Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Biochars
2.3. Characterization of Biochars
2.4. Adsorption Experiments
2.5. Data Analysis
3. Results and Discussion
3.1. Single-Metal Adsorption onto Biochars
3.1.1. Adsorption Kinetics
3.1.2. Adsorption Isotherms
3.1.3. Analysis of Thermodynamic Parameters
3.2. Binary Metal Adsorption onto Biochars
3.2.1. Competitive Adsorption of Cu2+ with Zn2+/Pb2+
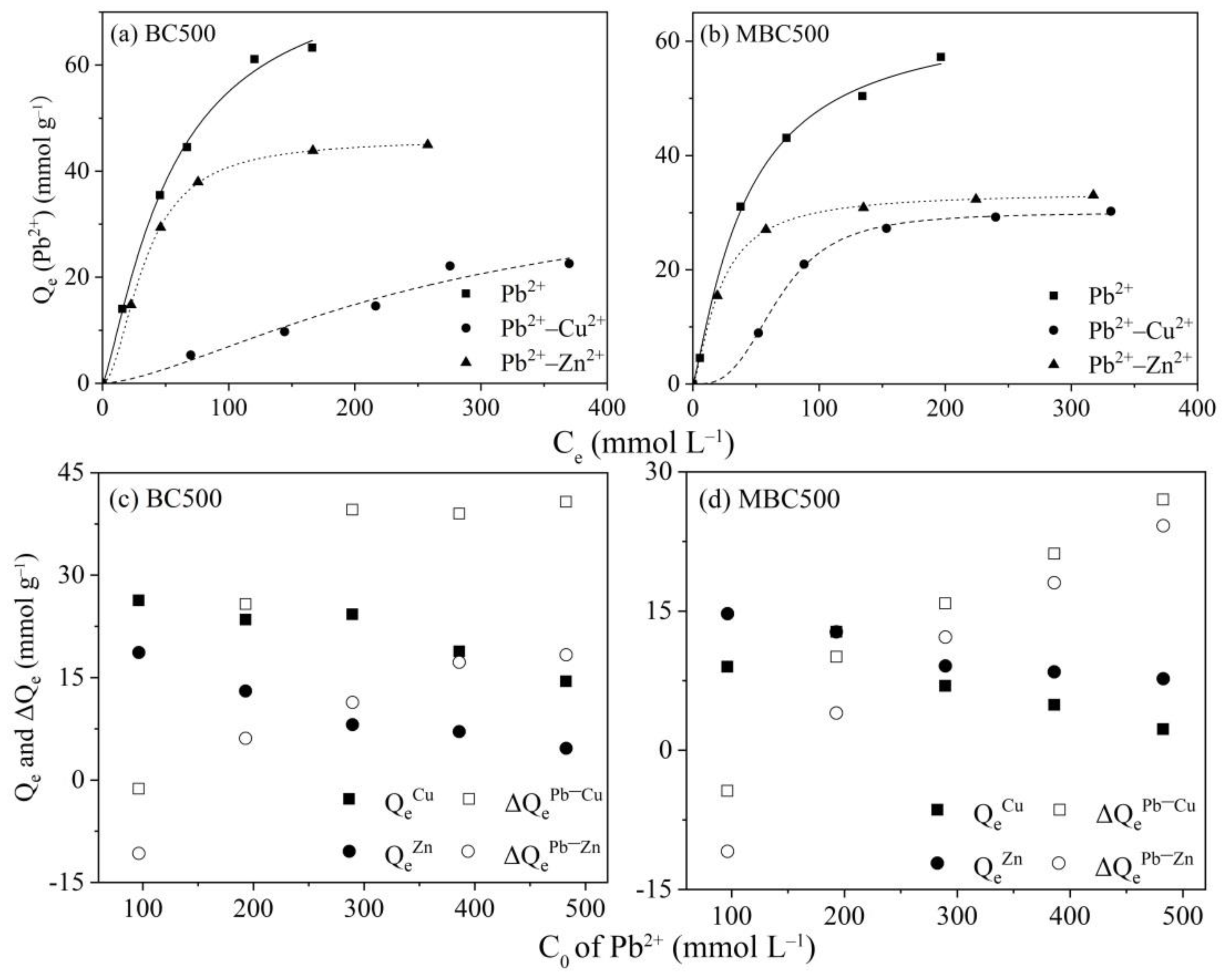
3.2.2. Competitive Adsorption of Pb2+ with Cu2+/Zn2+
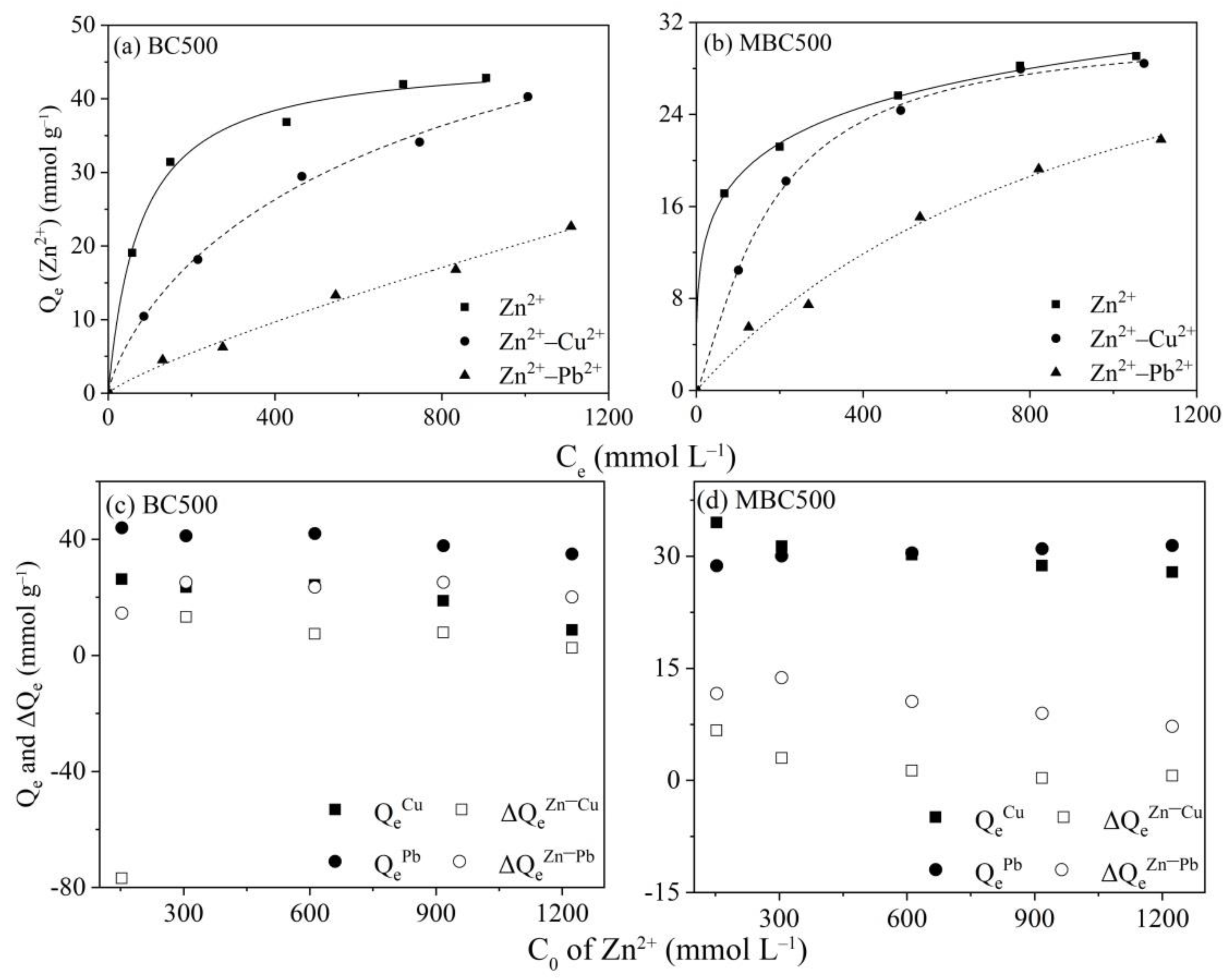
3.2.3. Competitive Adsorption of Zn2+ with Cu2+/Pb2+
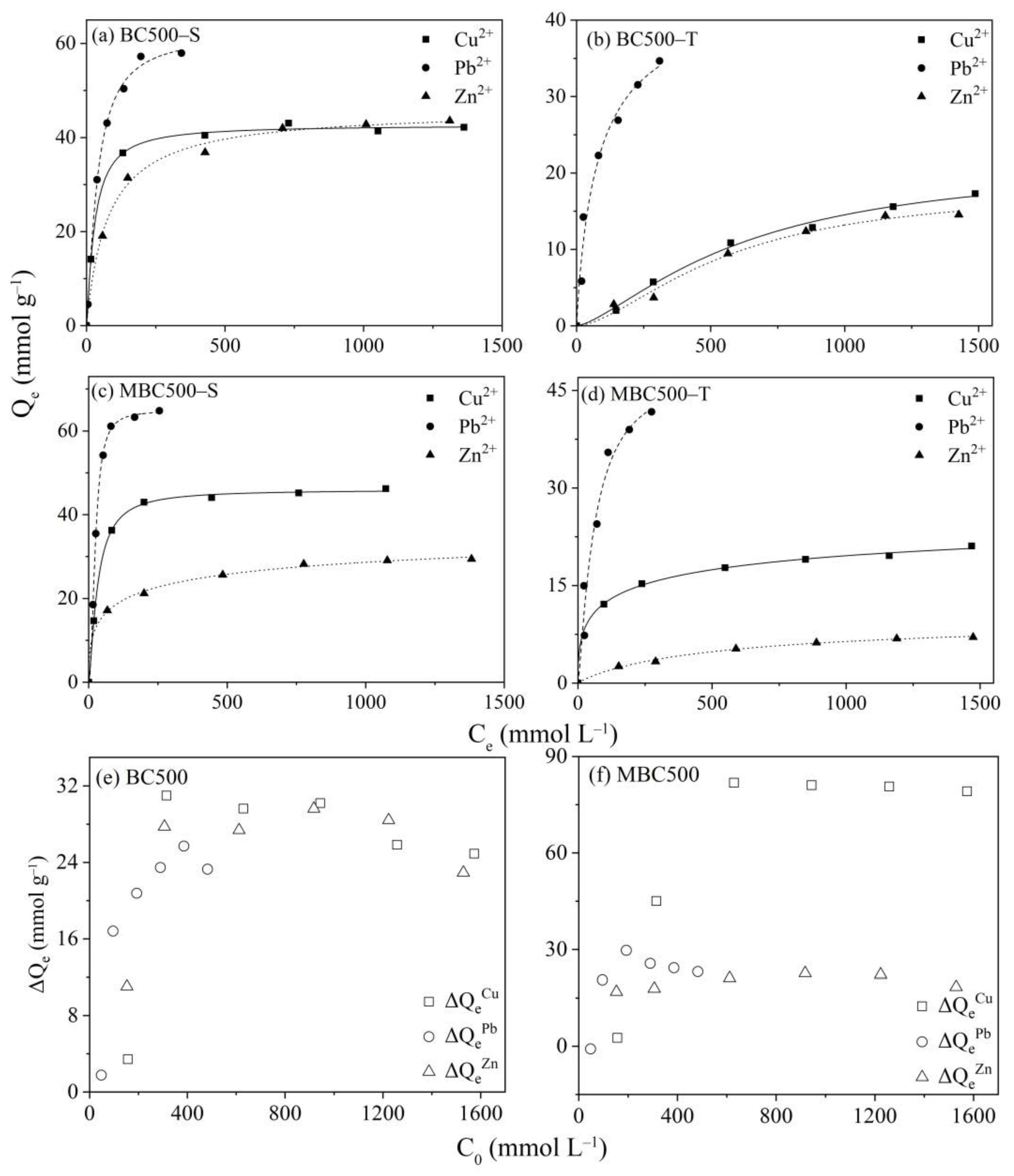
3.3. Ternary Metal Adsorption onto Biochars
3.4. Possible Mechanisms
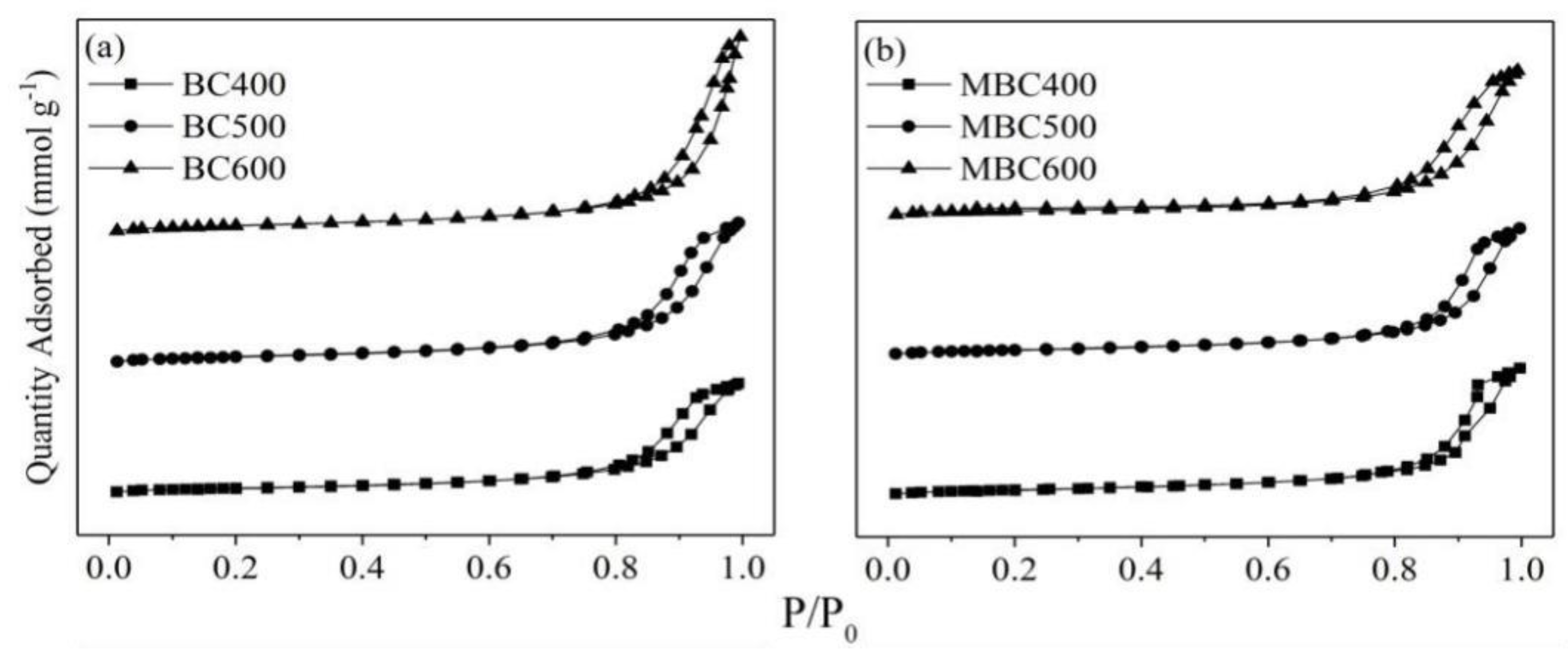
3.4.1. Surface Characteristics
3.4.2. FTIR Analysis
3.4.3. XRD Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, D.; Currell, M.J.; Cao, G. Deep challenges for China’s war on water pollution. Environ. Pollut. 2016, 218, 1222–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Wang, M.; Duan, M.; Ma, X.; Hong, J.; Xie, F.; Zhang, R.; Li, X. In search of key: Protecting human health and the ecosystem from water pollution in China. J. Clean. Prod. 2019, 228, 101–111. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, E.; Xia, P.; Feng, A.; Chi, Y.; Sun, Y. Distribution and pollution assessment of heavy metals in the intertidal zone environments of typical sea areas in China. Mar. Pollut. Bull. 2019, 138, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Xu, M.; Wang, Z. Inter-annual variability of heavy metals pollution in surface sediments of Jiangsu coastal region, China: Case study of the Dafeng Port. Mar. Pollut. Bull. 2010, 150, 110720. