Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Conductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios
Abstract
:1. Introduction
2. Materials and Methods
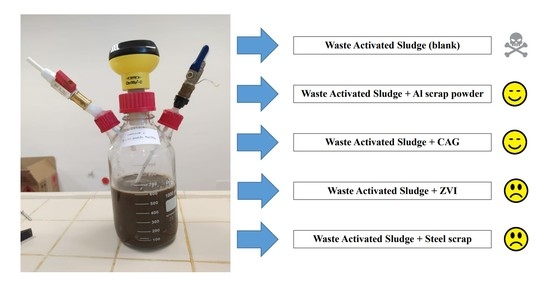
2.1. Substrate, Inoculum, and Additives for Semi-Continuous Test
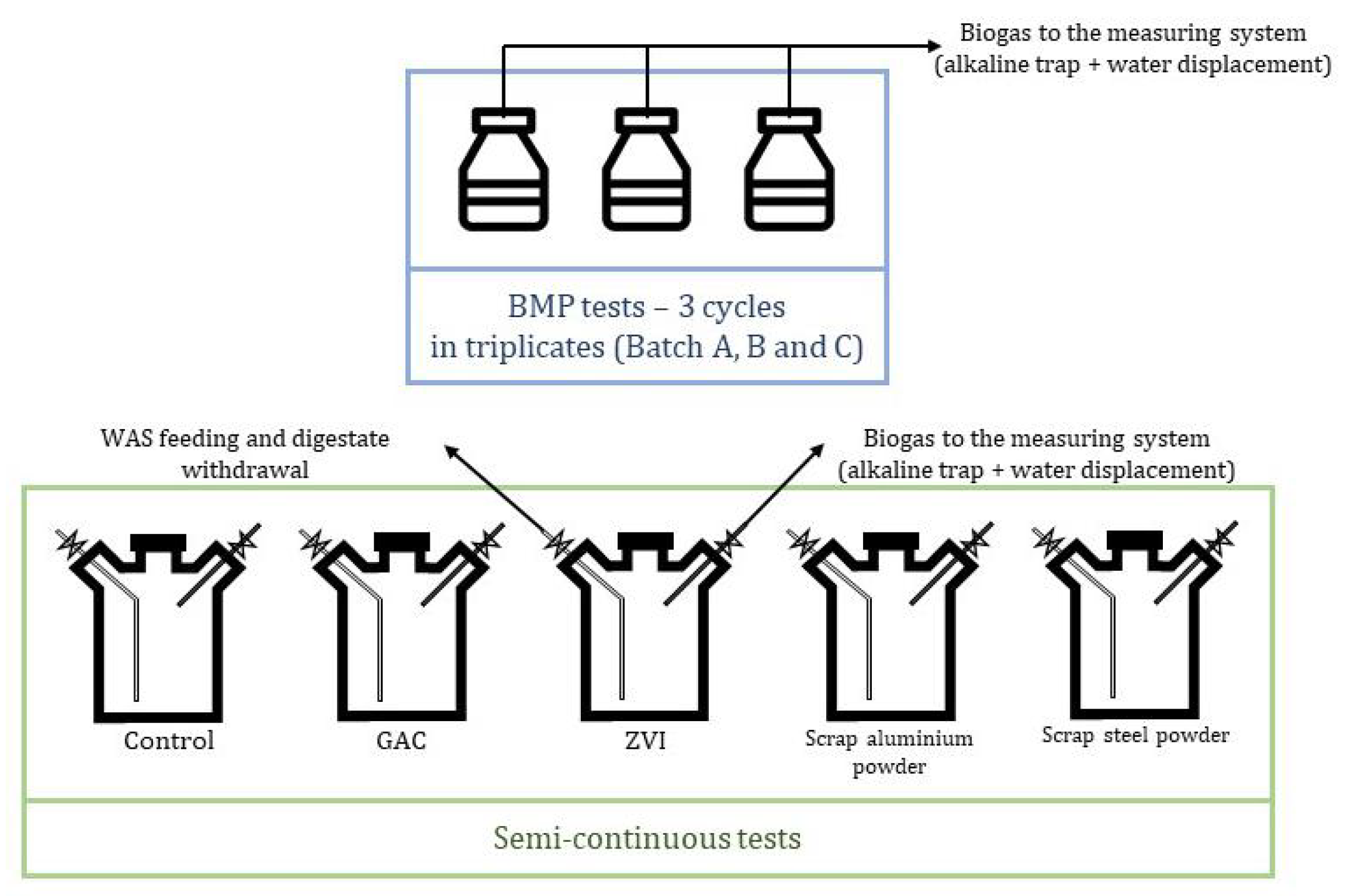
2.2. Batch Test Setup
2.3. Semi-Continuous Test Setup
2.4. Analytical Methods
3. Results
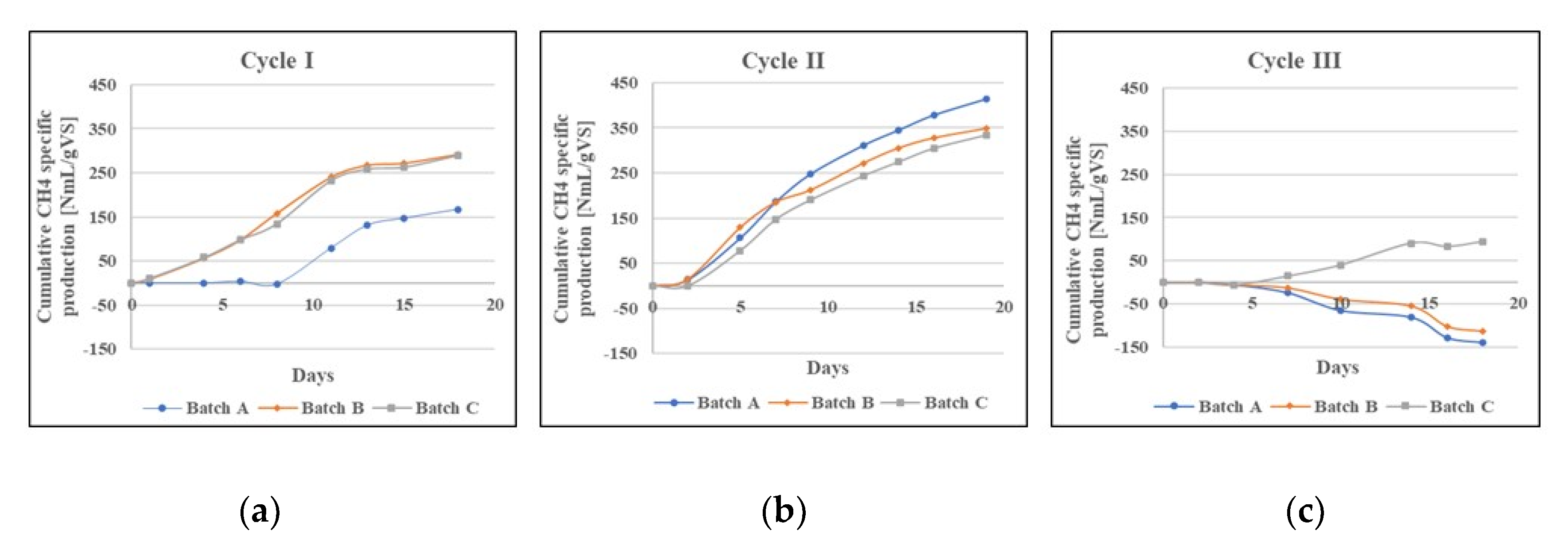
3.1. BMP Batch Tests
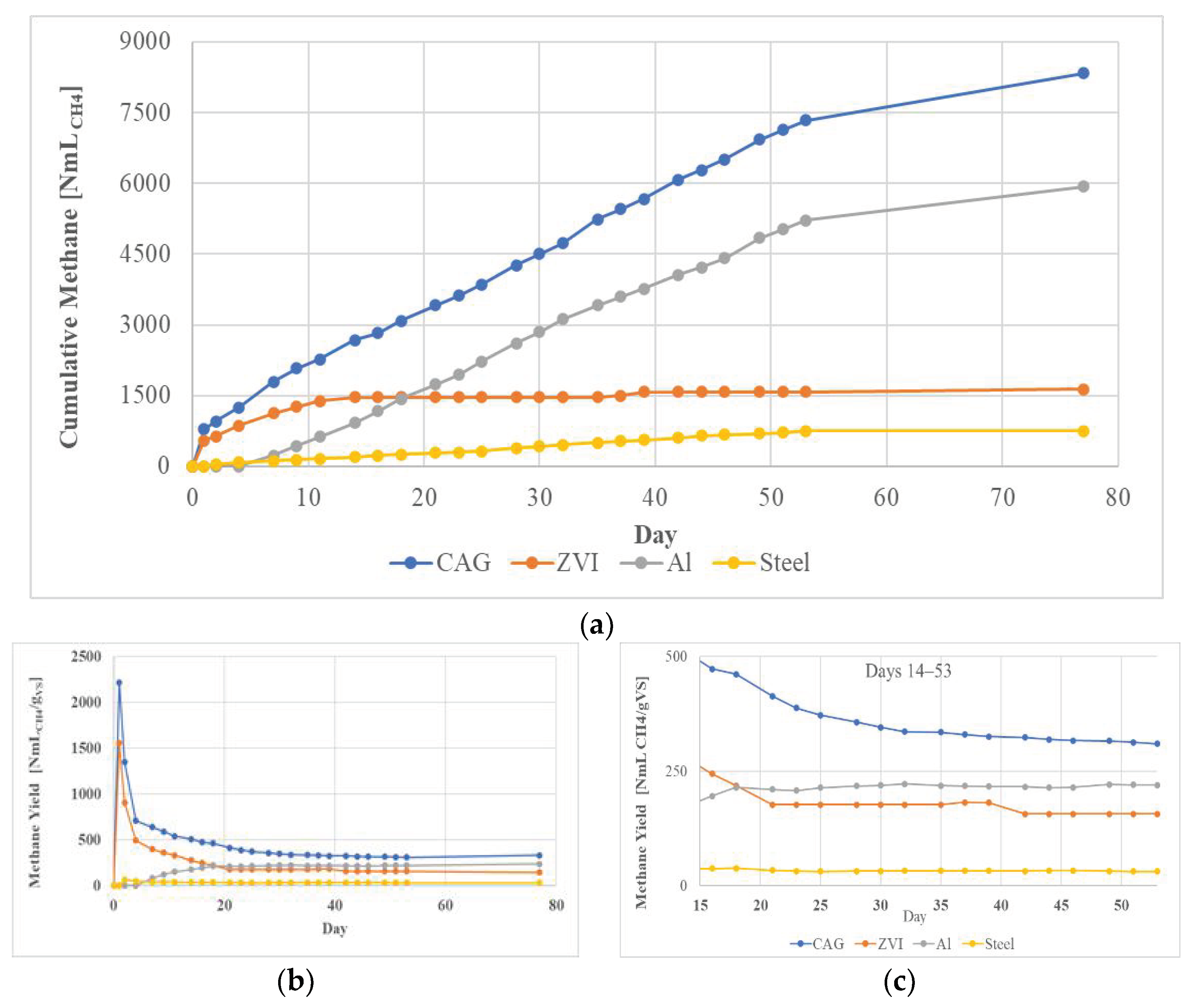
3.2. Semi-Continuous Tests
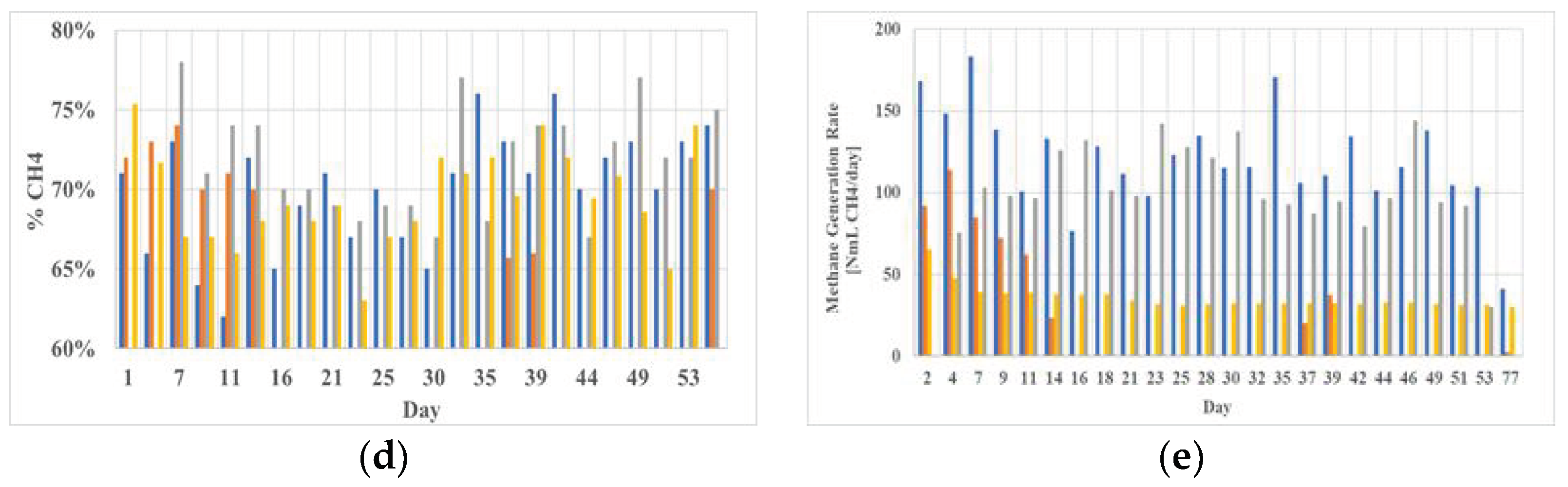
3.2.1. Biomethane Production
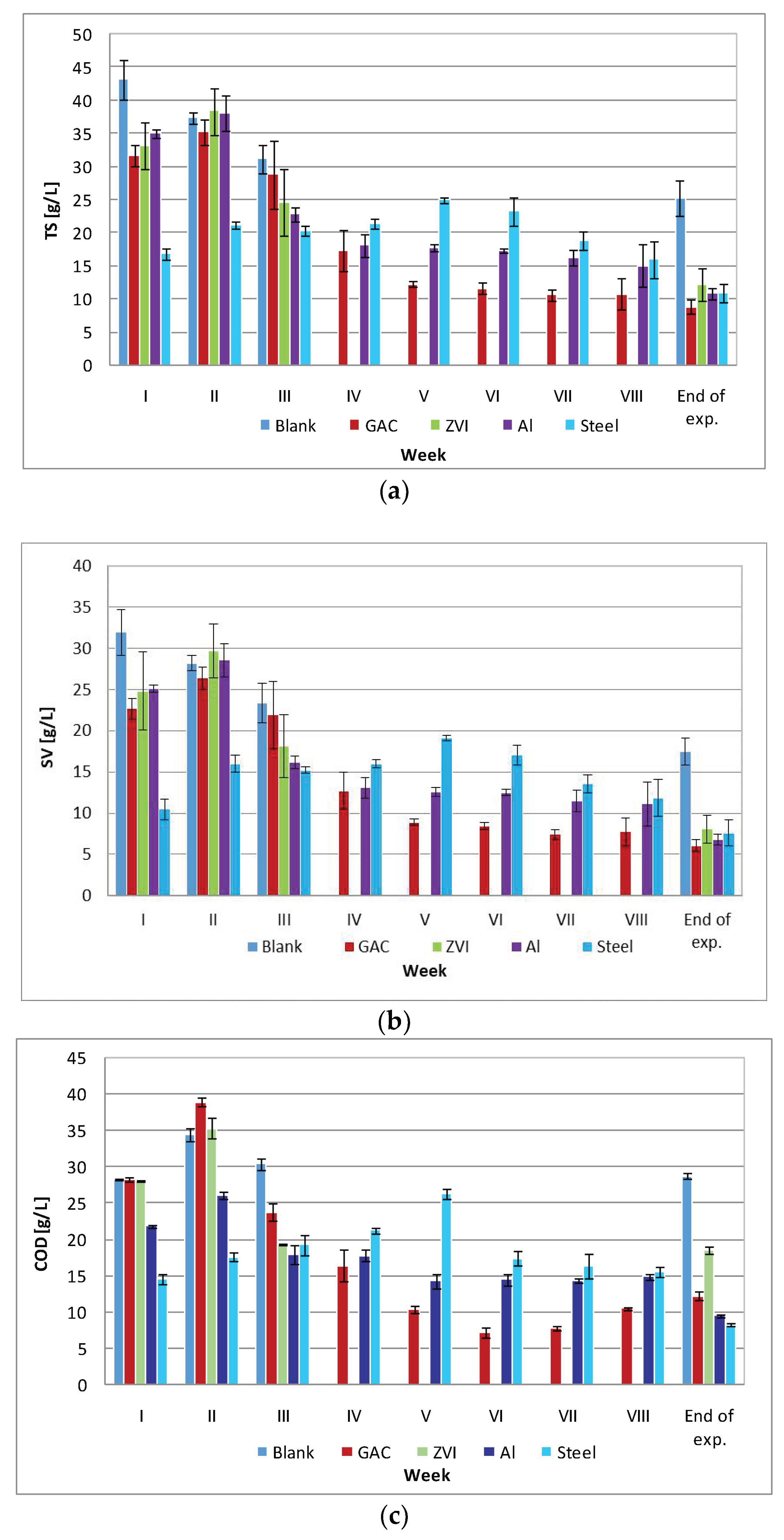
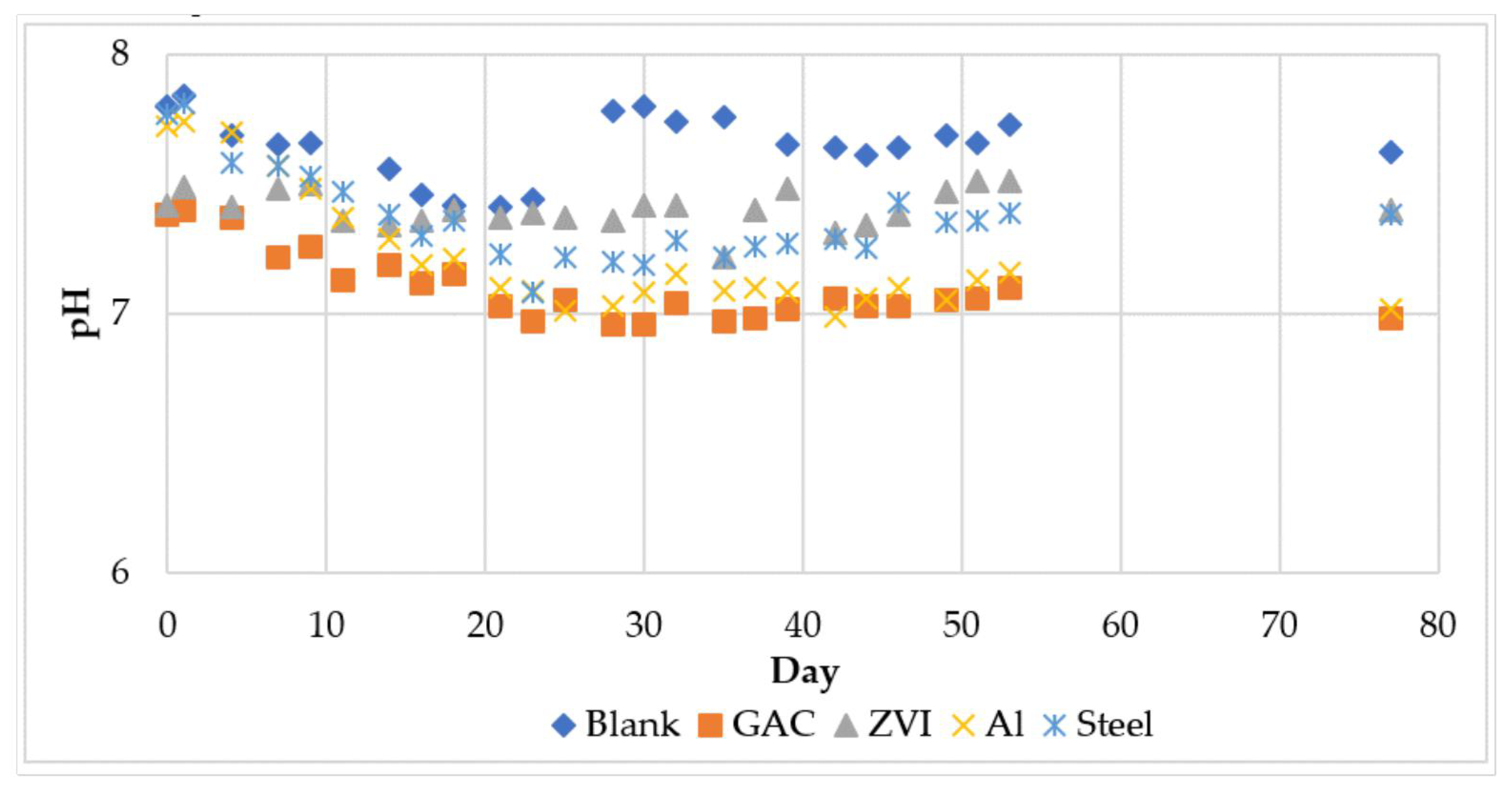
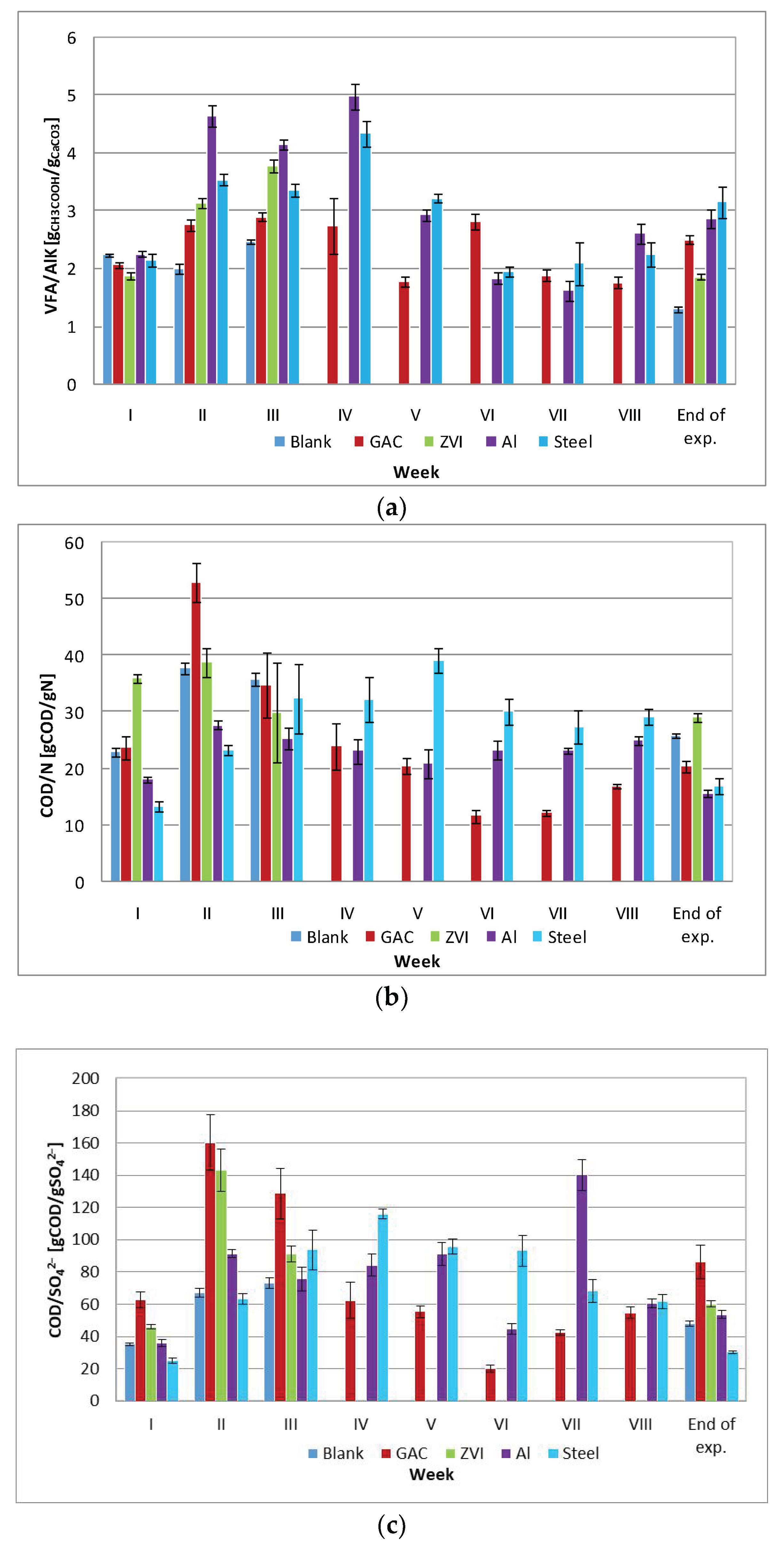
3.2.2. Other Process Parameters
3.2.3. Characterization of the Final Digestates
4. Discussion
4.1. BMP Batch Tests
4.2. Semi-Continuous Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ardern, E.; Lockett, W.T. Experiments on the oxidation of sewage without the aid of filters. J. Soc. Chem. Ind. 1914, 33, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Pariente, M.I.; Segura, Y.; Molina, R.; Martínez, F. Wastewater treatment as a process and a resource. Wastewater Treat. Residues Resour. Biorefinery Prod. Biofuels 2020, 19–45. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering. Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Schmidt, J.E.; Christensen, N.; Batstone, D.J.; Trably, E.; Lyberatos, G.; Stamatelatou, Κ.; Kornaros, M.; Metzger, L.; Amellal, N.; Watson, J.; et al. Safe recycling of sewage sludge on agricultural land—Biowaste. Process. Saf. Environ. Prot. 2006, 84, 253–257. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. Renew. Sustain. Energy Rev. 2019, 114, R713–R715. [Google Scholar] [CrossRef]
