Sugarcane Bagasse as a Co-Substrate with Oil-Refinery Biological Sludge for Biogas Production Using Batch Mesophilic Anaerobic Co-Digestion Technology: Effect of Carbon/Nitrogen Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods, Design and Operational Setup
3. Results and Discussion
3.1. Pre-Treatment Process Analysis, Characterization and Experimental Design
3.1.1. Thermo-Chemical Pre-Treatment of Raw Sugarcane Bagasse (RSCB)
3.1.2. Proximate and Ultimate Analysis for Treated Oily-Biological Sludge (TOBS) and Treated Sugarcane Bagasse (TSCB)
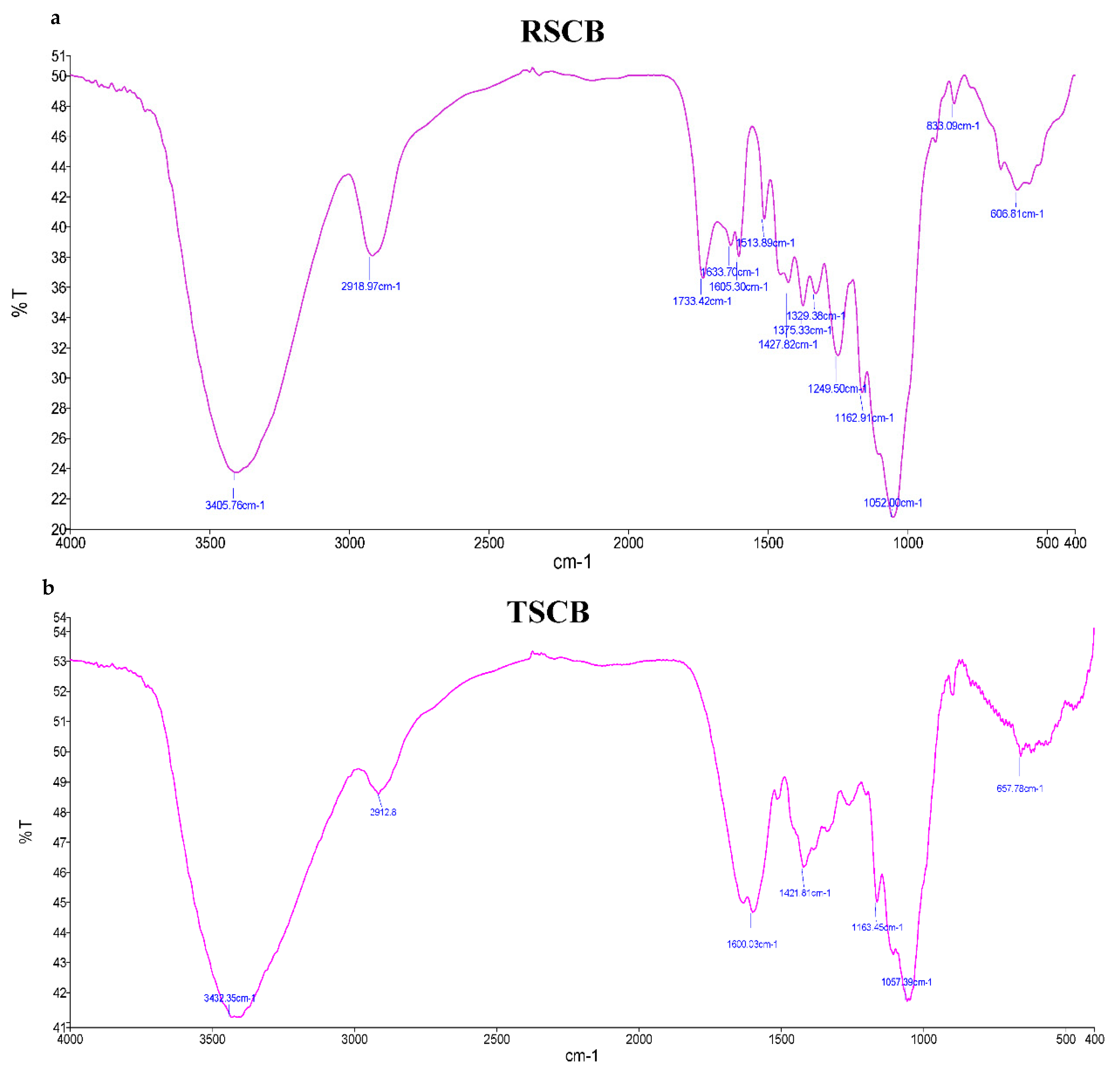
3.1.3. FTIR Analysis for Raw Sugarcane Bagasse (RSCB) and Treated Sugarcane Bagasse (TSCB)
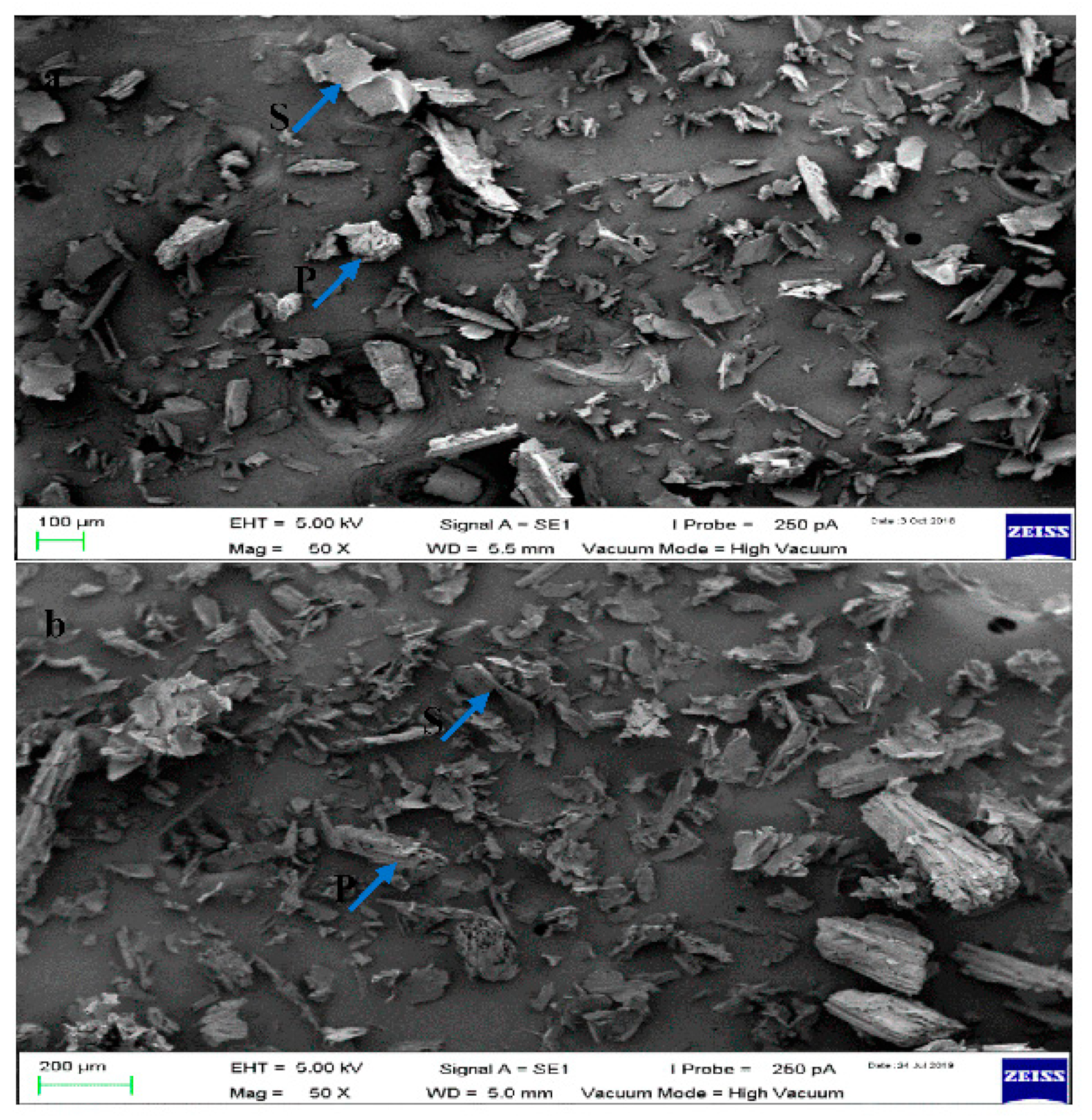
3.1.4. Surface Morphology of Raw Sugarcane Bagasse (RSCB) and Treated Sugarcane Bagasse (TSCB)
3.1.5. Scanning Electron Microscopy of ROBS and TOBS
3.1.6. Mixing Ratios of Treated Oily-Biological Sludge (TOBS) and Treated Sugarcane Bagasse (TSCB) to Obtain C/N Ratios between 20 and 30
3.2. Kinetic of Biogas and Methane Yield
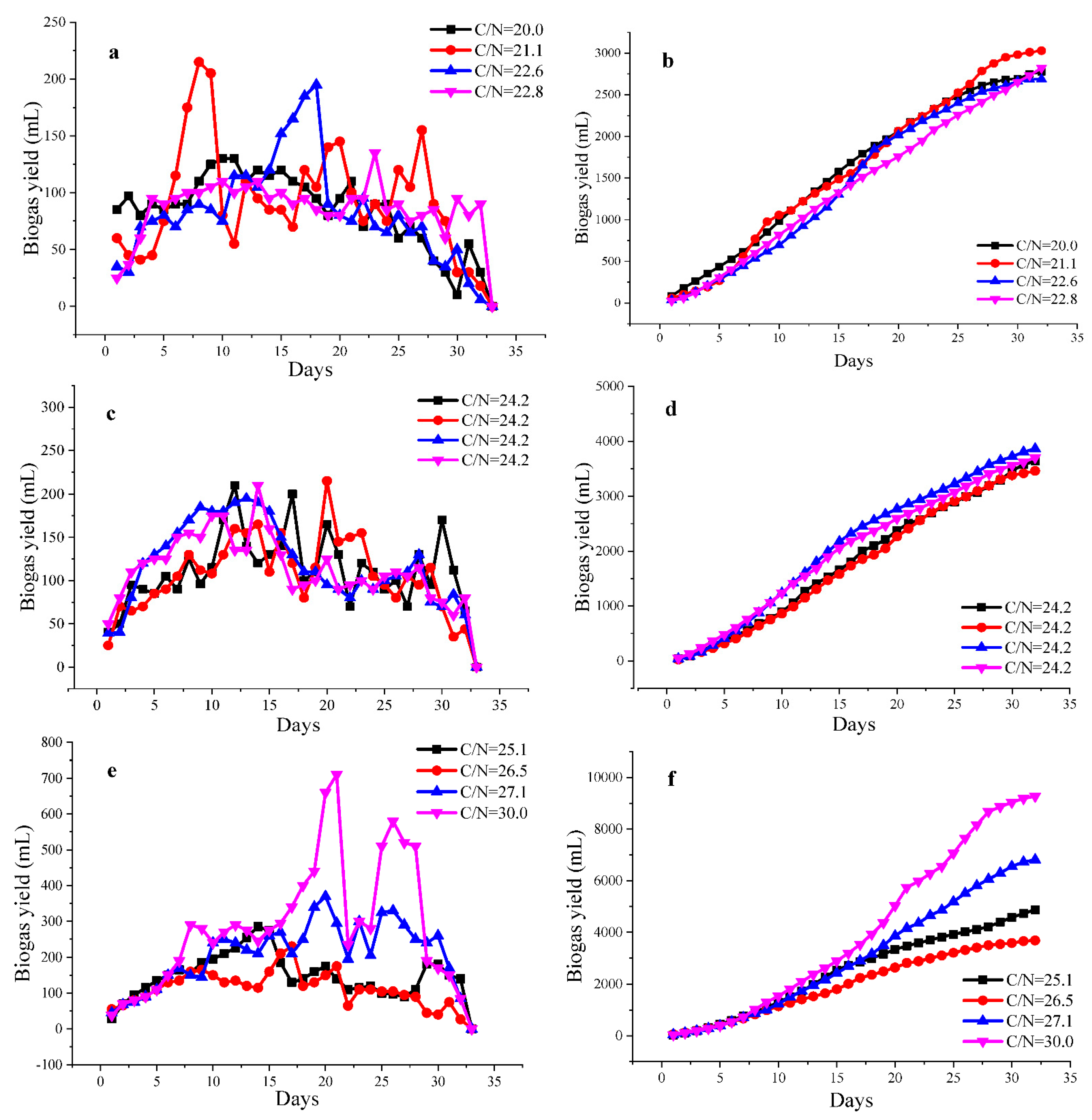
3.2.1. Effect of Carbon/Nitrogen (C/N) and Co-Substrate VS/Inoculum VS Ratios on Biogas Yield
ANOVA Analysis for Biogas Yield from C/N 20–30
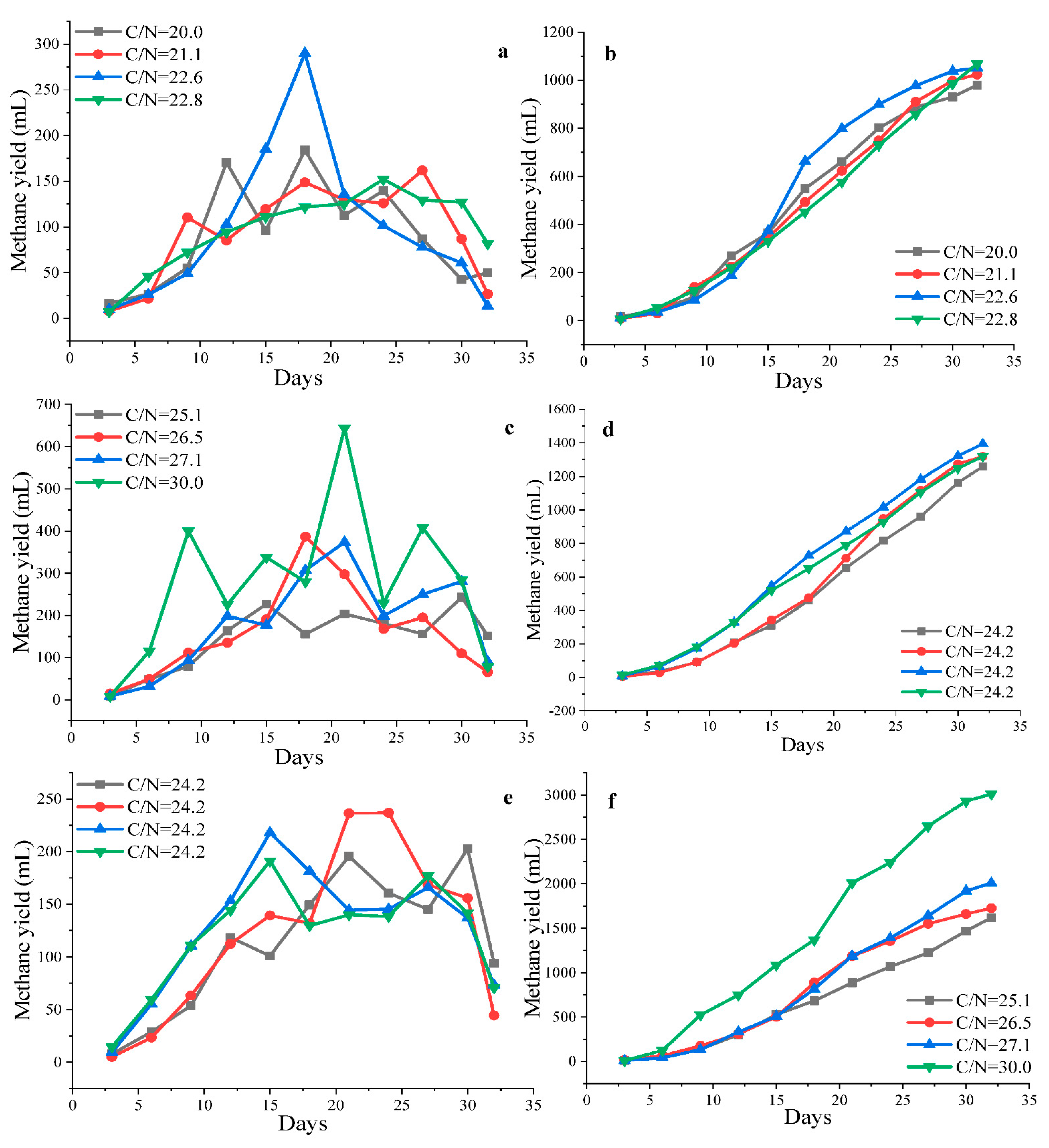
3.2.2. Effect of Carbon/Nitrogen (C/N) and Co-Substrate VS/Inoculum VS Ratios on Methane Yield
ANOVA Analysis for Methane Yield
3.2.3. Effect of Carbon/Nitrogen (C/N) and Co-Substrate VS/Inoculum VS Ratios on Volatile Solids Removed
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Novelty Statement
Appendix A
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 20.0 | 32 | 2777 | 86.78125 | 923.1442 | ||
| C/N = 21.1 | 32 | 3029 | 94.65625 | 2288.426 | ||
| C/N = 22.6 | 32 | 2688 | 84 | 1946.71 | ||
| C/N = 22.8 | 32 | 2822 | 88.1875 | 428.3508 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 1957.313 | 3 | 652.4375 | 0.467142 | 0.705731 | 2.677699 |
| Within Groups | 173,185.6 | 124 | 1396.658 | |||
| Total | 175,142.9 | 127 |
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 20.0 | 32 | 2777 | 86.78125 | 923.1442 | ||
| C/N = 21.1 | 32 | 3029 | 94.65625 | 2288.426 | ||
| C/N = 22.6 | 32 | 2688 | 84 | 1946.71 | ||
| C/N = 22.8 | 32 | 2822 | 88.1875 | 428.3508 | ||
| Ave C/N = 24.2 | 32 | 3665 | 114.5313 | 1083.051 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 19,429.71 | 4 | 4857.428 | 3.641424 | 0.007262 | 2.430002 |
| Within Groups | 206,760.2 | 155 | 1333.936 | |||
| Total | 226,189.9 | 159 |
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 25.1 | 32 | 4867 | 152.0938 | 3291.184 | ||
| C/N = 26.5 | 32 | 3683 | 115.0938 | 2190.152 | ||
