Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels)
Abstract
:1. Introduction
2. Material and Methods
2.1. Pollutant
2.2. Biosorbent
3. Characterization of Adsorbent
3.1. FT-IR Spectroscopy
3.1.1. FT-IR Analysis of TiO2 NPs
3.1.2. FT-IR Analysis of Biochar
3.1.3. FT-IR Analysis of TiO2 NCs
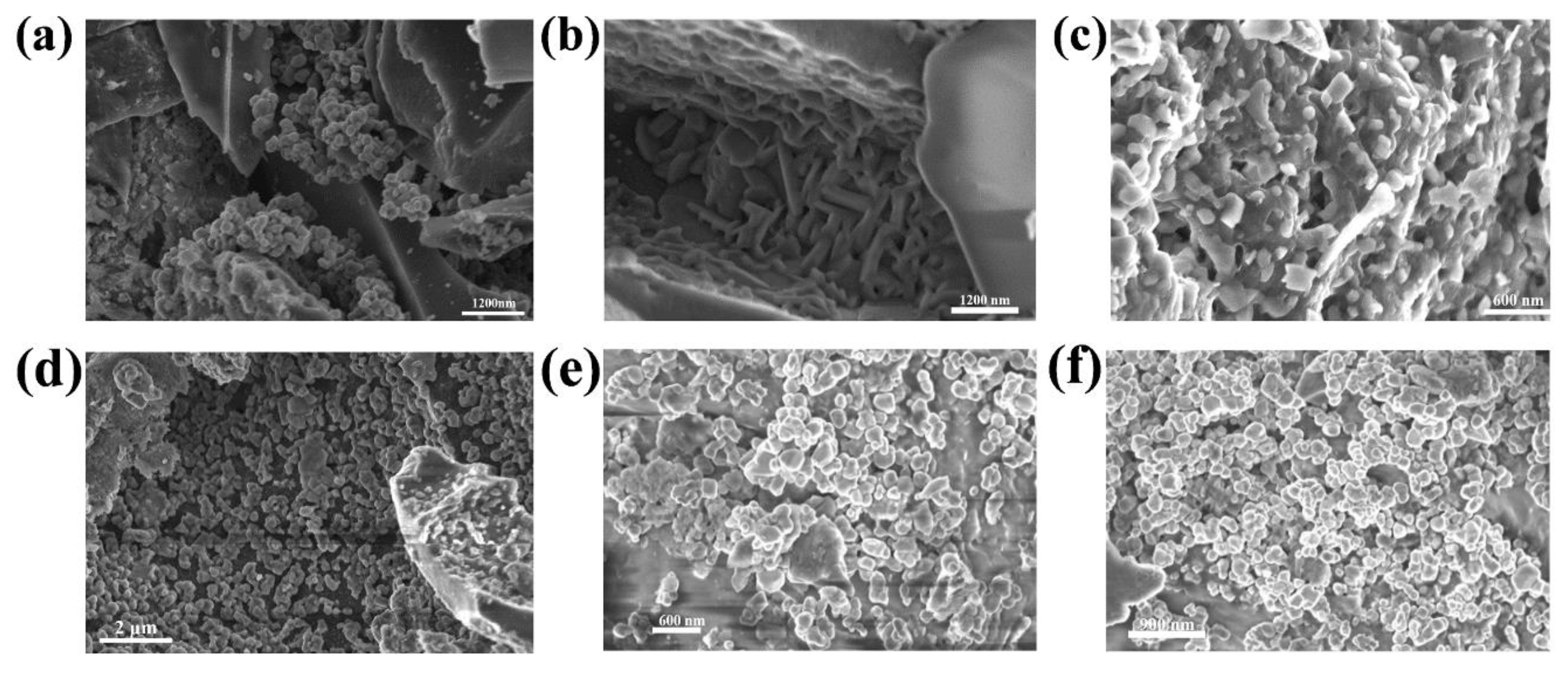
3.2. Scanning Electron Microscopy (SEM) Analysis
3.3. XRD Analysis of TiO2 NP
3.4. UV Analysis of TiO2 Nanoparticles (TiO2 NP)
4. Results and Discussion
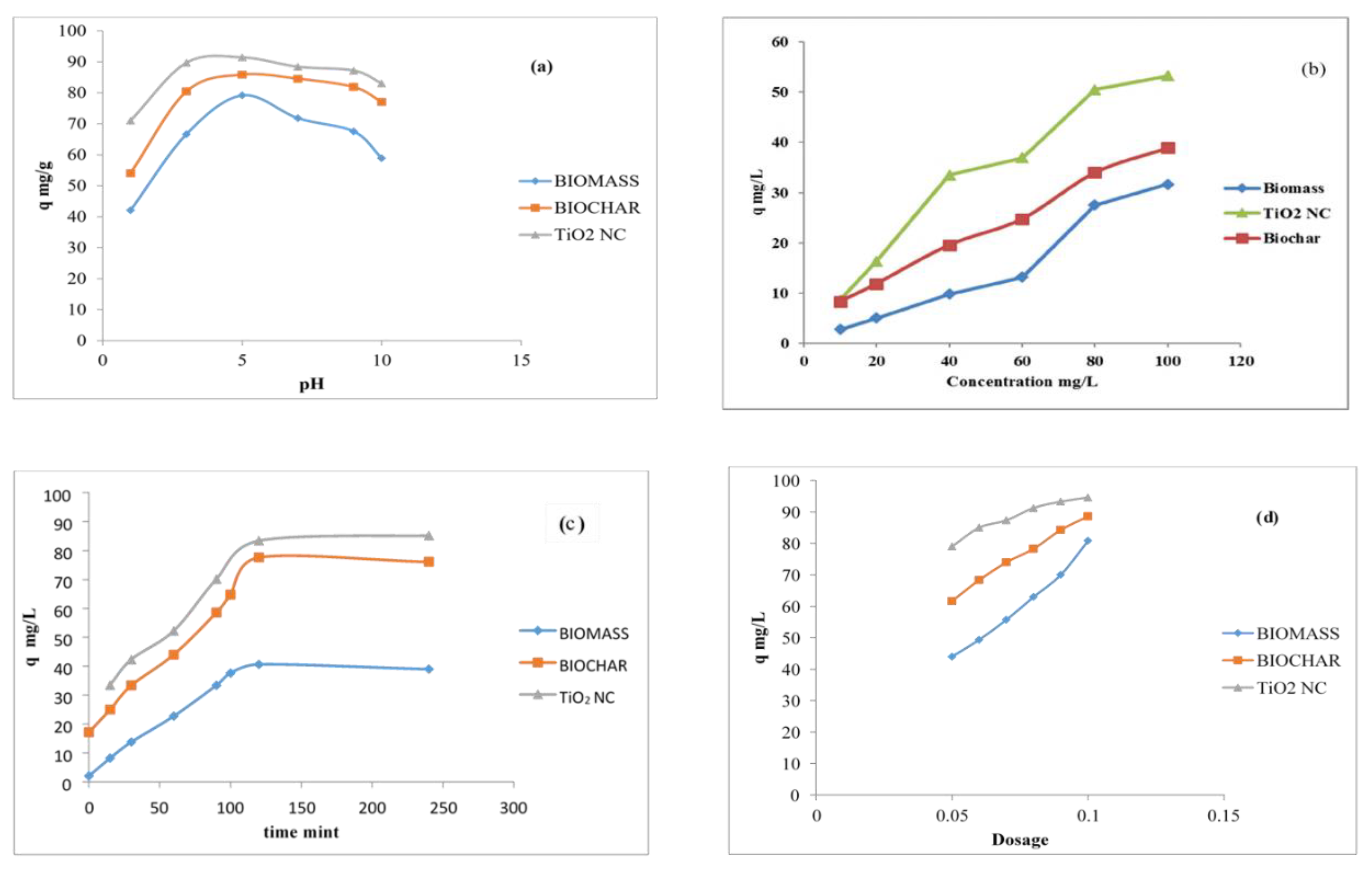
4.1. Effect of pH on Lead Adsorption
4.2. Effect of Initial Concentration on Lead Adsorption
4.3. Effect of Contact Time on Lead Adsorption
4.4. Effect of Adsorbent Dosage on Lead Adsorption
5. Comparison among Different Adsorbents
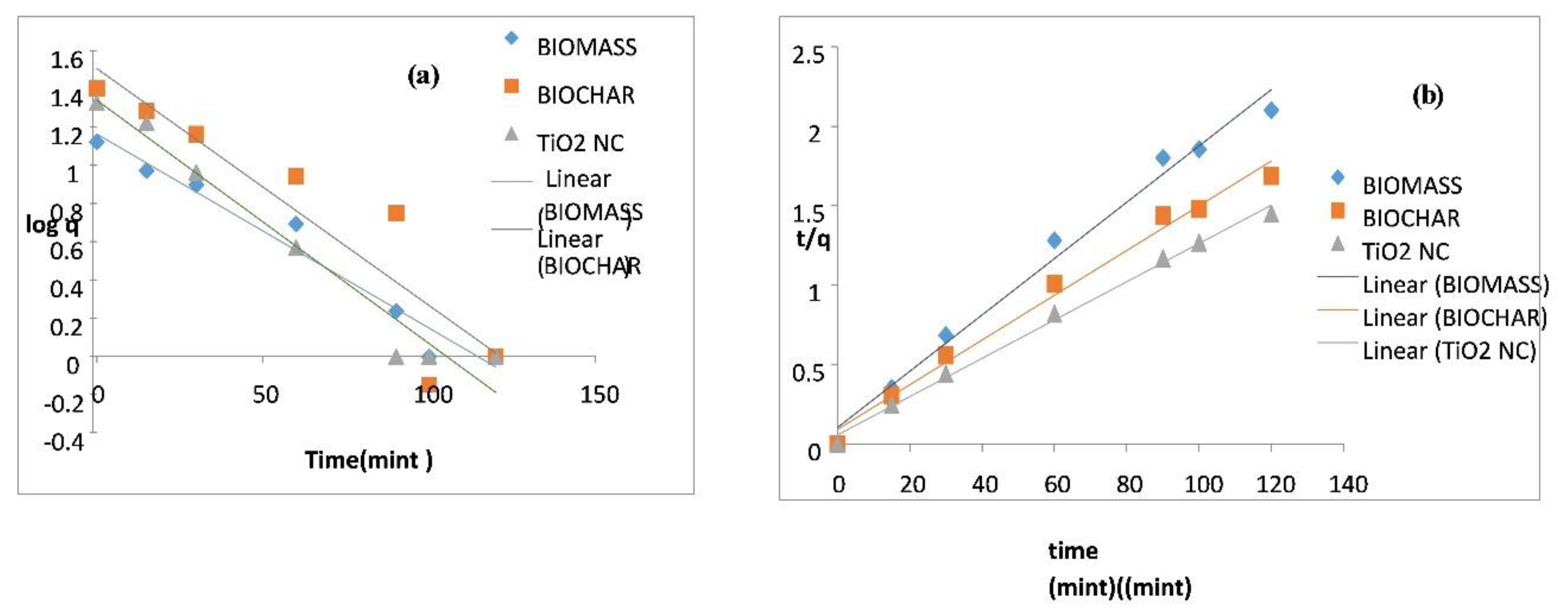
6. Adsorption Kinetics
6.1. Pseudo-First-Order Model
6.2. Pseudo-Second-Order Model
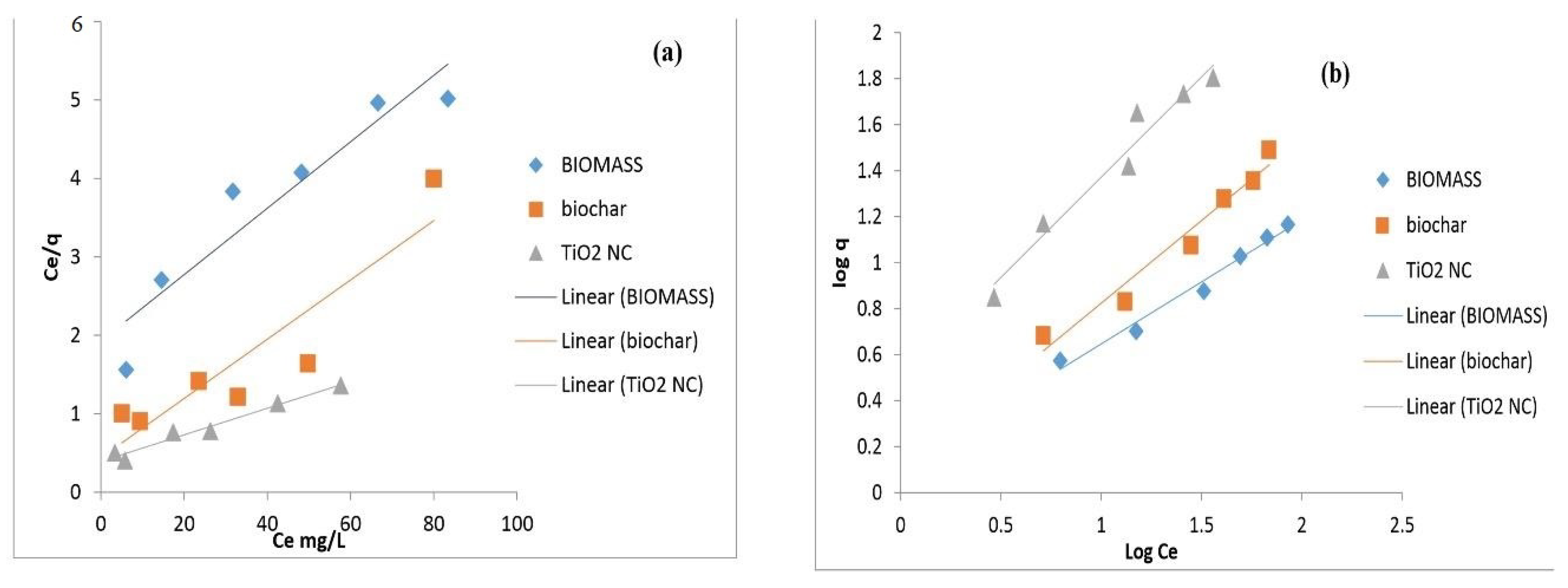
7. Equilibrium Modelling
7.1. Langmuir Isotherm
7.2. Freundlich Isotherm
