Hydrogeochemical Assessment of Groundwater and Suitability Analysis for Domestic and Agricultural Utility in Southern Punjab, Pakistan
Abstract
:1. Introduction
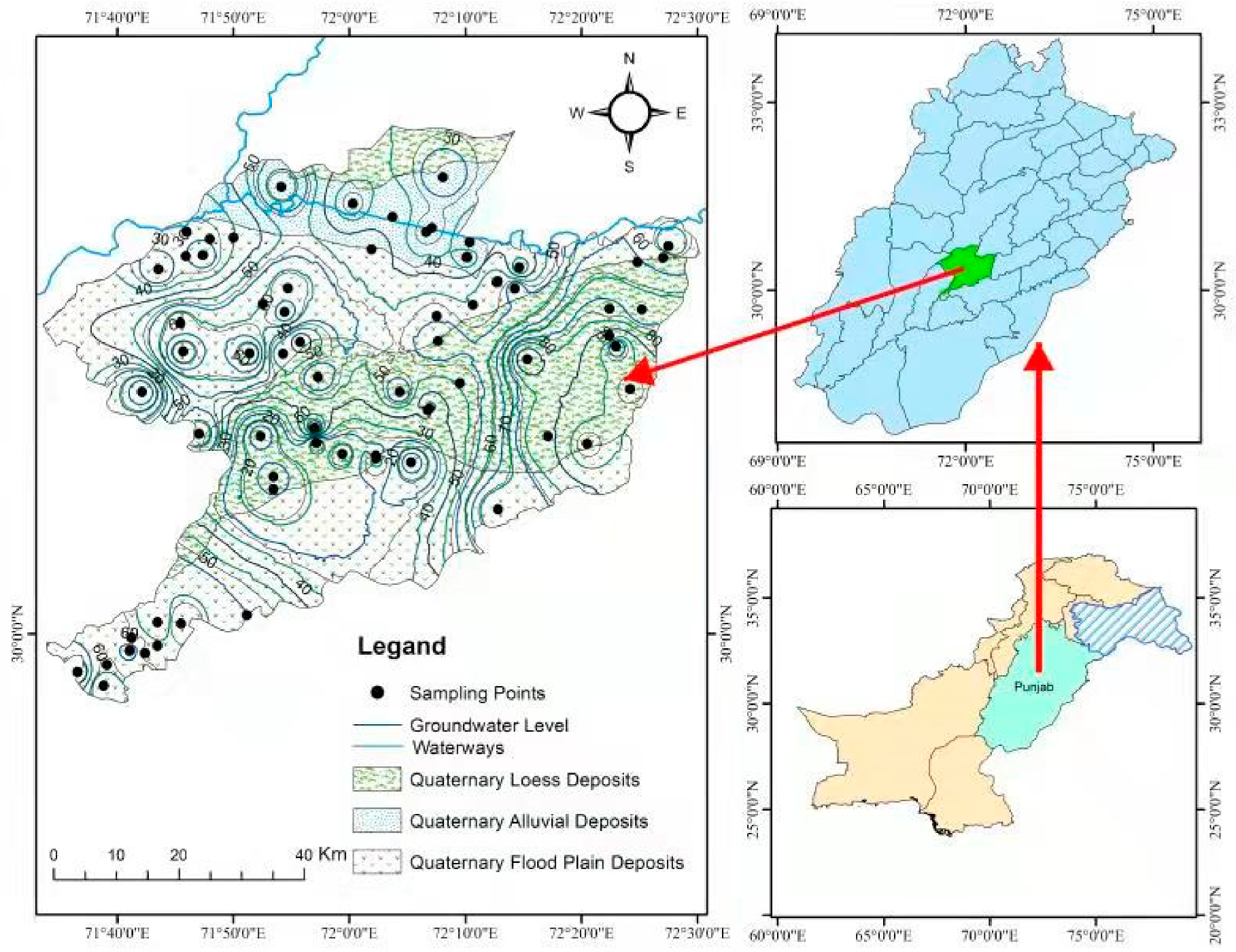
2. Study Area
Geology and Hydrogeology of the Study Area
3. Materials and Methods
3.1. Sampling and Analysis
3.2. Statistical Analysis
3.2.1. Correlation Analysis
3.2.2. Hierarchical Cluster Analysis
3.2.3. Principal Component Analysis
3.3. Hydrochemical Analysis
3.3.1. Geochemical Modeling
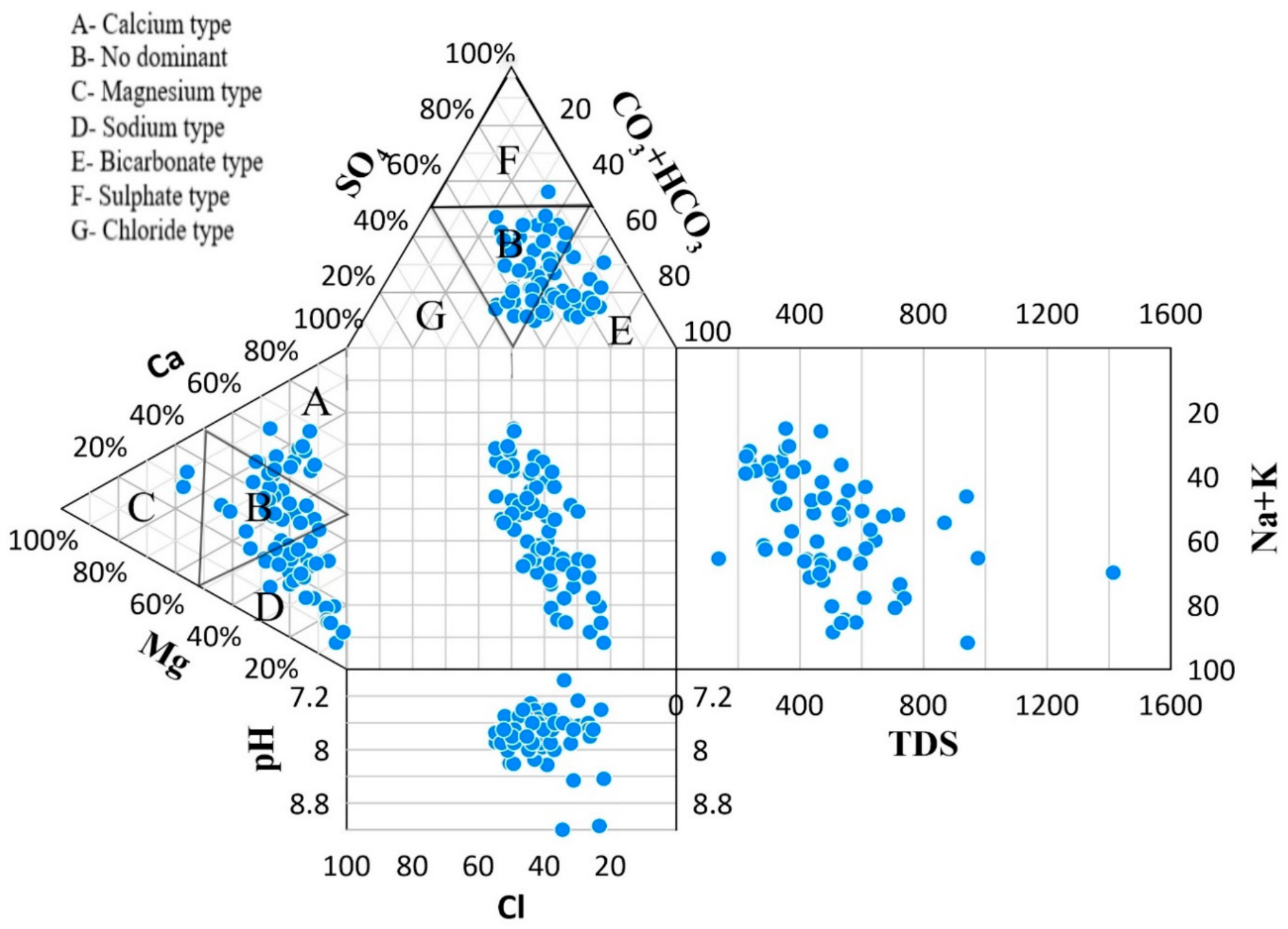
3.3.2. Water Type
3.3.3. Groundwater Classification
3.3.4. Ion Exchange Processes
3.4. Water Quality Analysis for Drinking
Water Quality Index (WQI)
3.5. Groundwater Quality Evaluation for Irrigation
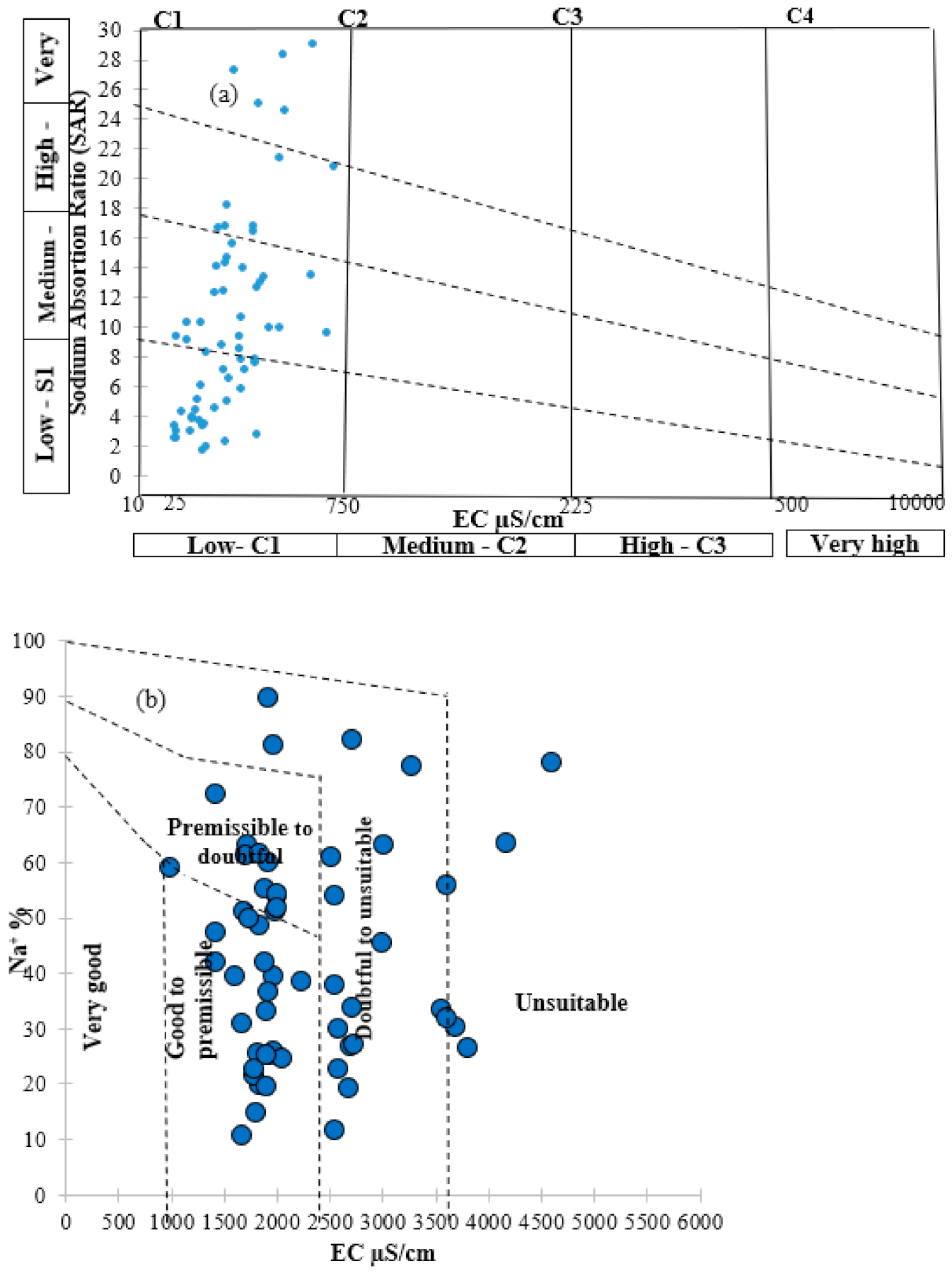
3.5.1. Sodium Adsorption Ratio
3.5.2. Soluble Sodium Percentage (%Na)
3.5.3. Kelly’s Ratio
3.5.4. Magnesium Hazard
3.5.5. Permeability Index
4. Results and Discussion
4.1. Groundwater Quality and Hydrochemical Characteristics
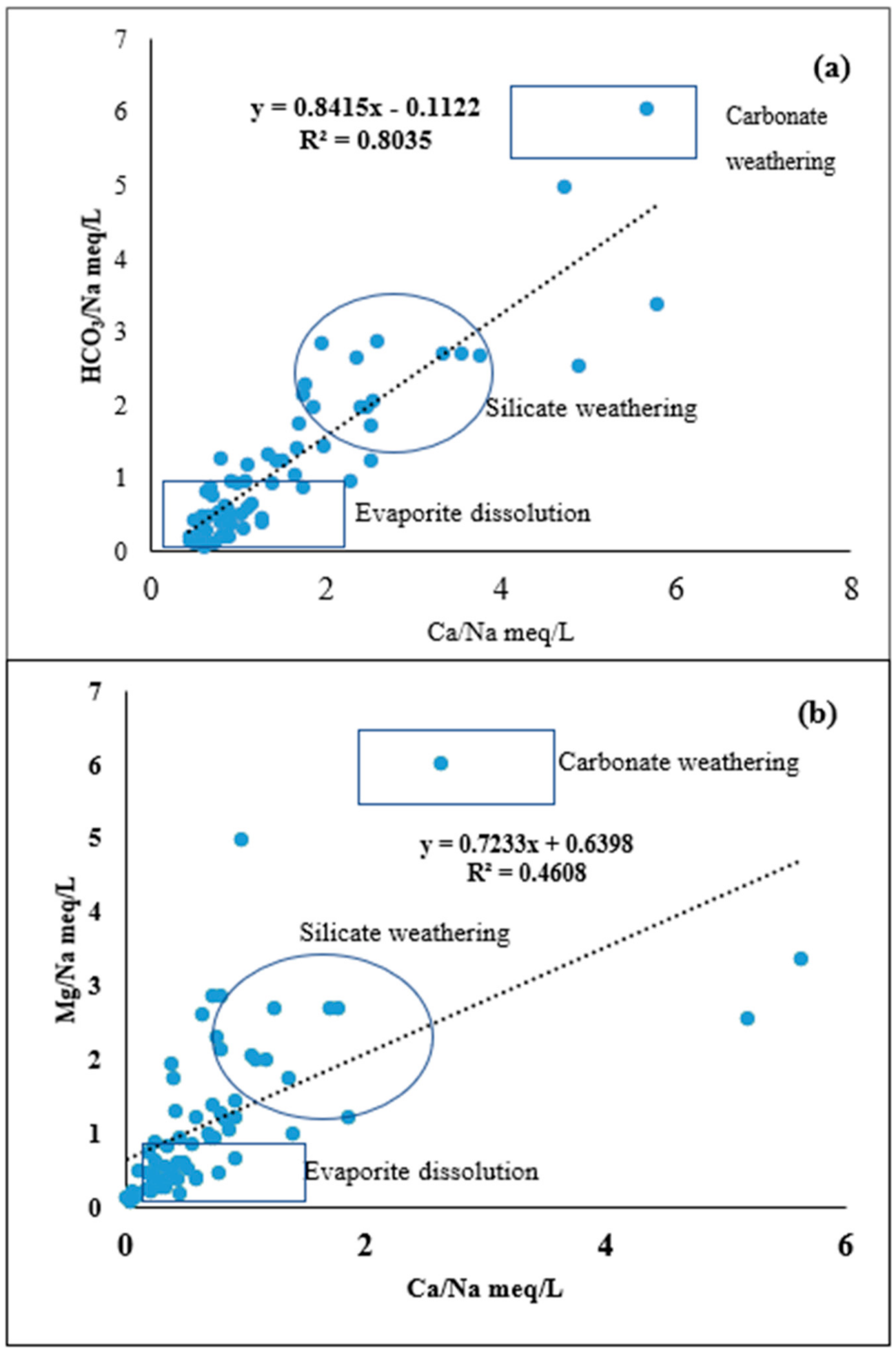
4.2. Silicate Weathering
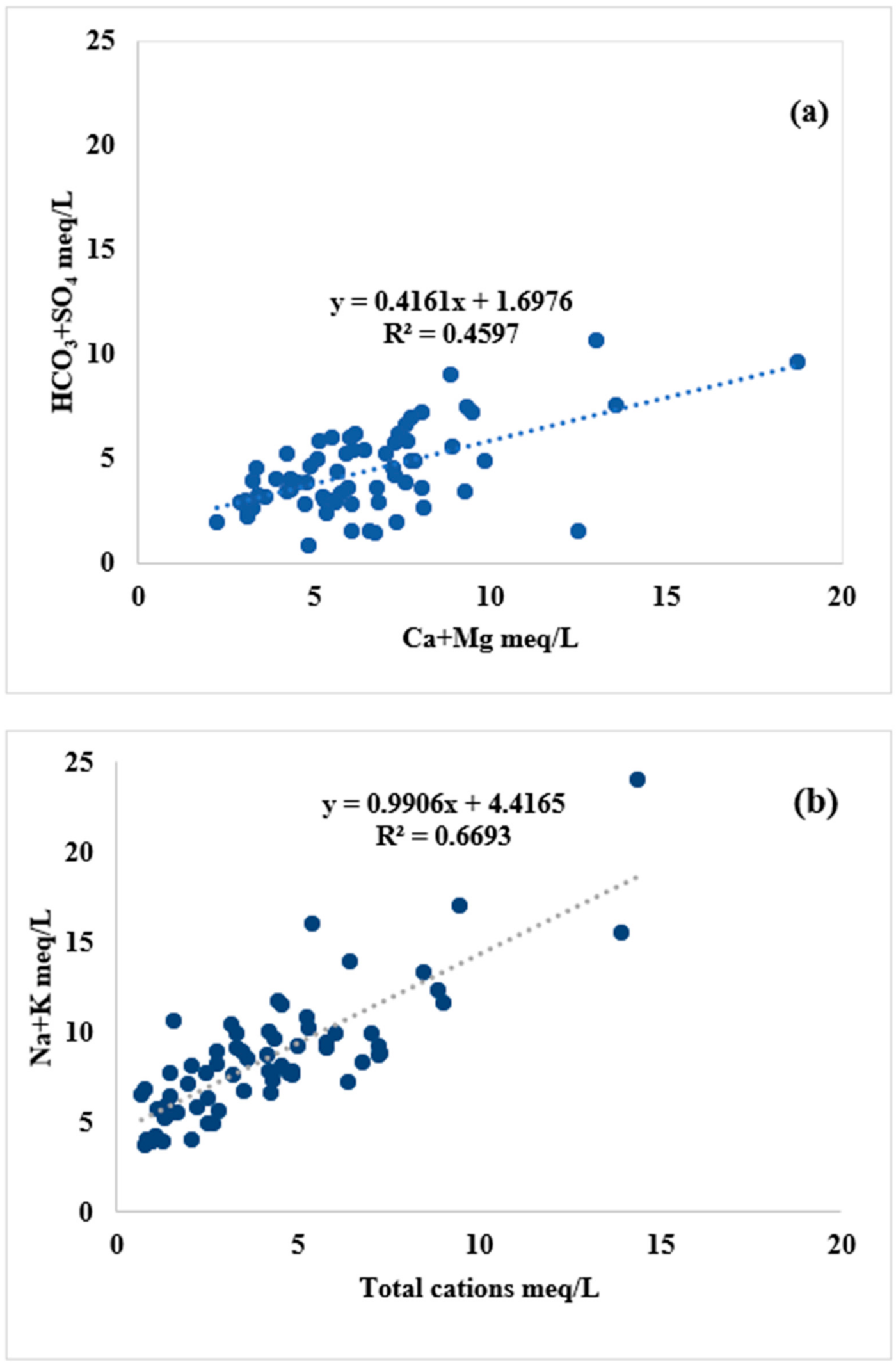
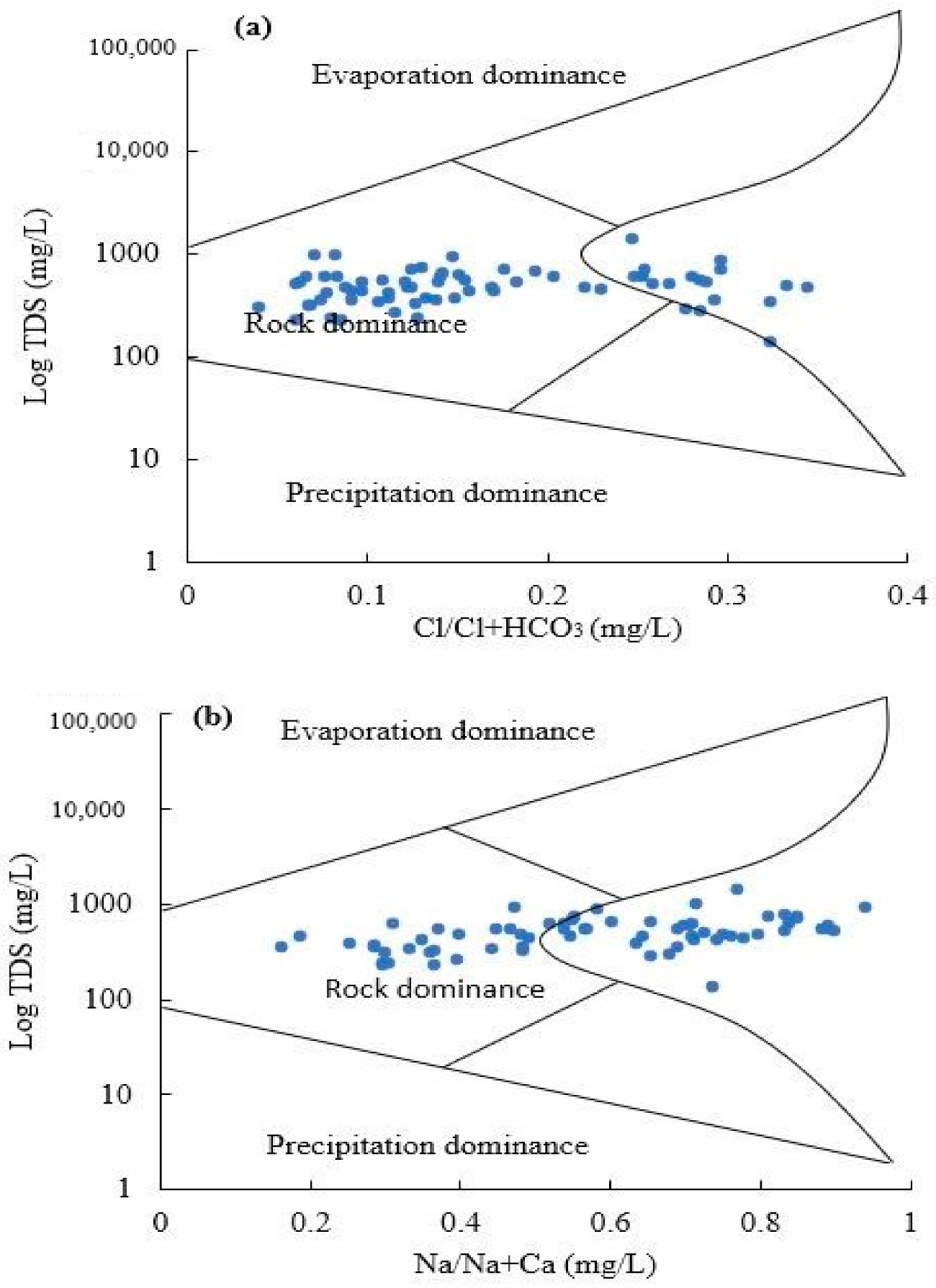
4.3. Hydrogeochemical Evolutional Processes
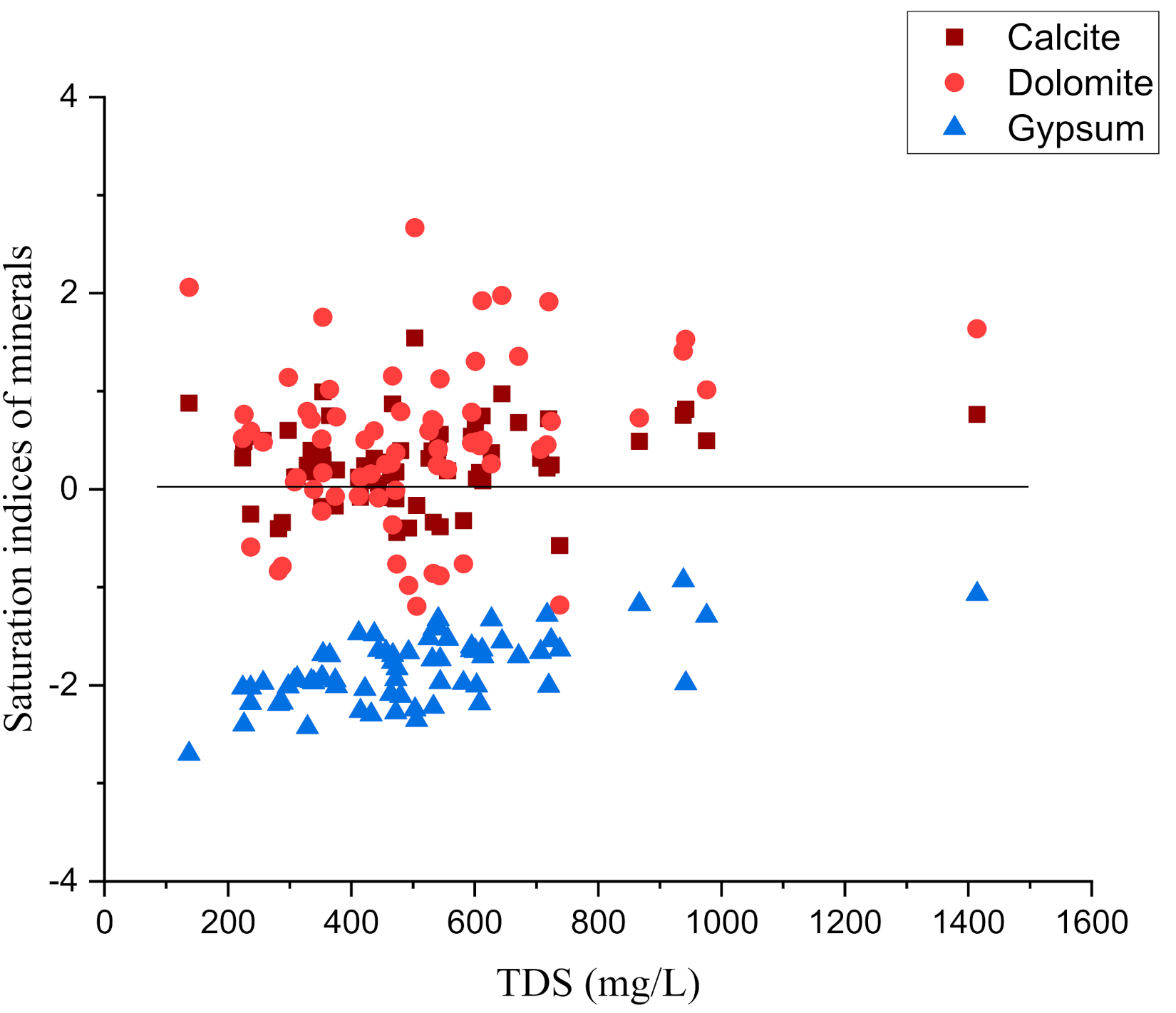
4.4. Mineral Phases
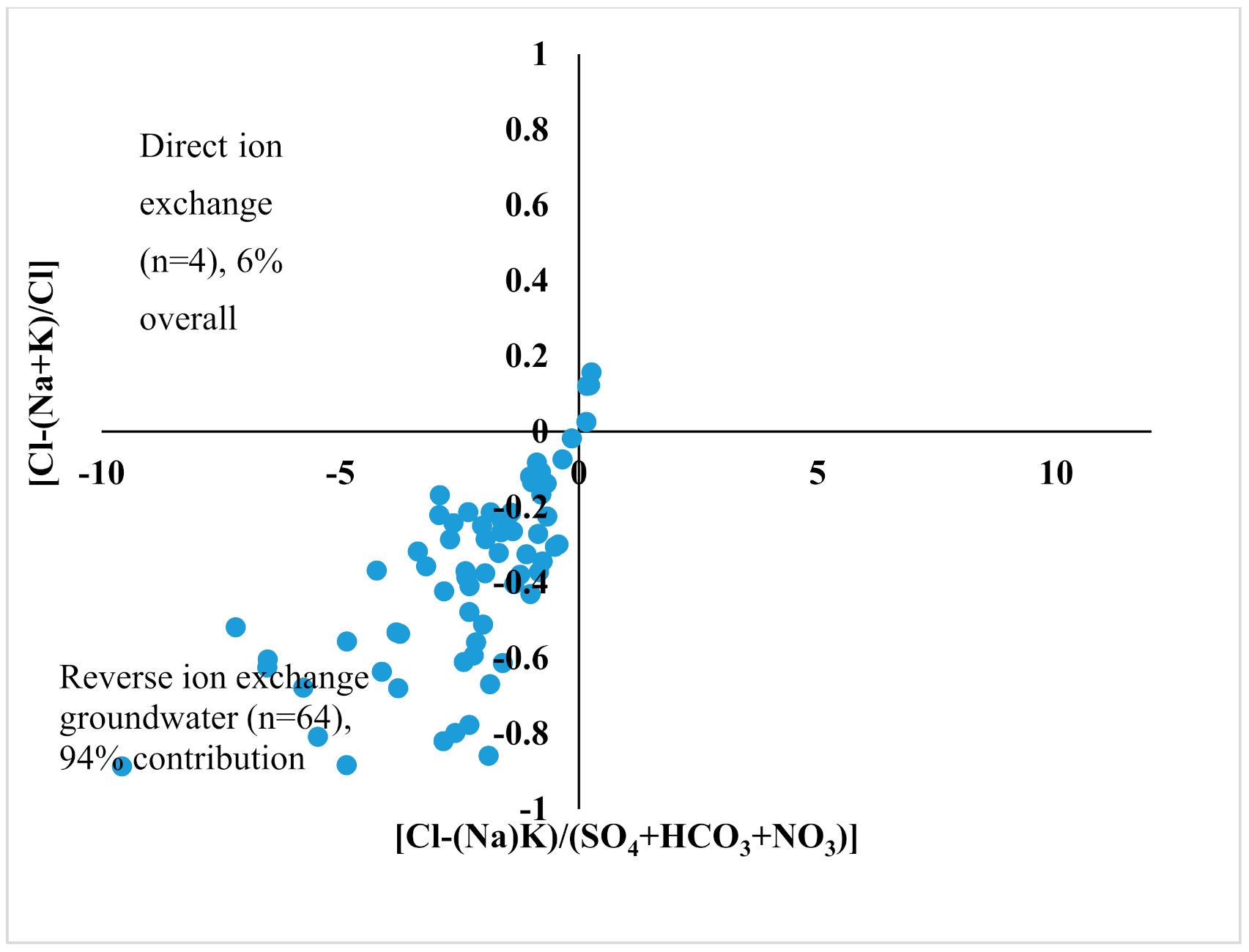
4.5. Ion Exchange Processes
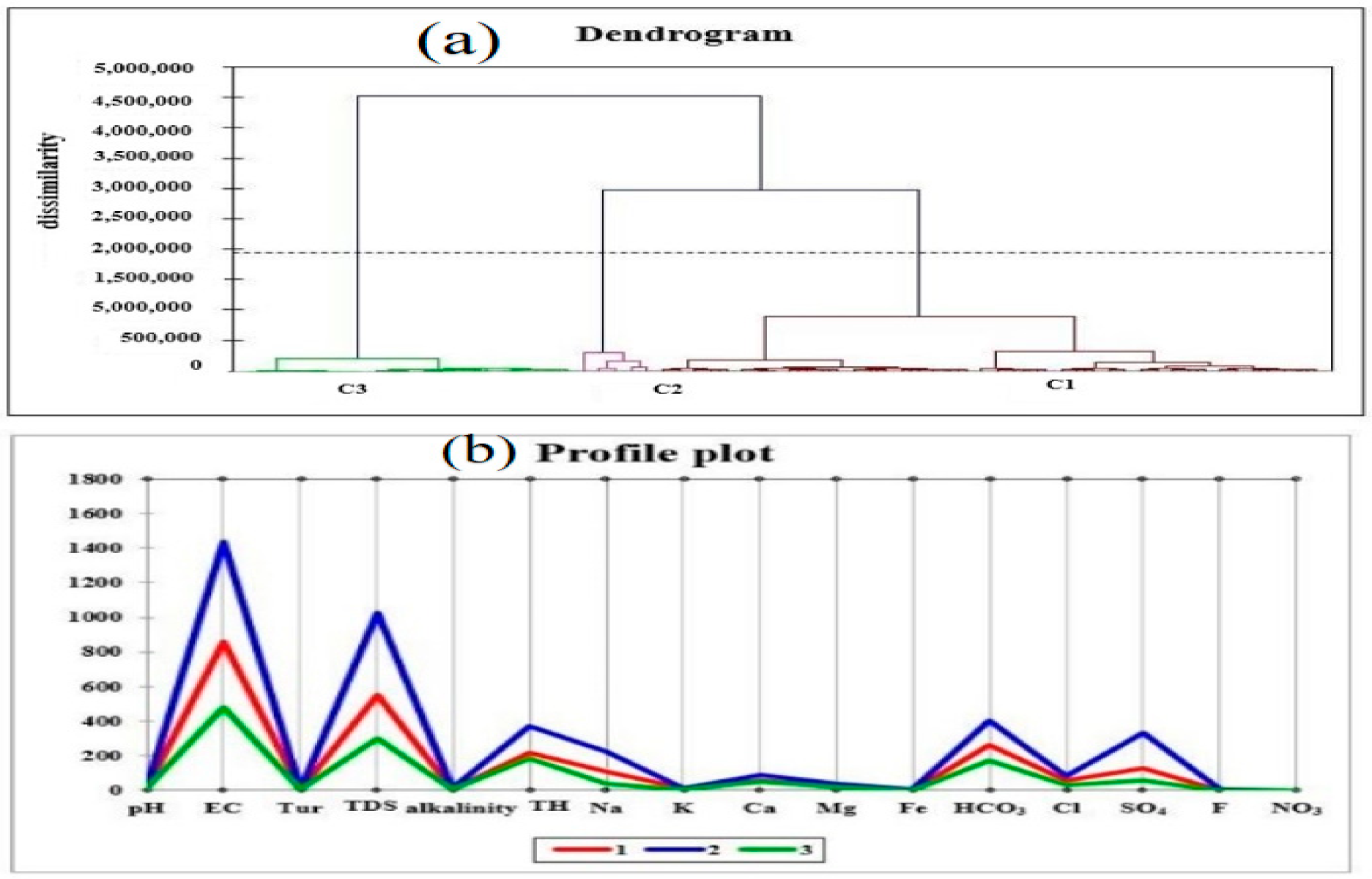
4.6. Cluster Analysis
4.7. Principal Component Analysis
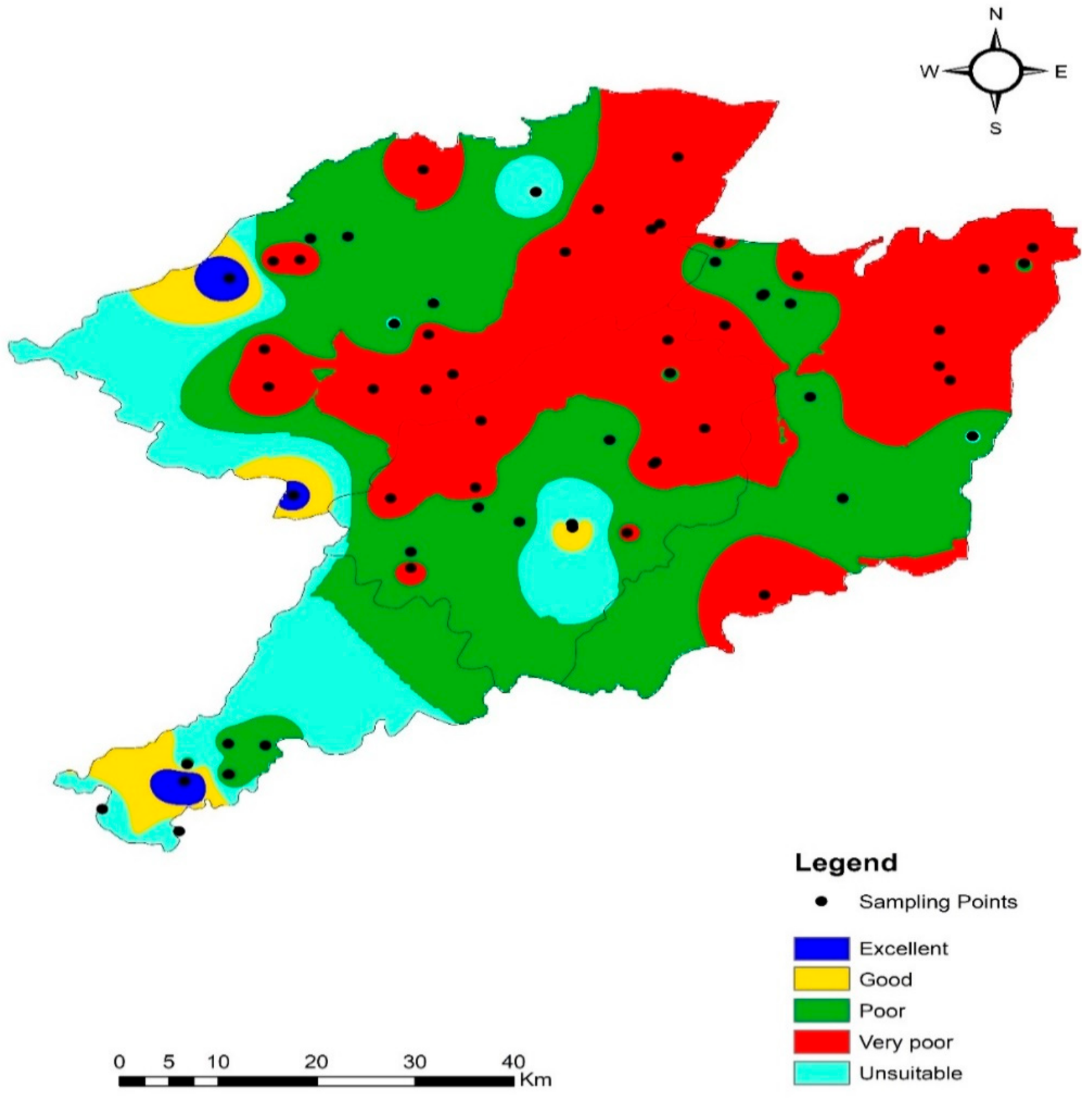
4.8. Groundwater Suitability Evaluation for Drinking Purposes
4.9. Assessment for Irrigation Purposes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrard, N.; Foster, T.; Willetts, J. Groundwater as a Source of Drinking Water in Southeast Asia and the Pacific: A Multi-Country Review of Current Reliance and Resource Concerns. Water 2019, 11, 1605. [Google Scholar] [CrossRef] [Green Version]
- Vincy, M.V.; Brilliant, R.; Pradeepkumar, A.P. Hydrochemical characterization and quality assessment of groundwater for drinking and irrigation purposes: A case study of Meenachil River Basin, Western Ghats, Kerala, India. Environ. Monit. Assess. 2014, 187, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Baloch, M.Y.J.; Talpur, S.A.; Talpur, H.A.; Iqbal, J.; Mangi, S.H.; Memon, S. Effects of Arsenic Toxicity on the Environment and Its Remediation Techniques: A Review. J. Water Environ. Technol. 2020, 18, 275–289. [Google Scholar] [CrossRef]
- Rashid, A.; Farooqi, A.; Gao, X.; Zahir, S.; Noor, S.; Khattak, J.A. Geochemical modeling, source apportionment, health risk exposure and control of higher fluoride in groundwater of sub-district Dargai, Pakistan. Chemosphere 2020, 243, 125409. [Google Scholar] [CrossRef]