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, S. Spatio-temporal variational characteristics analysis of heavy metals pollution in water of the typical northern rivers, China. J. Hydrol. 2018, 559, 787–793. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, G.; Liu, Z. Distribution, source and pollution level of heavy metals in river sediments from South China. Catena 2018, 170, 386–396. [Google Scholar] [CrossRef]
- Zhang, X.; Zha, T.; Guo, X.; Meng, G.; Zhou, J. Spatial distribution of metal pollution of soils of Chinese provincial capital cities. Sci. Total Environ. 2018, 643, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef] [Green Version]
- Boni, M.; Chiavola, A.; Marzeddu, S. Application of Biochar to the Remediation of Pb-Contaminated Solutions. Sustainability 2018, 10, 4440. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Kim, J.; Song, J.; Lee, S.M.; Jung, J. Application of iron-modified biochar for arsenite removal and toxicity reduction. J. Ind. Eng. Chem. 2019, 80, 17–22. [Google Scholar] [CrossRef]
- Szatyowicz, E.; Skoczko, I. Magnetic Field Usage Supported Filtration Through Different Filter Materials. Water 2019, 11, 1584. [Google Scholar] [CrossRef] [Green Version]
- Skoczko, I.; Szatyłowicz, E. Removal of heavy metal ions by filtration on activated alumina-assisted magnetic field. Desalin. Water Treat. 2018, 117, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhou, C.; Kuang, Y.J.; Jiang, Z.H.; Yang, M. Removal of hexavalent chromium in aquatic solutions by pomelo peel. Water Sci. Eng. 2020, 13, 65–73. [Google Scholar] [CrossRef]
