An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rock Sample Collection
2.2. Experiment Method
2.3. Test Method
3. Results
3.1. Analysis of Rock Sample Characteristics
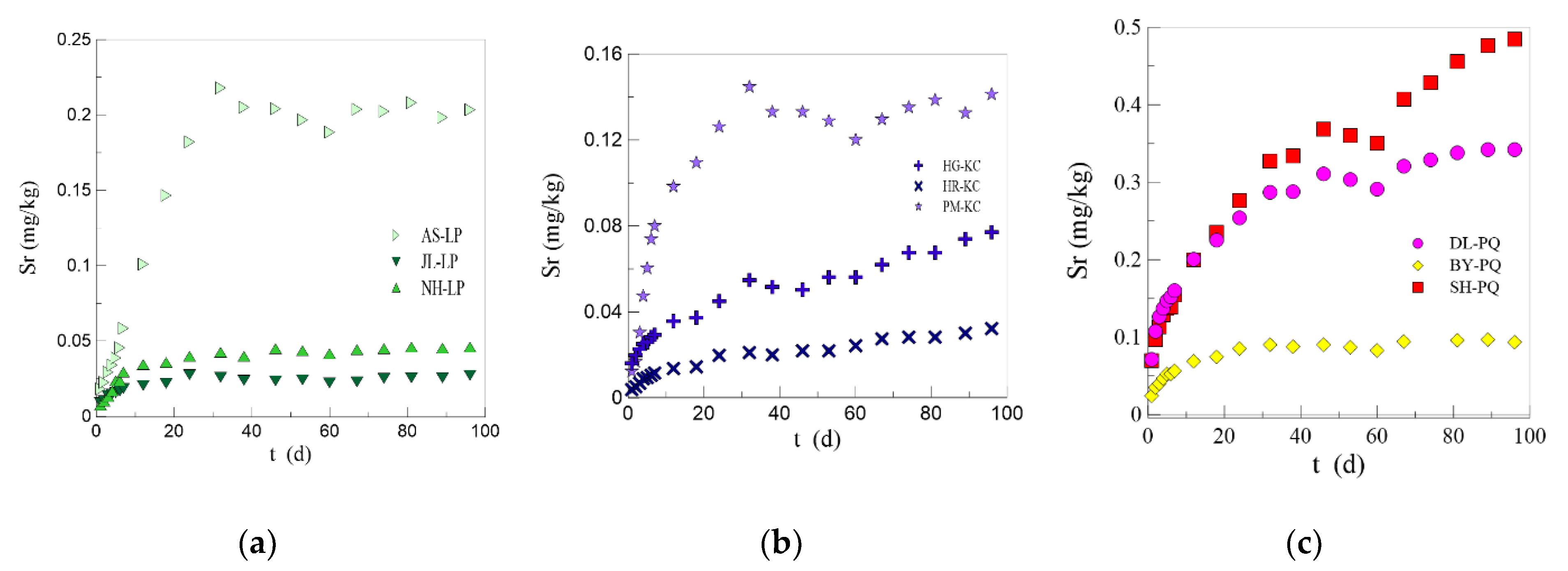
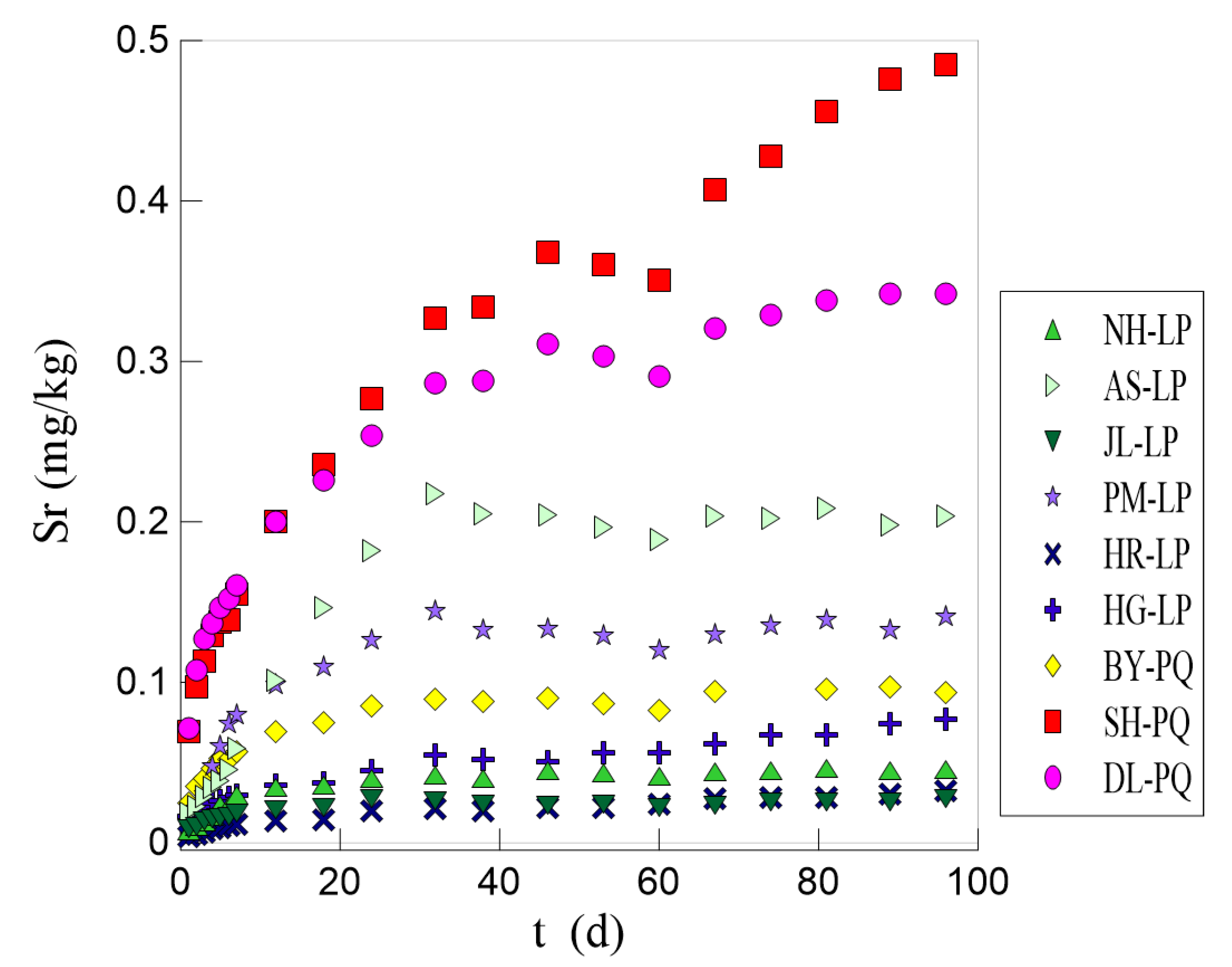
3.2. Analysis of Results of the Static Leaching Experiment
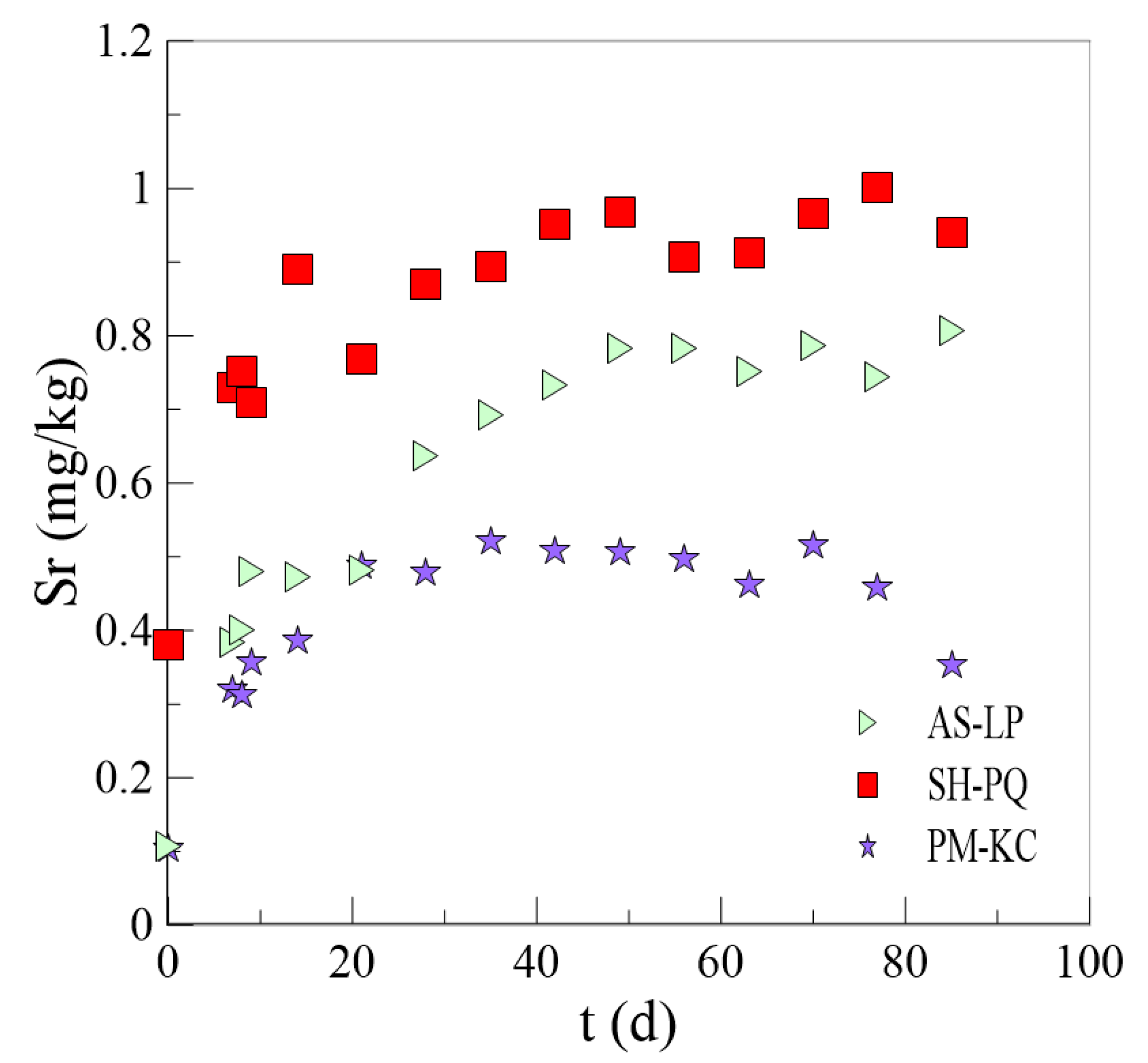
3.3. Analysis of Results of the CO2 Impact Experiment
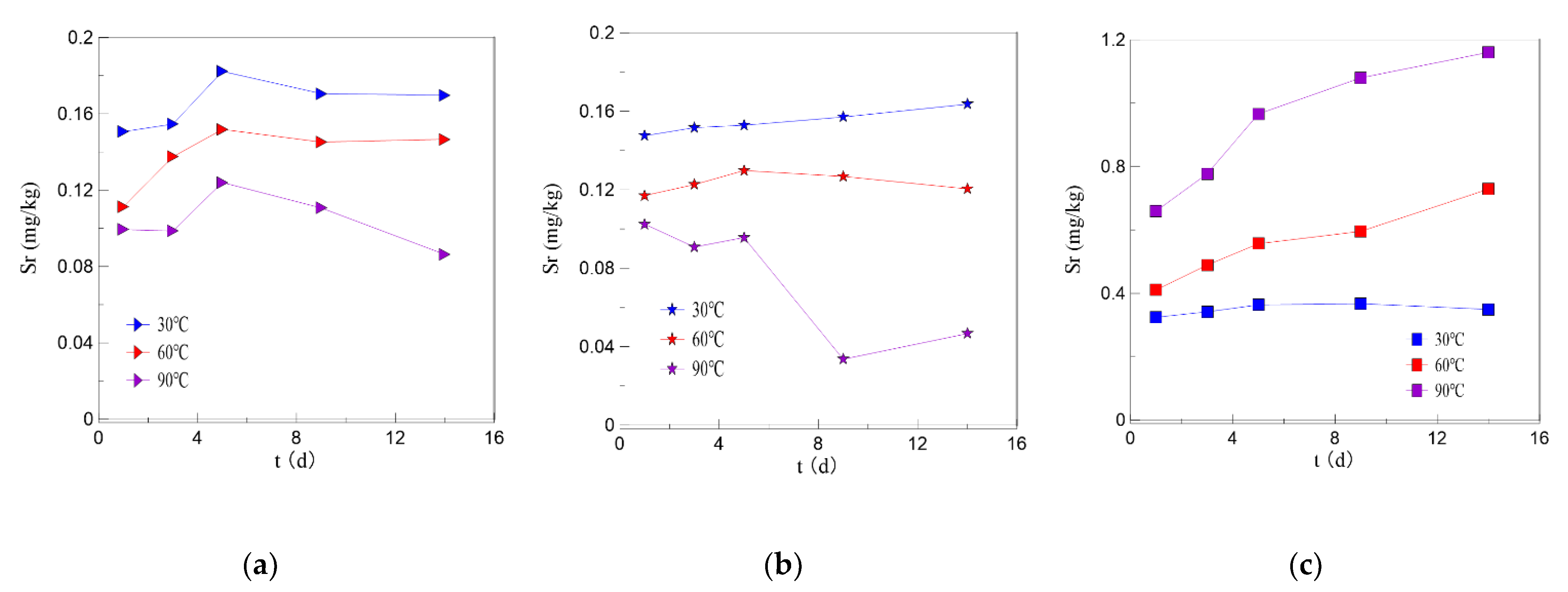
3.4. Analysis of Results of the Temperature Impact Experiment
4. Discussion
4.1. Sources of Sr
4.2. General Rules of Sr Dissolution under Different Conditions
5. Conclusions
- (1)
- Lithology has a great impact on the formation of Sr-bearing mineral water. It is noted that the higher the Sr content in rocks, the higher the Sr content in groundwater. The Sr content in the water is jointly controlled by the Sr content in rocks and the characteristics of the minerals in the rocks. The Sr content in groundwater may be more related to the minerals in rocks. A higher content of plagioclase (anorthite) in silicate rock areas is conducive to the leaching of Sr in water, while a higher content of calcite in carbonate rock areas boosts the leaching of Sr in water.
- (2)
- When the external conditions change, the response speeds and degrees of different rock samples are different. CO2 can promote the leaching of Sr from rocks, and CO2 has a greater impact on the leaching of Sr from carbonate minerals than from silicate minerals. The rise in temperature promotes the leaching of Sr from carbonate minerals but inhibits the leaching of Sr from silicate minerals.
- (3)
