Glucose Addition Enhanced the Advanced Treatment of Coking Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Procedures
2.2. Water Characteristics Measurement
2.3. DNA Extraction and Data Analysis
3. Results and Discussion
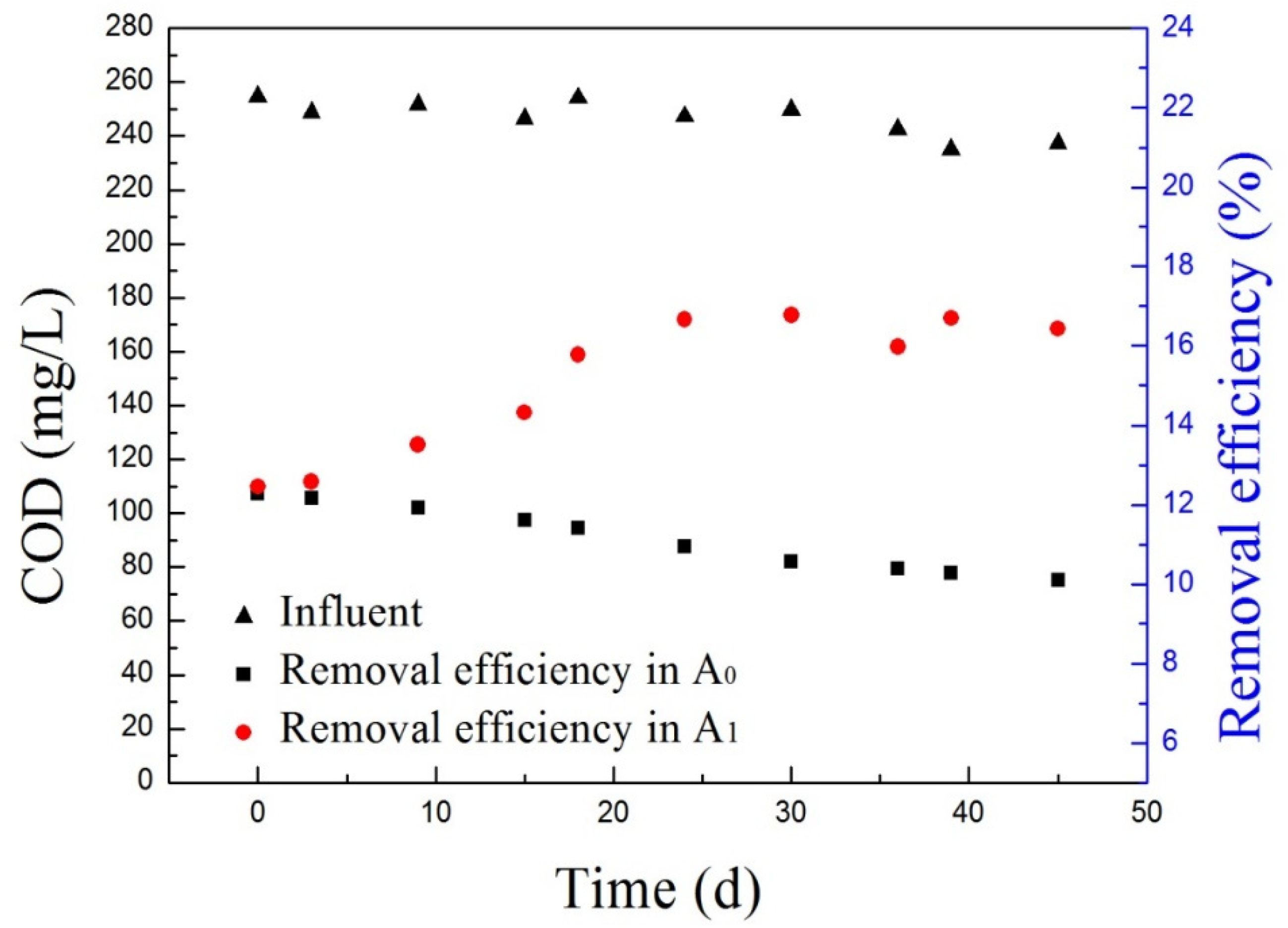
3.1. Influent and Effluent Characteristics during Acclimation
3.2. Changes of Concentration and Components of Organic Matter during Acclimation
3.2.1. UV-Vis Spectra
3.2.2. Characterization of Fluorescing Organic Matter Fractions of CWW
3.2.3. Organic Components Revealed by GC-MS
3.2.4. Molecular Weight Distribution of Organic Matter
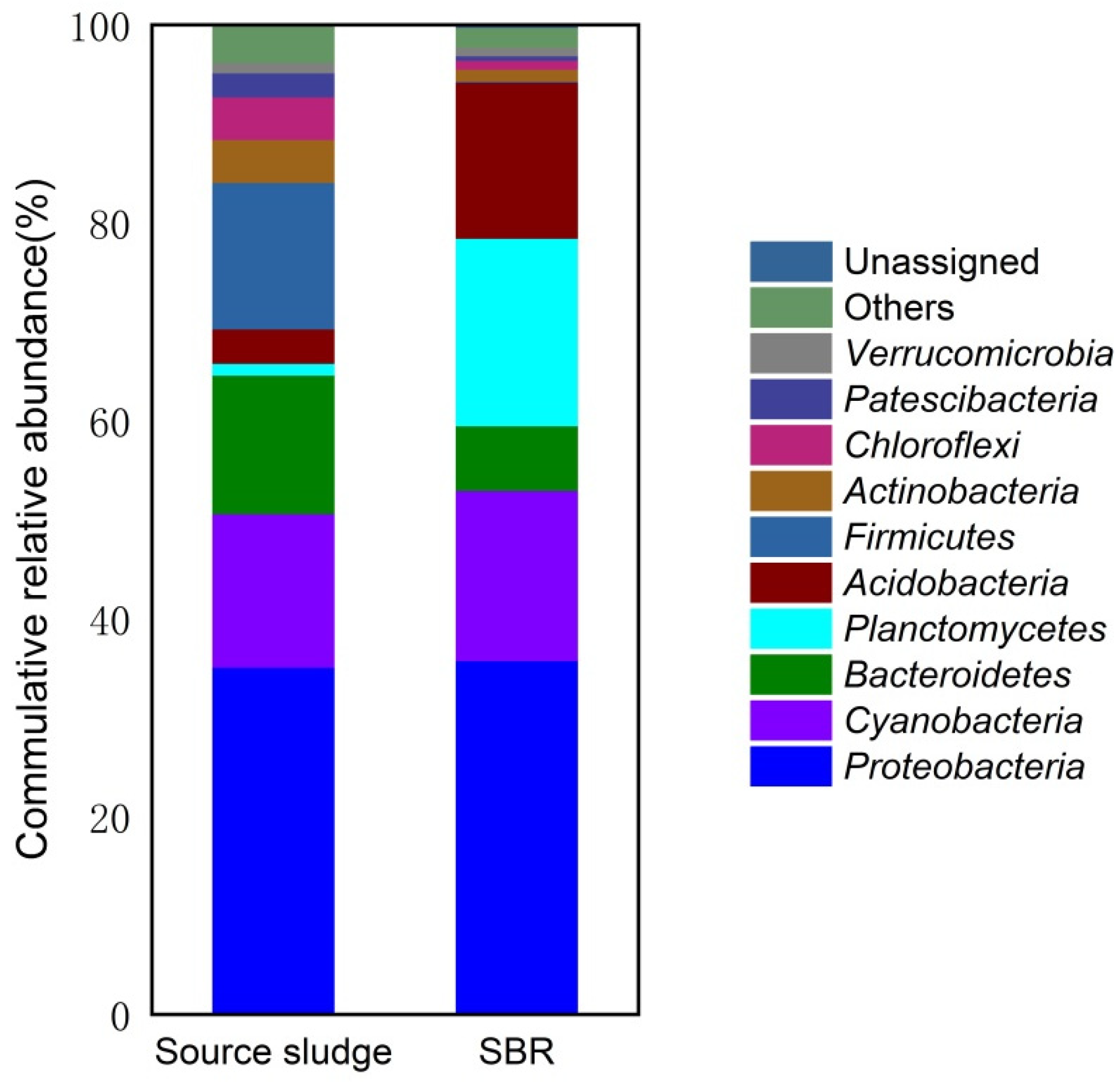
3.3. Microbial Diversity of the Source and Acclimated Sludge
3.4. Microbial Composition of the Source and Acclimated Sludge
3.5. Effects of Glucose Addition on CWW Treatment
3.6. Implications of Bioreactors with the Addition of a Co-Substrate in Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozyonar, F.; Karagozoglu, B. Treatment of pretreated coke wastewater by electrocoagulation and electrochemical peroxidation processes. Sep. Purif. Technol. 2015, 150, 268–277. [Google Scholar] [CrossRef]
- Turan, A.; Keyikoglu, R.; Kobya, M.; Khataee, A. Degradation of thiocyanate by electrochemical oxidation process in coke oven wastewater: Role of operative parameters and mechanistic study. Chemosphere 2020, 255, 127014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, R.; Liu, C.; Chen, L. Bioaugmentation with isolated strains for the removal of toxic and refractory organics from coking wastewater in a membrane bioreactor. Biodegeradation 2015, 26, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ni, J.; Lai, P. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using boron-doped diamond electrodes. Water Res. 2009, 43, 4347–4355. [Google Scholar] [CrossRef]
- Arora, P.K.; Bae, H. Bacterial degradation of chlorophenols and their derivatives. Microb. Cell Fact. 2014, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Beristain-Montiel, L.; Martinez-Hernandez, S.; de Maria Cuervo-Lopez, F.; Ramirez-Vives, F. Dynamics of a microbial community exposed to several concentrations of 2-chlorophenol in an anaerobic sequencing batch reactor. Environ. Technol. 2015, 36, 1776–1784. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Zhao, L.; Zhang, J.; Zeng, G.; Xie, Y.; Zhang, X.; Wang, Y. Removal of Gaseous Elemental Mercury by Cylindrical Activated Coke Loaded with CoOx-CeO2 from Simulated Coal Combustion Flue Gas. Energy Fuels 2015, 29, 6747–6757. [Google Scholar] [CrossRef]