- Low, S.K.; Tan, M.C. Dye adsorption characteristic of ultrasound pre-treated pomelo peel. J. Environ. Chem. Eng. 2018, 6, 3502–3509. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Liu, Y.; Guo, J.; Ren, J.; Zhou, F. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water. Fuel 2018, 233, 469–479. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; He, C.; Lyu, W.; Zhang, W.; Yan, W.; Yang, L. Development of rare earth element doped magnetic biochars with enhanced phosphate adsorption performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 236–243. [Google Scholar] [CrossRef]
- Akram, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S.; Sadaf, S. Biocomposite efficiency for Cr(VI) adsorption: Kinetic, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2017, 5, 400–411. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum ) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Tang, J.; Zheng, Z.; Wang, H.; Shao, Q.; Chen, G.; Li, Z.; Chen, Y.; Zhu, J.; et al. Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ. Sci. Pollut. Res. 2018, 25, 11854–11866. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ji, H.Y.; Lu, H.H.; Liu, Y.X.; Yang, R.Q.; He, L.L.; Yang, S.M. Simultaneous removal of Sb(III) and Cd(II) in water by adsorption onto a MnFe2O4–biochar nanocomposite. RSC Adv. 2018, 8, 3264–3273. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Wang, D.; Wang, L.; Ma, F. Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere 2018, 207, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Henriksen, S.W.; Andersen, M.N. Mechanism of orthophosphate (PO4-P) adsorption onto different biochars. Environ. Technol. Innov. 