Hydrogenotrophic Denitrification of Groundwater Using a Simplified Reactor for Drinking Water: A Case Study in the Kathmandu Valley, Nepal
Abstract
:1. Introduction
2. Materials and Methods
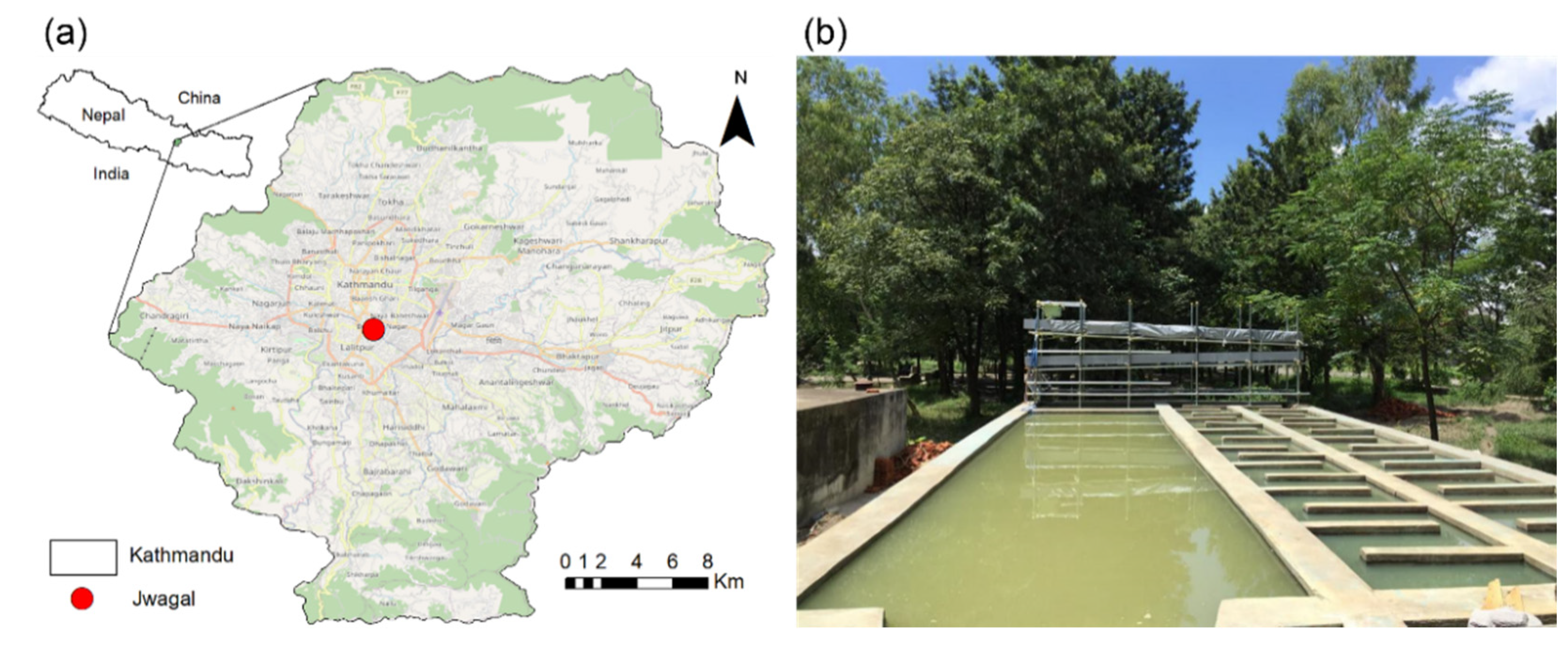
2.1. Description of the Study Area
2.2. Characteristics of Groundwater at Jwagal