- He, X.; Chai, Z.; Li, F.; Zhang, C.; Li, D.; Li, J.; Hu, J. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using Ti/RuO2-IrO2 electrodes. J. Chem. Technol. Biotechnol. 2013, 88, 1568–1575. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, O.; Lee, Y.Y.; Olvera-Vargas, H.; Deng, F.; Wang, Z.; Lefebvre, O. Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode. Electrochim. Acta 2018, 276, 12–20. [Google Scholar] [CrossRef]
- Restivo, J.; Orfao, J.J.M.; Armenise, S.; Garcia-Bordeje, E.; Pereira, M.F.R. Catalytic ozonation of metolachlor under continuous operation using nanocarbon materials grown on a ceramic monolith. J. Hazard. Mater 2012, 239, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Ma, J.; Xu, L.; Jia, L. Degradation of polycyclic aromatic hydrocarbons by Pseudomonas sp JM2 isolated from active sewage sludge of chemical plant. J. Environ. Sci. 2012, 24, 2141–2148. [Google Scholar] [CrossRef]
- Jexsen, H.L. Carbon Nutrition of Some Microorganisms Decomposing Halogen-substituted Aliphatic Acids. J. Acta Agric. Scand. 1963, 13, 404–412. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Z.; Sun, F.; Liu, H.; Cui, M.; Ye, Y.; Zhang, Y. Acclimating activated sludge with co-metabolic substrates for enhancing treatment of low-concentration polyether wastewater. Biochem. Eng. J. 2020, 159, 107583. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, H. Study of kinetics of degradation of cyclohexane carboxylic acid by acclimated activated sludge. Water Sci. Technol. 2016, 73, 2552–2558. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhang, J.; Yang, J.; Li, J.; Wang, J.; Hu, X. Acclimation of 2-chlorophenol-biodegrading activated sludge and microbial community analysis. Water Environ. Res. 2019, 91, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Miao, Y.; Sun, Y.; Zhang, Q.; Wu, L.; Peng, Y. Enhancing sewage nitrogen removal via anammox and endogenous denitrification: Significance of anaerobic/oxic/anoxic operation mode. Bioresour. Technol. 2019, 289, 121665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, H.; Chen, X.; Wan, D.; Mai, W.; Sun, C. Cometabolic degradation of low-strength coking wastewater and the bacterial community revealed by high-throughput sequencing. Bioresour. Technol. 