2020, 17, 100572. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Hua, Z.; Sun, L.; Bai, X.; Liang, L. Biosorption of nitroimidazole antibiotics onto chemically modified porous biochar prepared by experimental design: Kinetics, thermodynamics, and equilibrium analysis. Process Saf. Environ. 2016, 104, 422–435. [Google Scholar] [CrossRef]
- Moyo, M.; Lindiwe, S.T.; Sebata, E.; Nyamunda, B.C.; Guyo, U. Equilibrium, kinetic, and thermodynamic studies on biosorption of Cd(II) from aqueous solution by biocha. Res. Chem. Intermediat. 2015, 42, 1349–1362. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Am. Chem. Soc. 1960, 111, 3973–3993. [Google Scholar] [CrossRef]
- Dong, X.; Wang, C.; Li, H.; Wu, M.; Liao, S.; Zhang, D.; Pan, B. The sorption of heavy metals on thermally treated sediments with high organic matter content. Bioresour. Technol. 2014, 160, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.C.; Aldrich, C. Biosorption of heavy metals from aqueous solutions with tobacco dust. Bioresour. Technol. 2008, 99, 5595–5601. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Shreadah, M.A.; Ahmed, A.M.; Heiba, H.F. Multi-component adsorption of Pb(II), Cd(II), and Ni(II) onto Egyptian Na-activated bentonite; equilibrium, kinetics, thermodynamics, and application for seawater desalination. J. Environ. Chem. Eng. 2016, 4, 1166–1180. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zhu, J.; Wang, N.; Feng, J.; Yan, W. Adsorption of polythiophene/TiO2 composite for Zn (II), Pb (II) and Cu (II): Selectivity and synergistic effect investigation. Appl. Surf. Sci. 2018, 459, 318–326. [Google Scholar] [CrossRef]