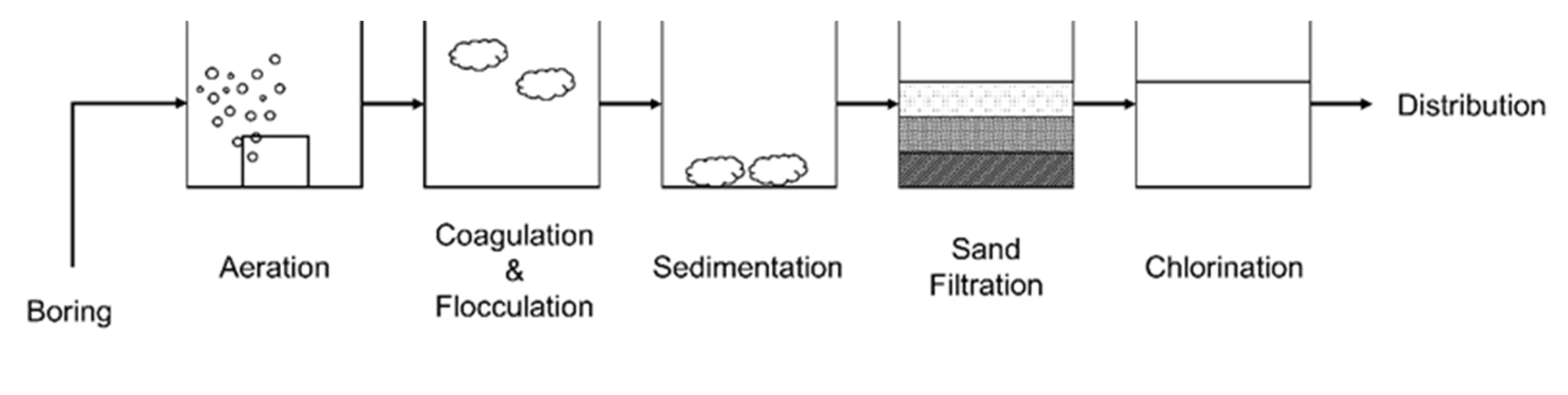
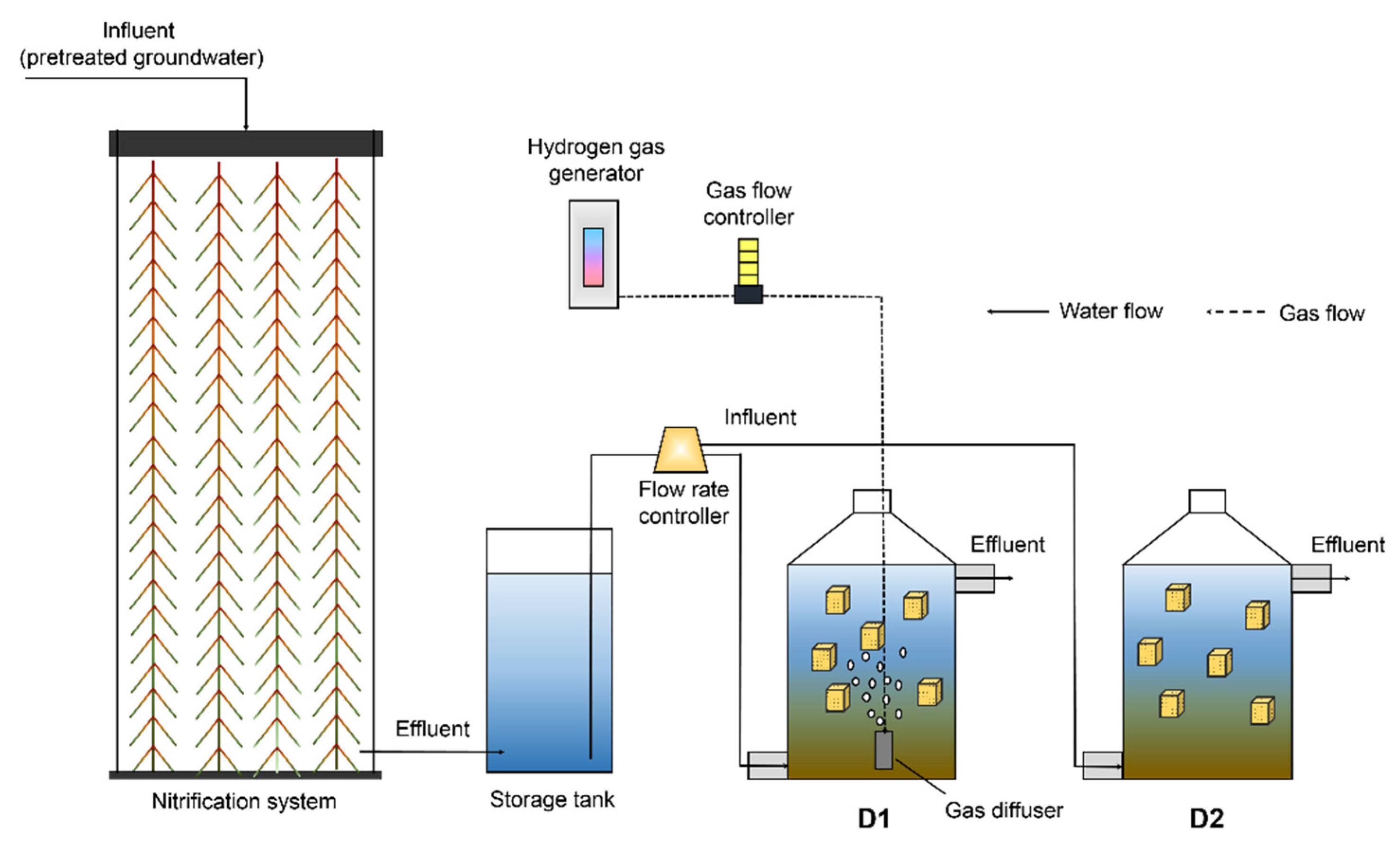
2.3. Simplified HD Reactor
2.4. Batch Test for the Determination of NO3−−N Removal and Denitrification Rate
2.5. Analytical Methods
2.6. Performance of the Simplified HD Reactor