2017, 245, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard methods for the examination of water and wastewater. Am. J. Public Health Nations Health 2005, 56, 387–388. [Google Scholar]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Knights, D.; Walters, W.A.; Peña, A.G.; Pirrung, M.; Stombaugh, J.; Caporaso, J.G.; Goodrich, J.K.; Bittinger, K.; Lozupone, C.A.; Costello, E.K.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahinkaya, E.; Dilek, F.B. Biodegradation kinetics of 2,4-dichlorophenol by acclimated mixed cultures. J. Biotechnol. 2007, 127, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, G.; Rodriguez-Abalde, A.; Fernandez, B.; Flotats, X.; Bonmati, A. Biomass adaptation over anaerobic co-digestion of sewage sludge and trapped grease waste. Bioresour. Technol. 2011, 102, 6830–6836. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S.; Petalas, A.V.; Mamais, D.; Thomaidis, N.S.; Gatidou, G.; Lekkas, T.D. Investigation of triclosan fate and toxicity in continuous-flow activated sludge systems. Chemosphere 2007, 68, 375–381. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Z.; Song, J.; Lv, L. Spectrum evolution of dissolved aromatic organic matters (DAOMs) during electro-peroxi-coagulation pretreatment of coking wastewater. Sep. Purif. Technol. 2020, 235, 116125. [Google Scholar] [CrossRef]
- Gao, L.; Li, S.; Wang, Y. Effect of different pH coking wastewater on adsorption of coking coal. Water Sci. Technol. 2016, 73, 582–587. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, K.; Puzey, R.; Ren, X. Analysis of Fluorescence and Biodegradability of Wastewater. Water Air Soil Pollut. 2019, 230, 108. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. J. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, J.; Ma, J.; Qiang, Z. Fluorescence spectroscopic characterization of DOM fractions isolated from a filtered river water after ozonation and catalytic ozonation. Chemosphere 2008, 71, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Roccaro, P.; Korshin, G.V.; Greco, V.; Sciuto, S.; Anumol, T.; Snyder, S.A.; Vagliasindi, F.G.A. Use of fluorescence EEM to monitor the removal of emerging contaminants in full scale wastewater treatment plants. J. Hazard. Mater. 2017, 323, 367–376. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Wei, C.; Yang, Q.; Wu, C.; Wu, H.; Hu, Y.; Ren, Y.; Lu, B. Analysis of COD composition in biological effluent: An example from coking wastewater treatment. Environ. Sci. 2012, 10, 1494–1501. [Google Scholar]
