Nutrient Control of Phytoplankton Abundance and Biomass, and Microplankton Assemblage Structure in the Lower Columbia River (Vancouver, Washington, USA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Nutrient Amendment Experiments
2.3. Sample Collection and Analyses
2.3.1. Nutrients
2.3.2. Chlorophyll
2.3.3. Microplankton
2.4. Statistical Analyses
2.4.1. Monthly Variability of Chl a Concentration and Microplankton Assemblage Structure
2.4.2. Effects of Nutrient Amendment
3. Results
3.1. Ambient Columbia River Temperature, Nutrient and Chl a Concentrations
3.2. Monthly Variability of Ambient Microplankton Assemblage Structure
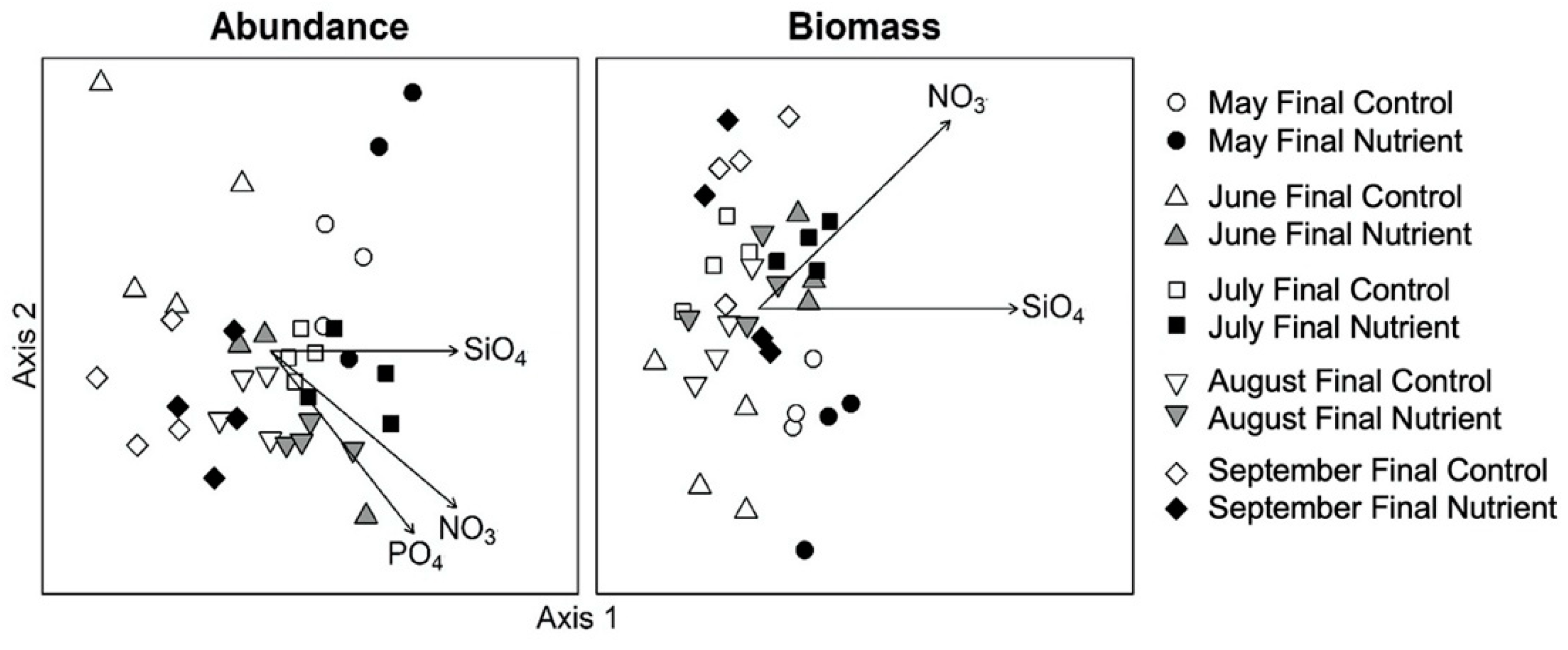
3.3. Effects of Nutrient Amendment on Chl a and Microplankton Assemblage Structure
4. Discussion
4.1. Ambient Columbia River Nutrient Concentrations, Chl a and Microplankton Composition
4.2. Effects of Nutrient Amendment on Abundance, Biomass and Assemblage Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecky, R.E.; Kilham, P. Nutrient Limitation of Phytoplankton in Freshwater and Marine Environments: A Review of Recent Evidence on the Effects of Enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W. Evolution of Phosphorus Limitation in Lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Howarth, R.W. Nitrogen Limitation on Land and in the Sea: How Can It Occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Falkowski, P.G. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryther, J.H.; Dunstan, W.M. Nitrogen, Phosphorus, and Eutrophication in the Coastal Marine Environment. Science 1971, 171, 1008–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyrrell, T. The Relative Influences of Nitrogen and Phosphorus on Oceanic Primary Production. Nature 1999, 400, 525–531. [Google Scholar] [CrossRef]
- Egge, J.; Aksnes, D. Silicate as Regulating Nutrient in Phytoplankton Competition. Mar. Ecol. Progr. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Eppley, R.W.; Rogers, J.N.; McCarthy, J.J. Half-Saturation Constants for Uptake of Nitrate and Ammonium by Marine Phytoplankton. Limnol. Oceanogr. 1969, 14, 912–920. [Google Scholar] [CrossRef]
- Jensen, J.P.; Jeppesen, E.; Olrik, K.; Kristensen, P. Impact of Nutrients and Physical Factors on the Shift from Cyanobacterial to Chlorophyte Dominance in Shallow Danish Lakes. Can. J. Fish. Aquat. Sci. 1994, 51, 1692–1699. [Google Scholar] [CrossRef]
- Officer, C.; Ryther, J. The Possible Importance of Silicon in Marine Eutrophication. Mar. Ecol. Progr. Ser. 1980, 3, 83–91. [Google Scholar] [CrossRef]
- Smith, V.H. Low Nitrogen to Phosphorus Ratios Favor Dominance by Blue-Green Algae in Lake Phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-Model of Seasonal Succession of Planktonic Events in Fresh Waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Tilman, D.; Kilham, S.S.; Kilham, P. Phytoplankton Community Ecology: The Role of Limiting Nutrients. Ann. Rev. Ecol. Syst. 1982, 13, 349–372. [Google Scholar] [CrossRef]
- Cira, E.K.; Paerl, H.W.; Wetz, M.S. Effects of Nitrogen Availability and Form on Phytoplankton Growth in a Eutrophied Estuary (Neuse River Estuary, NC, USA). PLoS ONE 2016, 11, e0160663. [Google Scholar] [CrossRef]
- Baker, P.D.; Brookes, J.D.; Burch, M.D.; Maier, H.R.; Ganf, G.G. Advection, Growth and Nutrient Status of Phytoplankton Populations in the Lower River Murray, South Australia. Regul. Rivers Res. Manag. 2000, 16, 327–344. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Li, Y.; Huang, Y.; Wei, J.; Norgbey, E.; Lai, Q.; Sarpong, L.; Wang, K.; Ji, D.; Yang, Z.; et al. Nutrient Addition Bioassay and Phytoplankton Community Structure Monitored during Autumn in Xiangxi Bay of Three Gorges Reservoir, China. Chemosphere 2020, 247, 125960. [Google Scholar] [CrossRef]
- Dodds, W.; Smith, V. Nitrogen, Phosphorus, and Eutrophication in Streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Desortová, B.; Punčochář, P. Variability of Phytoplankton Biomass in a Lowland River: Response to Climate Conditions. Limnologica 2011, 41, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Hardenbicker, P.; Rolinski, S.; Weitere, M.; Fischer, H. Contrasting Long-Term Trends and Shifts in Phytoplankton Dynamics in Two Large Rivers: Long-Term Phytoplankton Dynamics. Int. Rev. Hydrobiol. 2014, 99, 287–299. [Google Scholar] [CrossRef]