- Sakram, G.; Adimalla, N. Hydrogeochemical characterization and assessment of water suitability for drinking and irrigation in crystalline rocks of Mothkur region, Telangana State, South India. Appl. Water Sci. 2018, 8, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Nlend, B.; Celle-Jeanton, H.; Huneau, F.; Garel, E.; Boum-Nkot, S.N.; Etame, J. Shallow urban aquifers under hyper-recharge equatorial conditions and strong anthropogenic constrains. Implications in terms of groundwater resources potential and integrated water resources management strategies. Sci. Total. Environ. 2020, 757, 143887. [Google Scholar] [CrossRef]
- Medici, G.; Baják, P.; West, L.; Chapman, P.; Banwart, S. DOC and nitrate fluxes from farmland; impact on a dolostone aquifer KCZ. J. Hydrol. 2021, 595, 125658. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Chai, J.; Li, S.; Alqurashi, M.; Rehman, G.; Tariq, A.; Talpur, S.A.; Iqbal, J.; Munir, M.; et al. Shallow Groundwater Quality Assessment and Its Suitability Analysis for Drinking and Irrigation Purposes. Water 2021, 13, 3361. [Google Scholar] [CrossRef]
- Hussein, E.E.; Fouad, M.; Gad, M.I. Prediction of the pollutants movements from the polluted industrial zone in 10th of Ramadan city to the Quaternary aquifer. Appl. Water Sci. 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, M.B.; Jonoski, A.; Solomatine, D.; Uhlenbrook, S. A review of seawater intrusion in the Nile Delta groundwater system–the basis for assessing impacts due to climate changes and water resources development. Hydrol. Earth Syst. Sci. Discuss. 2013, 10, 10873–10911. [Google Scholar]
- Shah, S.H.I.A.; Yan, J.; Ullah, I.; Aslam, B.; Tariq, A.; Zhang, L.; Mumtaz, F. Classification of Aquifer Vulnerability by Using the DRASTIC Index and Geo-Electrical Techniques. Water 2021, 13, 2144. [Google Scholar] [CrossRef]
- Akhter, G.; Hasan, M. Determination of aquifer parameters using geoelectrical sounding and pumping test data in Khanewal District, Pakistan. Open Geosci. 2016, 8, 630–638. [Google Scholar] [CrossRef]
- Guo, X.; Zuo, R.; Shan, D.; Cao, Y.; Wang, J.; Teng, Y.; Fu, Q.; Zheng, B. Source apportionment of pollution in groundwater source area using factor analysis and positive matrix factorization methods. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1417–1436. [Google Scholar] [CrossRef]
- Tariq, M.I.; Afzal, S.; Hussain, I. Degradation and persistence of cotton pesticides in sandy loam soils from Punjab, Pakistan. Environ. Res. 2006, 100, 184–196. [Google Scholar] [CrossRef]
- Alam, F. Evaluation of hydrogeochemical parameters of groundwater for suitability of domestic and irrigational purposes: A case study from central Ganga Plain, India. Arab. J. Geosci. 2014, 7, 4121–4131. [Google Scholar] [CrossRef]
