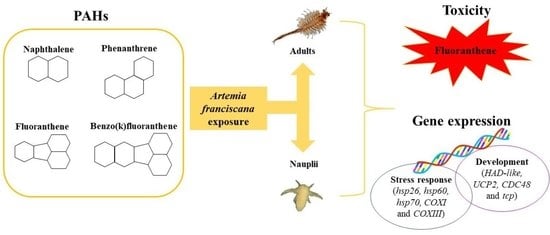
Genotoxicity Set Up in Artemia franciscana Nauplii and Adults Exposed to Phenanthrene, Naphthalene, Fluoranthene, and Benzo(k)fluoranthene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ecotoxicity Test
2.2. Chemicals
2.3. Acute Toxicity Test
2.4. Organisms Exposures for RNA Extraction
2.5. cDNA Synthesis and Real Time q-PCR
2.6. LC50 Calculation and Statistical Analyses
3. Results
3.1. PAHs Analysis
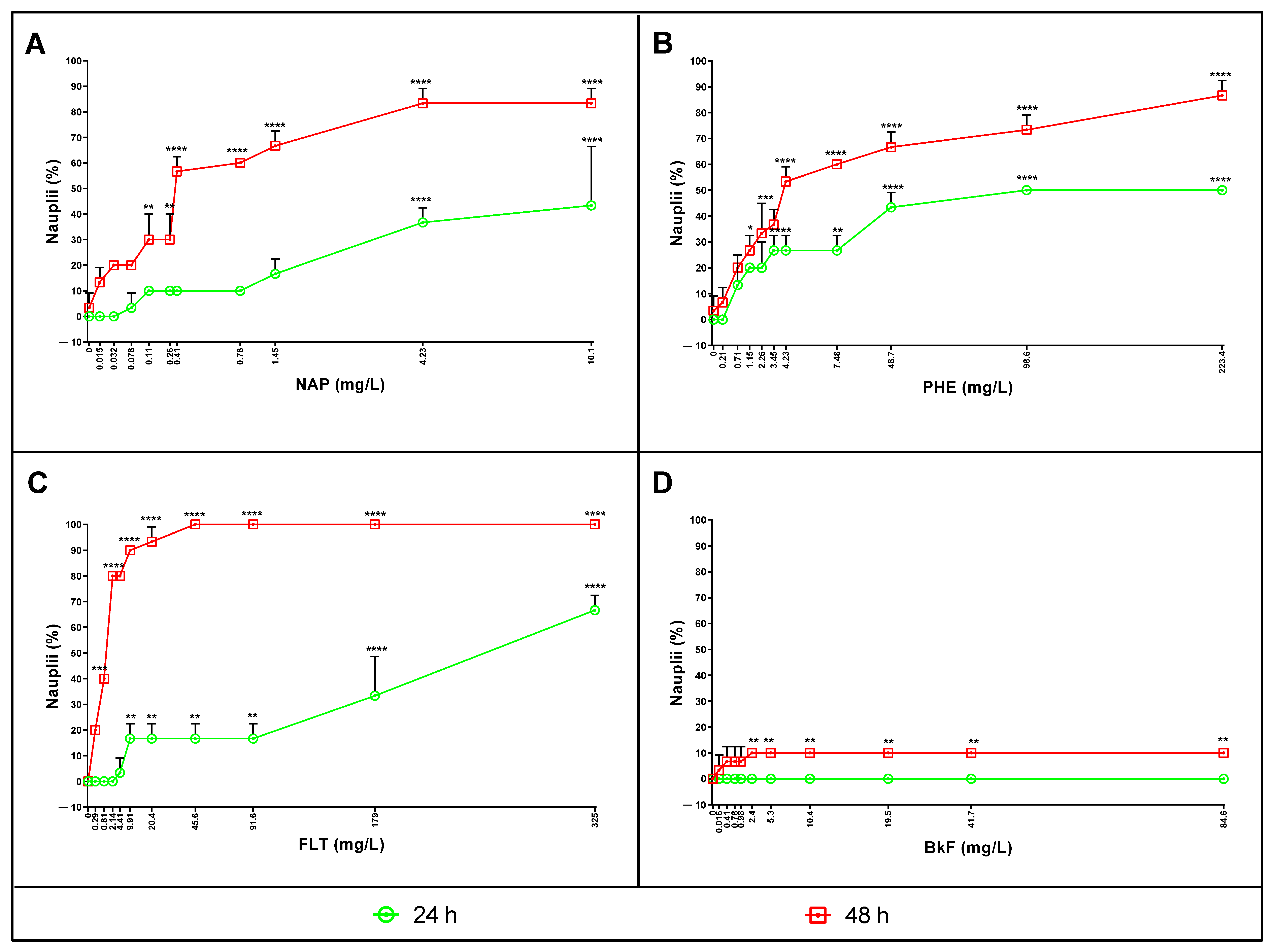
3.2. Naphthalene, Phenanthrene, Fluoranthene, and Benzo(k)fluoranthene Toxicity on Nauplii
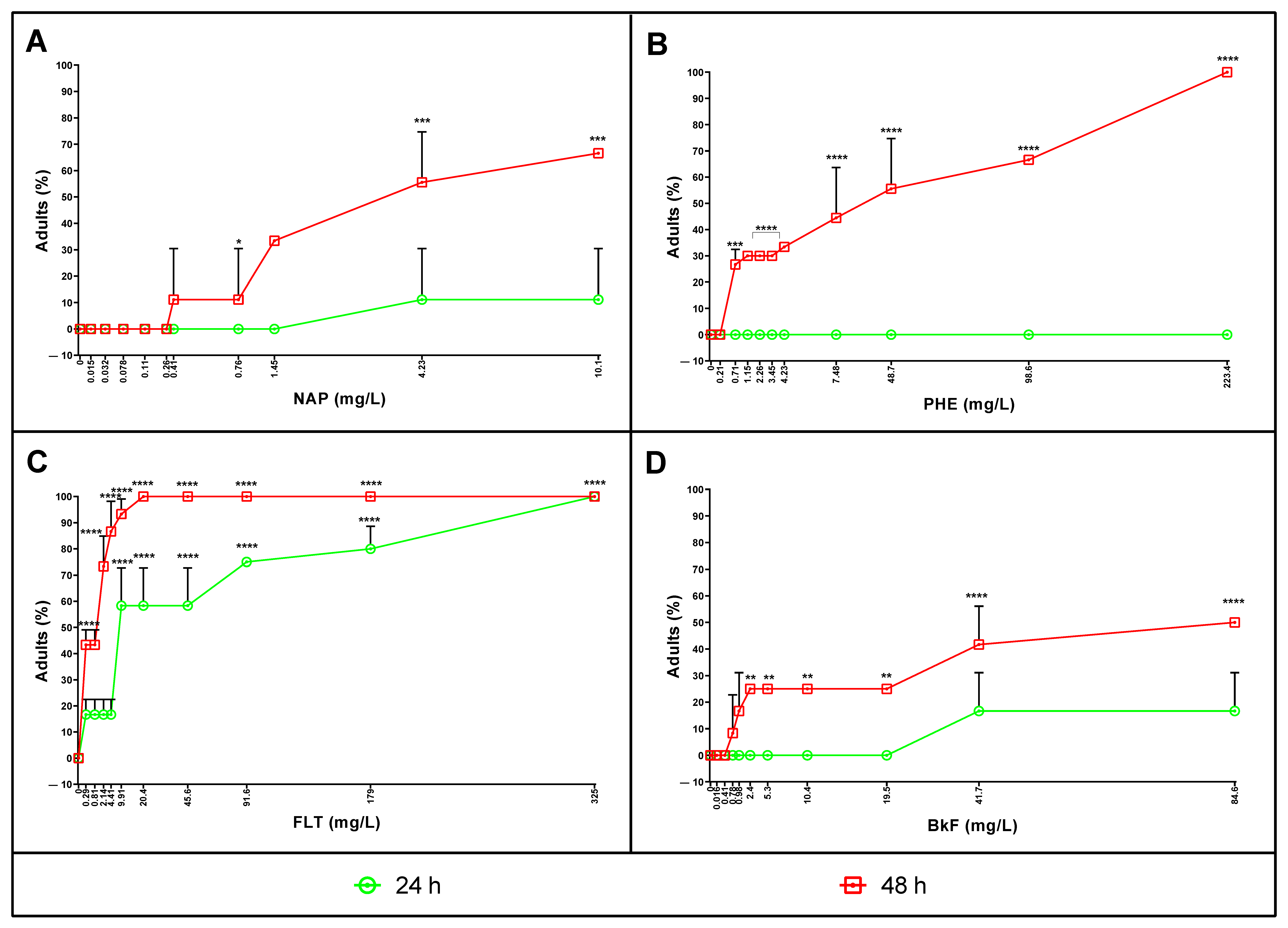
3.3. Naphthalene, Phenanthrene, Fluoranthene, and Benzo(k)fluoranthene Toxicity on Adults
3.4. Lethal Concentrations after 24 and 48 h of Exposure
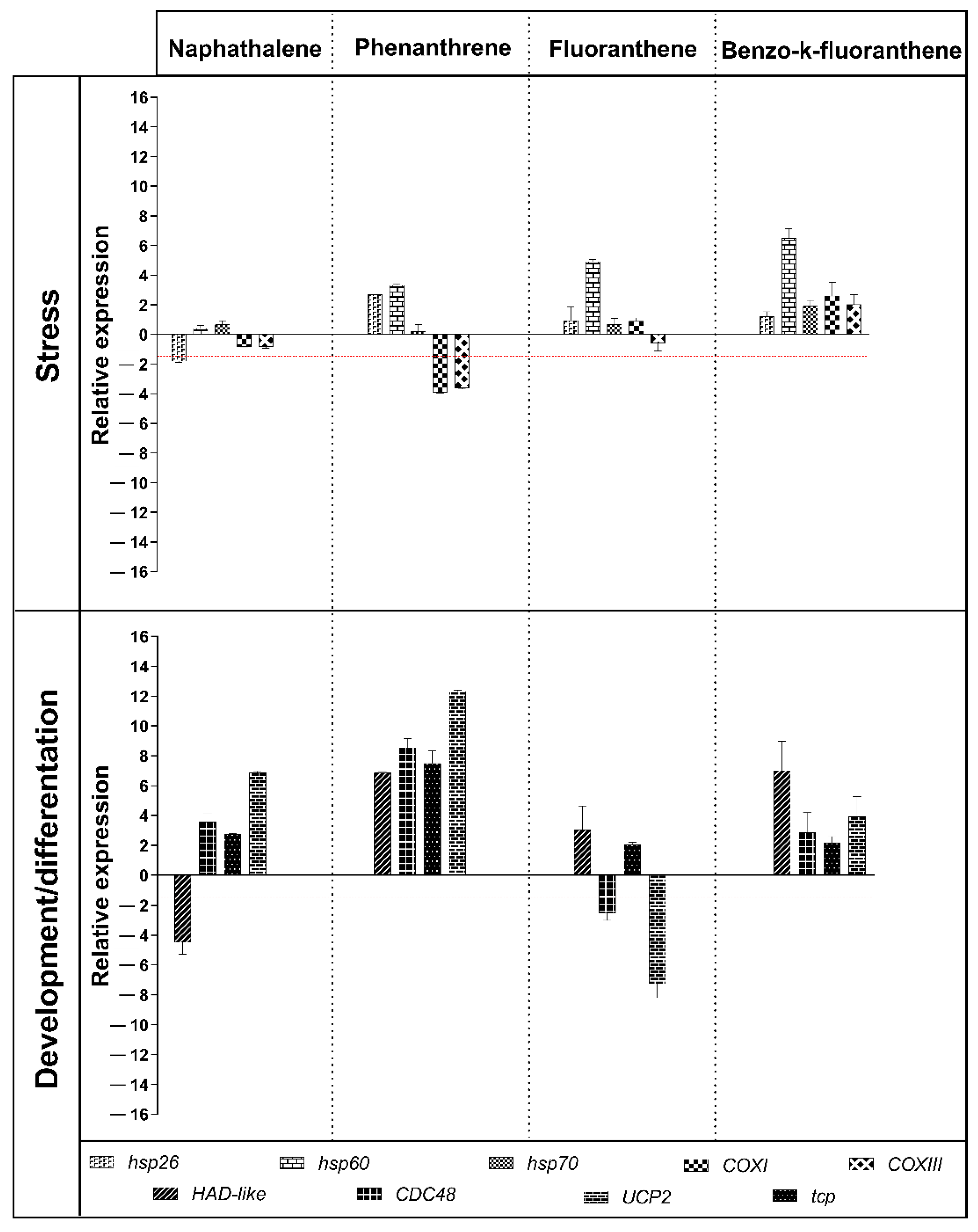
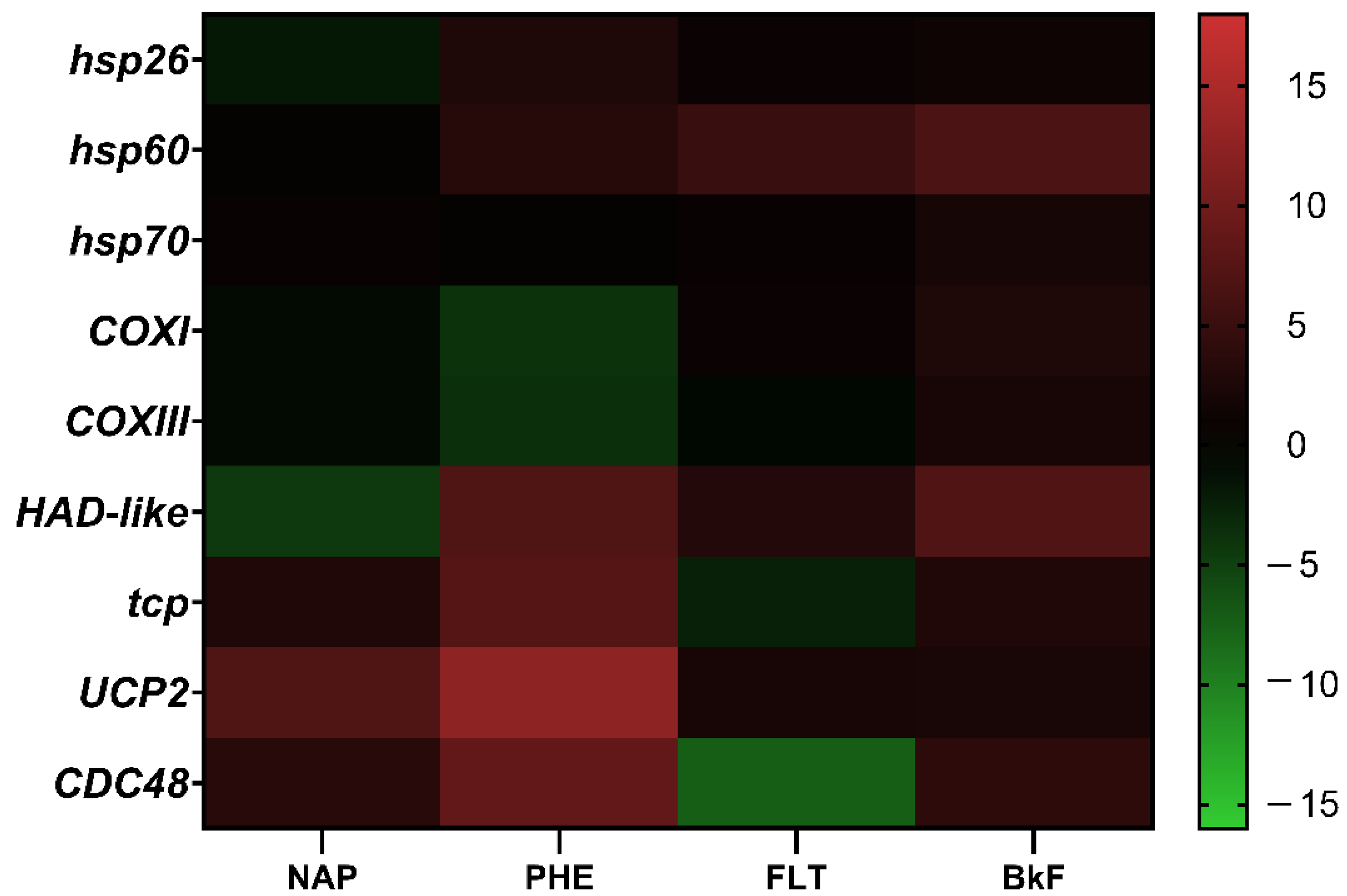
3.5. Gene Response to NAP, PHE, FLT, and BkF Exposure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medeiros, P.M.; Caruso Bícego, M. Investigation of natural and anthropogenic hydrocarbon inputs in sediments using geochemical markers. I. Santos, SP—Brazil. Mar. Pollut. Bull. 2004, 49, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Millemann, R.E.; Birge, W.J.; Black, J.A.; Cushman, R.M.; Daniels, K.L.; Franco, P.J.; Giddings, J.M.; McCarthy, J.F.; Stewart, A.J. Comparative Acute Toxicity to Aquatic Organisms of Components of Coal-Derived Synthetic Fuels. Trans. Am. Fish. Soc. 2014, 113, 37–41. [Google Scholar] [CrossRef]
- Giddings, J.M. Acute toxicity to Selenastrum capricornutum of aromatic compounds from coal conversion. Bull. Environ. Contam. Toxicol. 1979, 23, 360–364. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 2675. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.W.; Zhang, G.; Liu, G.Q.; Guo, L.L.; Li, X.D.; Wai, O. Polycyclic aromatic hydrocarbons (PAHs) in the water column and sediment core of Deep Bay, South China. Estuar. Coast. Shelf Sci. 2009, 83, 60–66. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Berrojalbiz, N.; Wollgast, J.; Dachs, J. Polycyclic aromatic hydrocarbons (PAHs) in the Mediterranean Sea: Atmospheric occurrence, deposition and decoupling with settling fluxes in the water column. Environ. Pollut. 2012, 166, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Chen, L.; He, Y.; Baumann, Z.; Mason, R.P.; Shen, H.; Yu, C.; Zhang, W.; Zhang, Q.; Wang, X. Impacts of farmed fish consumption and food trade on methylmercury exposure in China. Environ. Int. 2018, 120, 333–344. [Google Scholar] [CrossRef]
- Gregorin, C.; Albarano, L.; Somma, E.; Costantini, M.; Zupo, V. Assessing the ecotoxicity of copper and polycyclic aromatic hydrocarbons: Comparison of effects on Paracentrotus lividus and Botryllus schlosseri, as alternative bioassay methods. Water 2021, 13, 711. [Google Scholar] [CrossRef]
- Albarano, L.; Zupo, V.; Guida, M.; Libralato, G.; Caramiello, D.; Ruocco, N.; Costantini, M. PAHs and PCBs affect functionally intercorrelated genes in the sea urchin paracentrotus lividus embryos. Int. J. Mol. Sci. 2021, 22, 12498. [Google Scholar] [CrossRef]
- MacRae, J.D.; Hall, K.J. Biodegradation of polycyclic aromatic hydrocarbons (PAH) in marine sediment under denitrifying conditions. Water Sci. Technol. 1998, 38, 177–185. [Google Scholar] [CrossRef]