- Friedrich, G.; Pohlmann, M. Long-Term Plankton Studies at the Lower Rhine/Germany. Limnologica 2009, 39, 14–39. [Google Scholar] [CrossRef] [Green Version]
- Karadžić, V.; Simić, G.S.; Natić, D.; Ržaničanin, A.; Ćirić, M.; Gačić, Z. Changes in the Phytoplankton Community and Dominance of Cylindrospermopsis raciborskii (Wolosz.) Subba Raju in a Temperate Lowland River (Ponjavica, Serbia). Hydrobiologia 2013, 711, 43–60. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wang, X.; Wu, N.; Yang, W.; Lai, Z.; Lek, S. Spatio-Temporal Patterns and Predictions of Phytoplankton Assemblages in a Subtropical River Delta System. Fundam. Appl. Limnol. 2016, 187, 335–349. [Google Scholar] [CrossRef]
- Peterson, C.G.; Stevenson, R.J. Seasonality in River Phytoplankton: Multivariate Analyses of Data from the Ohio River and Six Kentucky Tributaries. Hydrobiologia 1989, 182, 99–114. [Google Scholar] [CrossRef]
- Scott, D.; Harvey, J.; Alexander, R.; Schwarz, G. Dominance of Organic Nitrogen from Headwater Streams to Large Rivers across the Conterminous United States: Organic Nitrogen in U.S. Rivers. Glob. Biogeochem. Cycles 2007, 21, GB1003. [Google Scholar] [CrossRef] [Green Version]
- Gaulke, A.K.; Wetz, M.S.; Paerl, H.W. Picophytoplankton: A Major Contributor to Planktonic Biomass and Primary Production in a Eutrophic, River-Dominated Estuary. Estuar. Coast. Shelf Sci. 2010, 90, 45–54. [Google Scholar] [CrossRef]
- Tavernini, S.; Pierobon, E.; Viaroli, P. Physical Factors and Dissolved Reactive Silica Affect Phytoplankton Community Structure and Dynamics in a Lowland Eutrophic River (Po River, Italy). Hydrobiologia 2011, 669, 213–225. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. Phytoplankton, Dissolved Oxygen and Nutrient Patterns along a Eutrophic River-Estuary Continuum: Observation and Modeling. J. Environ. Manag. 2020, 261, 110233. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, J.-Z.; Guéguen, C. Speciation and Fluxes of Nutrients (N, P, Si) from the Upper Yukon River. Glob. Biogeochem. Cycles 2004, 18, GB1038. [Google Scholar] [CrossRef]
- Li, M.; Xu, K.; Watanabe, M.; Chen, Z. Long-Term Variations in Dissolved Silicate, Nitrogen, and Phosphorus Flux from the Yangtze River into the East China Sea and Impacts on Estuarine Ecosystem. Estuar. Coast. Shelf Sci. 2007, 71, 3–12. [Google Scholar] [CrossRef]
- Bowes, M.J.; Loewenthal, M.; Read, D.S.; Hutchins, M.G.; Prudhomme, C.; Armstrong, L.K.; Harman, S.A.; Wickham, H.D.; Gozzard, E.; Carvalho, L. Identifying Multiple Stressor Controls on Phytoplankton Dynamics in the River Thames (UK) Using High-Frequency Water Quality Data. Sci. Total Environ. 2016, 569–570, 1489–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triplett, L.D.; Engstrom, D.R.; Conley, D.J.; Schellhaass, S.M. Silica Fluxes and Trapping in Two Contrasting Natural Impoundments of the Upper Mississippi River. Biogeochemistry 2008, 87, 217–230. [Google Scholar] [CrossRef]
- Hartmann, J.; Levy, J.; Kempe, S. Increasing Dissolved Silica Trends in the Rhine River: An Effect of Recovery from High P Loads? Limnology 2011, 12, 63–73. [Google Scholar] [CrossRef]
- Sullivan, B.E.; Prahl, F.G.; Small, L.F.; Covert, P.A. Seasonality of Phytoplankton Production in the Columbia River: A Natural or Anthropogenic Pattern? Geochim. Cosmochim. Acta 2001, 65, 1125–1139. [Google Scholar] [CrossRef]
- Maier, M.A.; Peterson, T.D. Prevalence of Chytrid Parasitism among Diatom Populations in the Lower Columbia River (2009–2013). Freshw. Biol. 2017, 62, 414–428. [Google Scholar] [CrossRef]
- Rose, V.; Rollwagen-Bollens, G.; Bollens, S.; Zimmerman, J. Seasonal and Interannual Variation in Lower Columbia River Phytoplankton (2005–2018): Environmental Variability and a Decline in Large Bloom-Forming Diatoms. Aquat. Microb. Ecol. 2021, 87, 29–46. [Google Scholar] [CrossRef]
- Simenstad, C.A.; Small, L.F.; David McIntire, C.; Jay, D.A.; Sherwood, C. Columbia River Estuary Studies: An Introduction to the Estuary, a Brief History, and Prior Studies. Progr. Oceanogr. 1990, 25, 1–13. [Google Scholar] [CrossRef]
