Induced Allelopathic Effects of Thalassiosira weissflogii on Colony Formation in Phaeocystis globosa
Abstract
:1. Introduction
2. Materials and Methods
3. Results
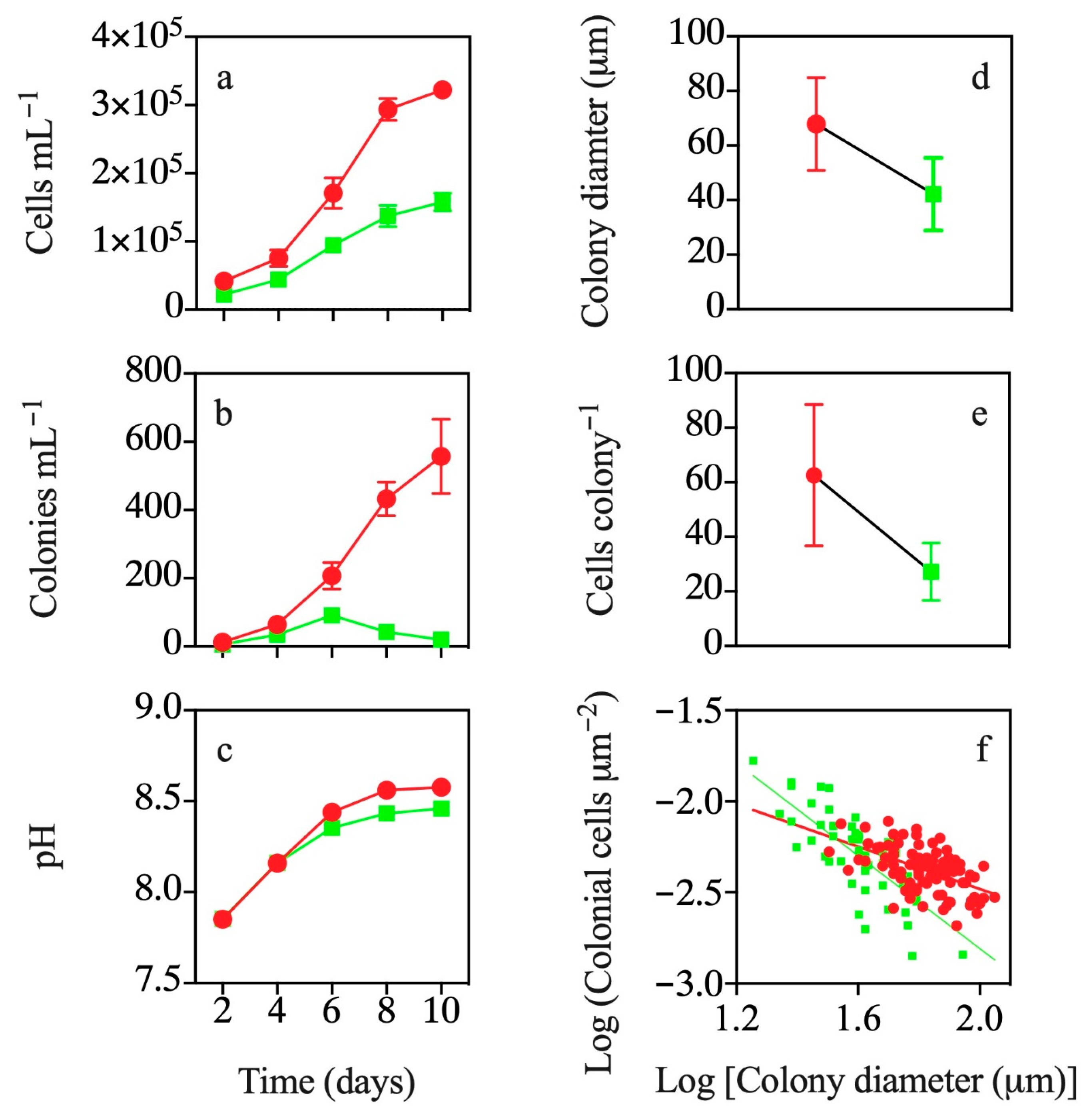
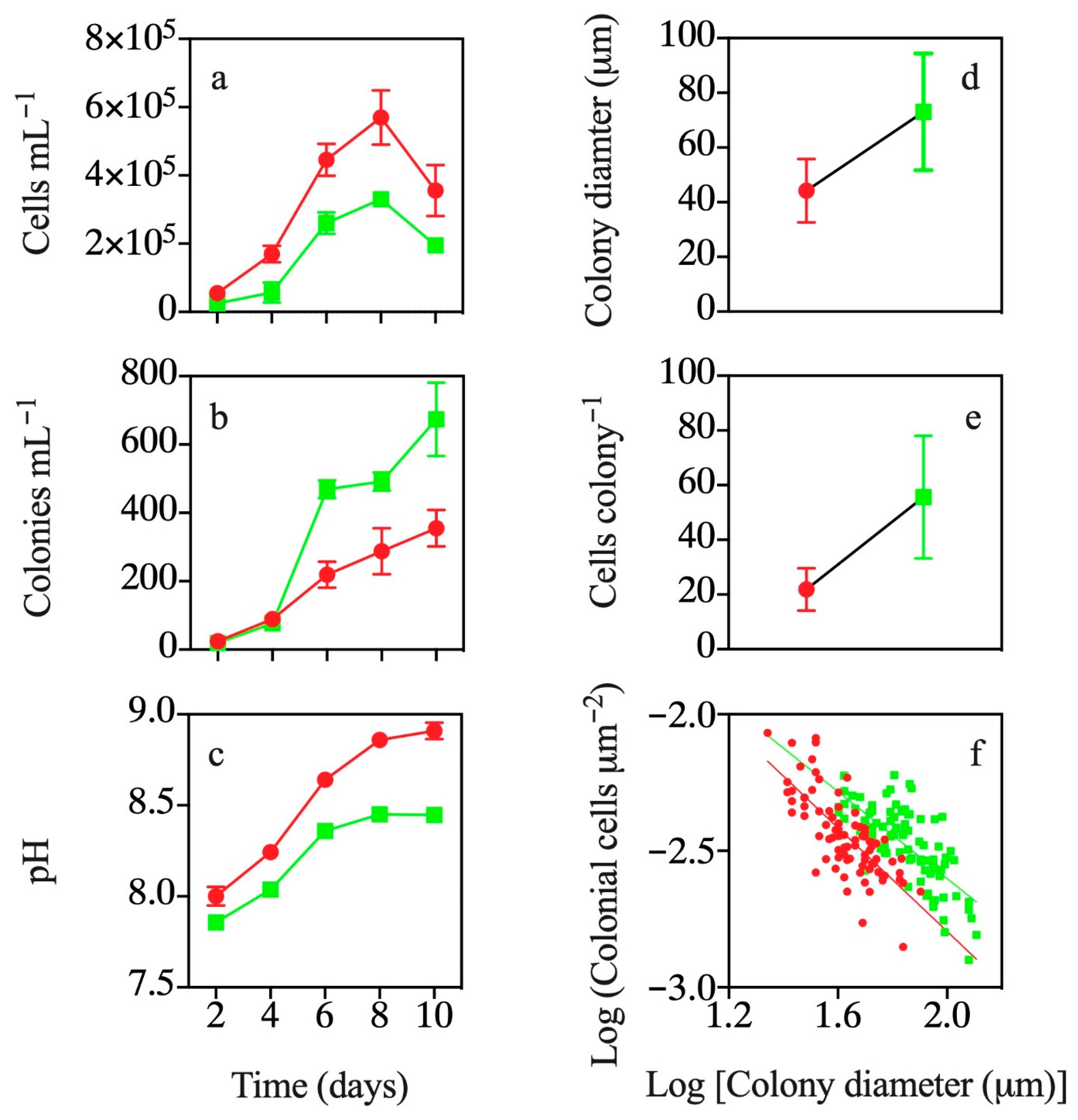
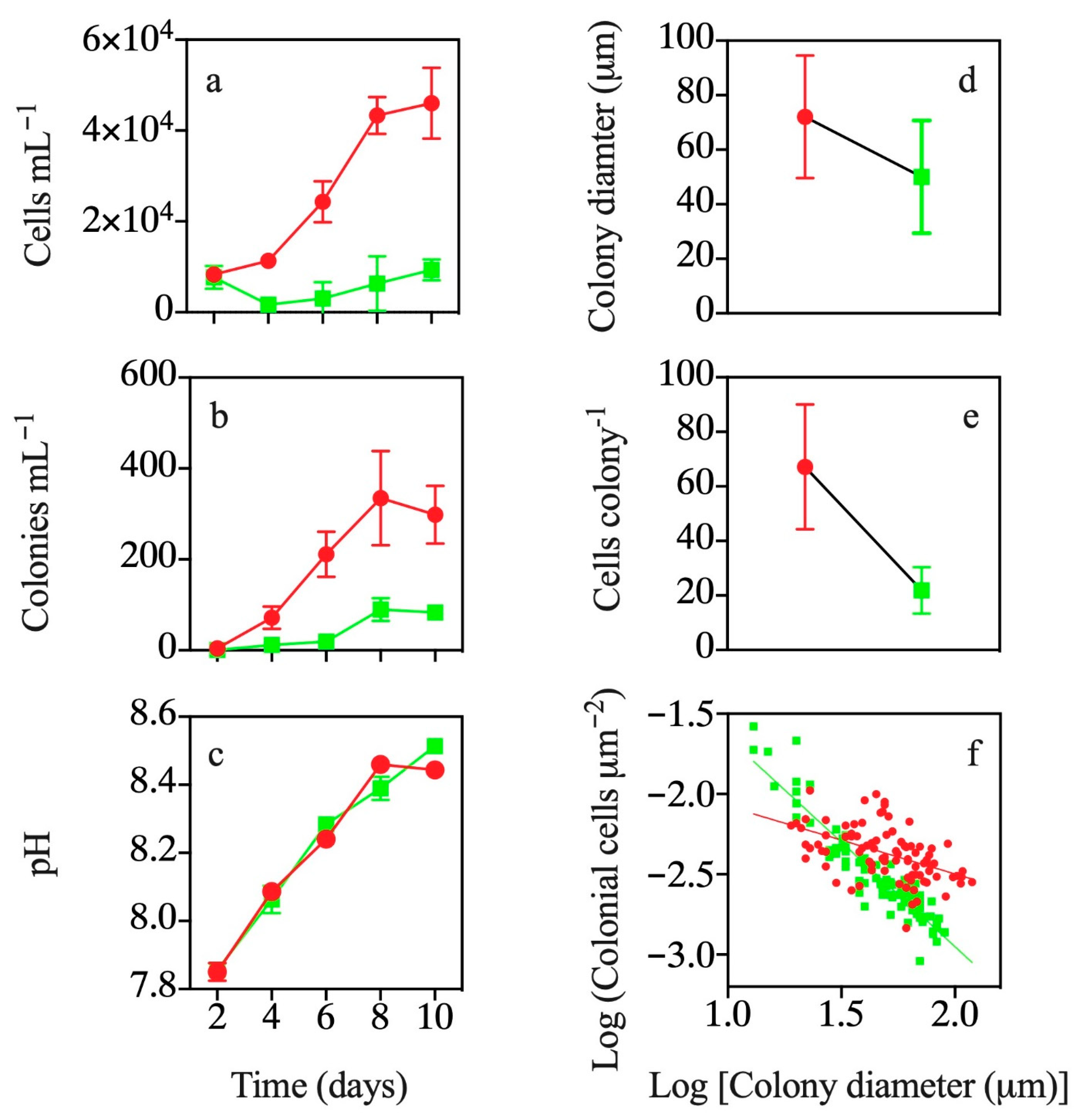
3.1. Co-occurrence with T. weissflogii
3.2. Effects of Exposure to T. weissflogii Exudates
3.3. Effects of Continuous Exposure to T. weissflogii Exudates
3.4. Effects of Exudates of Pre-Exposed T. weissflogii
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisman, J.; Jonker, R.R.; Zonneveld, C.; Weissing, F.J. Competition for light between phytoplankton species: Experimental tests of mechanistic theory. Ecology 1999, 80, 211–222. [Google Scholar] [CrossRef]
- Sommer, U. Phytoplankton succession in microcosm experiments under simultaneous grazing pressure and resource limitation. Limnol. Oceanogr. 1988, 33, 1037–1054. [Google Scholar] [CrossRef]
- Cermeño, P.; Lee, J.B.; Wyman, K.; Schofield, O.; Falkowski, P.G. Competitive dynamics in two species of marine phytoplankton under non-equilibrium conditions. Mar. Ecol. Prog. Ser. 2011, 429, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Marañón, E. Cell size as a key determinant of phytoplankton metabolism and community structure. Ann. Rev. Mar. Sci. 2015, 7, 242–264. [Google Scholar] [CrossRef] [Green Version]
- Legrand, C.; Rengefors, K.; Fistarol, G.O.; Granéli, E. Allelopathy in phytoplankton—Biochemical, ecological and evolutionary aspects. Phycologia 2003, 42, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Granéli, E.; Hansen, P.J. Allelopathy in harmful algae: A mechanism to compete for resources? In Ecology of Harmful Algae, Ecological Studies; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–201. [Google Scholar]