- Tariq, S.R.; Shaheen, N.; Khalique, A.; Shah, M.H. Distribution, correlation, and source apportionment of selected metals in tannery effluents, related soils, and groundwater—a case study from Multan, Pakistan. Environ. Monit. Assess. 2010, 166, 303–312. [Google Scholar] [CrossRef]
- Talib, M.A.; Tang, Z.; Shahab, A.; Siddique, J.; Faheem, M.; Fatima, M. Hydrogeochemical Characterization and Suitability Assessment of Groundwater: A Case Study in Central Sindh, Pakistan. Int. J. Environ. Res. Public Health 2019, 16, 886. [Google Scholar] [CrossRef] [Green Version]
- Shahab, A.; Shihua, Q.; Rashid, A.; Hasan, F.U.; Sohail, M.T. Evaluation of Water Quality for Drinking and Agricultural Suitability in the Lower Indus Plain in Sindh Province, Pakistan. Pol. J. Environ. Stud. 2016, 25, 2563–2574. [Google Scholar] [CrossRef]
- Adams, T.E. Water Resources Forecasting Within the Indus River Basin: A Call for Comprehensive Modeling. Indus River Basin 2019, 267–308. [Google Scholar]
- Raza, M.; Hussain, F.; Lee, J.-Y.; Shakoor, M.B.; Kwon, K.D. Groundwater status in Pakistan: A review of contamination, health risks, and potential needs. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1713–1762. [Google Scholar] [CrossRef]
- Ahmad, F. Detection of change in vegetation cover using multi-spectral and multi-temporal information for district Sargodha, Pakistan. Soc. Nat. 2012, 24, 557–571. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcusspecies. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Ashraf, A.; Ahmad, Z.; Akhter, G. Monitoring Groundwater Flow Dynamics and Vulnerability to Climate Change in Chaj Doab, Indus Basin, Through Modeling Approach. In Springer Hydrogeology; Springer: Singapore, 2018; pp. 593–611. [Google Scholar]
- Boateng, T.K.; Opoku, F.; Acquaah, S.O.; Akoto, O. Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environ. Earth Sci. 2016, 75, 489. [Google Scholar] [CrossRef]
- Ferroud, A.; Rafini, S.; Chesnaux, R. Using flow dimension sequences to interpret non-uniform aquifers with constant-rate pumping-tests: A review. J. Hydrol. X 2019, 2, 100003. [Google Scholar] [CrossRef]
- Bozdağ, A. Combining AHP with GIS for assessment of irrigation water quality in Çumra irrigation district (Konya), Central Anatolia, Turkey. Environ. Earth Sci. 2015, 73, 8217–8236. [Google Scholar] [CrossRef]