- Pitás, V.; Somogyi, V.; Kárpáti, Á.; Thury, P.; Fráter, T. Reduction of chemical oxygen demand in a conventional activated sludge system treating coke oven wastewater. J. Clean. Prod. 2020, 273, 122482. [Google Scholar] [CrossRef]
- Wu, H.; Yin, Z.H.; Quan, Y.; Fang, Y.Y.; Yin, C.R. Removal of methyl acrylate by ceramic-packed biotrickling filter and their response to bacterial community. Bioresour. Technol. 2016, 209, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Yang, Y.; Wu, G.M.; Mao, J.; Zhou, T. Simulation and optimi-zation of a coking wastewater biological treatment process by activated sludge models (ASM). J. Environ. Manag. 2016, 165, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Zhao, Z.Q.; Qin, Z.; Lu, D.; Liang, S.X. Distribution and degradation of organic matter with different molecular weight in an A2/O treatment system. Appl. Water Sci. 2017, 7, 3497–3502. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tu, X.D.; Liu, Y. Characteristics of residual organics MW distribu-tion in the secondary effluent and experimental study of applicable pretreatment for membrane. Environ. Sci. 2016, 29, 75–80. [Google Scholar]
- Xia, Y. Microbial Communities in Municipal Wastewater Treatment Plants of Different Scales as Revealed by Clone Library. Ph.D. Thesis, Tsinghua University, Beijing, China, 2016. [Google Scholar]
- Ivanova, A.A.; Wegner, C.; Kim, Y.; Liesack, W.; Dedysh, S.N. Metatranscriptomics reveals the hydrolytic potential of peat-inhabiting Planctomycetes. Anton. Leeuw. 2018, 111, 801–809. [Google Scholar] [CrossRef]
- Ma, Q.; Qu, Y.; Shen, W.; Zhang, Z.; Wang, J.; Liu, Z.; Li, D.; Li, H.; Zhou, J. Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing. Bioresour. Technol. 2015, 179, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jiang, B.; Huang, F.; Hu, X. Nitrogen removal mechanism and microbial community changes of bioaugmentation subsurface wastewater infiltration system. Bioresour. Technol. 2019, 294, 122140. [Google Scholar] [CrossRef]
