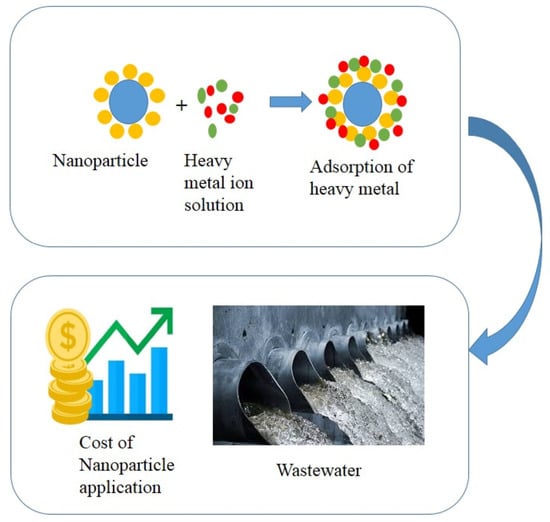
A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment
Abstract
:1. Introduction
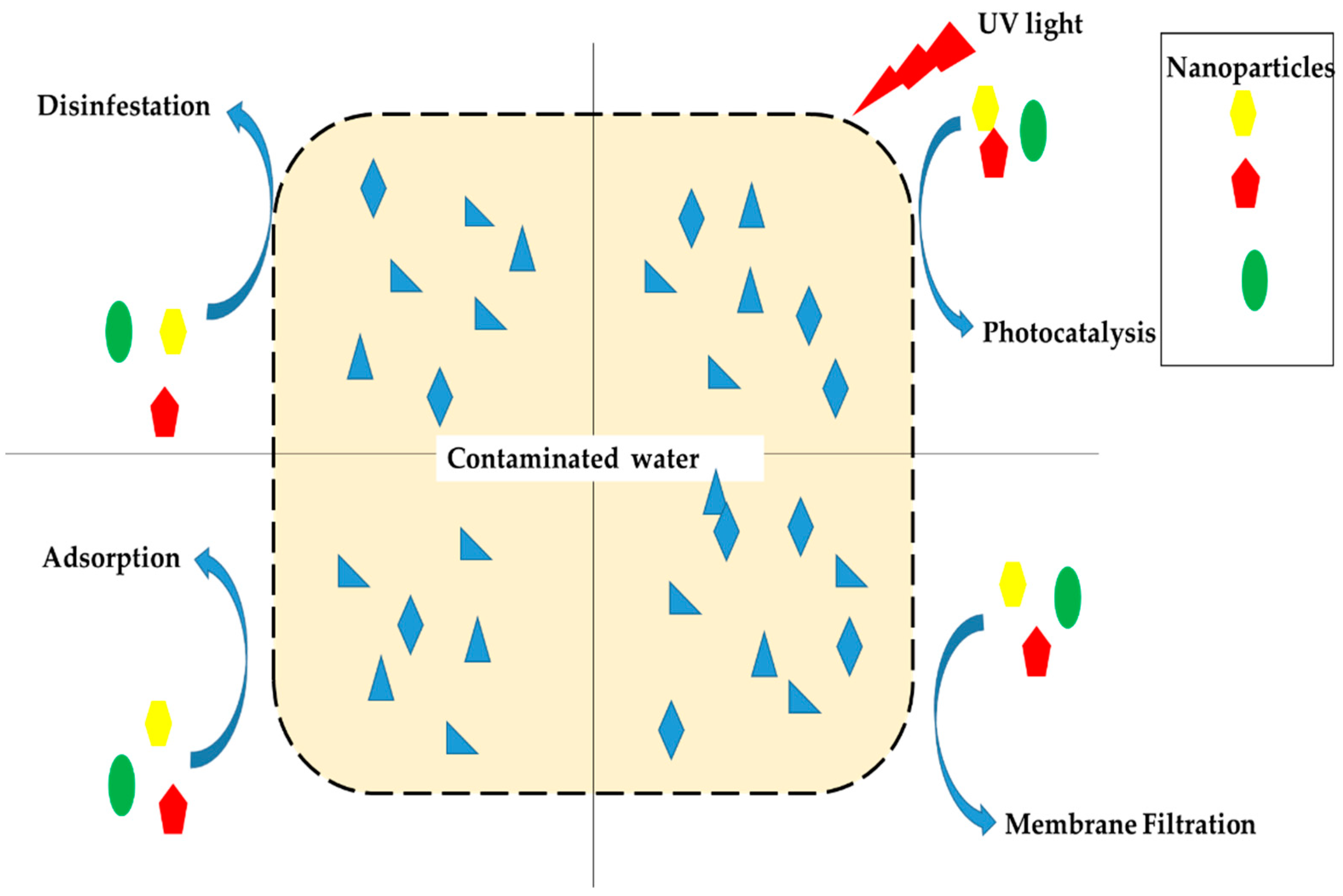
2. Overview of Nanotechnology Application in Wastewater Treatment
2.1. Photocatalysis Technology
2.2. Adsorption Technology
2.3. Nano-Membrane Technology
2.4. Nanotechnology Disinfection
3. Market Assessment of Nanoparticles in the Wastewater Treatment Sector
4. Contributing Factors to the Production Cost of Nanoparticles
5. Challenges of Nanoparticle Application for Heavy Metal Removal in Wastewater Treatment
5.1. Using Graphene Oxide Nano-Sheets in WWTPs
5.2. Using Magnetic Nanoparticles in WWTPs
5.3. Using Polymeric Hydrogels in WWTPs
5.4. Using Activated Carbon in WWTPs
6. Emerging Research in Nanotechnology for Heavy Metal Removal
7. Economic Evaluation of Nanoparticle Application for Heavy Metal Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokarram, M.; Saber, A.; Sheykhi, V. Effects of heavy metal contamination on river water quality due to release of industrial effluents. J. Clean. Prod. 2020, 277, 123380. [Google Scholar] [CrossRef]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Li, Y.; Yang, J.; Lei, K.; Li, Y.; Li, F.; Zheng, D.; Fang, X.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Gu, M.; Hao, L.; Wang, Y.; Li, X.; Chen, Y.; Li, W.; Jiang, L. The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem. Phys. 2020, 534, 110750. [Google Scholar] [CrossRef]
- Kumar, V.; Al-Gheethi, A.; Asharuddin, S.M.; Othman, N. Potential of cassava peels as a sustainable coagulant aid for institutional wastewater treatment: Characterisation, optimisation and techno-economic analysis. Chem. Eng. J. 2021, 420, 127642. [Google Scholar] [CrossRef]
- Moradi, G.; Zinadini, S.; Rajabi, L.; Derakhshan, A.A. Removal of heavy metal ions using a new high performance nanofiltration membrane modified with curcumin boehmite nanoparticles. Chem. Eng. J. 2020, 390, 124546. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of Granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu, G. Fabrication of highly durable and robust superhydrophobic-superoleophilic nanofibrous membranes based on a fluorine-free system for efficient oil/water separation. J. Membr. Sci. 2019, 570, 303–313. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Gao, S.; Cui, J.; Qu, Q.; Wang, Y.; Huang, C.; Fu, G. Self-healing and superwettable nanofibrous membranes with excellent stability toward multifunctional applications in water purification. ACS Appl. Mater. Interfaces 2020, 12, 23644–23654. [Google Scholar] [CrossRef] [PubMed]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Kulal, P.; Badalamoole, V. Efficient removal of dyes and heavy metal ions from waste water using Gum ghatti–graft–poly(4-acryloylmorpholine) hydrogel incorporated with magnetite nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104207. [Google Scholar] [CrossRef]