- Mannina, G.; Ekama, G.; Caniani, D.; Cosenza, A.; Esposito, G.; Gori, R.; Garrido-Baserba, M.; Rosso, D.; Olsson, G. Greenhouse gases from wastewater treatment—A review of modelling tools. Sci. Total. Environ. 2016, 551–552, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Gori, M.; Lubello, C. European trends in greenhouse gases emissions from integrated solid waste management. Environ. Technol. 2015, 36, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Odnell, A.; Recktenwald, M.; Stensén, K.; Jonsson, B.-H.; Karlsson, M. Activity, life time and effect of hydrolytic enzymes for enhanced biogas production from sludge anaerobic digestion. Water Res. 2016, 103, 462–471. [Google Scholar] [CrossRef]
- Speece, R.E. Anaerobic biotechnology for industrial wastewater treatment. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Cavinato, C.; Bolzonella, D.; Valentino, F.; Fatone, F.; Cecchi, F. Mesophilic and thermophilic anaerobic co-digestion of waste activated sludge and source sorted biowaste in pilot- and full-scale reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Battistoni, P.; Cecchi, F. Mesophilic anaerobic digestion of waste activated sludge: Influence of the solid retention time in the wastewater treatment process. Process. Biochem. 2005, 40, 1453–1460. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.-J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; Wiley: Hoboken, NJ, USA, 2009; ISBN 0813823463. [Google Scholar]
- Abelleira-Pereira, J.M.; Pérez-Elvira, S.; Sánchez-Oneto, J.; de la Cruz, R.; Portela, J.R.; Nebot, E. Enhancement of methane production in mesophilic anaerobic digestion of secondary sewage sludge by advanced thermal hydrolysis pretreatment. Water Res. 2015, 71, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G.F.; Owen, W.F. Fundamentals of anaerobic digestion of wastewater sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Carrere, H.; Dumas, C.; Battimelli, A.; Batstone, D.; Delgenes, J.