- Felpeto, A.B.; Roy, S.; Vasconcelos, V.M. Allelopathy prevents competitive exclusion and promotes phytoplankton biodiversity. Oikos 2018, 127, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Fistarol, G.O.; Legrand, C.; Granéli, E. Allelopathic effect of Prymnesium parvumon a natural plankton community. Mar. Ecol. Prog. Ser. 2003, 255, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Kubanek, J.; Hicks, M.K.; Naar, J.; Villareal, T.A. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol. Oceanogr. 2005, 50, 883–895. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Nagasoe, S.; Matsubara, T.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Allelopathic interactions between the bacillariophyte Skeletonema costatum and the raphidophyte Heterosigma akashiwo. Mar. Ecol. Prog. Ser. 2007, 339, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Prince, E.K.; Myers, T.L.; Kubanek, J. Effects of harmful algal blooms on competitors: Allelopathic mechanisms of the red tide dinoflagellate Karenia brevis. Limnol. Oceanogr. 2008, 53, 531–541. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York USA on cooccurring phytoplankton. Mar. Ecol. Prog. Ser. 2010, 406, 19–31. [Google Scholar] [CrossRef]
- Granéli, E.; Weberg, M.; Salomon, P.S. Harmful algal blooms of allelopathic microalgal species: The role of eutrophication. Harmful Algae 2008, 8, 94–102. [Google Scholar] [CrossRef]
- Jonsson, P.R.; Pavia, H.; Toth, G. Formation of harmful algal blooms cannot be explained by allelopathic interactions. Proc. Natl. Acad. Sci. USA 2009, 106, 11177–11182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillmann, U.; John, U.; Cembella, A. On the allelochemical potency of the marine dinoflagellate Alexandrium ostenfeldii against heterotrophic and autotrophic protists. J. Plankton Res. 2007, 29, 527–543. [Google Scholar] [CrossRef]
- Suikkanen, S.; Hakanen, P.; Spilling, K.; Kremp, A. Allelopathic effects of Baltic Sea spring bloom dinoflagellates on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 2011, 439, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Toda, S.; Matsuyama, Y.; Yamaguchi, M.; Kotani, Y.; Honjo, T. Interactions between the red tide dinoflagellates Heterocapsa circularisquama and Gymnodinium mikimotoi in laboratory culture. J. Exp. Mar. Biol. Ecol. 1999, 241, 285–299. [Google Scholar] [CrossRef]