- Shi, D.; Bera, G.; Knap, A.H.; Quigg, A.; Al Atwah, I.; Gold-Bouchot, G.; Wade, T.L. A mesocosm experiment to determine half-lives of individual hydrocarbons in simulated oil spill scenarios with and without the dispersant, Corexit. Mar. Pollut. Bull. 2020, 151, 110804. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B.; Fuentes, C.; Sanchez, M.; Predoi, K.; Acevedo, M. Persistence profile of polyaromatic hydrocarbons in shallow and deep Gulf waters and sediments: Effect of water temperature and sediment-water partitioning characteristics. Mar. Pollut. Bull. 2011, 62, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Arvanitidis, C.; Borja, A.; Frid, C.; Hiddink, J.G.; Krause, J.; Lorance, P.; Ragnarsson, S.Á.; Sköld, M.; Trabucco, B.; et al. Indicators for sea-floor integrity under the european marine strategy framework directive. Ecol. Indic. 2012, 12, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Munné, A.; Ginebreda, A.; Prat, N. (Eds.) Experiences from Surface Water Quality Monitoring: The EU Water Framework Directive Implementation in the Catalan River Basin District (Part I); Springer: Cham, Switzerland, 2015. [Google Scholar]
- Bellas, J.; Saco-Álvarez, L.; Nieto, Ó.; Beiras, R. Ecotoxicological evaluation of polycyclic aromatic hydrocarbons using marine invertebrate embryo-larval bioassays. Mar. Pollut. Bull. 2008, 57, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, N.; Bertocci, I.; Munari, M.; Musco, L.; Caramiello, D.; Danovaro, R.; Zupo, V.; Costantini, M. Morphological and molecular responses of the sea urchin Paracentrotus lividus to highly contaminated marine sediments: The case study of Bagnoli-Coroglio brownfield (Mediterranean Sea). Mar. Environ. Res. 2020, 154, 104865. [Google Scholar] [CrossRef]
- Albarano, L.; Zupo, V.; Caramiello, D.; Toscanesi, M.; Trifuoggi, M.; Guida, M.; Libralato, G.; Costantini, M. Sub-chronic effects of slight pah-and pcb-contaminated mesocosms in Paracentrotus lividus lmk: A multi-endpoint approach and de novo transcriptomic. Int. J. Mol. Sci. 2021, 22, 6674. [Google Scholar] [CrossRef]