| C/N = 27.1 | 32 | 6808 | 212.75 | 7365.226 | ||
| C/N = 30.0 | 32 | 9268 | 289.625 | 30,224.18 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 558,965.5 | 3 | 186,321.8 | 17.3038 | 1.9 × 10−9 | 2.677699 |
| Within Groups | 1,335,193 | 124 | 10,767.68 | |||
| Total | 1,894,158 | 127 | ||||
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 20.0 | 32 | 2777 | 86.78125 | 923.1442 | ||
| C/N = 21.1 | 32 | 3029 | 94.65625 | 2288.426 | ||
| C/N = 22.6 | 32 | 2688 | 84 | 1946.71 | ||
| C/N = 22.8 | 32 | 2822 | 88.1875 | 428.3508 | ||
| Ave C/N = 24.2 | 32 | 3665 | 114.5313 | 1083.051 | ||
| C/N = 25.1 | 32 | 4867 | 152.0938 | 3291.184 | ||
| C/N = 26.5 | 32 | 3683 | 115.0938 | 2190.152 | ||
| C/N = 27.1 | 32 | 6808 | 212.75 | 7365.226 | ||
| C/N = 30.0 | 32 | 9268 | 289.625 | 30,224.18 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 1,271,971 | 8 | 158,996.4 | 28.76871 | 1.61 × 10−32 | 1.971665 |
| Within Groups | 1,541,953 | 279 | 5526.714 | |||
| Total | 2,813,924 | 287 | ||||
Appendix B
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 20.0 | 11 | 979.9306 | 89.0846 | 3286.609 | ||
| C/N = 21.1 | 11 | 1023.803 | 93.07296 | 2818.605 | ||
| C/N = 22.6 | 11 | 1051.351 | 95.57733 | 7029.023 | ||
| C/N = 22.8 | 11 | 1067.176 | 97.01602 | 1813.93 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 398.3631 | 3 | 132.7877 | 0.035533 | 0.990889 | 2.838745 |
| Within Groups | 149,481.7 | 40 | 3737.041 | |||
| Total | 149,880 | 43 | ||||
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 20.0 | 11 | 979.9306 | 89.0846 | 3286.609 | ||
| C/N = 21.1 | 11 | 1023.803 | 93.07296 | 2818.605 | ||
| C/N = 22.6 | 11 | 1051.351 | 95.57733 | 7029.023 | ||
| C/N = 22.8 | 11 | 1067.176 | 97.01602 | 1813.93 | ||
| Ave C/N = 24.2 | 11 | 1321.423 | 120.1293 | 3433.406 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 6550.961 | 4 | 1637.74 | 0.445484 | 0.775136 | 2.557179 |
| Within Groups | 183,815.7 | 50 | 3676.314 | |||
| Total | 190,366.7 | 54 |
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 25.1 | 11 | 1617.179 | 147.0163 | 5369.843 | ||
| C/N = 26.5 | 11 | 1723.404 | 156.6731 | 12,032.22 | ||
| C/N = 27.1 | 11 | 2007.842 | 182.5311 | 13,639.7 | ||
| C/N = 30.0 | 11 | 3009.458 | 273.587 | 31,249.45 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 110,009.3 | 3 | 36,669.78 | 2.354732 | 0.086381 | 2.838745 |
| Within Groups | 622,912.2 | 40 | 15,572.8 | |||
| Total | 732,921.5 | 43 |
| Anova: Single Factor | ||||||
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| C/N = 20.0 | 11 | 979.9306 | 89.0846 | 3286.609 | ||
| C/N = 21.1 | 11 | 1023.803 | 93.07296 | 2818.605 | ||
| C/N = 22.6 | 11 | 1051.351 | 95.57733 | 7029.023 | ||