- Caporale, A.G.; Pigna, M.; Sommella, A.; Conte, P. Effect of pruning-derived biochar on heavy metals removal and water dynamics. Biol. Fer. Soils 2014, 50, 1211–1222. [Google Scholar] [CrossRef] [Green Version]
- Chiban, M.; Soudani, A.; Sinan, F.; Persin, M. Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surf. B Biointerfaces 2011, 82, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Razmi, F.A.; Ngadi, N.; Wong, S.; Inuwa, I.M.; Opotu, L.A. Kinetics, thermodynamics, isotherm and regeneration analysis of chitosan modified pandan adsorbent. J. Clean. Prod. 2019, 231, 98–109. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Rodríguez-Jardón, L.; Arencibia, A.; Sanz, R.; Iglesias, M.; Maya, E.M. Bromine pre-functionalized porous polyphenylenes: New platforms for one-step grafting and applications in reversible CO2 capture. J. CO2 Util. 2019, 30, 183–192. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Luo, X.; Chen, P.; Liu, J. 2D magnetic scallion sheathing-based biochar composites design and application for effective removal of arsenite in aqueous solutions. Chem. Eng. Res. Des. 2019, 152, 384–392. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Huang, Z.; Wang, H.; Jiang, L.; Huang, J.; Tan, M.; Li, H. Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Xin, J.; Sun, P.; Wu, D. Adsorption behavior and mechanism of Cr(VI) by modified biochar derived from Enteromorpha prolifera. Ecotoxicol. Environ. Saf. 2018, 164, 440–447. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Y.X.; Lu, H.H.; Yang, R.Q.; Yang, S.M. Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions. J. Solid State Chem. 2018, 261, 53–61. [Google Scholar] [CrossRef]