2.7. Statistical Analysis
3. Results and Discussion
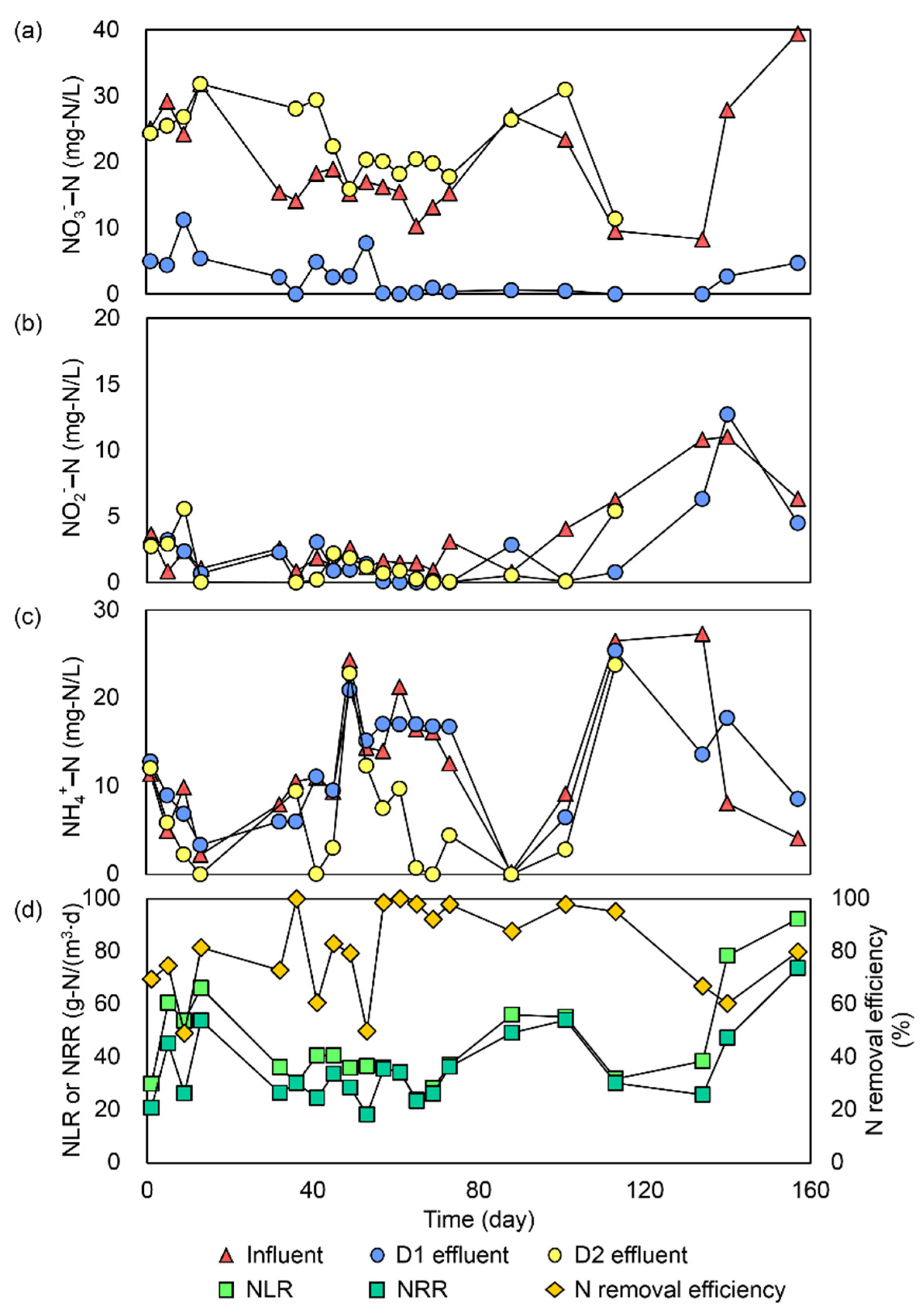
3.1. Variations in Nitrogen Concentrations
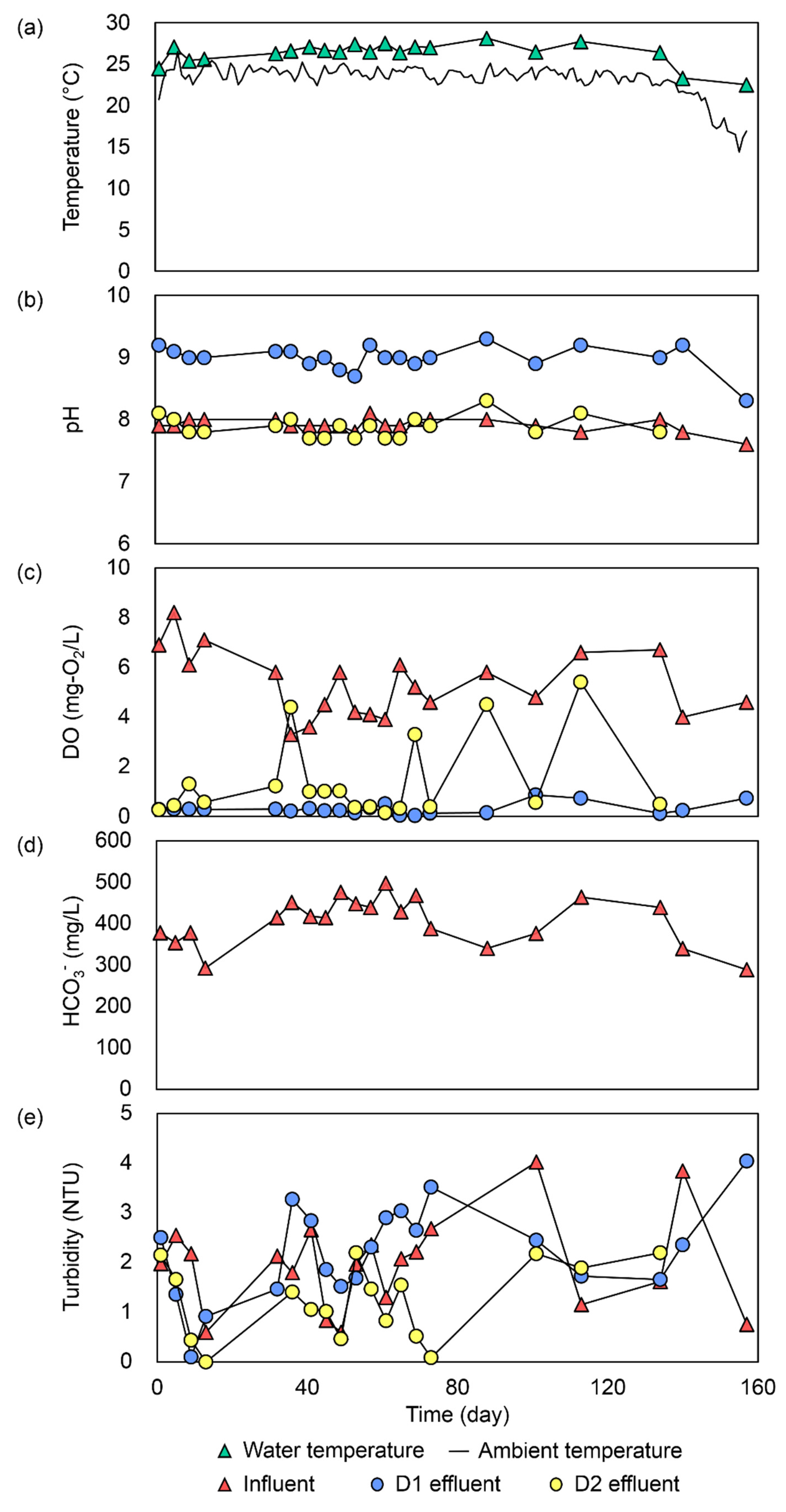
3.2. Ambient Condition and Changes in Operational Parameters
3.3. Comparison with Various HD Reactors