| C/N = 22.8 | 11 | 1067.176 | 97.01602 | 1813.93 | ||
| Ave C/N = 24.2 | 11 | 1321.423 | 120.1293 | 3433.406 | ||
| C/N = 25.1 | 11 | 1617.179 | 147.0163 | 5369.843 | ||
| C/N = 26.5 | 11 | 1723.404 | 156.6731 | 12,032.22 | ||
| C/N = 27.1 | 11 | 2007.842 | 182.5311 | 13,639.7 | ||
| C/N = 30.0 | 11 | 3009.458 | 273.587 | 31,249.45 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 318,877.3 | 8 | 39,859.66 | 4.446814 | 0.000144 | 2.042986 |
| Within Groups | 806,727.9 | 90 | 8963.643 | |||
| Total | 1,125,605 | 98 |
References
- Baykara, S.Z. Hydrogen: A brief overview on its sources, production and environmental impact. Int. J. Hydrog. Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and utilization of fuel pellets from biomass: A review. Fuel Process. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Alvarado, R.; Ponce, P.; Alvarado, R.; Ponce, K.; Huachizaca, V.; Toledo, E. Sustainable and non-sustainable energy and output in Latin America: A cointegration and causality approach with panel data. Energy Strategy Rev. 2019, 26, 100369. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J.; Chang, Y.; Lee, Y.-J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef]
- Obi, F.; Ugwuishiwu, B.; Nwakaire, J. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Mymrin, V.; Pedroso, D.E.; Pedroso, C.; Alekseev, K.; Avanci, M.A.; Winter, E., Jr.; Cechin, L.; Rolim, P.H.; Iarozinski, A.; Catai, R.E. Environmentally clean composites with hazardous aluminum anodizing sludge, concrete waste, and lime production waste. J. Clean. Prod. 2018, 174, 380–388. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R. Biogas production from co-digestion of dairy manure and food waste. Bioresour. Technol. 2010, 101, 4021–4028. [Google Scholar] [CrossRef]
- Pubule, J.; Blumberga, A.; Romagnoli, F.; Blumberga, D. Finding an optimal solution for biowaste management in the Baltic States. J. Clean. Prod. 2015, 88, 214–223. [Google Scholar] [CrossRef]
- Katinas, V.; Marčiukaitis, M.; Perednis, E.; Dzenajavičienė, E.F. Analysis of biodegradable waste use for energy generation in Lithuania. Renew. Sustain. Energy Rev. 2019, 101, 559–567. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A review of sludge-to-energy recovery methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Đurđević, D.; Blecich, P.; Lenić, K. Energy potential of digestate produced by anaerobic digestion in biogas power plants: The case study of Croatia. Environ. Eng. Sci. 2018, 35, 1286–1293. [Google Scholar] [CrossRef]
- Ülgüdür, N.; Ergüder, T.H.; Uludağ-Demirer, S.; Demirer, G.N. High-rate anaerobic treatment of digestate using fixed film reactors. Environ. Pollut. 2019, 252, 1622–1632. [Google Scholar] [CrossRef]