- Zhao, H.; Lang, Y. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J. Taiwan Inst. Chem. E. 2018, 88, 152–160. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Liu, H.; Cheng, W.; Bao, Y. Modification of calcium-rich biochar by loading Si/Mn binary oxide after NaOH activation and its adsorption mechanisms for removal of Cu(II) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2010, 601, 124960. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Wang, Z.; Feng, J.; Li, S.; Yan, W. Synthesis of Ce-doped magnetic biochar for effective Sb(V) removal: Performance and mechanism. Powder Technol. 2019, 345, 501–508. [Google Scholar] [CrossRef]

| Metal Ions | Biochar | ∆G0 | ∆H0 (kJ mol−1) | ∆S0 (J mol−1 K−1) | R2 | ||
|---|---|---|---|---|---|---|---|
| (kJ mol−1) | |||||||
| 25 °C | 35 °C | 45 °C | |||||
| Cu2+ | BC400 | −13.00 | −13.44 | −13.87 | 19.70 | 43.57 | 0.9934 |
| BC500 | −9.07 | −9.37 | −9.67 | 14.30 | 30.38 | 0.9787 | |
| BC600 | −17.78 | −18.38 | −18.98 | 27.78 | 59.76 | 0.9974 | |
| MBC400 | −28.56 | −29.51 | −30.47 | 31.09 | 95.72 | 0.9787 | |
| MBC500 | −9.11 | −9.42 | −9.73 | 10.87 | 30.55 | 0.9397 | |
| MBC600 | −7.22 | −7.46 | −7.71 | 7.71 | 20.98 | 0.9233 | |
| Pb2+ | BC400 | −46.29 | −47.85 | −49.40 | 50.08 | 155.18 | 0.9831 |
| BC500 | −16.34 | −16.89 | −17.43 | 19.16 | 54.76 | 0.9830 | |
| BC600 | −17.01 | −17.58 | −18.15 | 12.56 | 57.13 | 0.9978 | |
| MBC400 | −12.90 | −13.34 | −13.77 | 13.72 | 43.25 | 0.9810 | |