- Singh, S.; Raju, N.J.; Ramakrishna, C. Evaluation of Groundwater Quality and Its Suitability for Domestic and Irrigation Use in Parts of the Chandauli-Varanasi Region, Uttar Pradesh, India. J. Water Resour. Prot. 2015, 07, 572–587. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Ward, P.; McMullen, L.M.; Yang, X. A case of ‘blown pack’spoilage of vacuum-packaged pork likely associated with Clostridium estertheticum in Canada. Letters in applied microbiology. Lett. Appl. Microbiol. 2020, 70, 13–20. [Google Scholar] [CrossRef]
- Boonkaewwan, S.; Sonthiphand, P.; Chotpantarat, S. Mechanisms of arsenic contamination associated with hydrochemical characteristics in coastal alluvial aquifers using multivariate statistical technique and hydrogeochemical modeling: A case study in Rayong province, eastern Thailand. Environ. Geochem. Health 2021, 43, 537–566. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Javed, A.; Khan, S.; Gao, X.; Li, C.; Ullah, Z.; Sardar, T.; Muhammad, J.; Nazneen, S. Potentially harmful metals, and health risk evaluation in groundwater of Mardan, Pakistan: Application of geostatistical approach and geographic information system. Geosci. Front. 2021, 12, 101128. [Google Scholar] [CrossRef]
- Rojas, J.P.; Arevalo, A.; Foulds, I.G.; Hussain, M.M. Design and characterization of ultra-stretchable monolithic silicon fabric. Appl. Phys. Lett. 2014, 105, 154101. [Google Scholar] [CrossRef] [Green Version]
- Purushotham, D.; Prakash, M.R.; Rao, A.N. Groundwater depletion and quality deterioration due to environmental impacts in Maheshwaram watershed of R.R. district, AP (India). Environ. Earth Sci. 2010, 62, 1707–1721. [Google Scholar] [CrossRef]
- Sujatha, D.; Reddy, B.R. Quality characterization of groundwater in the south-eastern part of the Ranga Reddy district, Andhra Pradesh, India. Environ. Earth Sci. 2003, 44, 579–586. [Google Scholar] [CrossRef]
- Sunkari, E.D.; Abu, M.; Zango, M.S. Geochemical evolution and tracing of groundwater salinization using different ionic ratios, multivariate statistical and geochemical modeling approaches in a typical semi-arid basin. J. Contam. Hydrol. 2021, 236, 103742. [Google Scholar] [CrossRef]
- Brown, G.W.; Birley, J.L.T.; Wing, J.K. Influence of Family Life on the Course of Schizophrenic Disorders: A Replication. Br. J. Psychiatry 1972, 121, 241–258. [Google Scholar] [CrossRef]
- Odukoya, A.M. Geochemical and technology, Geochemical and quality assessment of groundwater in some Nigerian basement complex. Int. J. Environ. Sci. Technol. 2015, 12, 3643–3656. [Google Scholar] [CrossRef] [Green Version]
- Karanth, K. Ground Water Assessment: Development and Management; Tata McGraw-Hill Education: Berkshire, UK, 1987. [Google Scholar]