- Álvarez, J.; Otero, L.; Lema, J. A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresour. Technol. 2010, 101, 1153–1158. [Google Scholar] [CrossRef]
- Archer, S.A.; Steinberger-Wilckens, R. Systematic analysis of biomass derived fuels for fuel cells. Int. J. Hydrog. Energy 2018, 43, 23178–23192. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef]
- Fagerström, A.; Al Seadi, T.; Rasi, S.; Briseid, T. The Role of Anaerobic Digestion and Biogas in the Circular Economy; IEA Bioenergy: Paris, France, 2018. [Google Scholar]
- Kankia, M.U.; Baloo, L.; Mohammed, B.S.; Hassan, S.B.; Haruna, S.; Danlami, N.; Ishak, E.A.; Samahani, W.N. Effects of petroleum sludge ash in fly ash-based geopolymer mortar. Constr. Build. Mater. 2021, 272, 121939. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Shuaibu, A.; Umaru, I.; Musa, S.; Lawal, I.M.; Abubakar, S. Stabilization of soft soil by incinerated sewage sludge ash from municipal wastewater treatment plant for engineering construction. Sustain. Struct. Mater. Int. J. 2019, 2, 32–44. [Google Scholar]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Sathish, S.; Balaji, R.; Shafee, S.; Mageswaran, C. Experimental Analysis on Anaerobic Digestion of Industrial Waste Biomass. AIP Conf. Proc. 2020, 2225, 060002. [Google Scholar]
- Sun, J.; Sun, X.; Sun, R.; Su, Y. Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym. 2004, 56, 195–204. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Fernandes, T.; Bos, G.K.; Zeeman, G.; Sanders, J.; Van Lier, J. Effects of thermo-chemical pre-treatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 2575–2579. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, Z.; Luo, Y.; Zou, X.; Fang, C. Effect of alkaline treatment on anaerobic digestion of rice straw. Huan Jing Ke Xue Huanjing Kexue 2010, 31, 2208–2213. [Google Scholar]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.; Delgenès, J.; Steyer, J.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zheng, L.; Wang, Z.; Dai, X. Perspective on enhancing the anaerobic digestion of waste activated sludge. J. Hazard. Mater. 2020, 389, 121847. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- AZIM, K.; OUYIHYA, K.; AMELLOUK, A.; PERISSOL, C.; THAMI ALAMI, I.; SOUDI, B. Dynamic composting optimization through C/N ratio variation as a startup parameter. Build. Org. Bridges 2014, 3, 787–790. [Google Scholar]
- Ghaleb, A.A.S.; Kutty, S.R.M.; Ho, Y.-C.; Jagaba, A.H.; Noor, A.; Al-Sabaeei, A.M.; Almahbashi, N.M.Y. Response Surface Methodology to Optimize Methane Production from Mesophilic Anaerobic Co-Digestion of Oily-Biological Sludge and Sugarcane Bagasse. Sustainability 2020, 12, 2116. [Google Scholar] [CrossRef] [Green Version]