-P.; Steyer, J.-P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; Limonti, C. Energetic valorization of wet olive mill wastes through a suitable integrated treatment: H2O2 with lime and anaerobic digestion. Sustainability 2016, 8, 1150. [Google Scholar] [CrossRef] [Green Version]
- Ruffino, B.; Campo, G.; Genon, G.; Lorenzi, E.; Novarino, D.; Scibilia, G.; Zanetti, M. Improvement of anaerobic digestion of sewage sludge in a wastewater treatment plant by means of mechanical and thermal pre-treatments: Performance, energy and economical assessment. Bioresour. Technol. 2015, 175, 298–308. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zheng, L.; Wang, Z.; Dai, X. Perspective on enhancing the anaerobic digestion of waste activated sludge. J. Hazard Mater. 2020, 389, 121847. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabro, V. Biofuel production and phosphorus recovery through an integrated treatment of agro-industrial waste. Sustainability 2018, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Calabrò, V. Biogas generation through anaerobic digestion of compost leachate in semi-continuous completely stirred thank reactor. Processes 2019, 7, 635. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, S.; Liang, D.; Li, N. Conductive materials in anaerobic digestion: From mechanism to application. Bioresour. Technol. 2020, 298, 122403. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.-D.; Guiot, S.R. Direct interspecies electron transfer in anaerobic digestion: A Review. Adv. Biochem. Eng. Biotechnol. 2015, 151, 101–115. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Yin, Q.; Liu, Y.; Du, J.; Wu, G. New insights into the effect of direct interspecies electron transfer on syntrophic methanogenesis through thermodynamic analysis. Bioresour. Technol. Rep. 2019, 7, 100225. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between geobacter metallireducens and methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R. Syntrophy Goes electric: Direct interspecies electron transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Paone, E.; Komilis, D. Semi-continuous anaerobic digestion of orange peel waste: Effect of activated carbon addition and alkaline pretreatment on the process. Sustainability 2019, 11, 3386. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Pan, C.; Fu, X.; Lu, W.; Ye, R.; Guo, H.; Wang, H.; Chusov, A. Effects of conductive carbon materials on dry anaerobic digestion of sewage sludge: Process and mechanism. J. Hazard Mater. 2020, 384, 121339. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, L.; Wijaya, S.M.; Zhou, Y. Unveiling the role of activated carbon on hydrolysis process in anaerobic di-gestion. Bioresour. Technol. 2020, 296, 122366. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sustain. Energy Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Lizama, A.C.; Figueiras, C.C.; Pedreguera, A.Z.; Ruiz Espinoza, J.E. Enhancing the performance and stability of the an-aerobic digestion of sewage sludge by zero valent iron nanoparticles dosage. Bioresour. Technol. 2019, 275, 352–359. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Liu, Y.Y.; Zhao, Y. Influence of zero valent scrap iron (ZVSI) supply on methane production from waste activated sludge. Chem. Eng. J. 2015, 263, 461–470. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Yu, Q.; Xu, Z.; Quan, X. Enhanced high-solids anaerobic digestion of waste activated sludge by the addition of scrap iron. Bioresour. Technol. 2014, 159, 297–304. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Y.; Tan, D.; Zhao, Z.; Zhao, H.; Quan, X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour. Technol. 2018, 249, 666–672. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- American Public Health Association; American Water Works Association; Water Environment Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.E., Clesceri, L.S., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Calabrò, P.S.; Paone, E.; Komilis, D. Strategies for the sustainable management of orange peel waste through anaerobic digestion. J. Environ. Manag. 2018, 212, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Panzera, M. Biomethane production tests on ensiled orange peel waste. Int. J. Heat Technol. 2017, 35, S130–S136. [Google Scholar] [CrossRef]
- Schievano, A.; Pognani, M.; D’Imporzano, G.; Adani, F. Predicting anaerobic biogasification potential of ingestates and digestates of a full-scale biogas plant using chemical and biological parameters. Bioresour. Technol. 2008, 99, 8112–8117. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Erdirencelebi, D.; Berktay, A. Effect of COD/SO ratio on anaerobic treatment of landfill leachate during the start-up period. Environ. Technol. 2011, 33, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid–liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef]
- Ruffino, B.; Fiore, S.; Roati, C.; Campo, G.; Novarino, D.; Zanetti, M. Scale effect of anaerobic digestion tests in fed-batch and semi-continuous mode for the technical and economic feasibility of a full scale digester. Bioresour. Technol. 2015, 182, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.D.; Allen, E.; Murphy, J.D. Assessing the variability in biomethane production from the organic fraction of municipal solid waste in batch and continuous operation. Appl. Energy 2014, 128, 307–314. [Google Scholar] [CrossRef]