- Based on the shortcomings of the present experiments, leaching experiments under different redox conditions and selective sequential extraction experiments should be carried out in the future; the influence of artificially changing the chemical balance in the experimental process should be evaluate, and the mineral equivalence of Sr in different rocks should be studied so as to better understand the release mechanism of Sr in the process of water rock interaction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duo, X.; Mi, M.; Yang, L.; Guo, J.; Xue, J.; Zhou, W.; Zhong, X.; Chen, F.; Zhao, A. Identification characteristics and formation conditions of natural mineral water in Luanping County. Miner. Explor. 2019, 10, 1830–1837. [Google Scholar]
- Niu, X. What benefits does drinking natural mineral water have for human body. Shanghai Geol. 2003, 3, 21–32. [Google Scholar]
- Nebojša, Đ.P.; Simona, J.; Jana, Š.; Danijela, B.M.; Biljana, P.D.; Aleksandar, Ž.K. Assessment of spa mineral water quality from Vrnjačka Banja, Serbia: Geochemical, bacteriological, and health risk aspects. Environ. Monit. Assess. 2019, 191, 11. [Google Scholar]
- Olivera, K.; Suzana, E.; Dušan, P.; Petar, D.; Aleksandar, K. Hydrogeological conditions for the occurrence of two magnesium-rich natural mineral waters in Serbia and their physiological significance. Environ. Earth Sci. 2017, 76, 1–10. [Google Scholar]
- Suzan, P. Hydrogeochemistry of thermal and mineralized waters in the Diyadin (Ağri) area, Eastern Turkey. Appl. Geochem. 2013, 38, 70–81. [Google Scholar]
- Corral, M.M.; Galindo, E.; Ontiveros, C.; Díaz, J.A. Hydrogeochemical areas as background for specific mineral and thermal waters of Spain. Environ. Earth Sci. 2015, 73, 2683–2697. [Google Scholar] [CrossRef]