- Ikenaka, Y.; Sakamoto, M.; Nagata, T. Effects of polycyclic aromatic hydrocarbons (PAHs) on an aquatic ecosystem: Acute toxicity and community-level toxic impact tests of benzo[a]pyrene using lake zooplankton community. J. Toxicol. Sci. 2013, 38, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Sese, B.T.; Grant, A.; Reid, B.J. Toxicity of polycyclic aromatic hydrocarbons to the nematode caenorhabditis elegans. J. Toxicol. Environ. Health—Part A Curr. Issues 2009, 72, 1168–1180. [Google Scholar] [CrossRef]
- Verrhiest, G.; Clément, B.; Blake, G. Single and combined effects of sediment-associated PAHs on three species of freshwater macroinvertebrates. Ecotoxicology 2001, 10, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.; Kagan, E.D.; Kagan, I.A.; Kagan, P.A.; Quigley, S. The phototoxicity of non-carcinogenic polycyclic aromatic hydrocarbons in aquatic organisms. Chemosphere 1985, 14, 1829–1834. [Google Scholar] [CrossRef]
- Rojo-Nieto, E.; Smith, K.E.C.; Perales, J.A.; Mayer, P. Recreating the seawater mixture composition of HOCs in toxicity tests with Artemia franciscana by passive dosing. Aquat. Toxicol. 2012, 120–121, 27–34. [Google Scholar] [CrossRef]
- Colvin, K.A.; Parkerton, T.F.; Redman, A.D.; Lewis, C.; Galloway, T.S. Miniaturised marine tests as indicators of aromatic hydrocarbon toxicity: Potential applicability to oil spill assessment. Mar. Pollut. Bull. 2021, 165, 112151. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G. The case of Artemia spp. in nanoecotoxicology. Mar. Environ. Res. 2014, 101, 38–43. [Google Scholar] [CrossRef]
- Libralato, G.; Losso, C.; Ghirardini, A.V. Toxicity of untreated wood leachates towards two saltwater organisms (Crassostrea gigas and Artemia franciscana). J. Hazard. Mater. 2007, 144, 590–593. [Google Scholar] [CrossRef]