- Moeller, L.; Zehnsdorf, A.; Müller, R.A. Effect of triticale milling coarseness on biogas production. Chem. Ing. Tech. 2018, 90, 249–255. [Google Scholar] [CrossRef]
- Wang, T.; Qin, Y.; Cao, Y.; Han, B.; Ren, J. Simultaneous addition of zero-valent iron and activated carbon on enhanced mesophilic anaerobic digestion of waste-activated sludge. Environ. Sci. Pollut. Res. 2017, 24, 22371–22381. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Scibetta, S.; Sidari, R. Improvement of semi-continuous anaerobic digestion of pre-treated orange peel waste by the combined use of zero valent iron and granular activated carbon. Biomass Bioenergy 2019, 129, 105337. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, T.C. Effects of dissolved oxygen on formation of corrosion products and concomitant oxygen and nitrate reduction in zero-valent iron systems with or without aqueous Fe2+. Water Res. 2005, 39, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A. Removal of Cr(VI) from water using a new reactive material: Magnesium oxide supported nanoscale Zero-Valent iron. Materials 2016, 9, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the tolerance to polyphenols of the anaerobic digestion of olive wastewater through microbial adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Tajarudin, H. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Sci. Total. Environ. 2020, 707, 134533. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.F.; Mendes, M.F.A.; Coelho, G.L.V. Thermodynamic study of fatty acids adsorption on different adsorbents. J. Chem. Thermodyn. 2007, 39, 1027–1037. [Google Scholar] [CrossRef]