- Payne, J.T.; Wood, A.W.; Hamlet, A.F.; Palmer, R.N.; Lettenmaier, D.P. Mitigating the Effects of Climate Change on the Water Resources of the Columbia River Basin. Clim. Chang. 2004, 62, 233–256. [Google Scholar] [CrossRef]
- Chawla, A.; Jay, D.A.; Baptista, A.M.; Wilkin, M.; Seaton, C. Seasonal Variability and Estuary–Shelf Interactions in Circulation Dynamics of a River-Dominated Estuary. Estuaries Coasts 2008, 31, 269–288. [Google Scholar] [CrossRef] [Green Version]
- Jay, D.A.; Leffler, K.; Diefenderfer, H.L.; Borde, A.B. Tidal-Fluvial and Estuarine Processes in the Lower Columbia River: I. Along-Channel Water Level Variations, Pacific Ocean to Bonneville Dam. Estuaries Coasts 2015, 38, 415–433. [Google Scholar] [CrossRef]
- Dexter, E.; Katz, S.L.; Bollens, S.M.; Rollwagen-Bollens, G.; Hampton, S.E. Modeling the Trophic Impacts of Invasive Zooplankton in a Highly Invaded River. PLoS ONE 2020, 15, e0243002. [Google Scholar] [CrossRef] [PubMed]
- Dexter, E.; Bollens, S.M.; Rollwagen-Bollens, G.; Emerson, J.; Zimmerman, J. Persistent vs. Ephemeral Invasions: 8.5 Years of Zooplankton Community Dynamics in the Columbia River. Limnol. Oceanogr. 2015, 60, 527–539. [Google Scholar] [CrossRef]
- Bestion, E.; García-Carreras, B.; Schaum, C.-E.; Pawar, S.; Yvon-Durocher, G. Metabolic Traits Predict the Effects of Warming on Phytoplankton Competition. Ecol. Lett. 2018, 21, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilham, S.S.; Kreeger, D.A.; Lynn, S.G.; Goulden, C.E.; Herrera, L. COMBO: A Defined Freshwater Culture Medium for Algae and Zooplankton. Hydrobiologia 1998, 377, 147–159. [Google Scholar] [CrossRef]
- Prahl, F.G.; Small, L.F.; Sullivan, B.A.; Cordell, J.; Simenstad, C.A.; Crump, B.C.; Baross, J.A. Biogeochemical Gradients in the Lower Columbia River. Hydrobiologia 1998, 361, 37–52. [Google Scholar] [CrossRef]
- Moss, B. Ecology of Fresh Waters: Man and Medium, Past to Future, 3rd ed.; Blackwell Publishing: Malden, MA, USA, 1998; ISBN 978-0-632-03512-0. [Google Scholar]
- Wetzel, R.G. Limnology, 2nd ed.; Saunders College Publishing: Philadelphia, PA, USA, 1983; ISBN 978-0-03-033016-2. [Google Scholar]
- Kalff, J. Limnology: Inland Water Ecosystems, 2nd ed.; Prentice Hall Publishers: Hoboken, NJ, USA, 2001; ISBN 978-0-13-033775-7. [Google Scholar]
- Armengol, J. Sau Reservoir’s Light Climate: Relationships between Secchi Depth and Light Extinction Coefficient. Limnetica 2003, 22, 195–210. [Google Scholar] [CrossRef]
- Gordon, L.I.; Jennings, J.C.; Ross, A.A.; Krest, J.M. A Suggested Protocol for Continuous Flow Automated Analysis of Seawater Nutrients (Phosphate, Nitrate, Nitrite and Silicic Acid) in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study; WOCE Hydrographic Program Office: Southampton, UK, 1993; p. 56. [Google Scholar]
- Arar, E.J.; Collins, G. In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence; US Environmental Protection Agency: Cincinnati, OH, USA, 1997; p. 22.
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. SIL Commun. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Kirchman, D. Statistical Analysis of Direct Counts of Microbial Abundance. In Handbook of Methods in Aquatic Microbial Ecology; Lewis Publishers: Boca Raton, FL, USA, 1993; ISBN 978-0-87371-564-5. [Google Scholar]
- Patterson, D.J.; Hedley, S. Free-Living Freshwater Protozoa: A Colour Guide; Wolfe Publishing: Prescott, AZ, USA, 1992; ISBN 978-0-7234-1683-8. [Google Scholar]
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. Freshwater Algae of North America: Ecology and Classification, 2nd ed.; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2015; ISBN 978-0-12-385876-4. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to Volume Relationships for Dinoflagellates, Diatoms, and Other Protist Plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Statist. 1979, 6, 65–70. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Ogle, D.; Doll, J.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. 2020. Available online: https://cloud.r-project.org/web/packages/FSA/index.html (accessed on 1 May 2020).