- Migliore, L.; Brambilla, G.; Grassitellis, A.; Delupis, G.D.; Vergata, U.T.; Rm, S.L.; Pubblica, S.I. Toxicity and bioaccumulation of sulphadimethoxine in Artemia (Crustacea, Anostraca). Int. J. Salt Lake Res. 1993, 2, 141–152. [Google Scholar] [CrossRef]
- Persoone, G.; Baudo, R.; Cotman, M.; Blaise, C.; Thompson, K.C.; Moreira-Santos, M.; Vollat, B.; Törökne, A.; Han, T. Review on the acute Daphnia magna toxicity test ? Evaluation of the sensitivity and the precision of assays performed with organisms from laboratory cultures or hatched from dormant eggs. Knowl. Manag. Aquat. Ecosyst. 2009, 393, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Manfra, L.; Canepa, S.; Piazza, V.; Faimali, M. Lethal and sublethal endpoints observed for Artemia exposed to two reference toxicants and an ecotoxicological concern organic compound. Ecotoxicol. Environ. Saf. 2016, 123, 60–64. [Google Scholar] [CrossRef]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.A.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2017, 189, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Comeche, A.; Martín-Villamil, M.; Picó, Y.; Varó, I. Effect of methylparaben in Artemia franciscana. Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2017, 199, 98–105. [Google Scholar] [CrossRef] [PubMed]
- De Vos, S.; Van Stappen, G.; Sorgeloos, P.; Vuylsteke, M.; Rombauts, S.; Bossier, P. Identification of salt stress response genes using the Artemia transcriptome. Aquaculture 2019, 500, 305–314. [Google Scholar] [CrossRef]
