fe0-based permeable reactive barriers with Faraday’s law. Sci. Rep. 2021, 11, 16998. [Google Scholar] [CrossRef]
- Schreier, C.G.; Reinhard, M. Transformation of chlorinated organic compounds by iron and manganese powders in buffered water and in landfill leachate. Chemosphere 1994, 29, 1743–1753. [Google Scholar] [CrossRef]
- McGeough, K.L.; Kalin, R.M.; Myles, P. Carbon Disulfide Removal by Zero Valent Iron. Environ. Sci. Technol. 2007, 41, 4607–4612. [Google Scholar] [CrossRef]
- Augustos de Lemos Chernicharo, C.; Von Sperling, M. Biological Wastewater Treatment in Warm Climate Regions; IWA Publishing: London, UK, 2005. [Google Scholar]


| Name | Elemental Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Ni | Cr | Mn | Sn | Nb | Mo | |
| Steel wool (SW) | 99.25 | 0.09 | 0.09 | 0.04 | 0.5 | <LOD | <LOD | <LOD |
| Steel scraps (SI) | 98.68 | 0.33 | 0.15 | 0.27 | 0.4 | 0.07 | 0.01 | 0.02 |
| System | Medium | Fe0 Material | Fe0 Dosage (g/L) | Fe0 to Inoculum Ratio (g Fe0/gTS) |
|---|---|---|---|---|
| I | DS | none | 0 | 0.00 |
| II | DS | SI | 1 | 0.08 |
| III | DS | SI | 4 | 0.31 |
| IV | DS | SI | 10 | 0.78 |
| V | DS | SI | 15 | 1.17 |
| VI | DS | SI | 30 | 2.33 |
| VII | DS | SW | 10 | 0.78 |
| VIII | DW | SI | 10 | - |
| IX | DW | SW | 10 | - |
| System | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COD (mg COD/L) | NO3− + NH4+ (mg NO3−+ NH4+/L) | PO43− (mg PO43−/L) | Total-Fe (mg Fe/L) | pH | ||||||
| Avrg | S.D | Avrg | S.D | Avrg | S.D | Avrg | S.D | Avrg | S.D | |
| I | 235 | 115 | 89.1 | 8.6 | 19.21 | 2.96 | 0.53 | 0.25 | 7.01 | 0.16 |
| II | 177 | 120 | 79.7 | 5.6 | 15.62 | 4.40 | 1.38 | 0.29 | 7.05 | 0.12 |
| III | 166 | 124 | 73.8 | 6.1 | 10.70 | 5.26 | 2.19 | 0.87 | 7.09 | 0.12 |
| IV | 150 | 130 | 62.0 | 11.4 | 4.53 | 6.26 | 3.99 | 1.62 | 7.38 | 0.15 |
| V | 153 | 128 | 68.3 | 10.4 | 7.83 | 6.03 | 3.18 | 1.70 | 7.22 | 0.13 |
| VI | 159 | 125 | 69.9 | 8.54 | 9.74 | 5.83 | 2.52 | 1.02 | 7.18 | 0.14 |
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A: Time | is in range | 0 days | 76 days | 1 | 1 | 3 |
| B: Fe0 Dosage | is in range | 0 g Fe0/L | 30 g Fe0/L | 1 | 1 | 3 |
| COD | minimise | 48 mg COD/L | 408 mg COD/L | 1 | 1 | 3 |
| PO4 −3 | minimise | 0.3 mg PO43−/L | 26.2 mg PO43−/L | 1 | 1 | 3 |
| NO3− + NH4+ | minimise | 43.8 mg NO3−+ NH4+/L | 105.2 mg NO3−+ NH4+/L | 1 | 1 | 3 |
| Time (Days) | System | COD (mg COD/L) | PO43− (mg PO43−/L) | NO3−+ NH4+ (mg NO3−+ NH4+/L) | Desirability | |||
|---|---|---|---|---|---|---|---|---|
| Avrg | S.D | Avrg | S.D | (mg/L) | S.D | |||
| 60 | IV | 57.9 | 3.2 | 0.5 | 0.1 | 43.8 | 10.4 | 0.985 |
| 53 | VII | 54.5 | 3.2 | 0.3 | 0.5 | 54.6 | 4.5 | 0.932 |
| 53 | V | 56.7 | 3.1 | 3.6 | 0.4 | 50.8 | 9.6 | 0.907 |
| 51 | VI | 65.3 | 3.1 | 6.7 | 0.7 | 52.5 | 9.8 | 0.848 |
| 58 | III | 66.1 | 3.0 | 6.3 | 0.6 | 65.3 | 6.0 | 0.777 |
| 58 | II | 81.2 | 3.0 | 11.4 | 1.1 | 71.7 | 5.7 | 0.654 |
| 49 | I | 142.3 | 2.8 | 15.4 | 1.5 | 82.7 | 9.0 | 0.482 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakari, O.; Njau, K.N.; Noubactep, C. Fe0-Supported Anaerobic Digestion for Organics and Nutrients Removal from Domestic Sewage. Water 2022, 14, 1623. https://doi.org/10.3390/w14101623
Bakari O, Njau KN, Noubactep C. Fe0-Supported Anaerobic Digestion for Organics and Nutrients Removal from Domestic Sewage. Water. 2022; 14(10):1623. https://doi.org/10.3390/w14101623
Chicago/Turabian StyleBakari, Omari, Karoli N. Njau, and Chicgoua Noubactep. 2022. "Fe0-Supported Anaerobic Digestion for Organics and Nutrients Removal from Domestic Sewage" Water 14, no. 10: 1623. https://doi.org/10.3390/w14101623
APA StyleBakari, O., Njau, K. N., & Noubactep, C. (2022). Fe0-Supported Anaerobic Digestion for Organics and Nutrients Removal from Domestic Sewage. Water, 14(10), 1623. https://doi.org/10.3390/w14101623