- López-Velandia, C.; Moreno-Barbosa, J.J.; Sierra-Ramirez, R.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of volatile carboxylic acids on activated carbon synthesized from watermelon shells. Adsorpt. Sci. Technol. 2014, 32, 227–242. [Google Scholar] [CrossRef]
| Thickened WAS–I * | Thickened WAS–II ** | Thickened WAS–III *** | |
|---|---|---|---|
| TS (%) | 1.38 ± 0.16 | 1.39 ± 0.14 | 1.39 ± 0.24 |
| VS (%) | 1.11 ± 0.26 | 1.08 ± 0.12 | 1.10 ± 0.31 |
| pH | 6.60 | 6.50 | 6.70 |
| Conductivity (mS/cm) | 7.30 | 1.50 | 1.43 |
| COD (g/L) | 15.29 ± 0.19 | 15.80 ± 0.64 | 14.91 ± 0.49 |
| VFA (gCH3COOH/L) | 1.27 ± 0.03 | 1.23 ± 0.05 | 1.20 ± 0.02 |
| ALK (gCaCO3/L) | 0.19 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 |
| TN (g/L) | 0.46 ± 0.02 | 0.47 ± 0.02 | 0.46 ± 0.01 |
| N-NH4+ (g/L) | 0.16 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.00 |
| P-PO43− (g/L) | 0.10 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.00 |
| SO42− (g/L) | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 |
| Fe (mg/L) | 49.88 ± 3.75 | 39.81 ± 4.94 | 37.24 ± 3.78 |
| Al (mg/L) | 173.34 ± 4.43 | 145.84 ± 13.91 | 143.57 ± 2.85 |
| Mn (mg/L) | 0.82 ± 0.15 | 0.68 ± 0.19 | 0.61 ± 0.18 |
| Zn (mg/L) | 9.06 ± 0.11 | 8.71 ± 0.24 | 7.60 ± 0.2 |
| Pb (mg/L) | N.D. | N.D. | N.D. |
| Cu (mg/L) | 3.03 ± 0.39 | 2.64 ± 0.2 | 2.47 ± 0.26 |
| Cr (mg/L) | N.D. | N.D. | N.D. |
| Ni (mg/L) | N.D. | N.D. | N.D. |
| Fe [mg/L] | Al [mg/L] | Mn [mg/L] | Zn [mg/L] | Pb [mg/L] | Cu [mg/L] | Cr [mg/L] | Ni [mg/L] | |
|---|---|---|---|---|---|---|---|---|
| BlankDigestate | 58.1 ± 8.3 | 147.9 ± 20.6 | 4.6 ± 0.2 | 6.4 ± 0.0 | 0.97 ± 0.07 | 4.26 ± 0.22 | - | 2.20 ± 0.17 |
| Blank Liquid phase | 3.9 ± 0.2 | 11.1 ± 0.4 | 0.10 ± 0.0 | 0.4 ± 0.0 | 0.49 ± 0.05 | 0.18 ± 0.09 | - | 0.25 ± 0.03 |
| GAC Digestate | 55.9 ± 6.9 | 135.5 ± 7.7 | 1.7 ± 0.1 | 7.2 ± 0.1 | 0.68 ± 0.05 | 2.98 ± 0.25 | - | 0.21 ± 0.04 |