- Chu, H.; Liu, X.; Ma, J.; Li, T.; Fan, H.; Zhou, X.; Zhang, Y.; Li, E.; Zhang, X. Two-stage anoxic-oxic (A/O) system for the treatment of coking wastewater: Full-scale performance and microbial community analysis. Chem. Eng. J. 2021, 417, 129204. [Google Scholar] [CrossRef]
- Yu, N.; Yang, J.; Yin, G.; Li, R.; Zou, W.; He, C. Identification and characterization of a novel esterase from Thauera sp. Biotechnol. Appl. Bioc. 2018, 65, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, S.; Zhong, H.; Hua, W.; Chen, R.; Cao, Y.; Zhao, L. A diverse bacterial community in an anoxic quinoline-degrading bioreactor determined by using pyrosequencing and clone library analysis. Appl. Microbiol. Biot. 2011, 91, 425–434. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Chen, X.; Li, Y. Effects of carbon sources on sludge performance and microbial community for 4-chlorophenol wastewater treatment in sequencing batch reactors. Bioresour. Technol. 2018, 255, 22–28. [Google Scholar] [CrossRef]
- Foesel, B.U.; Rohde, M.; Overmann, J. Blastocatella fastidiosa gen. nov., sp nov., isolated from semiarid savanna soil—The first described species of Acidobacteria subdivision 4. Syst. Appl. Microbiol. 2013, 36, 82–89. [Google Scholar] [CrossRef]
- Durruty, I.; Okada, E.; Gonzalez, J.F.; Murialdo, S.E. Degradation of chlorophenol mixtures in a fed-batch system by two soil bacteria. Water SA 2011, 37, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Kaczorek, E.; Smulek, W.; Zdarta, A.; Sawczuk, A.; Zgola-Grzeskowiak, A. Influence of saponins on the biodegradation of halogenated phenols. Ecotoxicology 2016, 131, 127–134. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Li, J.; Wang, Y.; Wang, C.; Wang, P. Experimental and kinetic study on the cometabolic biodegradation of phenol and 4-chlorophenol by psychrotrophic Pseudomonas putida LY1. Environ. Sci. Pollut. Res. 2015, 22, 565–573. [Google Scholar] [CrossRef]
- He, C.; Liu, L.Z.; He, W.L. Advanced treatment of coking wastewater by ozone catalytic oxidation technology. Clean Coal Technol. 2016, 22, 53–58. [Google Scholar]
- He, C.; Wang, J.B.; Wang, C.R.; Zhang, C.H.; Hou, P.; Xu, X.Y. Catalytic ozonation of bio-treated coking wastewater in continuous pilot- and full-scale system: Efficiency, catalyst deactivation and in-situ regeneration. Water Res. 2020, 183, 116090. [Google Scholar] [CrossRef] [PubMed]




| Wastewater | COD (mg/L) | NH4+-N (mg/L) | TOC (mg/L) | UV254 (Abs) |