- Council, N.R. Methane Generation from Human, Animal, and Agricultural Wastes: Report of an Ad Hoc Panel of the Advisory Committee on Technology Innovation, Board on Science and Technology for International Development, Commission on International Relations, National Research Council; National Academies: Washington, DC, USA, 1977; Volume 276. [Google Scholar]
- Ghaleb, A.; Kutty, S.; Ho, Y.; Jagaba, A.; Noor, A.; Al-Sabaeei, A.; Kumar, V.; Saeed, A. Anaerobic Co-Digestion for Oily-Biological Sludge with Sugarcane Bagasse for Biogas Production under Mesophilic Condition. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kuala Lumpur, Malaysia, 9–11 August 2020; p. 012084. [Google Scholar]
- Szymczycha-Madeja, A.; Welna, M.; Zyrnicki, W. Multi-element analysis, bioavailability and fractionation of herbal tea products. J. Braz. Chem. Soc. 2013, 24, 777–787. [Google Scholar] [CrossRef]
- Sun, X.; Xu, F.; Sun, R.; Fowler, P.; Baird, M. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.O.; Shukla, M.; Maheswari, C.U.; Rajulu, A.V. Mechanical and physical characterization of sodium hydroxide treated Borassus fruit fibers. J. For. Res. 2012, 23, 667–674. [Google Scholar] [CrossRef]
- Pandey, K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Rao, P.S.; Reddy, K.S.; Kalyani, S.; Krishnaiah, A. Comparative sorption of copper and nickel from aqueous solutions by natural neem (Azadirachta indica) sawdust and acid treated sawdust. Wood Sci. Technol. 2007, 41, 427–442. [Google Scholar]
- Liu, D.; Han, G.; Huang, J.; Zhang, Y. Composition and structure study of natural Nelumbo nucifera fiber. Carbohydr. Polym. 2009, 75, 39–43. [Google Scholar] [CrossRef]
- Zhang, Z.; Rackemann, D.W.; Doherty, W.O.; O’Hara, I.M. Glycerol carbonate as green solvent for pretreatment of sugarcane bagasse. Biotechnol. Biofuels 2013, 6, 153. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B. Characterization of alkali-treated jute fibers for physical and mechanical properties. J. Appl. Polym. Sci. 2001, 80, 1013–1020. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 33–46. [Google Scholar]
- Karatzos, S.K.; Edye, L.A.; Doherty, W.O.S. Sugarcane bagasse pretreatment using three imidazolium-based ionic liquids; mass balances and enzyme kinetics. Biotechnol. Biofuels 2012, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sun, J.; Li, M.; Wang, S.; Pei, H.; Zhang, J. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 2009, 100, 5853–5858. [Google Scholar] [CrossRef]