- Seth, R.; Mohan, M.; Singh, P.; Singh, R.; Dobhal, R.; Singh, K.P.; Gupta, S. Water quality evaluation of Himalayan Rivers of Kumaun region, Uttarakhand, India. Appl. Water Sci. 2014, 6, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Yahiaoui, R.; Tan, S.; Cong, L.; Singh, R.; Yan, F.; Zhang, W. Multispectral terahertz sensing with highly flexible ultrathin metamaterial absorber. J. Appl. Phys. 2015, 118, 083103. [Google Scholar] [CrossRef]
- Brooks, N.; Adger, W.N.; Kelly, P.M. The determinants of vulnerability and adaptive capacity at the national level and the implications for adaptation. Glob. Environ. Change 2005, 15, 151–163. [Google Scholar] [CrossRef]
- Szabolcs, I. The influence of irrigation water of high sodium carbonate content on soils. Agrokémia Talajt. 1964, 13, 237–246. [Google Scholar]
- Tank, D.K.; Chandel, C.S.J.N. Analysis of the major ion constituents in groundwater of Jaipur city. Nat. Sci. 2010, 8, 1–7. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Dahiphale, P.; Kasal, Y.; Madane, D. Groundwater Potential Zones Identification Using Geographical Information System. Eur. J. Mol. Clin. Med. 2020, 7, 2741–2748. [Google Scholar]
- Driessens, F.C.; Verbeeck, R. Biominerals; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Balakrishnan, P. Groundwater quality mapping using geographic information system (GIS): A case study of Gulbarga City, Karnataka, India. Afr. J. Environ. Sci. Technol. 2011, 5, 1069–1084. [Google Scholar] [CrossRef]
- Gu, X.; Xiao, Y.; Yin, S.; Hao, Q.; Liu, H.; Hao, Z.; Meng, G.; Pei, Q.; Yan, H. Hydrogeochemical Characterization and Quality Assessment of Groundwater in a Long-Term Reclaimed Water Irrigation Area, North China Plain. Water 2018, 10, 1209. [Google Scholar] [CrossRef] [Green Version]
- Talpur, S.A.; Noonari, T.M.; Rashid, A.; Ahmed, A.; Baloch, M.Y.J.; Talpur, H.A.; Soomro, M.H. Hydrogeochemical signatures and suitability assessment of groundwater with elevated fluoride in unconfined aquifers Badin district, Sindh, Pakistan. SN Appl. Sci. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304. [Google Scholar]
- Spitz, K.; Moreno, J. A Practical Guide to Groundwater and Solute Transport Modeling; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Jehan, S.; Khan, S.; Khattak, S.A.; Muhammad, S.; Rashid, A.; Muhammad, N. Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur agency, Pakistan. Measurement 2019, 139, 249–257. [Google Scholar] [CrossRef]
- Prasanth, S.V.S.; Magesh, N.S.; Jitheshlal, K.V.; Chandrasekar, N.; Gangadhar, K. Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl. Water Sci. 2012, 2, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Burkart, M.; Stoner, J. Nitrogen in Groundwater Associated with Agricultural Systems. In Nitrogen in the Environment; Elsevier: Amsterdam, The Netherlands, 2008; pp. 177–202. [Google Scholar]