- Tameishi, M.; Yamasaki, Y.; Nagasoe, S.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Allelopathic effects of the dinophyte Prorocentrum minimum on the growth of the bacillariophyte Skeletonema costatum. Harmful Algae 2009, 8, 421–429. [Google Scholar] [CrossRef]
- Poulson, K.L.; Sieg, R.D.; Prince, E.K.; Kubanek, J. Allelopathic compounds of a red tide dinoflagellate have species-specific and context-dependent impacts on phytoplankton. Mar. Ecol. Prog. Ser. 2010, 416, 69–78. [Google Scholar] [CrossRef]
- Uchida, T.; Yamaguchi, M.; Matsuyama, Y.; Honjo, T. The red-tide dinoflagellate Heterocapsa sp. kills Gyrodinium instriatum by cell contact. Mar. Ecol. Prog. Ser. 1995, 118, 301–303. [Google Scholar] [CrossRef]
- Corcoran, A.A.; Seger, M.; Niu, R.; Nirmalakhandan, N.; Lammers, P.J.; Holguin, F.O.; Boeing, W.J. Evidence for induced allelopathy in an isolate of Coelastrella following co-culture with Chlorella sorokiniana. Algal. Res. 2019, 41, 101535. [Google Scholar] [CrossRef]
- Paul, C.; Barofsky, A.; Vidoudez, C.; Pohnert, G. Diatom exudates influence metabolism and cell growth of co-cultured diatom species. Mar. Ecol. Prog. Ser. 2009, 389, 61–70. [Google Scholar] [CrossRef]
- Smith, W.O., Jr.; Codispoti, L.A.; Nelson, D.M.; Manley, T.; Buskey, E.J.; Niebauer, H.J.; Cota, G.F. Importance of Phaeocystis blooms in the high-latitude ocean carbon cycle. Nature 1991, 352, 514–516. [Google Scholar] [CrossRef]
- Lancelot, C.; Keller, M.D.; Rousseau, V.; Smith, W.O.; Mathot, S. Autecology of the marine haptophyte Phaeocystiss pp. In Physiological Ecology of Harmful Algal Blooms; NATO ASI Series; Anderson, D.M., Cembella, A.D., Hallegraef, G.M., Eds.; Springer: Berlin, Germany, 1998; Volume 41, pp. 209–224. [Google Scholar]