- Sachan, D.A.R.; Gopal, D. Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100467. [Google Scholar] [CrossRef]
- Goutam, S.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green synthesis of nanoparticles and their applications in water and wastewater treatment. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; pp. 349–379. [Google Scholar]
- Saikia, J.; Gogoi, A.; Baruah, S. Nanotechnology for water remediation. In Environmental Nanotechnology; Springer: Cham, Denmark, 2019; pp. 195–211. [Google Scholar]
- Simeonidis, K.; Martinez-Boubeta, C.; Zamora-Pérez, P.; Rivera-Gil, P.; Kaprara, E.; Kokkinos, E.; Mitrakas, M. Implementing nanoparticles for competitive drinking water purification. Environ. Chem. Lett. 2019, 17, 705–719. [Google Scholar] [CrossRef]
- Sharma, R. Nanotechnology: An approach for water purification-review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012007. [Google Scholar] [CrossRef]
- Gao, X.; Meng, X. Photocatalysis for Heavy Metal Treatment: A Review. Processes 2021, 9, 1729. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Simonsen, G.; Strand, M.; Øye, G. Potential applications of magnetic nanoparticles within separation in the petroleum industry. J. Pet. Sci. Eng. 2018, 165, 488–495. [Google Scholar] [CrossRef]
- Din, M.I.; Nabi, A.G.; Hussain, Z.; Arshad, M.; Intisar, A.; Sharif, A.; Ahmed, E.; Mehmood, H.A.; Mirza, M.L. Innovative seizure of metal/metal oxide nanoparticles in water purification: A critical review of potential risks. Crit. Rev. Anal. Chem. 2019, 49, 534–541. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Muharrem, I.N.C.E.; Ince, O.K. An overview of adsorption technique for heavy metal removal from water/wastewater: A critical review. Int. J. Pure Appl. Sci. 2017, 3, 10–19. [Google Scholar]
- Ali, S.; Rehman, S.A.U.; Shah, I.A.; Farid, M.U.; An, A.K.; Huang, H. Efficient removal of zinc from water and wastewater effluents by hydroxylated and carboxylated carbon nanotube membranes: Behaviors and mechanisms of dynamic filtration. J. Hazard. Mater. 2019, 365, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Amini, Z. Using nanomembrane to heavy metal removal from wastewater: A mini-review. Adv. Appl. NanoBio-Technol. 2022, 3, 7–13. [Google Scholar]
- Esakkimuthu, T.; Sivakumar, D.; Akila, S. Application of nanoparticles in wastewater treatment. Pollut. Res. 2014, 33, 567–571. [Google Scholar]
- Najafpoor, A.; Norouzian-Ostad, R.; Alidadi, H.; Rohani-Bastami, T.; Davoudi, M.; Barjasteh-Askari, F.; Zanganeh, J. Effect of magnetic nanoparticles and silver-loaded magnetic nanoparticles on advanced wastewater treatment and disinfection. J. Mol. Liq. 2020, 303, 112640. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.H.; Banat, F.; Abu Haija, M. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, Y.A.; Balasubramanyam, J.; Hanumanthappa, H. Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, Y.; Deng, S.; Zhao, B.; Fu, Y.; Liu, Z. Synthesis of proanthocyanidins-functionalized Fe3O4 magnetic nanoparticles with high solubility for removal of heavy-metal ions. Chem. Phys. Lett. 2020, 753, 137600. [Google Scholar] [CrossRef]