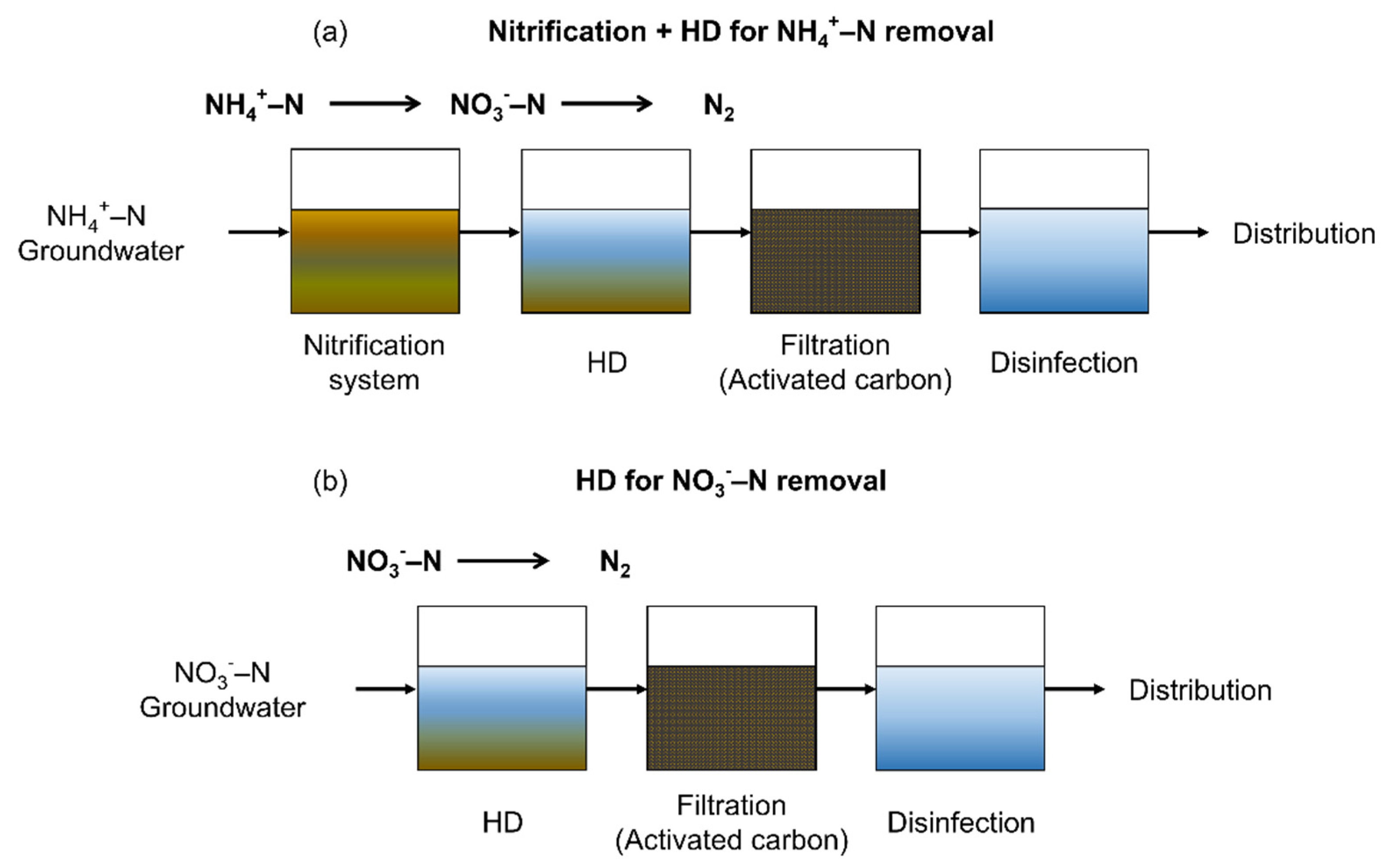
3.4. Application and Implementation of the Simplified HD Reactor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations (UN). Sustainable Development Goals. 2016. Available online: http://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 18 November 2020).