- Schoemann, V.; Becquevort, S.; Stefels, J.; Rousseau, W.; Lancelot, C. Phaeocystis blooms in the global ocean and their controlling mechanisms: A review. J. Sea Res. 2005, 53, 43–66. [Google Scholar] [CrossRef]
- Rousseau, V.; Chrétiennot-Dinet, M.J.; Jacobsen, A.; Verity, P.; Whipple, S. The life cycle of Phaeocystis: State of knowledge and presumptive role in ecology. Biogeochemistry 2007, 83, 29–47. [Google Scholar] [CrossRef]
- Qi, Y.Z.; Chen, J.F.; Wang, Z.H.; Xu, N.; Wang, Y.; Shen, P.P.; Lu, S.H.; Hodgkiss, I.J. Some observations on harmful algal bloom (HAB) events along the coast of Guangdong, southern China in 1998. Hydrobiologia 2004, 51, 209–214. [Google Scholar] [CrossRef]
- Peperzak, L.; Colijn, F.; Gieskes, W.W.C.; Peeters, J.H.C. Development of the diatom-Phaeocystis spring bloom in the Dutch coastal zone of the North Sea: The silicon depletion versus the daily irradiance threshold hypothesis. J. Plankton Res. 1998, 20, 517–537. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, V.; Leynaert, A.; Daoud, N.; Lancelot, C. Diatom succession, silicification and silicic acid availability in Belgian coastal waters (southern North Sea). Mar. Ecol. Prog. Ser. 2002, 236, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Breton, E.; Rousseau, V.; Parent, J.Y.; Ozer, J.; Lancelot, C. Hydroclimatic modulation of diatom/Phaeocystis blooms in nutrient-enriched Belgian coastal waters (North Sea). Limnol. Oceanogr. 2006, 51, 1401–1409. [Google Scholar] [CrossRef]
- Gypens, N.; Lacroix, G.; Lancelot, C. Causes of variability in diatom and Phaeocystis blooms in Belgian coastal waters between 1989 and 2003: A model study. J. Sea Res. 2007, 57, 19–35. [Google Scholar] [CrossRef]
- He, C.; Song, S.; Li, C. The spatial-temporal distribution of Phaeocytis globosa colonies and related affecting factors in Guangxi Beibu Gulf (in Chinese, with English abstract). Oceanol. Limnol. Sin. 2019, 50, 630–643. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.T. Grazing and colony size development in Phaeocystis globosa (Prymnesiophyceae): The role of a chemical signal. J. Plankton Res. 2003, 25, 831–842. [Google Scholar] [CrossRef]