- Khoso, W.A.; Haleem, N.; Baig, M.A.; Jamal, Y. Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci. Rep 2021, 11, 3790. [Google Scholar] [CrossRef]
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial-based membrane for water purification. Appl. Mater. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- Kumar, M.; Khan, M.A.; Arafat, H.A. Recent developments in the rational fabrication of thin film nanocomposite membranes for water purification and desalination. ACS Omega 2020, 5, 3792–3800. [Google Scholar] [CrossRef]
- Yabe, J.; Ishizuka, M.; Umemura, T. Current levels of heavy metal pollution in Africa. J. Vet. Med. Sci. 2010, 72, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Álvarez, E.; Mochón, M.; Sánchez, J.; Rodríguez, M. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Ngo, H.; Guo, W.; Jin, P.; Dzakpasu, M.; Yang, S.; Wang, Q.; Wang, X.; Ao, D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tytła, M. Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland—Case study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef] [Green Version]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Jamkhande, G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Manikam, V.R.; Cheong, K.Y.; Razak, K.A. Chemical reduction methods for synthesizing Ag and Al nanoparticles and their respective nanoalloys. Mater. Sci. Eng. B 2011, 176, 187–203. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver nanoparticles: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Nandatamadini, F.; Karina, S.; Nandiyanto, A.B.D.; Ragadhita, R. Feasibility study based on economic perspective of cobalt nanoparticle synthesis with chemical reduction method. Cakra Kim. 2019, 7, 61–68. [Google Scholar]
- Mahdavian, A.R.; Mirrahimi, M.A.S. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 2010, 159, 264–271. [Google Scholar] [CrossRef]
- Singh, A.; Jain, D.; Upadhyay, M.K.; Khandelwal, N.; Verma, H.N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostructures 2010, 5, 483–489. [Google Scholar]
- Parveen, K.; Banse, V.; Ledwani, L. April. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Noman, E.; Al-Gheethi, A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Inactivating pathogenic bacteria in greywater by biosynthesized Cu/Zn nanoparticles from secondary metabolite of Aspergillus iizukae; optimization, mechanism and techno economic analysis. PLoS ONE 2019, 14, e0221522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meramo-Hurtado, S.I.; González-Delgado, A.D. Application of techno-economic and sensitivity analyses as decision-making tools for assessing emerging large-scale technologies for production of chitosan-based adsorbents. ACS Omega 2020, 5, 17601–17610. [Google Scholar] [CrossRef] [PubMed]