- Fordyce, F.M.; Vrana, K.; Zhovinsky, E.; Povoroznuk, V.; Toth, G.; Hope, B.C.; Iljinsky, U.; Baker, J. A health risk assessment for fluoride in Central Europe. Environ. Geochem. Health 2007, 29, 83–102. [Google Scholar] [CrossRef] [Green Version]
- Rashid, A.; Guan, D.-X.; Farooqi, A.; Khan, S.; Zahir, S.; Jehan, S.; Khattak, S.A.; Khan, M.S.; Khan, R. Fluoride prevalence in groundwater around a fluorite mining area in the flood plain of the River Swat, Pakistan. Sci. Total. Environ. 2018, 635, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Rafique, T.; Bashir, E.; Bhanger, M.I.; Laghari, A.; Usmani, T.H. Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 2010, 78, 1313–1321. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Lin, Y.; Fang, Y.; Qian, H.; Liu, R.; Ma, H. Hydrogeochemical Characteristics and Quality Assessment of Groundwater in an Irrigated Region, Northwest China. Water 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Subramani, T.; Rajmohan, N.; Elango, L. Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ. Monit. Assess. 2009, 162, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Elango, L.; Kannan, R. Rock–water interaction and its control on chemical composition of groundwater. Dev. Environ. Sci. 2007, 5, 229–243. [Google Scholar]
- Richter, D.D.; Markewitz, D.J.B. How deep is soil? BioScience 1995, 45, 600–609. [Google Scholar] [CrossRef]
- Salem, Z.E.; Atwia, M.G.; El-Horiny, M. Hydrogeochemical analysis and evaluation of groundwater in the reclaimed small basin of Abu Mina, Egypt. Hydrogeol. J. 2015, 23, 1781–1797. [Google Scholar] [CrossRef]
- Lawal, B.; Shittu, O.K.; Rotimi, A.A.; Olalekan, I.A.; Kamooru, A.A.; Ossai, P.C. Effect of Methanol Extract of Telfairia occcidentalis on Haematological Parameters in Wister Rats. J. Med. Sci. 2015, 15, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Zhou, Y.; Zeng, Y.; Ji, Y.; Sun, Y.; Lei, M. Hydrogeochemical characteristics and groundwater quality assessment in the plain area of Yarkant River Basin in Xinjiang, P.R. China. Environ. Sci. Pollut. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Thin, P.P.; Hendrayana, H.; Wilopo, W.; Kawasaki, S. Assessment of groundwater facies in Wates Coastal Area, Kulon Progo, Yogyakarta, Indonesia. J. Degrad. Min. Lands Manag. 2018, 5, 1389–1401. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Chen, K.; Jiao, J.J.; Huang, J.; Huang, R. Multivariate statistical evaluation of trace elements in groundwater in a coastal area in Shenzhen, China. Environ. Pollut. 2007, 147, 771–780. [Google Scholar] [CrossRef]
- Helena, B. Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res. 2000, 34, 807–816. [Google Scholar] [CrossRef]