|---|---|---|---|---|
| Influent50% | 112 ± 3 | 1.3 ± 0.4 | 23.23 ± 2.58 | 0.986 |
| Effluent50% | 97 ± 3 | 1.1 ± 0.2 | 21.82 ± 2.12 | 0.953 |
| Influent100% | 249 ± 4 | 4.3 ± 0.4 | 55.88 ± 2.94 | 2.416 |
| Effluent100% | 222 ± 3 | 3.2 ± 0.3 | 52.53 ± 2.33 | 2.296 |
| Fluorescence Region | Fluorescence Intensity (a.u.) (Peak Position) | |||
|---|---|---|---|---|
| Influent50% | Effluent50% | Influent100% | Effluent100% | |
| Region I | 230/350 (6463) | 230/355 (5979) | 230/345 (**** a) | 235/355 (4227) |
| Region II | 275/305 (2169) | 275/305 (2306) | 275/305 (8397) | 275/305 (1548) |
| Region III | 280/355 (4351) | 280/355 (4168) | 275/345 (**** a) | 280/355 (4918) |
| Region IV | 310/380 (3344) | 310/385 (3491) | 315/370 (6721) | 320/375 (3387) |
| Region V | 250/410 (2771) | 250/410 (2900) | 255/430 (5839) | 255/435 (3487) |
| Type of Organic Matters | Number of the Investigated Organic Matters in Influent | Number of the Investigated Organic Matters in Effluent |
|---|---|---|
| Phenols | 5 | 2 |
| Quinolines | 2 | 1 |
| Furans | 6 | 2 |
| Indoles | 5 | 1 |
| Long chain alkanes | 9 | 16 |
| Benzenes | 8 | 7 |
| Amines | 3 | 2 |
| Halogens | 8 | 6 |
| Pyridines, Piperidines | 3 | 2 |
| Naphthalenes | 6 | 2 |
| Fluorenes | 2 | 1 |
| Indenes | 2 | 0 |
| Alcohols | 1 | 3 |
| Lipids | 4 | 1 |
| Ethers | 5 | 4 |
| Ketones | 1 | 1 |
| Pyrazines, carbazoles | 5 | 2 |
| The Sample Name | Number of Valid Sequence | No. of OTUs | ACE | Chao1 | Simpson | Shannon | Coverage a |
|---|---|---|---|---|---|---|---|
| In SBR | 48376 | 357 | 385 | 397 | 0.08 | 3.40 | 0.999 |
| Source sludge | 34853 | 833 | 835 | 840 | 0.03 | 5.00 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Xia, Y.; He, X.; Li, W.; Yuan, L.; Wu, X.; Qin, Y.; Yuan, R.; Gong, X. Glucose Addition Enhanced the Advanced Treatment of Coking Wastewater. Water 2021, 13, 3365. https://doi.org/10.3390/w13233365
Li N, Xia Y, He X, Li W, Yuan L, Wu X, Qin Y, Yuan R, Gong X. Glucose Addition Enhanced the Advanced Treatment of Coking Wastewater. Water. 2021; 13(23):3365. https://doi.org/10.3390/w13233365
Chicago/Turabian StyleLi, Na, Yu Xia, Xuwen He, Weijia Li, Lianhua Yuan, Xu Wu, Yihe Qin, Run Yuan, and Xun Gong. 2021. "Glucose Addition Enhanced the Advanced Treatment of Coking Wastewater" Water 13, no. 23: 3365. https://doi.org/10.3390/w13233365