- Wang, X.; Tang, K.W.; Wang, Y.; Smith, J.R.W.O. Temperature effects on growth, colony development and carbon partitioning in three Phaeocystis species. Aquat. Biol. 2010, 9, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Liu, D.Y. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef] [Green Version]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis; John Wiley & Sons: Weinheim, Germany, 1999. [Google Scholar]
- Wang, X.; Wang, Y.; Ou, L.; He, X.; Chen, D. Allocation costs associated with induced defense in Phaeocystis globosa (Prymnesiophyceae): The effects of nutrient availability. Sci. Rep. 2015, 5, 10850. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.F.; Hansen, P.J. Effects of high pH on the growth and survival of six marine heterotrophic protists. Mar. Ecol. Prog. Ser. 2003, 260, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Lundholm, N.; Hansen, P.J.; Kotaki, Y. Lack of allelopathic effects of the domoic acid-producing marine diatom Pseudo-nitzschia multiseries. Mar. Ecol. Prog. Ser. 2005, 288, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Riisgaard, K.; Nielsen, T.G.; Hansen, P.J. Impact of elevated pH on succession in the Arctic spring bloom. Mar. Ecol. Prog. Ser. 2015, 530, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, Y.; Zou, Y.; Go, J.; Shikata, T.; Matsuyama, Y.; Nagai, K.; Shimasaki, Y.; Yamaguchi, K.; Oshima, Y.; Oda, T.; et al. Cell contact-dependent lethal effect of the dinoflagellate Heterocapsa circularisquama on phytoplankton–phytoplankton interactions. J. Sea Res. 2011, 65, 76–83. [Google Scholar] [CrossRef]
- Hulot, F.D.; Huisman, J. Allelopathic interactions between phytoplankton species: The roles of heterotrophic bacteria and mixing intensity. Limnol. Oceanogr. 2004, 49, 1424–1434. [Google Scholar] [CrossRef]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Allelopathic effects of the Baltic cyanobacteria Nodularia spumdigena, Aphanizomenon flos-aquae and Anabaena lemmermannii on algal monocultures. J. Exp. Mar. Biol. Ecol. 2004, 308, 85–101. [Google Scholar] [CrossRef]