- Avvannavar, S.M.; Shrihari, S. Evaluation of water quality index for drinking purposes for river Netravathi, Mangalore, South India. Environ. Monit. Assess. 2007, 143, 279–290. [Google Scholar] [CrossRef]
- Sahu, P.; Sikdar, P.K. Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ. Earth Sci. 2008, 55, 823–835. [Google Scholar] [CrossRef]
- Bouderbala, A. Assessment of Groundwater Quality and its Suitability for Agricultural Uses in the Nador Plain, North of Algeria. Water Qual. Expo. Health 2015, 7, 445–457. [Google Scholar] [CrossRef]

| Parameters | Minimum | Maximum | Mean | Standard Deviation | WHO Standards | NSBL * | NSBL % |
|---|---|---|---|---|---|---|---|
| Turbidity (NTU) | 0 | 7 | 2.52 | 1.66 | 5 | 3 | 4.41 |
| pH | 6.96 | 9.2 | 7.79 | 0.36 | 6.5–8.5 | 2 | 2.94 |
| EC (μS/cm) | 350 | 1525 | 783.6 | 282.2 | 400 | 63 | 92.6 |
| TDS (mg/L) | 137 | 1414 | 507.28 | 209.24 | 1000 | 1 | 1.47 |
| TH (mg/L) | 35 | 530 | 215.7 | 97.13 | 300 | 10 | 14.7 |
| Na+ (mg/L) | 15 | 324 | 90.87 | 65.04 | 200 | 4 | 5.88 |
| K+ (mg/L) | 0.2 | 34 | 6.42 | 5.07 | 12 | 6 | 8.82 |
| Mg2+ (mg/L) | 1.0 | 73 | 20.78 | 13.53 | 50 | 3 | 4.41 |
| Ca2+ (mg/L) | 16 | 132 | 52.93 | 24.97 | 100 | 4 | 5.88 |
| Fe2+ (mg/L) | 0.1 | 4.16 | 0.54 | 0.67 | 0.3 | 37 | 54.4 |
| F− (mg/L) | 0.01 | 3.95 | 0.54 | 0.52 | 1.5 | 2 | 2.94 |
| Cl− (mg/L) | 9.6 | 167 | 46.78 | 29.25 | 250 | 0 | 0.0 |
| SO42− (mg/L) | 22 | 498 | 117.5 | 86.32 | 250 | 4 | 5.88 |
| HCO3− (mg/L) NO3-N (mg/L) | 100 <0.1 | 510 4.2 | 243.65 0.30 | 90.93 0.55 | 250 10 | 26 0 | 38.23 0.0 |
| Parameter | pH | EC | TDS | Na+ | K+ | Ca2+ | Mg2+ | Fe | HCO3 | Cl− | SO42− | F− | NO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||||
| EC | −0.10 | 1 | |||||||||||
| TDS | −0.11 | 0.95 | 1 | ||||||||||
| Na+ | 0.00 | 0.80 | 0.83 | 1 | |||||||||
| K+ | −0.04 | 0.33 | 0.31 | 0.20 | 1 | ||||||||
| Ca | −0.13 | 0.35 | 0.38 | −0.09 | 0.22 | 1 | |||||||
| Mg | −0.13 | 0.43 | 0.48 | 0.054 | 0.19 | 0.40 | 1 | ||||||
| Fe | 0.14 | 0.28 | 0.45 | 0.33 | 0.11 | 0.30 | 0.31 | 1 | |||||
| HCO3 | −0.01 | 0.72 | 0.74 | 0.62 | 0.40 | 0.26 | 0.46 | 0.39 | 1 | ||||
| Cl | 0.00 | 0.52 | 0.60 | 0.56 | 0.12 | 0.07 | 0.22 | 0.38 | 0.23 | 1 | |||
| SO4 | −0.11 | 0.79 | 0.86 | 0.67 | 0.22 | 0.47 | 0.45 | 0.40 | 0.42 | 0.43 | 1 | ||
| F | −0.01 | 0.53 | 0.49 | 0.58 | 0.06 | 0.01 | 0.02 | 0.08 | 0.51 | 0.07 | 0.36 | 1 | |
| NO3− | −0.10 | −0.01 | −0.02 | 0.05 | −0.10 | −0.14 | −0.06 | −0.04 | −0.08 | 0.25 | −0.09 | −0.10 | 1 |
| Loading Factors | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Depth | 0.11 | 0.16 | −0.39 | −0.35 |
| pH | −0.08 | −0.12 | −0.46 | 0.51 |
| EC | 0.91 | −0.23 | 0.10 | −0.18 |