- Kurchavov, A.M.; Tolmacheva, E.V.; Bogatikov, O.A.; Kotov, A.B. Formation of granitoids of Caucasus Mineral Waters: Evidence from study of melt and fluid inclusions in minerals. Dokl. Earth Sci. 2013, 452, 308–312. [Google Scholar] [CrossRef]
- Wang, L. Origin of the Enrichment Mineral Water in Danjiamiao and the Research on Mining Scheme. Ph.D. Thesis, Hefei University of Technology, Hefei, China, 2017. [Google Scholar]
- Jose, M.M.; Hans, G.M.; Paula, M.C.; Silva, M.A. Origin and Evolution of Cl In CO2-rich thermal and mineral waters from northern Portugal. Appl. Geochem. 2020, 116, 1–9. [Google Scholar]
- Ignacio, S.M. Between chemistry, medicine and leisure: Antonio Casares and the study of mineral waters and Spanish spas in the nineteenth century. Ann. Sci. 2016, 73, 289–302. [Google Scholar]
- Yu, K.; Zheng, S. A discussion on the formational mechanism of compounded, high-quality, Maquanxiang natural mineral water for drinking in Shanxi Province. J. Hebei GEO Univ. 1996, Z1, 373–379. [Google Scholar]
- Liu, Q.; Wang, G.; Zhang, F. Geochemical environment of trace element strontium (Sr) enriched in mineral waters. Hydrogeol. Eng. Geol. 2004, 31, 19–23. [Google Scholar]
- Tanja, P.; Milena, Z.M.; Nebojsa, V.; Dragana, V. Hydrogeological conditions for the forming and quality of mineral waters in Serbia. J. Geochem. Explor. 2010, 107, 373–381. [Google Scholar]
- Ma, Z. Experimental Study on Formation Mechanism of Mineral Water in Jingyu County. Ph.D. Thesis, Jilin University, Changchun, China, 2016. [Google Scholar]
- Baizhong, Y.; Changlai, X.; Xiujuan, L.; Runchu, W.; Shili, W. Characteristics and genesis of mineral water from Changbai Mountain, Northeast China. Environ. Earth Sci. 2015, 73, 4819–4829. [Google Scholar]