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Herv, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. 2019. Available online: https://cran.r-project.org/web/packages/RVAideMemoire/index.html (accessed on 9 April 2022).
- Kruskal, J.B. Multidimensional Scaling by Optimizing Goodness of Fit to a Nonmetric Hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Dexter, E.; Rollwagen-Bollens, G.; Bollens, S.M. The Trouble with Stress: A Flexible Method for the Evaluation of Nonmetric Multidimensional Scaling. Limnol. Oceanogr. Methods 2018, 16, 434–443. [Google Scholar] [CrossRef]
- Clarke, K.R.; Ainsworth, M. A Method of Linking Multivariate Community Structure to Environmental Variables. Mar. Ecol. Progr. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Hedges, L.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Cambridge, MA, USA, 1985; ISBN 978-0-08-057065-5. [Google Scholar]
- McGrath, R.E.; Meyer, G.J. When Effect Sizes Disagree: The Case of r and d. Psychol. Meth. 2006, 11, 386–401. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Chen, S.H.A.; Lüdecke, D. Indices of Effect Existence and Significance in the Bayesian Framework. Front. Psychol. 2019, 10, 2767. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2017. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 9 April 2022).
- Humborg, C.; Conley, D.J.; Rahm, L.; Wulff, F.; Cociasu, A.; Ittekkot, V. Silicon Retention in River Basins: Far-Reaching Effects on Biogeochemistry and Aquatic Food Webs in Coastal Marine Environments. Ambio 2000, 29, 45–50. [Google Scholar] [CrossRef]
- US EPA. National Rivers and Streams Assessment 2008–2009: A Collaborative Survey; US EPA: Washington, DC, USA, 2016.
- Knowlton, M.F.; Jones, J.R. Seston, Light, Nutrients and Chlorophyll in the Lower Missouri River, 1994–1998. J. Freshw. Ecol. 2000, 15, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Rubin, M.A.; Leff, L.G. Nutrients and Other Abiotic Factors Affecting Bacterial Communities in an Ohio River (USA). Microb. Ecol. 2007, 54, 374–383. [Google Scholar] [CrossRef]
- Houser, J.N.; Richardson, W.B. Nitrogen and Phosphorus in the Upper Mississippi River: Transport, Processing, and Effects on the River Ecosystem. Hydrobiologia 2010, 640, 71–88. [Google Scholar] [CrossRef]
- Rose, V.; Rollwagen-Bollens, G.; Bollens, S.M.; Zimmerman, J. The Effects of Run-of-river Dam Spill on Columbia River Microplankton. River Res. Appl. 2019, 35, 1478–1488. [Google Scholar] [CrossRef]