- Yashni, G.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Dai-Viet, N.V.; Al-Kahtani, A.A.; Al-Sahari, M.; Hazhar, N.J.N.; Noman, E.; Alkhadher, S. Bio-inspired ZnO NPs synthesized from Citrus sinensis peels extract for Congo red removal from textile wastewater via photocatalysis: Optimization, mechanisms, techno-economic analysis. Chemosphere 2021, 281, 130661. [Google Scholar] [CrossRef]
- Liu, J.; Martin, F.; McGrail, B. Rare-earth element extraction from geothermal brine using magnetic core-shell nanoparticles-techno-economic analysis. Geothermics 2021, 89, 101938. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. A prototype of textile wastewater treatment using coagulation and adsorption by Fe/Cu nanoparticles: Techno-economic and scaling-up studies. Nanomater. Nanotechnol. 2021, 11, 18479804211041181. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Tang, S.C.; Yan, D.Y.; Lo, I.M. Sustainable wastewater treatment using microsized magnetic hydrogel with magnetic separation technology. Ind. Eng. Chem. Res. 2014, 53, 15718–15724. [Google Scholar] [CrossRef]
- Mirshahghassemi, S.; Ebner, A.D.; Cai, B.; Lead, J.R. Application of high gradient magnetic separation for oil remediation using polymer-coated magnetic nanoparticles. Sep. Purif. Technol. 2017, 179, 328–334. [Google Scholar] [CrossRef]
- Lv, L.; Wu, X.; Han, X.; Li, C. Amino acid modified graphene oxide for assembly of nanoparticles for wastewater treatment. Appl. Surf. Sci. 2020, 534, 147620. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Pan, G.; Wang, L.; Song, S.; Xu, Z.; Fu, D.; Zhang, G. Preparation of modified graphene oxide nanomaterials for water and wastewater treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 032074. [Google Scholar] [CrossRef]
- Gao, F. An overview of surface-functionalized magnetic nanoparticles: Preparation and application for wastewater treatment. ChemistrySelect 2019, 4, 6805–6811. [Google Scholar] [CrossRef]
- Lai, L.; Xie, Q.; Chi, L.; Gu, W.; Wu, D. Adsorption of phosphate from water by easily separable Fe3O4@ SiO2 core/shell magnetic nanoparticles functionalized with hydrous lanthanum oxide. J. Colloid Interface Sci. 2016, 465, 76–82. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Znad, H.; Hasan, M.N. Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J. Mol. Liq. 2021, 329, 115541. [Google Scholar] [CrossRef]
- Hasan, M.M.; Shenashen, M.A.; Hasan, M.N.; Znad, H.; Salman, M.S.; Awual, M.R. Natural biodegradable polymeric bioadsorbents for efficient cationic dye encapsulation from wastewater. J. Mol. Liq. 2021, 323, 114587. [Google Scholar] [CrossRef]
- Tanveer, M.; Farooq, A.; Ata, S.; Bibi, I.; Sultan, M.; Iqbal, M.; Jabeen, S.; Gull, N.; Islam, A.; Khan, R.U.; et al. Aluminum nanoparticles, chitosan, acrylic acid and vinyltrimethoxysilane based hybrid hydrogel as a remarkable water super-absorbent and antimicrobial activity. Surf. Interfaces 2021, 25, 101285. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Surface modified magnetic nanoparticles as efficient adsorbents for heavy metal removal from wastewater: Progress and prospects. Mater. Express 2017, 7, 439–456. [Google Scholar] [CrossRef]