- Fistarol, G.O.; Legrand, C.; Granéli, E. Allelopathic effect on a nutrient-limited phytoplankton species. Aquat. Microb. Ecol. 2005, 41, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Myers, T.L.; Prince, E.K.; Naar, J.; Kubanek, J. Loss of waterborne brevetoxins from exposure to phytoplankton competitors. Harmful Algae 2008, 7, 762–771. [Google Scholar] [CrossRef]
- Davidson, A.T.; Marchant, H.J. Binding of manganese by Antarctic Phaeocystis pouchetii and the role of bacteria in its release. Mar. Biol. 1987, 95, 481–487. [Google Scholar] [CrossRef]
- Lancelot, C. The mucilage phenomenon in the continental coastal waters of the North-Sea. Sci. Total Environ. 1995, 165, 83–102. [Google Scholar] [CrossRef]
- Poulson-Ellestad, K.L.; Jones, C.M.; Roy, J.; Viant, M.R.; Fernandez, F.M.; Kubanek, J.; Nunn, B.L. Metabolomics and proteomics reveal impacts of chemically mediated competition on marine plankton. Proc. Natl. Acad. Sci. USA 2014, 111, 9009–9014. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W.M., Jr. Evolutionary interpretations of allelochemical interactions in phytoplankton algae. Am. Nat. 1986, 127, 184–194. [Google Scholar] [CrossRef]
- Hamm, C.E. Architecture, ecology and biogeochemistry of Phaeocystis colonies. J. Sea Res. 2000, 43, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Nejstgaard, J.C.; Tang, K.W.; Steinke, M.; Dutz, J.; Koski, M.; Antajan, E.; Long, J.D. Zooplankton grazing on Phaeocystis: A quantitative review and future challenges. Biogeochemistry 2007, 83, 147–172. [Google Scholar] [CrossRef] [Green Version]