- Sommer, U. Trophic Cascades in Marine and Freshwater Plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Rollwagen-Bollens, G.; Bollens, S.; Gonzalez, A.; Zimmerman, J.; Lee, T.; Emerson, J. Feeding Dynamics of the Copepod Diacyclops thomasi before, during and Following Filamentous Cyanobacteria Blooms in a Large, Shallow Temperate Lake. Hydrobiologia 2013, 705, 101–118. [Google Scholar] [CrossRef]
- Rollwagen-Bollens, G.; Lee, T.; Rose, V.; Bollens, S. Beyond Eutrophication: Vancouver Lake, WA, USA as a Model System for Assessing Multiple, Interacting Biotic and Sbiotic Drivers of Harmful Cyanobacterial Blooms. Water 2018, 10, 757. [Google Scholar] [CrossRef] [Green Version]
- Dexter, E.; Bollens, S.M. Zooplankton Invasions in the Early 21st Century: A Global Survey of Recent Studies and Recommendations for Future Research. Hydrobiologia 2020, 847, 309–319. [Google Scholar] [CrossRef]
- Hassett, W.; Bollens, S.M.; Counihan, T.D.; Rollwagen-Bollens, G.; Zimmerman, J.; Katz, S.; Emerson, J. Veligers of the Invasive Asian Clam Corbicula fluminea in the Columbia River Basin: Broadscale Distribution, Abundance, and Ecological Associations. Lake Reserv. Manag. 2017, 33, 234–248. [Google Scholar] [CrossRef]
- Bolam, B.A.; Rollwagen-Bollens, G.; Bollens, S.M. Feeding Rates and Prey Selection of the Invasive Asian Clam, Corbicula fluminea, on Microplankton in the Columbia River, USA. Hydrobiologia 2019, 833, 107–123. [Google Scholar] [CrossRef]
- Rollwagen-Bollens, G.; Bolam, B.A.; Bollens, S.M.; Henricksen, S.; Sandison, C.; Zimmerman, J. Temperature-Dependent Functional Response of the Invasive Asian Clam, Corbicula fluminea, Feeding on Natural Phytoplankton. Inland Waters 2021, 11, 250–256. [Google Scholar] [CrossRef]
- Henricksen, S.; Bollens, S. Abundance and Growth of the Invasive Asian Clam, Corbicula fluminea, in the Lower Columbia River, USA. Aquat. Invasions 2022, 17, 36–56. [Google Scholar] [CrossRef]
- Elser, J.J.; Marzolf, E.R.; Goldman, C.R. Phosphorus and Nitrogen Limitation of Phytoplankton Growth in the Freshwaters of North America: A Review and Critique of Experimental Enrichments. Can. J. Fish. Aquat. Sci. 1990, 47, 1468–1477. [Google Scholar] [CrossRef]
- Schindler, D.W. The Dilemma of Controlling Cultural Eutrophication of Lakes. Proc. R. Soc. B 2012, 279, 4322–4333. [Google Scholar] [CrossRef] [Green Version]