- Lee, H.; Hamid, S.; Zain, S. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Torres, M.L.; Lloréns, M.d.C.E. Effect of alkaline pretreatment on anaerobic digestion of solid wastes. Waste Manag. 2008, 28, 2229–2234. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef]
- Grady, C.L., Jr.; Daigger, G.T.; Love, N.G.; Filipe, C.D. Biological Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Fang, H.H.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Verma, S. Anaerobic digestion of biodegradable organics in municipal solid wastes. Columbia Univ. 2002, 7, 98–104. [Google Scholar]
- Yen, H.-W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Murto, M.; Björnsson, L.; Mattiasson, B. Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J. Environ. Manag. 2004, 70, 101–107. [Google Scholar] [CrossRef]
- Procházka, J.; Dolejš, P.; Máca, J.; Dohányos, M. Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl. Microbiol. Biotechnol. 2012, 93, 439–447. [Google Scholar] [CrossRef]
- Marchaim, U.; Krause, C. Propionic to acetic acid ratios in overloaded anaerobic digestion. Bioresour. Technol. 1993, 43, 195–203. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Optimization of process parameters for accelerated methane yield from anaerobic co-digestion of rice straw and food waste. Renew. Energy 2020, 149, 1352–1359. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Enhanced methane production from anaerobic co-digestion of rice straw and hydrilla verticillata and its kinetic analysis. Biomass Bioenergy 2019, 125, 8–16. [Google Scholar] [CrossRef]
- Stuckey, D.C.; McCarty, P.L. The effect of thermal pretreatment on the anaerobic biodegradability and toxicity of waste activated sludge. Water Res. 1984, 18, 1343–1353. [Google Scholar] [CrossRef]
- Vlyssides, A.; Karlis, P. Thermal-alkaline solubilization of waste activated sludge as a pre-treatment stage for anaerobic digestion. Bioresour. Technol. 2004, 91, 201–206. [Google Scholar] [CrossRef]
- Oosterhuis, M.; Ringoot, D.; Hendriks, A.; Roeleveld, P. Thermal hydrolysis of waste activated sludge at Hengelo wastewater treatment plant, The Netherlands. Water Sci. Technol. 2014, 70, 1–7. [Google Scholar] [CrossRef]
- Costa, A.G.; Pinheiro, G.C.; Pinheiro, F.G.C.; Dos Santos, A.; Santaella, S.T.; Leitão, R.C. The use of thermochemical pretreatments to improve the anaerobic biodegradability and biochemical methane potential of the sugarcane bagasse. Chem. Eng. J. 2014, 248, 363–372. [Google Scholar] [CrossRef]
- Alagöz, B.A.; Yenigün, O.; Erdinçler, A. Enhancement of anaerobic digestion efficiency of wastewater sludge and olive waste: Synergistic effect of co-digestion and ultrasonic/microwave sludge pre-treatment. Waste Manag. 2015, 46, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Maragkaki, A.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef]