- Varó, I.; Perini, A.; Torreblanca, A.; Garcia, Y.; Bergami, E.; Vannuccini, M.L.; Corsi, I. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Sci. Total Environ. 2019, 675, 570–580. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, K.; Liu, R.; Giesy, J.P.; Li, Z.; Li, W.; Zhan, J.; Liu, L.; Gong, Y. Transcriptomic responses of Artemia salina exposed to an environmentally relevant dose of Alexandrium minutum cells or Gonyautoxin2/3. Chemosphere 2020, 238, 124661. [Google Scholar] [CrossRef]
- Lee, J.M.; Cho, B.C.; Park, J.S. Transcriptomic analysis of brine shrimp Artemia franciscana across a wide range of salinities. Mar. Genomics 2022, 61, 100919. [Google Scholar] [CrossRef]
- Arienzo, M.; Donadio, C.; Mangoni, O.; Bolinesi, F.; Stanislao, C.; Trifuoggi, M.; Toscanesi, M.; Di Natale, G.; Ferrara, L. Characterization and source apportionment of polycyclic aromatic hydrocarbons (pahs) in the sediments of gulf of Pozzuoli (Campania, Italy). Mar. Pollut. Bull. 2017, 124, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Walker, T.R.; Willis, R. Spatiotemporal characterization of petroleum hydrocarbons and polychlorinated biphenyls in small craft harbour sediments in Nova Scotia, Canada. Mar. Pollut. Bull. 2022, 177, 113524. [Google Scholar] [CrossRef]
- Armiento, G.; Caprioli, R.; Cerbone, A.; Chiavarini, S.; Crovato, C.; De Cassan, M.; De Rosa, L.; Montereali, M.R.; Nardi, E.; Nardi, L.; et al. Current status of coastal sediments contamination in the former industrial area of Bagnoli-Coroglio (Naples, Italy). Chem. Ecol. 2020, 36, 579–597. [Google Scholar] [CrossRef]
- APAT; IRSA; CNR. Metodo 8060 di Valutazione della Tossicità Acuta con Artemia sp. In Manuali e Linee Guida-Metodi Analitici per le Acque; APAT IRSA CNR: Rome, Italy, 2003; Volume 3, pp. 1043–1049. [Google Scholar]
- ISO 10253; Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum tricornutum. ISO: London, UK, 2016.