- Li, J.; Xing, X.; Li, J.; Shi, M.; Lin, A.; Xu, C.; Zheng, J.; Li, R. Preparation of thiol-functionalized activated carbon from sewage sludge with coal blending for heavy metal removal from contaminated water. Environ. Pollut. 2018, 234, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, A.R.O.; Abdul Latiff, A.A.; Daud, Z.; Mat Daud, N.F.; Aliyu, M.K. Heavy metal removal from wastewater of palm oil mill using developed activated carbon from coconut shell and cow bones. Key Eng. Mater. 2017, 737, 428–432. [Google Scholar]
- Marsh, H.; Reinoso-Rodriguez, F. Activated Carbon; Elsevier: London, UK, 2012. [Google Scholar]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem. 2015, 13, 699–708. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.; Pang, X.; Zhang, Y.; Zhang, Y.; Dong, W.F.; Yan, R. Chitosan-based carbon nanoparticles as a heavy metal indicator and for wastewater treatment. RSC Adv. 2021, 11, 12015–12021. [Google Scholar] [CrossRef]
- Masuku, M.; Ouma, L.; Pholosi, A. Microwave assisted synthesis of oleic acid modified magnetite nanoparticles for benzene adsorption. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100429. [Google Scholar] [CrossRef]
- Deng, N.; Wang, Y.; Luo, G. A novel method for fast and continuous preparation of superfine titanium dioxide nanoparticles in microfluidic system. Particuology 2022, 60, 61–67. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, Y.; Xue, J.; Zhu, F.; Zhong, Z.; Liu, R. An innovative alcohol-solution combustion-calcination process for the fabrication of NiFe2O4 nanorods and their adsorption characteristics of methyl blue in aqueous solution. Mater. Res. Express 2021, 8, 095003. [Google Scholar] [CrossRef]
- Li, T.; Yang, T.; Yu, Z.; Xu, G.; Du, M.; Guan, Y.; Guo, C. An innovative magnetic bar separator for removal of chromium ions in tanning wastewater. J. Water Process Eng. 2021, 40, 101916. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Du, X.; Gong, B.; Jegatheesan, V.; Haq, I.U. Recent advances in the prediction of fouling in membrane bioreactors. Membranes 2021, 11, 381. [Google Scholar] [CrossRef]
- Cao, W.; Ma, W.; Lu, T.; Jiang, Z.; Xiong, R.; Huang, C. Multifunctional nanofibrous membranes with sunlight-driven self-cleaning performance for complex oily wastewater remediation. J. Colloid Interface Sci. 2022, 608, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Liang, H.; Cao, W.; Deng, Y.; Qu, Q.; Ma, W.; Xiong, R.; Huang, C. Blow-spun nanofibrous composite Self-cleaning membrane for enhanced purification of oily wastewater. J. Colloid Interface Sci. 2022, 608, 2860–2869. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Nabavi, S.R.; Emadi, H.; Faraji, M. Development of a superhydrophilic nanofiber membrane for oil/water emulsion separation via modification of polyacrylonitrile/polyaniline composite. Polym. Adv. Technol. 2021, 32, 1301–1316. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, L.; Fu, K.; Zhu, F.; Yang, T.; Yang, T.; Luo, J.; Wu, M.; Yu, D. Ultrastable MOF-based foams for versatile applications. Nano Res. 2021, 15, 2961–2970. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.A.; Nirmalakhandan, N. Wastewater infrastructure for sustainable cities: Assessment based on UN sustainable development goals (SDGs). Int. J. Sustain. Dev. World Ecol. 2021, 28, 203–209. [Google Scholar] [CrossRef]
- Malik, O.A.; Hsu, A.; Johnson, L.A.; de Sherbinin, A. A Global indicator of wastewater treatment to inform the Sustainable Development Goals (SDGs). Environ. Sci. Policy 2015, 48, 172–185. [Google Scholar] [CrossRef]
- Bhaduri, A.; Bogardi, J.; Siddiqi, A.; Voigt, H.; Vörösmarty, C.; Pahl-Wostl, C.; Bunn, S.E.; Shrivastava, P.; Lawford, R.; Foster, S.; et al. Achieving Sustainable Development Goals from a Water Perspective. Front. Environ. Sci. 2016, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.; Abeysiriwardana-Arachchige, I.S.A.; Nirmalakhandan, N. Evaluating wastewater treatment infrastructure systems based on UN Sustainable Development Goals and targets. J. Clean. Prod. 2021, 298, 126795. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.; Gallardo, M.; Molina, R.; López-Muñoz, M.J. Life cycle assessment and techno-economic evaluation of alternatives for the treatment of wastewater in a chrome-plating industry. J. Clean. Prod. 2018, 172, 2351–2362. [Google Scholar] [CrossRef]
- Mahmudabadi, T.Z.; Ebrahimi, A.A.; Eslami, H.; Mokhtari, M.; Salmani, M.H.; Ghaneian, M.T.; Mohamadzadeh, M.; Pakdaman, M. Optimization and economic evaluation of modified coagulation–flocculation process for enhanced treatment of ceramic-tile industry wastewater. AMB Express 2018, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Qiu, P.; Qian, Y.; Kong, Z.; Zheng, X.; Tang, Z.; Guo, H. Textile wastewater treatment for water reuse: A case study. Processes 2019, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Al-Jabari, M.H.; Sulaiman, S.; Ali, S.; Barakat, R.; Mubarak, A.; Khan, S.A. Adsorption study of levofloxacin on reusable magnetic nanoparticles: Kinetics and antibacterial activity. J. Mol. Liq. 2019, 291, 111249. [Google Scholar] [CrossRef]
- Chalasani, R.; Vasudevan, S. Cyclodextrin-functionalized Fe3O4@TiO2: Reusable, magnetic nanoparticles for photocatalytic degradation of endocrine-disrupting chemicals in water supplies. ACS Nano 2013, 7, 4093–4104. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.K.; Shivalkar, S.; Banerjee, S. Synthesis of M. oleifera leaf extract capped magnetic nanoparticles for effective lead [Pb(II)] removal from solution: Kinetics, isotherm and reusability study. J. Mol. Liq. 2020, 305, 112811. [Google Scholar] [CrossRef]
- Yunus, Z.M.; Al-Gheethi, A.; Othman, N.; Hamdan, R.; Ruslan, N.N. Removal of heavy metals from mining effluents in tile and electroplating industries using honeydew peel activated carbon: A microstructure and techno-economic analysis. J. Clean. Prod. 2020, 251, 119738. [Google Scholar] [CrossRef]