- Vinograd, N.; Porowski, A. Application of isotopic and geochemical studies to explain the origin and formation of mineral waters of Staraya Russa Spa, NW Russia. Environ. Earth Sci. 2020, 79, 1–17. [Google Scholar] [CrossRef]
- Sun, H.; Wei, X.; Gan, F.; Wang, H.; Jia, F.; He, Z.; Li, D.; Li, J.; Zhang, J. Genetic type and formation mechanism of strontium-rich groundwater in the upper and middle reaches of Luanhe river basin. Acta Geosci. Sin. 2020, 41, 65–79. [Google Scholar]
- Yan, Z.; Hao, H. Cause analysis of strontium type mineral water in Jinan. Water Res. Sci. Technol. Shandong 2017, 11, 12–13. [Google Scholar]
- Su, H.; Yang, R.; Duo, X.; Zhao, Q.; Sun, Z.; Zhao, H. Distribution rules and geochemical conditions of mineral water resources in Chengde city. Geol. Chem. Miner. 2019, 41, 27–34. [Google Scholar]
- Qian, Y.; Chen, Y.; Chen, Q.; You, D.; Zou, S. General characteristics of burial dissolution for Ordovician carbonate reservoirs in the northwest of Tazhong area. Acta Petrolei Sin. 2006, 27, 47–52. [Google Scholar]
- Zhu, X.; Liu, W.; Li, Z.; Chen, T.; Ren, Y.; Shao, H.; Wang, L. Distribution and characterization analyses of strontium-bearing mineral spring water in the Chengde region. Hydrogeol. Eng. Geol. 2020, 47, 65–73. [Google Scholar]
- Li, G.; Liu, Z.; Xie, Z.; Duan, Y.; He, S.; Deng, M.; Wang, Y.; Li, Y.; Wu, Z. Discovery of non-hydrothermal saddle-shaped dolomite in Leikoupo formation, western Sichuan basin and its significance. Oil Gas Geol. 2020, 41, 164–176. [Google Scholar]
- Wen, Z.; Xu, X.; Zhao, R.; Wang, F.; Hu, W. Geologic and geochemical features of Devonian granites in Dangchuan area, western Qinling, and its tectonic significance. Geol. Rev. 2008, 54, 827–836. [Google Scholar]