- Weisse, T.; Lukić, D.; Lu, X. Container Volume May Affect Growth Rates of Ciliates and Clearance Rates of Their Microcrustacean Predators in Microcosm Experiments. J. Plankt. Res. 2021, 43, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.; Velzeboer, R.; Zammit, R. Influence of Nutrient Availability on Phytoplankton Growth and Community Structure in the Port Adelaide River, Australia: Bioassay Assessment of Potential Nutrient Limitation. Hydrobiologia 2000, 429, 89–103. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Bowling, L.C.; Buckney, R.T. Responses of Phytoplankton to In-Situ Nutrient Enrichment; Potential Influences on Species Dominance in a River. Int. Rev. Hydrobiol. 2001, 86, 285–298. [Google Scholar] [CrossRef]
- Domingues, R.B.; Anselmo, T.P.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Nutrient Limitation of Phytoplankton Growth in the Freshwater Tidal Zone of a Turbid, Mediterranean Estuary. Coast. Shelf Sci. 2011, 91, 282–297. [Google Scholar] [CrossRef]
- Wise, D.R.; Johnson, H.M. Surface-Water Nutrient Conditions and Sources in the United States Pacific Northwest. J. Am. Water Resour. Assoc. 2011, 47, 1110–1135. [Google Scholar] [CrossRef]
- Rose, V.J.; Forney, W.M.; Norton, R.A.; Harrison, J.A. Catchment Characteristics, Water Quality, and Cyanobacterial Blooms in Washington and Oregon Lakes. Lake Reserv. Manag. 2019, 35, 51–63. [Google Scholar] [CrossRef]
- Emerson, J.E.; Bollens, S.M.; Counihan, T.D. Seasonal Dynamics of Zooplankton in Columbia–Snake River Reservoirs, with Special Emphasis on the Invasive Copepod Pseudodiaptomus forbesi. Aquat. Invasions 2015, 10, 25–40. [Google Scholar] [CrossRef]
- Haskell, C.A.; Beauchamp, D.A.; Bollens, S.M. Trophic Interactions and Consumption Rates of Subyearling Chinook Salmon and Nonnative Juvenile American Shad in Columbia River Reservoirs. Trans. Am. Fish. Soc. 2017, 146, 291–298. [Google Scholar] [CrossRef]
- Haskell, C.A.; Beauchamp, D.A.; Bollens, S.M. Linking Functional Response and Bioenergetics to Estimate Juvenile Salmon Growth in a Reservoir Food Web. PLoS ONE 2017, 12, e0185933. [Google Scholar] [CrossRef]

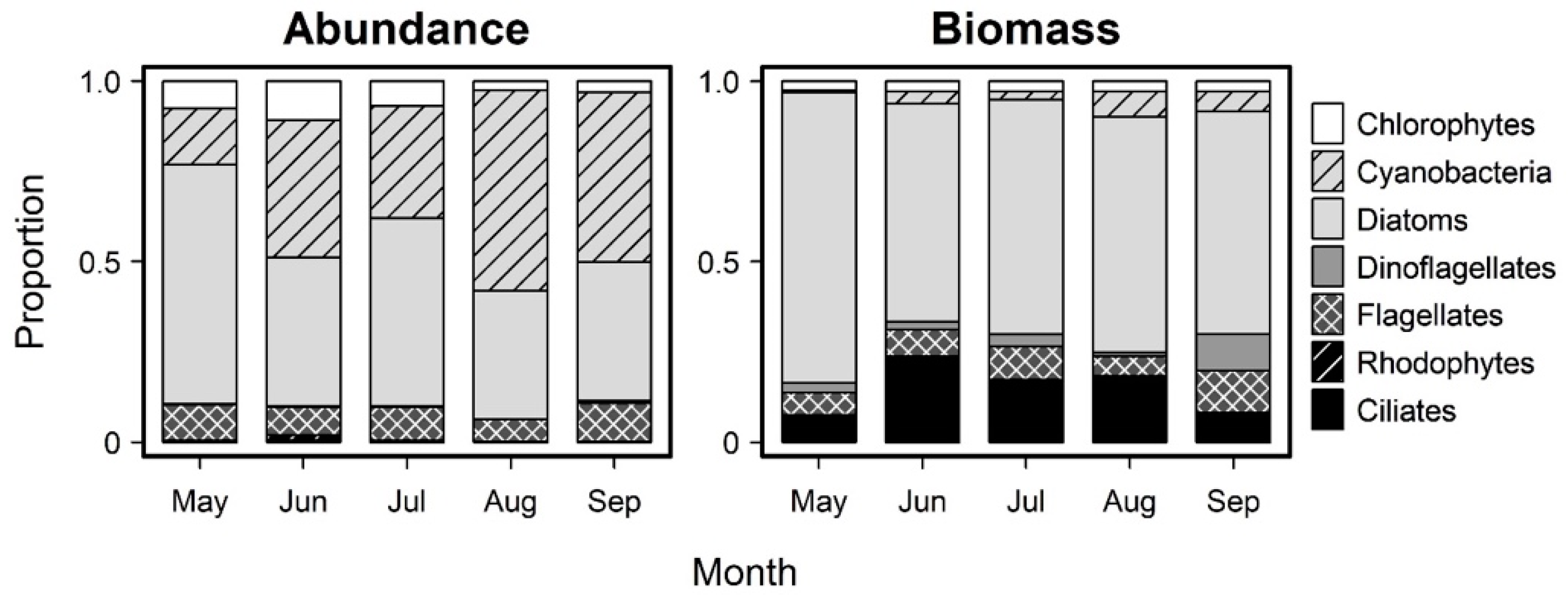
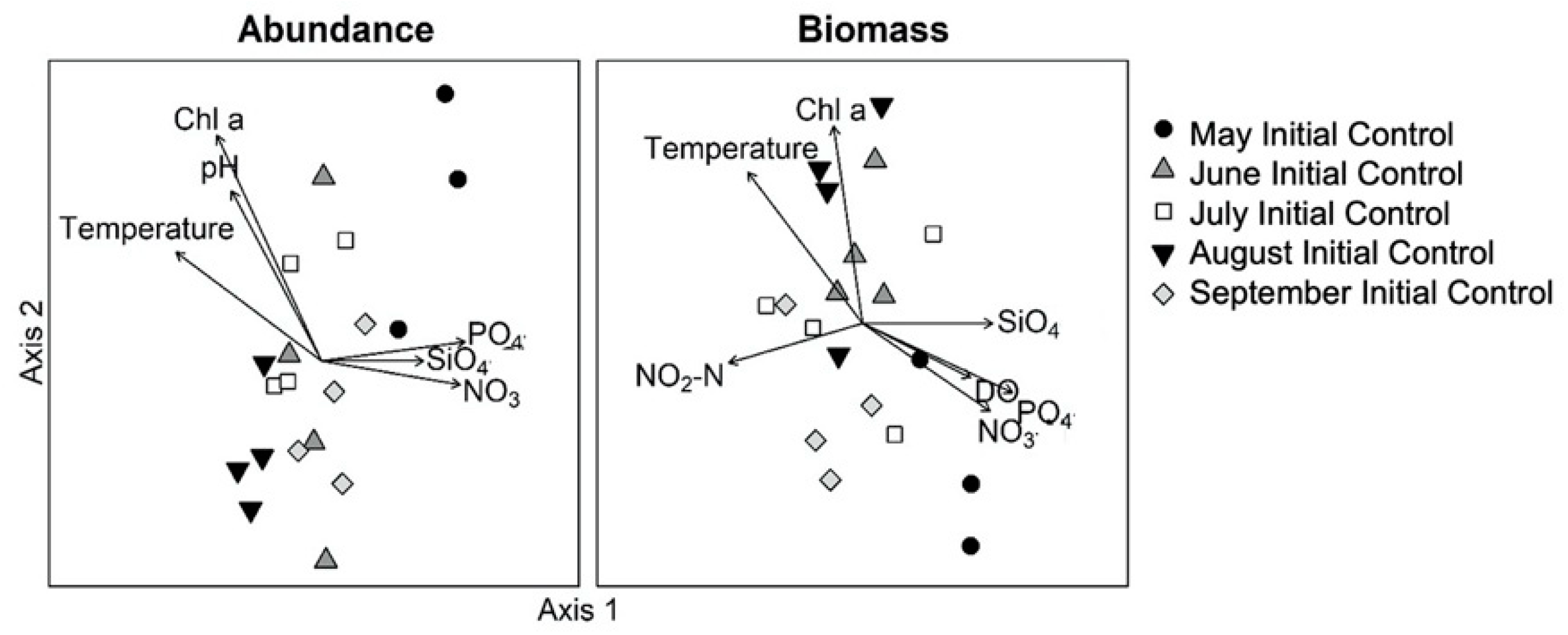
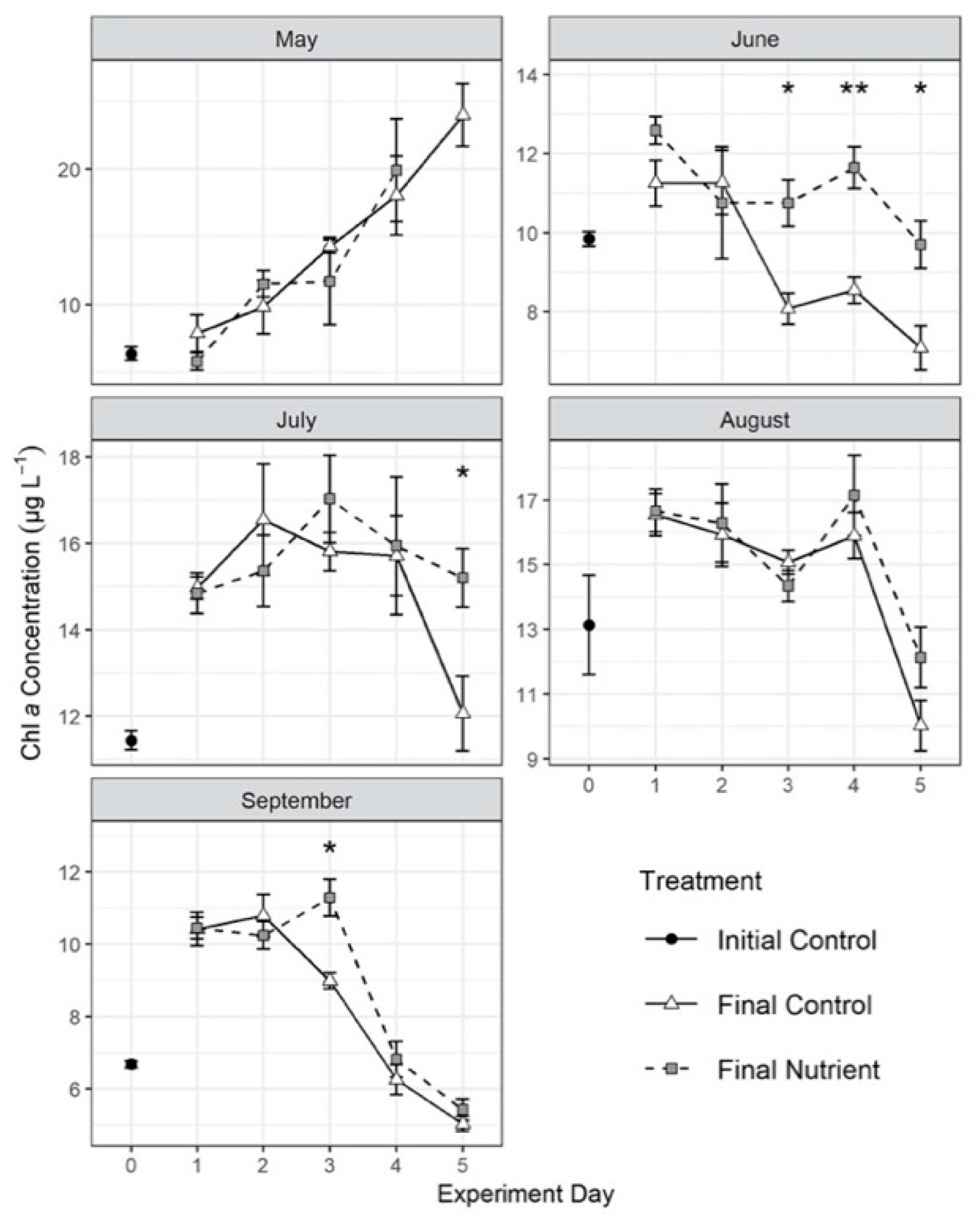
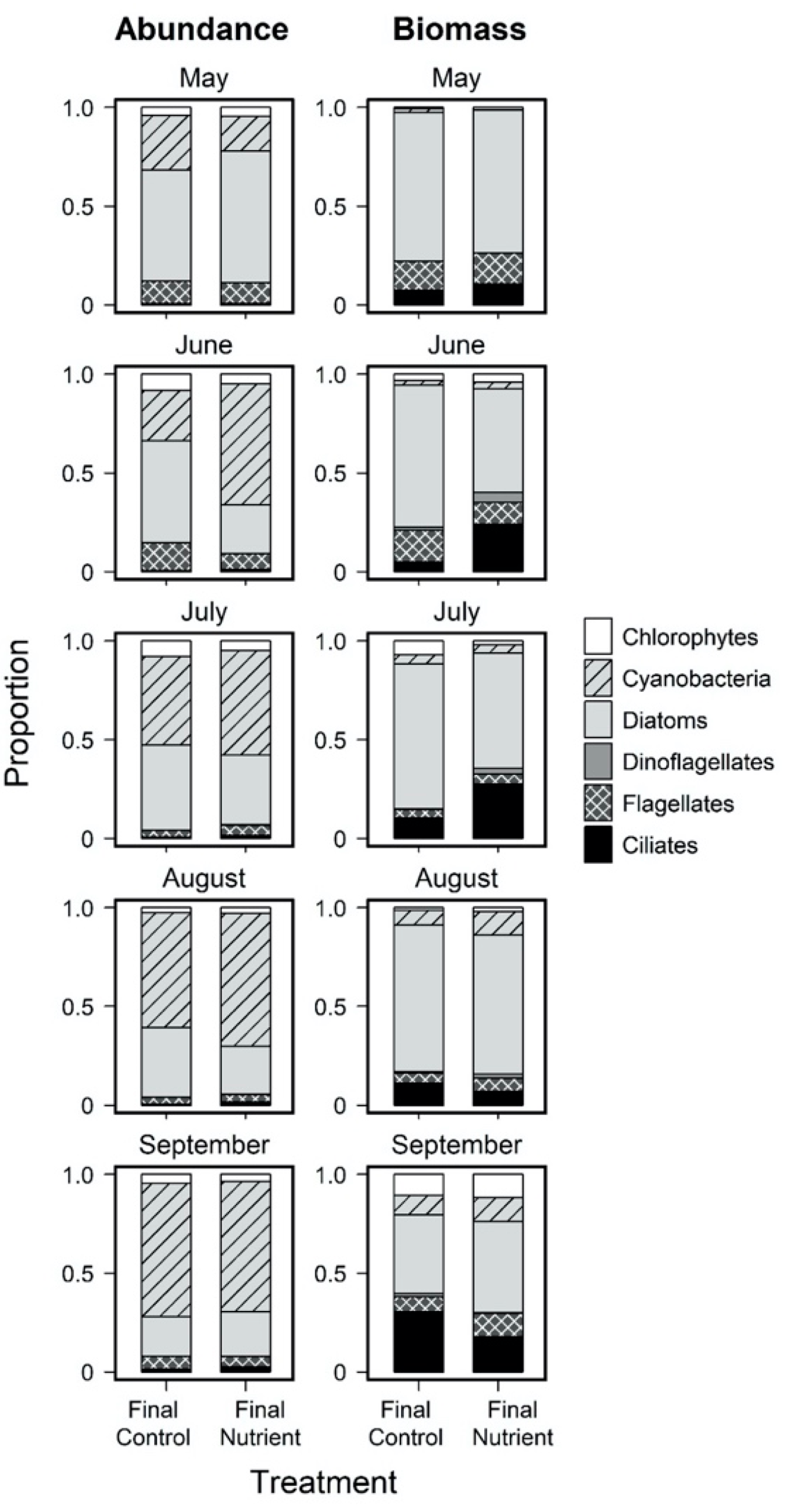

| Day 0 Initial Control: Abundance | Day 0 Initial Control: Carbon Biomass | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | SS | MS | F | R2 | p | df | SS | MS | F | R2 | p | ||