- Tamrakar, N.K.; Manandhar, K.C. Institutional Capacity Assessment of KUKL; Ministry of Urban Development (MOUD): Kathmandu, Nepal; Japan International Cooperation Agency (JICA): Tokyo, Japan, 2016.
- Pradhan, B.; Sharma, P.; Pradhan, K.P. Urban Growth and Environment and Health Hazards in Kathmandu Valley, Nepal. In Urban Health Risk and Resilience in Asian Cities; Singh, R.B., Srinagesh, B., Anand, S., Eds.; Springer: Singapore, 2020; pp. 293–324. [Google Scholar] [CrossRef]
- Kathmandu Upatyaka Khanepani Limited (KUKL). Annual Report of Kathmandu Upatyaka Khanepani Limited; Kathmandu Upatyaka Khanepani Limited: Kathmandu, Nepal, 2020. [Google Scholar]
- Shrestha, S.; Aihara, Y.; Bhattarai, A.P.; Bista, N.; Rajbhandari, S.; Kondo, N.; Kazama, F.; Nishida, K.; Shindo, J. Dynamics of domestic water consumption in the urban area of the Kathmandu Valley: Situation analysis pre and post 2015 Gorkha earthquake. Water 2017, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Ojha, R.; Thapa, B.R.; Shrestha, S.; Shindo, J.; Ishidaira, H.; Kazama, F. Water price optimization after the Melamchi Water Supply Project: Ensuring affordability and equitability for consumer’s water use and sustainability for utilities. Water 2018, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Ojha, R.; Thapa, B.R.; Shrestha, S.; Shindo, J.; Ishidaira, H.; Kazama, F. Water taxation and subsidy analysis based on consumer water use behavior and water sources inside the Kathmandu Valley. Water 2018, 10, 1802. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, K.B.; Thapa, B.R.; Aihara, Y.; Shrestha, S.; Bhattarai, A.P.; Bista, N.; Kazama, F.; Shindo, J. Hidden cost of drinking water treatment and its relation with socioeconomic status in Nepalese urban context. Water 2018, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, K.B.; Kamei, T.; Shrestha, S.; Aihara, Y.; Bhattarai, A.P.; Bista, N.; Thapa, B.R.; Kazama, F.; Shindo, J. Socioeconomic impacts of LCD-treated drinking water distribution in an urban community in the Kathmandu Valley, Nepal. Water 2019, 11, 1323. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Malla, S.S.; Bhattarai, A.P.; Haramoto, E.; Shindo, J.; Nishida, K. Waterborne diarrhoeal infection risk from multiple water sources and the impact of an earthquake. J. Water Health 2020, 18, 464–476. [Google Scholar] [CrossRef]
- Shakya, B.M.; Nakamura, T.; Kamei, T.; Shrestha, S.D.; Nishida, K. Seasonal groundwater quality status and nitrogen contamination in the shallow aquifer system of the Kathmandu Valley, Nepal. Water 2019, 11, 2184. [Google Scholar] [CrossRef] [Green Version]
- Khatiwada, N.R.; Takizawa, S.; Tran, T.V.N.; Inoue, M. Groundwater contamination assessment for sustainable water supply in Kathmandu Valley, Nepal. Water Sci. Technol. 2002, 46, 147–154. [Google Scholar] [CrossRef]
- Warner, N.R.; Levy, J.; Harpp, K.; Farruggia, F. Drinking water quality in Nepal’s Kathmandu Valley: A survey and assessment of selected controlling site characteristics. Hydrogeol. J. 2008, 16, 321–334. [Google Scholar] [CrossRef]
- Pathak, D.R.; Hiratsuka, A. An investigation of nitrate and iron concentrations and their relationship in shallow groundwater systems of Kathmandu. Desalin. Water Treat. 2010, 19, 191–197. [Google Scholar] [CrossRef]
- Chapagain, S.K.; Pandey, V.P.; Shrestha, S.; Nakamura, T.; Kazama, F. Assessment of deep groundwater quality in Kathmandu Valley using multivariate statistical techniques. Water Air Soil Pollut. 2009, 210, 277–288. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2017. [Google Scholar]
- Ward, M.; Jones, R.; Brender, J.; de Kok, T.; Weyer, P.; Nolan, B.; Villanueva, C.; van Breda, S. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanitchaidecha, W.; Shakya, M.; Nagano, Y.; Kazama, F. NH4-N removal from groundwater using attached growth reactor: Case study in Chyasal, Nepal. In IPCBEE Vol. 18; International Conference on Biotechnology and Environment Management, Singapore, 2011; IACSIT Press: Singapore, 2011. [Google Scholar]
- Khanitchaidecha, W.; Shakya, M.; Kamei, T.; Kazama, F. NH4-N removal through nitrification and hydrogenotrophic denitrification in simple attached growth reactors. Water Air Soil Pollut. 2012, 223, 3939–3953. [Google Scholar] [CrossRef]
- Maharjan, A.K.; Kamei, T.; Amatya, I.M.; Mori, K.; Kazama, F.; Toyama, T. Ammonium-nitrogen (NH4+–N) removal from groundwater by a dropping nitrification reactor: Characterization of NH4+–N transformation and bacterial community in the reactor. Water 2020, 12, 599. [Google Scholar] [CrossRef] [Green Version]