- Shi, H.; Miao, W.; Zhang, X.; Li, W.; Tang, Q.; Li, Y. Geochemical characteristics and ore-forming material source of celestite deposits in Dafeng Mountain, northwestern Qaidam basin. Acta Geol. Sin. 2018, 92, 1733–1752. [Google Scholar]
- Yan, B.; Xiao, C.; Liang, X.; Ma, Z.; Wei, R.; Wu, S. Experiment on the characteristics component (H2SiO3) of the mineral water in the basalt in Jingyu County: A case study of Wangdashan spring. J. Jilin Univ. 2015, 45, 892–898. [Google Scholar]
- George, S.; Legg, J.O.; Smith, S.J. Soil nitrogen availability evaluations based on nitrogen mineralization potentials of soils and uptake of labeled and unlabeled nitrogen by plants. Plant Soil. 1973, 39, 113–124. [Google Scholar]
- Haiyan, Z.; Yuesuo, Y.; Heyang, Q.; Ying, L.; Tong, Y. Hydrochemical evolution of rare cold mineral waters in the Wudalianchi UNESCO Global Geopark, China. Environ. Earth Sci. 2018, 77, 1–11. [Google Scholar]
- Yue, W. Analysis for Amphibole Characteristics and Provenances of Typical Rivers in Northern China. Ph.D. Thesis, Ludong University, Yantai, China, 2012. [Google Scholar]
- Huang, S.; Zhang, X.; Liu, L.; Huan, J.; Huang, K. Progress of research on carbonate diagenesis. Earth Sci. Front. 2009, 16, 219–231. [Google Scholar]
- Zhao, T.; Yan, Z.; Zhang, J.; Wang, Y.; Jiang, L. PHREEQC-based Simulation of Impact of Temperature and CO2 on Feldspar Mineral Solubility. Guangxi J. Light Ind. 2016, 3, 84–86. [Google Scholar]
- Carreira, P.M.; Marques, J.M.; Carvalho, M.R.; Nunes, D.; Silva, M.A. Carbon isotopes and geochemical processes in CO2-rich cold mineral water, N-Portugal. Environ. Earth Sci. 2014, 71, 2941–2953. [Google Scholar] [CrossRef]