| Month | 4 | 0.266 | 0.066 | 8.537 | 0.709 | 0.001 | Month | 4 | 0.120 | 0.030 | 4.103 | 0.540 | 0.002 |
| Residuals | 14 | 0.109 | 0.008 | 0.291 | Residuals | 14 | 0.103 | 0.007 | 0.460 | ||||
| Total | 18 | 0.375 | 1.000 | Total | 18 | 0.223 | 1.000 | ||||||
| p values for pairwise comparisons | p values for pairwise comparisons | ||||||||||||
| May | June | July | August | May | June | July | August | ||||||
| Jun. | 0.032 | Jun. | 0.027 | ||||||||||
| Jul. | 0.027 | 0.38 | Jul. | 0.094 | 0.738 | ||||||||
| Aug. | 0.031 | 0.031 | 0.024 | Aug. | 0.025 | 0.146 | 0.067 | ||||||
| Sep. | 0.033 | 0.034 | 0.088 | 0.026 | Sep. | 0.031 | 0.028 | 0.243 | 0.029 | ||||
| Experiment | Parameters | Abundance | Carbon Biomass | ||
|---|---|---|---|---|---|
| r2 | p | r2 | p | ||
| Day 0 Initial Control | Temperature | 0.833 | 0.001 | 0.545 | 0.002 |
| Dissolved oxygen | 0.272 | 0.088 | 0.324 | 0.055 | |
| pH | 0.410 | 0.015 | 0.202 | 0.181 | |
| Chlorophyll a | 0.597 | 0.001 | 0.375 | 0.029 | |
| PO4 | 0.768 | 0.001 | 0.617 | 0.001 | |
| SiO4 | 0.390 | 0.024 | 0.433 | 0.012 | |
| NO3 | 0.719 | 0.001 | 0.486 | 0.010 | |
| NO2 | 0.274 | 0.095 | 0.472 | 0.008 | |
| NH4 | 0.321 | 0.051 | 0.286 | 0.066 | |
| May Day 5: Abundance | May Day 5: Carbon Biomass | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | SS | MS | F | R2 | p | df | SS | MS | F | R2 | p | |||
| Treatment | 1 | 0.014 | 0.014 | 0.972 | 0.196 | 0.4 | Treatment | 1 | 0.012 | 0.012 | 2.197 | 0.355 | 0.2 | |
| Residuals | 4 | 0.057 | 0.014 | 0.804 | Residuals | 4 | 0.230 | 0.006 | 0.645 | |||||
| Total | 5 | 0.071 | 1.000 | Total | 5 | 0.035 | 1.000 | |||||||
| June Day 5: Abundance | June Day 5: Carbon Biomass | |||||||||||||
| df | SS | MS | F | R2 | p | df | SS | MS | F | R2 | p | |||
| Treatment | 1 | 0.099 | 0.099 | 4.648 | 0.482 | 0.023 | Treatment | 1 | 0.067 | 0.067 | 5.579 | 0.527 | 0.027 | |
| Residuals | 5 | 0.106 | 0.021 | 0.518 | Residuals | 5 | 0.060 | 0.012 | 0.473 | |||||
| Total | 6 | 0.205 | 1.000 | Total | 6 | 0.127 | 1.000 | |||||||
| July Day 5: Abundance | July Day 5: Carbon Biomass | |||||||||||||
| df | SS | MS | F | R2 | p | df | SS | MS | F | R2 | p | |||
| Treatment | 1 | 0.022 | 0.022 | 4.96 | 0.453 | 0.027 | Treatment | 1 | 0.053 | 0.053 | 8.040 | 0.573 | 0.033 | |
| Residuals | 6 | 0.027 | 0.005 | 0.547 | Residuals | 6 | 0.040 | 0.007 | 0.427 | |||||