- Febriani, L.I.; Nurhashiva, C.; Veronica, J.; Ragadhita, R.; Nandiyanto, A.B.D.; Kurniawan, T. Computation Application: Techno-Economic Analysis on the Production of Magnesium Oxide Nanoparticles by Precipitation Method. Int. J. Inform. Inf. Syst. Comput. Eng. 2020, 1, 117–128. [Google Scholar] [CrossRef]
- Ragadhita, R.I.; Nandiyanto, A.B.; Maulana, A.C.; Oktiani, R.O.; Sukmafitri, A.J.; Machmud, A.M.; Surachman, E. Techno-economic analysis for the production of titanium dioxide nanoparticle produced by liquid-phase synthesis method. J. Eng. Sci. Technol. 2019, 14, 1639–1652. [Google Scholar]
- Abd Elhafez, S.E.; Hamad, H.A.; Zaatout, A.A.; Malash, G.F. Management of agricultural waste for removal of heavy metals from aqueous solution: Adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ. Sci. Pollut. Res. 2017, 24, 1397–1415. [Google Scholar] [CrossRef]

| Ref | Optimum pH | Nanoparticle Used | Contaminant | Initial Contaminant Dose | Adsorbent Dosage | Removal Efficiency | Removal Efficiency |
|---|---|---|---|---|---|---|---|
| Abdi et al. [33] | pH 5 | NF membranes with different magnetic graphene-based hybrids | Copper dye retention | 20 mg/L | - | - | Copper removal 92% Dye retention of 99% |
| Arshad et al. [34] | pH 7 | Graphene oxide embedded calcium alginate | Pb(II) | - | 5 mg/mL | 602 mg/g for Pb(II) | 99.6% |
| Sahraei and Ghaemy [35] | pH 6 | Modified gum tragacanth/graphene oxide composite hydrogel | Pb(II), Cd(II), and Ag(I) | 60 mg/L | 20 mg | 142.50 mg/g for Pb(II) 112.50 mg/g for Cd(II) 132.12 mg g−1 for Ag(I) | 94% for Pb(II) 79.40% for Cd(II) 83.55% for Ag(I) |
| Kumar et al. [36] | pH 8 | ZnO and SnO2 | Malachite Green Oxalate (MGO) hexavalent Chromium (Cr) | 20 mg/L for MGO 3 mg/L for Cr | 80 mg/L of SnO2 and ZnO for MGO removal 300 mg/L SnO2 and ZnO for hexavalent Chromium (Cr) | - | Malachite Green Oxalate: 95% by ZnO 92% by SnO2 Adsorption of Cr: 95% by ZnO 87% by SnO2 |
| Fouda et al. [37] | pH 7.5 | MgO | Co, Pb, Cd, and Ni | - | 1.0 mg/mL | 149.1 for Co 148.6, for Pb 135 for Cd 149.9 for Ni | 94.2% ± 1.2% for Cr 63.4% ± 1.7% for Co 72.7% ± 1.3% for Pb 74.1% ± 1.8% for Cd 70.8% ± 1.5% for Ni |
| Gu et al. [7] | pH range of 3–7 | ZnO | Cr3+ | - | 1 g/L | 88.547 mg/g for Cr3+ | 99.5% for Cr3+ |
| Shi et al. [38] | pH of 8.0 | Fe3O4 | Cu2+, Cd2+, and Pb2+ | 1 mg/mL | 18.8 mg/g for Cu2+, 20.9 mg/g for Cd2+ 21.5 mg/g for Pb2+ | 96.2% for Cu2+, 87.4% for Cd2+ 91.1% for Pb2+ | |
| Khoso et al. [39] | Cr(VI) ions at pH 3 Pb(II) ions at pH 5 Cd(II) at pH 5 | Nickel-Ferrite Nanoparticles (NFNs) | Cr(VI), Pb(II), and Cd(II) | 30 mg for Cr(VI) ions 40 mg for Pb(II) 40 mg for Cd(II) | 10 mg | - | 85.8% for Cr(VI) ions 75.25% for Pb(II) ions 77.41% for Cd(II) ions |
| Type of Production | Type of Nanoparticles | Minimum Global Production (Tons) | Maximum Global Production (Tons) |
|---|---|---|---|