- Chi, E.; Lan, B.; Xiao, Y. Impact of temperature and CO2 in solution on feldspar solubility. J. Water Res. Water Eng. 2014, 25, 230–232. [Google Scholar]
- Liu, Z.; Han, J.; Li, H. Equilibrium chemistry of the CaCO3-CO2-H2O system and discussions. Carsol. Sin. 2005, 24, 1–14. [Google Scholar]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
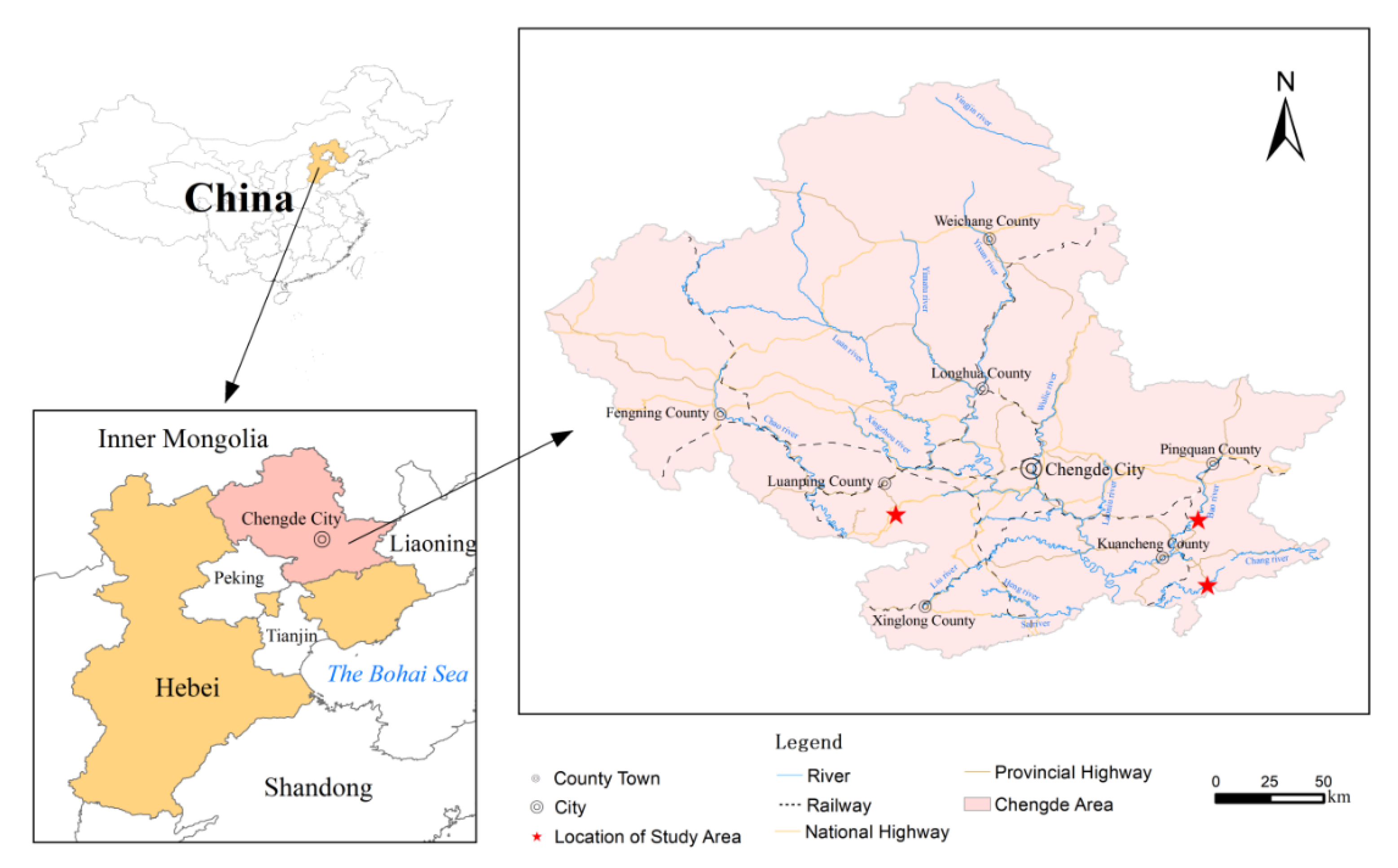

| Sampling Site | No. | Field Name | Name Used in Laboratory | Standard Symbol |
|---|---|---|---|---|
| Majiazhuang Village, Changshanyu Town, Luanping County | JL-LP | Breccia | Welded breccia | ib |
| AS-LP | Andesite | Trachyandensite | α | |
| NH-LP | Tuff | Crystal tuff | tf | |
| Miaoling Village, East Huanghuachuan Township, Kuancheng County | HG-KC | Granite | Plagiogranite | γ |
| HR-KC | Hybrid rock | Mica schist | sch | |
| PM-KC | Gneiss | Hornblende plagiogneiss | gn | |
| Yong’an Village, Dangba Town, Pingquan County | DL-PQ | Marble | Dolomitic marble | mb |
| SH-PQ | Limestone | Micrite | ls | |
| BY-PQ | Dolomite | Fine crystalline dolomite | dol |
| No. | HG-KC | HR-KC | PM-KC | JL-LP | AS-LP | NH-LP | SH-PQ | BY-PQ | DL-PQ |
|---|---|---|---|---|---|---|---|---|---|
| Chlorite | 1 | 1 | 14 | 0 | 3 | 0 | 0 | 0 | 0 |
| Plagioclase | 52 | 27 | 26 | 37 | 68 | 36 | 0 | 0 | 0 |
| Orthoclase | 26 | 6 | 4 | 22 | 19 | 20 | 0 | 0 | 0 |
| Quartz | 17 | 26 | 0 | 36 | 6 | 39 | 2 | 1 | 1 |
| Mica | 4 | 35 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Amphibole | 0 | 5 | 56 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematite | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Pyroxene | 0 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 0 |
| Calcite | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 1 | 74 |