- Shinoda, K.; Rujakom, S.; Singhopon, T.; Eamrat, R.; Kamei, T.; Kazama, F. Effects of Carrier Filling Ratio on Hydrogenotrophic Denitrification (HD) Performance. Jpn. J. Water Treat. Biol. 2020, 56, 67–78. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.-H.; Ebrahimi, S.; Oh, H.-M. Nitrate removal from drinking water with a focus on biological methods: A review. Environ. Sci. Pollut. Res. 2019, 26, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Khanitchaidecha, W.; Kazama, F. Hydrogenotrophic denitrification in an attached growth reactor under various operating conditions. Water Supply 2012, 12, 72–81. [Google Scholar] [CrossRef]
- Eamrat, R.; Tsutsumi, Y.; Kamei, T.; Khanichaidecha, W.; Tanaka, Y.; Kazama, F. Optimization of hydrogenotrophic denitrification behavior using continuous and intermittent hydrogen gas supply. J. Water Environ. Technol. 2017, 15, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Rujakom, S.; Shinoda, K.; Singhopon, T.; Nakano, M.; Kamei, T.; Kazama, F. Effect of Bicarbonate on the Performance of Hydrogen-based Denitrification at Different Hydraulic Retention Times. Jpn. J. Water Treat. Biol. 2020, 56, 33–45. [Google Scholar] [CrossRef]
- Ergas, S.J.; Reuss, A.F. Hydrogenotrophic denitrification of drinking water using a hollow fibre membrane bioreactor. J. Water Supply Res. Tech. Aqua 2001, 50, 161–171. [Google Scholar] [CrossRef]
- Mo, H.; Oleszkiewicz, J.A.; Cicek, N.; Rezania, B. Incorporating membrane gas diffusion into a membrane bioreactor for hydrogenotrophic denitrification of groundwater. Water Sci. Technol. 2005, 51, 357–364. [Google Scholar] [CrossRef]
- Rezania, B.; Oleszkiewicz, J.A.; Cicek, N. Hydrogen-dependent denitrification of water in an anaerobic submerged membrane bioreactor coupled with a novel hydrogen delivery system. Water Res. 2007, 41, 1074–1080. [Google Scholar] [CrossRef]
- Komori, M.; Sakakibara, Y. High-rate hydrogenotrophic denitrification in a fluidized-bed biofilm reactor using solidpolymer-electrolyte membrane electrode (SPEME). Water Sci. Technol. 2008, 58, 1441–1446. [Google Scholar] [CrossRef]
- Xia, S.; Zhong, F.; Zhang, Y.; Li, H.; Yang, X. Bio-reduction of nitrate from groundwater using a hydrogen-based membrane biofilm reactor. J. Environ. Sci. 2010, 22, 257–262. [Google Scholar] [CrossRef]
- Chen, D.; Yang, K.; Wang, H.; Lv, B. Nitrate removal from groundwater by hydrogen-fed autotrophic denitrification in a bio-ceramsite reactor. Water Sci. Technol. 2014, 69, 2417–2422. [Google Scholar] [CrossRef]
- Peng, T.; Feng, C.; Hu, W.; Chen, N.; He, Q.; Dong, S.; Xu, Y.; Gao, Y.; Li, M. Treatment of nitrate-contaminated groundwater by heterotrophic denitrification coupled with electro-autotrophic denitrifying packed bed reactor. Biochem. Eng. J. 2018, 134, 12–21. [Google Scholar] [CrossRef]
- Epsztein, R.; Beliavski, M.; Tarre, S.; Green, M. Pressurized hydrogenotrophic denitrification reactor for small water systems. J. Environ. Manag. 2018, 216, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Eamrat, R.; Tsutsumi, Y.; Kamei, T.; Khanitchaidecha, W.; Ito, T.; Kazama, F. Microbubble application to enhance hydrogenotrophic denitrification for groundwater treatment. Env. Nat. Res. J. 2020, 18, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, F.; Sarrafzadeh, M.-H.; Oh, H.-M. Hydrogen producer microalgae in interaction with hydrogen consumer denitrifiers as a novel strategy for nitrate removal from groundwater and biomass production. Algal Res. 2020, 45, 101747. [Google Scholar] [CrossRef]
- Inagaki, Y.; Yamada, D.; Komori, M.; Sakakibara, Y. Field application of hydrogenotrophic denitrification with two-stage injection of electrolytic hydrogen. J. Water Process Eng. 2020, 38, 101685. [Google Scholar] [CrossRef]
- Shakya, B.M.; Nakamura, T.; Shrestha, S.D.; Nishida, K. Identifying the deep groundwater recharge processes in an intermountain basin using the hydrochemical and water isotope characteristics. Hydrol. Res. 2019, 50, 1216–1229. [Google Scholar] [CrossRef] [Green Version]
- Japan International Cooperation Agency (JICA). Report of JICA WaSH-Mia/SATREPS Project for Symposium on Hydro-Microbiological Approach for Water Security in Kathmandu Valley, Nepal; Japan International Cooperation Agency (JICA): Kathmandu, Nepal, 2019.