- Mehryar, E.; Ding, W.; Hemmat, A.; Talha, Z.; Hassan, M.; Mamat, T.; Hei, K. Anaerobic co-digestion of oil refinery wastewater with bagasse; evaluating and modeling by neural network algorithms and mathematical equations. BioResources 2017, 12, 7325–7340. [Google Scholar]
- Alagöz, B.A.; Yenigün, O.; Erdinçler, A. Ultrasound assisted biogas production from co-digestion of wastewater sludges and agricultural wastes: Comparison with microwave pre-treatment. Ultrason. Sonochem. 2018, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]



| Hemicellulose (%) | Cellulose (%) | Lignin (%) | |
|---|---|---|---|
| RSCB | 24.32 | 36.20 | 28.03 |
| TSCB 1%, 45 min | 8.03 | 50.40 | 25.30 |
| TSCB 1%, 60 min | 10.25 | 62.05 | 13.50 |
| TSCB 1%, 75 min | 8.17 | 58.17 | 16.43 |
| TSCB 2%, 45 min | 8.37 | 56.80 | 17.87 |
| TSCB 2%, 60 min | 7.90 | 51.70 | 23.40 |
| TSCB 2%, 75 min | 4.95 | 64.35 | 14.45 |
| Parameter | Unit | TOBS | Unit | TSCB |
|---|---|---|---|---|
| Moisture Content | % | 94.20 | % | 0 |
| pH | N/A | 8.70 | N/A | 7.21 |
| TS | g/L | 58.00 | % | 100 |
| VS | g/L | 50.46 | % | 87.80 |
| C | % of TS | 4.31 | % | 34.70 |
| N | % of TS | 0.30 | % | 0.26 |
| C/N | N/A | 14.42 | N/A | 132.69 |
| Bagasse | Frequency (cm−1) | Possible Assignment |
|---|---|---|
| RSCB | 3405.76 | O-H Stretching and NH group (cellulose and lignin) |
| 2918.97 | C-H Stretching (cellulose and hemicellulose) | |
| 1733.42 | C=O stretching (Hemicellulose) | |
| 1633.70 | C=O stretching (lignin) | |
| 1605.30 | Aromatic skeleton C=C (lignin) | |
| 1513.89 | Aromatic skeleton C=C (lignin) | |
| 1427.82 | CH2 asymmetric stretching (lignin) | |
| 1375.33 | C-H bend (cellulose) | |
| 1329.38 | C-F stretch | |
| 1249.50 | C-O stretching (hemicellulose) | |
| 1162.91 | C-O-C Asymmetrical stretching (cellulose and hemicellulose) | |
| 1052.00 | C-O stretching (cellulose) | |
| 833.09 | C-H bending | |
| 606.81 | C-Br stretch |
| Bagasse | Frequency (cm−1) | Possible Assignment |
|---|---|---|
| TSCB | 3432.35 | O-H Stretching and NH (cellulose and lignin) |
| 2912.80 | C-H Stretching (cellulose and hemicellulose) | |
| 1600.03 | Aromatic skeletal C=C (lignin) | |
| 1421.81 | C-H bending (cellulose) | |
| 1163.45 | C-O-C asymmetrical stretching (cellulose and hemicellulose) | |
| 1057.39 | C-O stretching (cellulose) | |
| 657.78 | ≡C-H bend |
| Run No. | TSCB (g) | TOBS (g) | C/N | Co-Substrate VS/Inoculum VS |
|---|---|---|---|---|
| 1 | 1.0 | 294.0 | 20.0 | 0.06 |
| 2 | 1.0 | 243.5 | 21.1 | 0.07 |
| 3 | 1.5 | 294.0 | 22.6 | 0.09 |
| 4 | 1.0 | 193.0 | 22.8 | 0.09 |