- Kwon, H.C.; Kwon, J.H. Measuring aqueous solubility in the presence of small cosolvent volume fractions by passive dosing. Environ. Sci. Technol. 2012, 46, 12550–12556. [Google Scholar] [CrossRef]
- Vemula, V.R.; Lagishetty, V.; Lingala, S. Solubility enhancement techniques. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 41–51. [Google Scholar]
- Miyako, Y.; Khalef, N.; Matsuzaki, K.; Pinal, R. Solubility enhancement of hydrophobic compounds by cosolvents: Role of solute hydrophobicity on the solubilization effect. Int. J. Pharm. 2010, 393, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Barahona-Gomariz, M.V.; Sanz-Barrera, F.; Sánchez-Fortún, S. Acute toxicity of organic solvents on Artemia salina. Bull. Environ. Contam. Toxicol. 1994, 52, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, Y.; Vitiello, V.; Gallo, A.; Libralato, G.; Trifuoggi, M.; Toscanesi, M.; Lofrano, G.; Esposito, F.; Buttino, I. Assessment of the relative sensitivity of the copepods Acartia tonsa and Acartia clausi exposed to sediment-derived elutriates from the Bagnoli-Coroglio industrial area: Sensitivity of Acartia tonsa and Acartia clausi to sediment elutriates. Mar. Environ. Res. 2020, 155, 104878. [Google Scholar] [CrossRef]
- Riesgo, A.; Pérez-Porro, A.R.; Carmona, S.; Leys, S.P.; Giribet, G. Optimization of preservation and storage time of sponge tissues to obtain quality mRNA for next-generation sequencing. Mol. Ecol. Resour. 2012, 12, 312–322. [Google Scholar] [CrossRef]
- Chen, W.H.; Ge, X.; Wang, W.; Yu, J.; Hu, S. A gene catalogue for post-diapause development of an anhydrobiotic arthropod Artemia franciscana. BMC Genom. 2009, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.X.; Xu, Q.S.; Xu, B.J.; Zhao, J. Lanthanum- and cerium-induced oxidative stress in submerged Hydrilla verticillata plants. Russ. J. Plant Physiol. 2007, 54, 693–697. [Google Scholar] [CrossRef]
- Dambroski, H.R.; Feder, J.L. Host plant and latitude-related diapause variation in Rhagoletis pomonella: A test for multifaceted life history adaptation on different stages of diapause development. J. Evol. Biol. 2007, 20, 2101–2112. [Google Scholar] [CrossRef]
- Ragland, G.J.; Fuller, J.; Feder, J.L.; Hahn, D.A. Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J. Insect Physiol. 2009, 55, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Roccheri, M.C. Marine Invertebrates as Bioindicators of Heavy Metal Pollution. Open J. Met. 2014, 4, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Ferrarese, E.; Andreottola, G.; Oprea, I.A. Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater. 2008, 152, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Depledge, M.H.; Fossi, M.C. The role of biomarkers in environmental assessment (2). Invertebrates. Ecotoxicology 1994, 3, 161–172. [Google Scholar] [CrossRef]
- Balcıoğlu, E.B. Potential effects of polycyclic aromatic hydrocarbons (PAHs) in marine foods on human health: A critical review. Toxin Rev. 2016, 35, 98–105. [Google Scholar] [CrossRef]
- Tongo, I.; Ogbeide, O.; Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017, 4, 55–61. [Google Scholar] [CrossRef]
- Eganhouse, R.P.; Calder, J.A. The solubility of medium molecular weight aromatic hydrocarbons and the effects of hydrocarbon co-solutes and salinity. Geochim. Cosmochim. Acta 1976, 40, 555–561. [Google Scholar] [CrossRef]
- May, W.E.; Wasik, S.P.; Freeman, D.H. Determination of the solubility behavior of some polycyclic aromatic hydrocarbons in water. Anal. Chem. 1978, 50, 997–1000. [Google Scholar] [CrossRef]
- NOAA Cameo Chemicals. Available online: https://cameochemicals.noaa.gov/ (accessed on 1 February 2021).