| Dolomite | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 98 | 25 |
| Study Area | No. | Sr (mg/kg) | SD-Sr (mg/kg) | Zn (mg/kg) | SD-Zn (mg/kg) | Fe (mg/kg) | SD-Fe (mg/kg) | Ca (mg/kg) | SD-Ca (mg/kg) | K (mg/kg) | SD-K (mg/kg) | Ba (mg/kg) | SD-Ba |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detection limits | 3 | 10 | 25 | 40 | 45 | 45 | |||||||
| Luanping | JL-LP | 119 | 1.25 | 61 | 2.81 | 17,100 | 77.81 | 2060 | 28.84 | 14,200 | 90.03 | 594 | 12.18 |
| AS-LP | 454 | 2.53 | 90 | 3.48 | 44,000 | 181.28 | 12,500 | 160 | 12,200 | 106.75 | 1650 | 16.38 | |
| NH-LP | 86.1 | 1.00 | 47.5 | 2.42 | 6100 | 35.87 | 817 | 19.61 | 13,200 | 81.84 | - | - | |
| Kuancheng | HG-KC | 820 | 3.20 | 51.3 | 2.78 | 14,500 | 80.91 | 6360 | 41.91 | 13,100 | 92.35 | 2150 | 16.06 |
| HR-KC | 394 | 2.19 | 52.5 | 2.73 | 18,800 | 82.34 | 3500 | 34.3 | 13,700 | 96.59 | 1420 | 15.05 | |
| PM-KC | 671 | 3.33 | 146 | 4.53 | 83,600 | 237.42 | 32,700 | 283.51 | 45,600 | 72.50 | 937 | 16.96 | |
| Pingquan | DL-PQ | 178 | 1.57 | 22.1 | 2.48 | 971 | 18.25 | 320,000 | 736 | 232 | 52.43 | 303 | 15.42 |
| SH-PQ | 298 | 1.11 | 23.1 | 1.37 | 1190 | 10.96 | 302,000 | 398.64 | 778 | 31.66 | 50.2 | 10.74 | |
| BY-PQ | 52.8 | 0.70 | 19.4 | 1.77 | 5090 | 28.35 | 138,000 | 387.78 | 1000 | 40.4 | 80 | 13.04 | |
| Study Area | Sample No.—Sr Equilibrium Concentration (mg/kg) | ||
|---|---|---|---|
| Luanping | JL-LP—0.02449 | AS-LP—0.209 | NH-LP—0.0435 |
| Kuancheng | HG-KC—0.0625 | HR-KC—0.0275 | PM-KC—0.133 |
| Pingquan | DL-PQ—0.311 | SH-PQ—0.432 | BY-PQ—0.0891 |
| Sample No. | M0 (mg/kg) | km (d−1) | Fitted Equation | Coefficient of Determination R2 |
|---|---|---|---|---|