| 5 | 1.5 | 243.5 | 24.2 | 0.11 |
| 6 | 1.5 | 243.5 | 24.2 | 0.11 |
| 7 | 1.5 | 243.5 | 24.2 | 0.11 |
| 8 | 1.5 | 243.5 | 24.2 | 0.11 |
| 9 | 2.0 | 294.0 | 25.1 | 0.12 |
| 10 | 1.5 | 193.0 | 26.5 | 0.13 |
| 11 | 2.0 | 243.5 | 27.1 | 0.14 |
| 12 | 2.0 | 193.0 | 30.0 | 0.18 |
| Run No. | C/N | Volatile Solids Removed (g) | Biogas Yield (mL) | Methane Yield (mL) | Biogas Yield/VS Removed mL/g.VSremoved |
|---|---|---|---|---|---|
| 1 | 20.0 | 32.3 | 2777 | 980.0 | 86.0 |
| 2 | 21.1 | 32.5 | 3029 | 1023.8 | 93.2 |
| 3 | 22.6 | 33.2 | 2688 | 1051.3 | 81.0 |
| 4 | 22.8 | 33.4 | 2822 | 1067.3 | 84.5 |
| 5 | 24.2 | 34.8 | 3638 | 1257.3 | 104.5 |
| 6 | 24.2 | 35.1 | 3459 | 1317.5 | 98.5 |
| 7 | 24.2 | 36.1 | 3863 | 1393.4 | 107.0 |
| 8 | 24.2 | 35.6 | 3700 | 1317.6 | 103.9 |
| 9 | 25.1 | 39.2 | 4867 | 1617.3 | 124.2 |
| 10 | 26.5 | 39.8 | 3683 | 1723.3 | 92.5 |
| 11 | 27.1 | 42.2 | 6808 | 2007.9 | 161.3 |
| 12 | 30.0 | 46.2 | 9268 | 3009.3 | 200.6 |
| Substrate | Co-Substrate | Biogas and Methane Yield | Digestion Time (Batch) | Reference |
|---|---|---|---|---|
| Oil refinery wastewater | Sugarcane bagasse | 154.72 mL/g VS, 97.13 mL of CH4/g VS | 34 days | [66] |
| Waste activated sludge | Grape pomace | 180 mL/gVSremoved, 110 mL of CH4/gVSremoved | 30 days | [67] |
| Oil refinery biological sludge | Sugarcane bagasse | 200.6 mL/g VSremoved, 63.52 mL of CH4/gVSremoved | 33 days | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaleb, A.A.S.; Kutty, S.R.M.; Salih, G.H.A.; Jagaba, A.H.; Noor, A.; Kumar, V.; Almahbashi, N.M.Y.; Saeed, A.A.H.; Saleh Al-dhawi, B.N. Sugarcane Bagasse as a Co-Substrate with Oil-Refinery Biological Sludge for Biogas Production Using Batch Mesophilic Anaerobic Co-Digestion Technology: Effect of Carbon/Nitrogen Ratio. Water 2021, 13, 590. https://doi.org/10.3390/w13050590
Ghaleb AAS, Kutty SRM, Salih GHA, Jagaba AH, Noor A, Kumar V, Almahbashi NMY, Saeed AAH, Saleh Al-dhawi BN. Sugarcane Bagasse as a Co-Substrate with Oil-Refinery Biological Sludge for Biogas Production Using Batch Mesophilic Anaerobic Co-Digestion Technology: Effect of Carbon/Nitrogen Ratio. Water. 2021; 13(5):590. https://doi.org/10.3390/w13050590
Chicago/Turabian StyleGhaleb, Aiban Abdulhakim Saeed, Shamsul Rahman Mohamed Kutty, Gasim Hayder Ahmed Salih, Ahmad Hussaini Jagaba, Azmatullah Noor, Vicky Kumar, Najib Mohammed Yahya Almahbashi, Anwar Ameen Hezam Saeed, and Baker Nasser Saleh Al-dhawi. 2021. "Sugarcane Bagasse as a Co-Substrate with Oil-Refinery Biological Sludge for Biogas Production Using Batch Mesophilic Anaerobic Co-Digestion Technology: Effect of Carbon/Nitrogen Ratio" Water 13, no. 5: 590. https://doi.org/10.3390/w13050590