| Total | 7 | 0.049 | 1.000 | Total | 7 | 0.093 | 1.000 | |||||||
| August Day 5: Abundance | August Day 5: Carbon Biomass | |||||||||||||
| df | SS | MS | F | R2 | p | df | SS | MS | F | R2 | p | |||
| Treatment | 1 | 0.025 | 0.025 | 5.974 | 0.499 | 0.034 | Treatment | 1 | 0.015 | 0.015 | 1.962 | 0.246 | 0.222 | |
| Residuals | 6 | 0.026 | 0.004 | 0.501 | Residuals | 6 | 0.047 | 0.008 | 0.754 | |||||
| Total | 7 | 0.051 | 1.000 | Total | 7 | 0.062 | 1.000 | |||||||
| September Day 5: Abundance | September Day 5: Carbon Biomass | |||||||||||||
| df | SS | MS | F | R2 | p | df | SS | MS | F | R2 | p | |||
| Treatment | 1 | 0.022 | 0.022 | 2.650 | 0.306 | 0.125 | Treatment | 1 | 0.012 | 0.012 | 0.893 | 0.130 | 0.423 | |
| Residuals | 6 | 0.050 | 0.008 | 0.694 | Residuals | 6 | 0.081 | 0.013 | 0.870 | |||||
| Total | 7 | 0.072 | 1.000 | Total | 7 | 0.093 | 1.000 | |||||||
| Experiment | Parameters | Abundance | Biomass | ||
|---|---|---|---|---|---|
| r2 | p | r2 | p | ||
| Day 5 | PO4 | 0.266 | 0.006 | 0.143 | 0.080 |
| SiO4 | 0.222 | 0.012 | 0.402 | 0.001 | |
| NO3 | 0.320 | 0.002 | 0.274 | 0.007 | |
| NO2 | 0.138 | 0.083 | 0.012 | 0.848 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollwagen-Bollens, G.; Connelly, K.A.; Bollens, S.M.; Zimmerman, J.; Coker, A. Nutrient Control of Phytoplankton Abundance and Biomass, and Microplankton Assemblage Structure in the Lower Columbia River (Vancouver, Washington, USA). Water 2022, 14, 1599. https://doi.org/10.3390/w14101599
Rollwagen-Bollens G, Connelly KA, Bollens SM, Zimmerman J, Coker A. Nutrient Control of Phytoplankton Abundance and Biomass, and Microplankton Assemblage Structure in the Lower Columbia River (Vancouver, Washington, USA). Water. 2022; 14(10):1599. https://doi.org/10.3390/w14101599
Chicago/Turabian StyleRollwagen-Bollens, Gretchen, Kristin A. Connelly, Stephen M. Bollens, Julie Zimmerman, and Alixandra Coker. 2022. "Nutrient Control of Phytoplankton Abundance and Biomass, and Microplankton Assemblage Structure in the Lower Columbia River (Vancouver, Washington, USA)" Water 14, no. 10: 1599. https://doi.org/10.3390/w14101599
APA StyleRollwagen-Bollens, G., Connelly, K. A., Bollens, S. M., Zimmerman, J., & Coker, A. (2022). Nutrient Control of Phytoplankton Abundance and Biomass, and Microplankton Assemblage Structure in the Lower Columbia River (Vancouver, Washington, USA). Water, 14(10), 1599. https://doi.org/10.3390/w14101599