| GAC Liquid phase | 6.2 ± 0.7 | 12.3 ± 0.5 | 0.2 ± 0.1 | 0.9 ± 0.1 | 0.30 ± 0.07 | 0.39 ± 0.02 | - | 0.14 ± 0.03 |
| ZVI Digestate | 140.1 ± 19.5 | 115.5 ± 3.3 | 1.8 ± 0.1 | 8.0 ± 0.5 | 0.63 ± 0.04 | 5.44 ± 0.58 | - | 0.18 ± 0.09 |
| ZVI Liquid phase | 19.8 ± 1.8 | 18.9 ± 1.1 | 0.3 ± 0.0 | 1.2 ± 0.0 | 0.28 ± 0.05 | 0.58 ± 0.02 | - | 0.04 ± 0.00 |
| Al Digestate | 43.7 ± 0.6 | 189.5 ± 4.5 | 0.7 ± 0.1 | 7.9 ± 0.2 | 1.10 ± 0.09 | 3.65 ± 0.33 | - | 1.02 ± 0.06 |
| Al Liquid phase | 16.6 ± 4.2 | 67.4 ± 0.5 | 0.3 ± 0.0 | 3.1 ± 0.1 | 0.92 ± 0.04 | 1.36 ± 0.12 | - | 0.31 ± 0.01 |
| Steel Digestate | 62.5 ± 4.5 | 103.2 ± 5.9 | 1.1 ± 0.2 | 6.9 ± 0.1 | 1.41 ± 0.04 | 2.07 ± 0.26 | - | 0.91 ± 0.07 |
| Steel Liquid phase | 27.4 ± 3.5 | 45.0 ± 2.7 | 0.4 ± 0.1 | 2.6 ± 0.1 | 0.57 ± 0.06 | 0.95 ± 0.06 | - | 0.42 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, P.S.; Fazzino, F.; Limonti, C.; Siciliano, A. Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Conductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios. Water 2021, 13, 391. https://doi.org/10.3390/w13040391
Calabrò PS, Fazzino F, Limonti C, Siciliano A. Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Conductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios. Water. 2021; 13(4):391. https://doi.org/10.3390/w13040391
Chicago/Turabian StyleCalabrò, Paolo S., Filippo Fazzino, Carlo Limonti, and Alessio Siciliano. 2021. "Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Conductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios" Water 13, no. 4: 391. https://doi.org/10.3390/w13040391
APA StyleCalabrò, P. S., Fazzino, F., Limonti, C., & Siciliano, A. (2021). Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Conductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios. Water, 13(4), 391. https://doi.org/10.3390/w13040391