| MBC500 | −17.81 | −18.40 | −19.00 | 18.04 | 59.69 | 0.9872 | |
| MBC600 | −34.76 | −35.93 | −37.10 | 35.95 | 116.77 | 0.9724 | |
| Zn2+ | BC400 | −35.02 | −36.20 | −37.37 | 40.77 | 117.39 | 0.9160 |
| BC500 | −48.86 | −50.49 | −52.13 | 54.71 | 163.76 | 0.9747 | |
| BC600 | −9.99 | −10.32 | −10.66 | 10.38 | 33.54 | 0.9678 | |
| MBC400 | −2.33 | −2.41 | −2.49 | 11.23 | 7.79 | 0.9577 | |
| MBC500 | −2.03 | −2.10 | −2.16 | 9.97 | 6.77 | 0.9110 | |
| MBC600 | −33.46 | −34.58 | −35.70 | 39.71 | 112.40 | 0.9723 | |
| Biochar | BET Surface Area | Micropore Area | Total Pore Volume | Average Pore Size |
|---|---|---|---|---|
| (m2 g−1) | (m2 g−1) | (cm3 g−1) | (nm) | |
| BC400 | 21.65 | 4.22 | 0.11 | 19.98 |
| BC500 | 26.53 | 4.27 | 0.14 | 20.77 |
| BC600 | 32.13 | 5.34 | 0.17 | 23.08 |
| MBC400 | 19.97 | 4.92 | 0.10 | 18.82 |
| MBC500 | 22.44 | 2.33 | 0.12 | 19.77 |
| MBC600 | 26.48 | 1.76 | 0.14 | 21.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Dong, S.; Wang, L.; Li, X. Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution. Water 2021, 13, 868. https://doi.org/10.3390/w13060868
Wu Q, Dong S, Wang L, Li X. Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution. Water. 2021; 13(6):868. https://doi.org/10.3390/w13060868
Chicago/Turabian StyleWu, Qianlan, Shuzhen Dong, Lijun Wang, and Xiaoyun Li. 2021. "Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution" Water 13, no. 6: 868. https://doi.org/10.3390/w13060868
APA StyleWu, Q., Dong, S., Wang, L., & Li, X. (2021). Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution. Water, 13(6), 868. https://doi.org/10.3390/w13060868