| AS-LP | 0.765 | 0.0784 | Mt = 765[1 − e(−0.0784t)] | 0.911 |
| PM-KC | 0.494 | 0.135 | Mt = 494[1 − e(−0.135t)] | 0.904 |
| SH-PQ | 0.923 | 0.195 | Mt = 923[1 − e(−0.195t)] | 0.472 |
| No. | Static Leaching Experiment | CO2 Injection Experiment | ||
|---|---|---|---|---|
| Dissolution Potential (mg/kg) | Rate Constant (d−1) | Dissolution Potential (mg/kg) | Rate Constant (d−1) | |
| AS-LP | 0.21 | 0.0622 | 0.765 | 0.0784 |
| PM-KC | 0.134 | 0.114 | 0.494 | 0.135 |
| SH-PQ | 0.433 | 0.0495 | 0.923 | 0.195 |
| Study Area | No. | Element Content | Static Leaching Experiment | |||
|---|---|---|---|---|---|---|
| Sr (mg/kg) | Ca (mg/kg) | Sr Equilibrium Concentration (mg/kg) | Overall Ranking | Ranking among Samples in the Study Area | ||
| Luanping | JL-LP | 119 | 2060 | 0.02449 | 9 | 3 |
| AS-LP | 454 | 12,500 | 0.209 | 3 | 1 | |
| NH-LP | 86.1 | 817 | 0.0435 | 7 | 2 | |
| Kuancheng | HG-KC | 820 | 6360 | 0.0625 | 6 | 2 |
| HR-KC | 394 | 3500 | 0.0275 | 8 | 3 | |
| PM-KC | 671 | 32,700 | 0.133 | 4 | 1 | |
| Pingquan | DL-PQ | 178 | 320,000 | 0.311 | 2 | 2 |
| SH-PQ | 298 | 302,000 | 0.432 | 1 | 1 | |
| BY-PQ | 52.8 | 138,000 | 0.0891 | 5 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wu, X.; Zhai, Y.; Su, Y.; Liu, C. An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei. Water 2021, 13, 699. https://doi.org/10.3390/w13050699
Wang R, Wu X, Zhai Y, Su Y, Liu C. An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei. Water. 2021; 13(5):699. https://doi.org/10.3390/w13050699
Chicago/Turabian StyleWang, Ruifeng, Xiong Wu, Yanliang Zhai, Yuxuan Su, and Chenhui Liu. 2021. "An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei" Water 13, no. 5: 699. https://doi.org/10.3390/w13050699