| Nanoparticles produced in Large volume | TIO2 | 60,000 | 15,000 |
| ZnO | 32,000 | 36,000 | |
| Silicon dioxide (SiO2) | 185,000 | 1,400,000 | |
| Aluminium oxide (AL2O3) | 5000 | 10,100 | |
| CNT | 1550 | 1950 | |
| Nanoclays | 25,000 | 51,000 | |
| CeO2 | 880 | 1400 | |
| Nanoparticles produced in Large volume Low volume | Quantum dots | 4.5 | 9 |
| Antimony tin oxide (ATO) | 120 | 225 | |
| Copper oxide (CuO) | 290 | 570 | |
| Ag | 135 | 420 | |
| cellulose nanofibers (CNF) | 400 | 1350 | |
| Bismuth oxide (Bi2O3) | 35 | 55 | |
| cobaltic oxide | 5 | <10 | |
| Dendrimers | 0.3 | 1.25 | |
| Fullerenes and POSS | 40 | 100 | |
| Graphene | 60 | 80 | |
| Gold (Au) | 1 | 3 | |
| Iron oxide (FE2O3) | 9 | 45 | |
| Magnesium oxide (MgO) | 15 | 30 | |
| Manganese oxide (MnO2) | 2 | 3.5 | |
| Nickel (Ni) | 5 | 20 | |
| Zirconium oxide (ZrO2) | 80 | 300 |
| Name of Nanoparticle | Production Technology | Total Production Cost | Ref |
|---|---|---|---|
| Cu/Zn | biosynthesized | USD/year 131,387.20 | Noman et al. [56] |
| chitosan microbeads | topologies CM process | USD/year 37,838,536.68 | Meramo-Hurtado et al. [57] |
| chitosan microbeads modified with TiO2 nanoparticles | topologies CMTiO2 process | USD/year 64,792,191.25 | Meramo-Hurtado et al. [57] |
| ZnO | - | USD/year 57,124.32 | Yashni et al. [58] |
| rare earth elements | - | USD/year 1,006,002.00 | Liu et al. [59] |
| copper oxide | green synthesis | USD/year 2,219,500 | Mahmoud et al. [60] |
| Technology | Catalyst | Targeted Contaminant | Removal Efficiency | Cost USD/Year | Ref |
|---|---|---|---|---|---|
| adsorption | honeydew peel activated carbon | Cr3+ Zn2+ | 83.49% Cr3+ and 88.88% Zn2+ | 97,050.00 | Yunus et al. [100] |
| coagulant | cassava peel | Alum | 83.44% alum, | 21,370.00 | Kumar et al. [5] |
| CPS | 76.83% CPS, | ||||
| mixture of CPS | 32.87% mixture of CPS | ||||
| reduction–precipitation–settling process | - | Cr(VI) | 85% Cr(VI) | 43,875.98 | Rodríguez et al. [94] |
| ionic exchange and photocatalytic process | - | Cr(VI) | 85% Cr(VI) | 53,767.78 | Rodríguez et al. [94] |
| pathogen disinfection biosynthesized by Aspergillus iizukae | Cu/Zn | pathogen disinfection | inactivation | 131,387.20 | Noman et al. [56] |
| E. coli | (6 log10) of E. coli | ||||
| S. aureus | (5.21 log10) of S. aureus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpongwana, N.; Rathilal, S. A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment. Water 2022, 14, 1550. https://doi.org/10.3390/w14101550
Mpongwana N, Rathilal S. A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment. Water. 2022; 14(10):1550. https://doi.org/10.3390/w14101550
Chicago/Turabian StyleMpongwana, Ncumisa, and Sudesh Rathilal. 2022. "A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment" Water 14, no. 10: 1550. https://doi.org/10.3390/w14101550
APA StyleMpongwana, N., & Rathilal, S. (2022). A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment. Water, 14(10), 1550. https://doi.org/10.3390/w14101550