- Raina, A.; Zhao, J.; Wu, X.; Kunwar, L.; Whittington, D. The structure of water vending markets in Kathmandu, Nepal. Water Policy 2019, 21, 50–75. [Google Scholar] [CrossRef]
- Interdisciplinary Centre for River Basin Environment (ICRE). WaSH-Mia/SATREPS: Manual No. 4-2 Handbook for Installation, Operation, and Maintenance of Locally Fitted, Compact, and Distributed (LCD) Water Treatment System; Japan International Cooperation Agency (JICA): Kathmandu, Nepal, 2019.
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Historical Weather and Climate Data. Available online: https://meteostat.net/en/place/NP-0W5S?t=2017-05-30/2017-11-23 (accessed on 7 December 2020).
- Sunger, N.; Bose, P. Autotrophic denitrification using hydrogen generated from metallic iron corrosion. Bioresour. Technol. 2009, 100, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Q.; Chen, D.; Wei, L.; Zou, Z.; Zhou, J.; Yang, K.; Zhang, H. Microbial community in a hydrogenotrophic denitrification reactor based on pyrosequencing. Environ. Biotech. 2015, 99, 10829–10837. [Google Scholar] [CrossRef]
- Karanasios, K.A.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Hydrogenotrophic denitrification of potable water: A review. J. Hazard. Mater. 2010, 180, 20–37. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Zuo, J.; Wang, R.; Zhao, J.; Du, Y. Nitrogen removal and N2O accumulation during hydrogenotrophic denitrification: Influence of environmental factors and microbial community characteristics. Environ. Sci. Technol. 2017, 51, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Grießmeier, V.; Leberecht, K.; Gescher, J. NO3− removal efficiency in field denitrification beds: Key controlling factors and main implications. Environ. Microbiol. Rep. 2019, 11, 316–329. [Google Scholar] [CrossRef]
- Singhopon, T.; Shinoda, K.; Rujakom, S.; Kazama, F. Hydrogenotrophic denitrification for treating nitrate contaminated without/with reactive black 5 dye. J. Water Reuse Desalin. 2020, 10, 200–213. [Google Scholar] [CrossRef]
- Rujakom, S.; Shinoda, K.; Kamei, T.; Kazama, F. Investigation of hydrogen-based denitrification performance on nitrite accumulation under various bicarbonate doses. Env. Asia 2019, 12, 54–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Feng, C.; Huang, F.; Zhang, P.; Zhang, Z.; Yang, Y.; Sugiura, N. Behavior of autotrophic denitrification and heterotrophic denitrification in an intensified biofilm-electrode reactor for nitrate-contaminated drinking water treatment. Bioresour. Technol. 2012, 107, 159–165. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Water Quality and Health-Review of Turbidity: Information for Regulators and Water Suppliers; World Health Organization (WHO): Geneva, Switzerland, 2017. [Google Scholar]
- Gleick, P.H. Basic water requirements for human activities: Meeting basic needs. Water Int. 1996, 21, 83–92. [Google Scholar] [CrossRef]
- Central Bureau of Statistics—National Planning Commission Secretariat, Government of Nepal. Annual Household Survey 2015–2016; Central Bureau of Statistics: Kathmandu, Nepal, 2017.
- Pasakhala, B.; Harada, H.; Fujii, S.; Tanaka, S.; Shivakoti, B.R.; Shrestha, S. Household coping measures with water scarcity: A case study in Kathmandu, Nepal. J. Japan Soc. Civ. Eng. 2013, 69, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.S. Electricity crisis (Load Shedding) in Nepal, Its Manifestations and Ramifications. Hydro Nepal 2010, 6, 7–17. [Google Scholar] [CrossRef]
| Parameter | Concentrations (mg/L) | Reference |
|---|---|---|
| NH4+–N | 41.2–57.3 | [11] |
| NO3−–N | 0.4–3.4 | [11] |
| Na+ | 61 | [37] |
| K+ | 12 | [37] |
| Ca2+ | 68.8 | [37] |
| Mg2+ | 14 | [37] |
| Cl− | 2.1 | [37] |
| SO42- | Not detected | [37] |
| HCO3- | 579.5 | [37] |
| Fe2+ | 9.9–10.9 | This study |
| DOC (dissolved organic carbon) | 10.6–14.0 | This study |
| pH | 6.29–6.79 | This study |
| Day | NO3−–N Removal Rate (mg-N/L/d) | Denitrification Rate (mg-N/L/d) |
|---|---|---|
| Before Operation | 7.5 | 4.2 |
| Day 112 | 20.3 | 26.7 |