| Compounds | Nominal Concentration | Analytical Concentration | Nominal/Analytical Concentration Ratio |
|---|---|---|---|
| NAP | 0.025 | 0.015 | 1.67 |
| 0.05 | 0.032 | 1.56 | |
| 0.1 | 0.078 | 1.28 | |
| 0.2 | 0.11 | 1.82 | |
| 0.4 | 0.26 | 1.54 | |
| 0.5 | 0.41 | 1.22 | |
| 1 | 0.76 | 1.32 | |
| 2.5 | 1.45 | 1.72 | |
| 5 | 4.23 | 1.18 | |
| 10 | 10.1 | 0.99 | |
| PHE | 0.36 | 0.21 | 1.71 |
| 1 | 0.71 | 1.41 | |
| 2 | 1.15 | 1.74 | |
| 3 | 2.26 | 1.33 | |
| 4 | 3.45 | 1.16 | |
| 5 | 4.23 | 1.18 | |
| 10 | 7.48 | 1.34 | |
| 57.5 | 48.7 | 1.18 | |
| 115 | 98.6 | 1.17 | |
| 230 | 223.4 | 1.03 | |
| FLT | 0.41 | 0.29 | 1.41 |
| 1 | 0.81 | 1.23 | |
| 2.5 | 2.14 | 1.17 | |
| 5 | 4.41 | 1.13 | |
| 12.5 | 9.91 | 1.26 | |
| 25 | 20.4 | 1.23 | |
| 50 | 45.6 | 1.10 | |
| 97.5 | 91.6 | 1.06 | |
| 195 | 179 | 1.09 | |
| 390 | 325 | 1.20 | |
| BkF | 0.025 | 0.016 | 1.56 |
| 0.5 | 0.41 | 1.22 | |
| 1 | 0.78 | 1.28 | |
| 1.5 | 0.98 | 1.53 | |
| 3 | 2.4 | 1.25 | |
| 6 | 5.3 | 1.13 | |
| 12 | 10.4 | 1.15 | |
| 23.5 | 19.5 | 1.21 | |
| 47 | 41.7 | 1.13 | |
| 94 | 84.6 | 1.11 |
| PAHs | References | ||||
|---|---|---|---|---|---|
| NAP | PHE | FLT | BkF | ||
| D. magna | 7.924 (24 h) | 0.458 (24 h); 0.8 (48 h) | [17] | ||
| M. galloprovincialis | 0.009 (24 h) | 0.0002 (24 h) | 0.036 (24 h) | [16] | |
| P. lividus | 0.012 (24 h) | 0.069 (24 h) | 0.036 (24 h) | [16] | |
| C. intestinalis | 0.001 (24 h) | 0.069 (24 h) | 0.036 (24 h) | [16] | |
| C. elegans | 4.7 (48 h) | [17] | |||
| E. fetidas | 0.1 (48 h) | [17] | |||
| C. tentans | 2.81 (48 h) | 0.49 (48 h) | [2] | ||
| S. capricornutum | 2.96 (48 h) | 0.94 (48 h) | [2] | ||
| N. palea | 2.82 (48 h) | 0.87 (48 h) | [2] | ||
| P. gyrina | 5.02 (48 h) | [2] | |||
| G. minus | 3.93 (48 h) | 0.46 (48 h) | [2] | ||
| P. promelas | 1.99 (48 h) | [2] | |||
| S. gairdneri | 0.12 (48 h) | 0.03 (48 h) | [2] | ||
| M. salmonid | 0.68 (48 h) | 0.25 (48 h) | [2] | ||
| A. franciscana | 1.73 (24 h); 0.40 (48 h) | 4.44 (24 h); 3.07 (48 h) | 1.30 (24 h); 0.09 (48 h) | This study | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarano, L.; Serafini, S.; Toscanesi, M.; Trifuoggi, M.; Zupo, V.; Costantini, M.; Vignati, D.A.L.; Guida, M.; Libralato, G. Genotoxicity Set Up in Artemia franciscana Nauplii and Adults Exposed to Phenanthrene, Naphthalene, Fluoranthene, and Benzo(k)fluoranthene. Water 2022, 14, 1594. https://doi.org/10.3390/w14101594
Albarano L, Serafini S, Toscanesi M, Trifuoggi M, Zupo V, Costantini M, Vignati DAL, Guida M, Libralato G. Genotoxicity Set Up in Artemia franciscana Nauplii and Adults Exposed to Phenanthrene, Naphthalene, Fluoranthene, and Benzo(k)fluoranthene. Water. 2022; 14(10):1594. https://doi.org/10.3390/w14101594
Chicago/Turabian StyleAlbarano, Luisa, Sara Serafini, Maria Toscanesi, Marco Trifuoggi, Valerio Zupo, Maria Costantini, Davide A. L. Vignati, Marco Guida, and Giovanni Libralato. 2022. "Genotoxicity Set Up in Artemia franciscana Nauplii and Adults Exposed to Phenanthrene, Naphthalene, Fluoranthene, and Benzo(k)fluoranthene" Water 14, no. 10: 1594. https://doi.org/10.3390/w14101594