| Tur | 0.47 | 0.21 | −0.50 | 0.40 |
| TDS | 0.97 | −0.18 | 0.09 | −0.06 |
| TH | 0.66 | 0.69 | 0.19 | −0.02 |
| Na | 0.72 | −0.66 | −0.03 | −0.02 |
| K | 0.38 | 0.06 | −0.18 | −0.15 |
| Ca | 0.50 | 0.66 | 0.10 | −0.06 |
| Mg | 0.60 | 0.53 | 0.19 | −0.02 |
| Fe | 0.56 | 0.14 | −0.20 | 0.63 |
| HCO3 | 0.78 | −0.11 | −0.18 | −0.07 |
| Cl | 0.54 | −0.30 | 0.40 | 0.39 |
| SO4 | 0.84 | −0.01 | 0.09 | −0.11 |
| F | 0.48 | −0.46 | −0.29 | −0.31 |
| NO3 | −0.07 | −0.25 | 0.64 | 0.30 |
| Eigenvalue | 5.86 | 2.20 | 1.48 | 1.37 |
| Variability (%) | 36.60 | 13.75 | 9.24 | 8.54 |
| Cumulative % | 36.60 | 50.35 | 59.59 | 68.13 |
| WQI | Water Type | No. of Samples | % of Samples |
|---|---|---|---|
| (<50) | Excellent | 2 | 2.94 |
| (>50) | Good | 8 | 11.76 |
| (>100) | Poor | 27 | 39.7 |
| (>200) | Very poor | 19 | 27.94 |
| (>300) | Unsuitable for drinking | 12 | 17.64 |
| Indices | Minimum | Maximum | Mean | S.D | Permissible Limit | Unsuitable Samples | % of Suitable Samples |
|---|---|---|---|---|---|---|---|
| PI | 34.78 | 113.5 | 70.29 | 18.13 | >25 | - | 100% |
| MH | 9.44 | 68.96 | 38.43 | 13.47 | 50 | 13 | 80.88% |
| KR | 0.11 | 8.68 | 1.25 | 1.55 | 1 | 29 | 57.35% |
| SAR | 1.07 | 7.99 | 2.81 | 1.13 | 18 | 13 | 80% |
| Na% | 10.64 | 89.78 | 45.66 | 20.71 | 60 | 15 | 77.94% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, J.; Su, C.; Rashid, A.; Yang, N.; Baloch, M.Y.J.; Talpur, S.A.; Ullah, Z.; Rahman, G.; Rahman, N.U.; Earjh; et al. Hydrogeochemical Assessment of Groundwater and Suitability Analysis for Domestic and Agricultural Utility in Southern Punjab, Pakistan. Water 2021, 13, 3589. https://doi.org/10.3390/w13243589
Iqbal J, Su C, Rashid A, Yang N, Baloch MYJ, Talpur SA, Ullah Z, Rahman G, Rahman NU, Earjh, et al. Hydrogeochemical Assessment of Groundwater and Suitability Analysis for Domestic and Agricultural Utility in Southern Punjab, Pakistan. Water. 2021; 13(24):3589. https://doi.org/10.3390/w13243589
Chicago/Turabian StyleIqbal, Javed, Chunli Su, Abdur Rashid, Nan Yang, Muhammad Yousuf Jat Baloch, Shakeel Ahmed Talpur, Zahid Ullah, Gohar Rahman, Naveed Ur Rahman, Earjh, and et al. 2021. "Hydrogeochemical Assessment of Groundwater and Suitability Analysis for Domestic and Agricultural Utility in Southern Punjab, Pakistan" Water 13, no. 24: 3589. https://doi.org/10.3390/w13243589
APA StyleIqbal, J., Su, C., Rashid, A., Yang, N., Baloch, M. Y. J., Talpur, S. A., Ullah, Z., Rahman, G., Rahman, N. U., Earjh, & Sajjad, M. M. (2021). Hydrogeochemical Assessment of Groundwater and Suitability Analysis for Domestic and Agricultural Utility in Southern Punjab, Pakistan. Water, 13(24), 3589. https://doi.org/10.3390/w13243589