| Reactor | Substrate | Bacteria Inoculum | Temperature (°C) | HRT (d or h) Flow Rate (mL/min) | H2 Flow Rate (mL/min) H2 Pressure (MPa or atm) DH (mg/L) Applied Current (A) | NO3−–N (mg-N/L) | Carrier Materials | Reactor Volume (L) | NLR (g-N/(m3∙d)) | NRR (g-N/(m3∙d)) | Max or Average Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hollow fiber | Well water and synthetic groundwater | Biomass from an anoxic rotating biological reactor in a wastewater treatment plant | 4.1 h | 0.3–0.6 atm | 145 | Polypropylene hollow fiber | 1.2 | 770 | 770 | 100 | [26] | |
| Suspended growth membrane | Synthetic groundwater | Enriched autotrophic denitrifying biomass | 16.0 ± 1.1 | 12 h | DH 1.6 | 48 | 7 | 37.7 | 37.7 | 100 | [27] | |
| Submerged membrane | Synthetic groundwater | HD bacteria | 25–28 | 3 h | DH 1.6 | 25 | Hollow fiber membrane | 5.6 | 110 | 110 | 100 | [28] |
| Fluidized-bed biofilm using solid-polymer electrolyte membrane | Synthetic groundwater | 30 | 1 h | 4.0 A | 20–90 | Polyvinyl alcohol | 2.2 | 2160 | 2160 | 100 | [29] | |
| Membrane biofilm | Synthetic drinking water | Anaerobic activated sludge | 0.5 h | 0.05 MPa | 10 | Hollow fiber | 0.024 | 480 | 384 | 80 | [30] | |
| Attached growth | Synthetic groundwater | Activated sludge | 30 | 6.7 h | 70 mL/min | 20 | Fiber | 3 | 71.7 | 69.1 | 96.4 | [23] |
| Attached growth | Synthetic groundwater | Activated sludge | 2.7 h | 70 mL/min | 20 | Fiber carrier | 3 | 176 | 167 | 90 | [19] | |
| Bio-ceramite | Synthetic wastewater | Anaerobic activated sludge | 30 | 24 h | 0.01 MPa | 30 | Ceramite | 2.3 | 30 | 28.9 | 96.2 | [31] |
| Suspended growth | Synthetic groundwater | Enriched HD bacteria in lab | 32 ± 0.5 | 12 h | 15 mL/min | 40 | 2 | 80 | 77.2 | 96.5 | [24] | |
| Heterotrophic denitrification coupled with electro-autotrophic denitrifying packed bed | Synthetic groundwater | Activated sludge | Room temperature | 24 h | 0.1 A | 50 | Haycite Pine sawdust | 27.5 | 27.2 | 99 | [32] | |
| Unsaturated-flow pressurized | Synthetic groundwater | HD bacteria in lab | 25.5 ± 1 | 430 mL/min | DH1.5 | 25 | Plastic biofilm | 2100 | 2100 | 100 | [33] | |
| Suspended growth | Synthetic groundwater | Enriched HD bacteria in lab | 32 ± 0.5 | 12 h | 1 mL/min | 40 | 2 | 80 | 78.4 | 98 | [34] | |
| Attached growth | Synthetic groundwater | Enriched HD bacteria in lab | 32 ± 1 | 4 h | 40 mL/min | 40 | Polyolefin sponge | 2 | 210 | 209.9 | 98 | [21] |
| Attached growth | Raw groundwater treated by on-site nitrification reactor in Chyasal, Nepal | Bacteria from on-site nitrification | 13.3 h | 70 mL/min | 10 | Fiber carrier | 3 | 133 | 107 | >80 | 19 | |
| HD with two stage injection of electrolytic H2 | Raw groundwater in Saitama, Japan | Bacteria from their lab | 29.0 ± 3.1 | 4.2 d | 2 A | 8.1 ± 0.6 | Sand gravel | 1290 | 1.9 | 1.6 | 81.6 ± 4.4 | 36 |
| Attached growth | Raw groundwater treated by on-site dropping nitrification in Jwagal, Nepal | Bacteria from on-site HD reactor | 20.7–28.1 | 12 h | 120 mL/min | 8.3–45.9 | Polyolefin sponge | 20 | 73.7 | 73.7 | 100 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinoda, K.; Maharjan, A.K.; Maharjan, R.; Singhopon, T.; Rujakom, S.; Tsutsumi, Y.; Shakya, B.M.; Kamei, T.; Eamrat, R.; Kazama, F. Hydrogenotrophic Denitrification of Groundwater Using a Simplified Reactor for Drinking Water: A Case Study in the Kathmandu Valley, Nepal. Water 2021, 13, 444. https://doi.org/10.3390/w13040444
Shinoda K, Maharjan AK, Maharjan R, Singhopon T, Rujakom S, Tsutsumi Y, Shakya BM, Kamei T, Eamrat R, Kazama F. Hydrogenotrophic Denitrification of Groundwater Using a Simplified Reactor for Drinking Water: A Case Study in the Kathmandu Valley, Nepal. Water. 2021; 13(4):444. https://doi.org/10.3390/w13040444
Chicago/Turabian StyleShinoda, Kenta, Amit Kumar Maharjan, Rabin Maharjan, Tippawan Singhopon, Suphatchai Rujakom, Yuya Tsutsumi, Bijay Man Shakya, Tatsuru Kamei, Rawintra Eamrat, and Futaba Kazama. 2021. "Hydrogenotrophic Denitrification of Groundwater Using a Simplified Reactor for Drinking Water: A Case Study in the Kathmandu Valley, Nepal" Water 13, no. 4: 444. https://doi.org/10.3390/w13040444
APA StyleShinoda, K., Maharjan, A. K., Maharjan, R., Singhopon, T., Rujakom, S., Tsutsumi, Y., Shakya, B. M., Kamei, T., Eamrat, R., & Kazama, F. (2021). Hydrogenotrophic Denitrification of Groundwater Using a Simplified Reactor for Drinking Water: A Case Study in the Kathmandu Valley, Nepal. Water, 13(4), 444. https://doi.org/10.3390/w13040444