- Hamm, C.E.; Simson, D.A.; Merkel, R.; Smetacek, V. Colonies of Phaeocystis globosa are protected by a thin but tough skin. Mar. Ecol. Prog. Ser. 1999, 187, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Irigoien, X.; Flynn, K.J.; Harris, R.P. Phytoplankton blooms: A ‘loophole’ in microzooplankton grazing impact? J. Plankton Res. 2005, 27, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.O., Jr.; Liu, X.; Tang, K.W.; DeLizo, L.M.; Doan, N.H.; Nguyen, N.L.; Wang, X. Giantism and its role in the harmful algal bloom species Phaeocystis globosa. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 101, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Ou, L. The roles of light–dark cycles in the growth of Phaeocystis globosa from the South China Sea: The cost of colony enlargement. J. Sea. Res. 2014, 85, 518–523. [Google Scholar] [CrossRef]
- Jakobsen, H.H.; Tang, K.T. Effects of protozoan grazing on colony formation in Phaeocystis globosa (Prymnesiophyceae) and the potential costs and benefits. Aquat. Microb. Ecol. 2002, 27, 261–271. [Google Scholar] [CrossRef] [Green Version]

| Inorganic Nutrient (µmol L−1) | |||||
|---|---|---|---|---|---|
| Nitrate | Phosphate | Ammonium | Nitrite | Silicate | |
| Control | 771.6 ± 48.7 | 25.3 ± 0.6 | 2.4 ± 0.3 | 0.2 ± 0 | 105.7 ± 9.7 |
| Treatment | 753.7 ± 35.2 | 26.3 ± 2.6 | 1.8 ± 0.5 | 0.1 ± 0 | 79.4 ± 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Huo, Y.; Yang, F.; Wang, Y. Induced Allelopathic Effects of Thalassiosira weissflogii on Colony Formation in Phaeocystis globosa. Water 2021, 13, 581. https://doi.org/10.3390/w13050581
Wang X, Huo Y, Yang F, Wang Y. Induced Allelopathic Effects of Thalassiosira weissflogii on Colony Formation in Phaeocystis globosa. Water. 2021; 13(5):581. https://doi.org/10.3390/w13050581
Chicago/Turabian StyleWang, Xiaodong, Yiping Huo, Fan Yang, and Yan Wang. 2021. "Induced Allelopathic Effects of Thalassiosira weissflogii on Colony Formation in Phaeocystis globosa" Water 13, no. 5: 581. https://doi.org/10.3390/w13050581
APA StyleWang, X., Huo, Y., Yang, F., & Wang, Y. (2021). Induced Allelopathic Effects of Thalassiosira weissflogii on Colony Formation in Phaeocystis globosa. Water, 13(5), 581. https://doi.org/10.3390/w13050581