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. Chem. BioEng. Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Dai, Q.H.; Bian, X.Y.; Li, R.; Jiang, C.B.; Ge, J.M.; Li, B.L.; Ou, J. Biosorption of lead(II) from aqueous solution by lactic acid bacteria. Water Sci. Technol. 2019, 79, 627–634. [Google Scholar] [CrossRef]
- Davies, K.M.; Mercer, J.F.B.; Chen, N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef]
- Hashem, M.A.; Momen, M.A.; Hasan, M.; Nur-A-Tomal, M.S.; Sheikh, M.H.R. Chromium removal from tannery wastewater using Syzygium cumini bark adsorbent. Int. J. Environ. Sci. Technol. 2018, 16, 1395–1404. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Wang, B.; Bai, Z.; Jiang, H.; Prinsen, P.; Luque, R.; Zhao, S.; Xuan, J. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J. Hazard. Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, K.; Akhtar, M.W.; Khalid, A.M. Removal and recovery of zirconium from its aqueous solution by Candida tropicalis. J. Hazard. Mater. 2008, 156, 108–117. [Google Scholar] [CrossRef]
- Abdi, O.; Kazemi, M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399. [Google Scholar]
- Oyewo, O.A.; Boshielo, A.; Adeniyi, A.; Onyango, M.S. Evaluation of the efficiency of nanoadsorbent derived from orange peels in the removal of cerium from aqueous solution. Part. Sci. Technol. 2021, 39, 43–51. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Ai, X.; Liu, J.; Yin, Y.; Huang, Y.; Zhou, H.; Huang, K. Efficient removal of cadmium from soil-washing effluents by garlic peel biosorbent. Environ. Sci. Pollut. Res. 2018, 25, 19001–19011. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. Biosorption of chromium (VI) ion from aqueous solutions using walnut, hazelnut and almond shell. J. Hazard. Mater. 2018, 155, 378–384. [Google Scholar] [CrossRef]
- Çetinkaya, S.; Targan, Ş.; Tirtom, V.N. Comparative adsorption of Pb (II) and Cd (II) ions on chestnut shell in aqueous system. Chem. Ecol. 2018, 34, 640–654. [Google Scholar] [CrossRef]
- Zheng, L.; Peng, D.; Meng, P. Corncob-supported aluminium-manganese binary oxide composite enhanced removal of cadmium ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 109–119. [Google Scholar] [CrossRef]
- Elias, M.M.C.; Ferreira, G.M.D.; Almeida, F.T.R.; MartinsRosa, N.C.; AlmeidaSilva, I.; GonçalvesFilgueiras, J.; Azevedo, E.R.; Silva, L.H.M.; Melo, T.M.S.; FrédéricGil, L.; et al. Synthesis and application of sugarcane bagasse cellulose mixed esters. Part I: Removal of Co2+ and Ni2+ from single spiked aqueous solutions in batch mode using. J. Colloid Interface Sci. 2019, 533, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.; Ibrahim, R.; Naji, A. Experimental investigation and thermodynamic study of heavy metal removal from industrial wastewater using pomegranate peel. MATEC Web Conf. EDP Sci. 2018, 162, 05007. [Google Scholar] [CrossRef]
- Chamarthy, S.; Seo, C.W.; Marshall, W.E. Adsorption of selected toxic metals by modified peanut shells. J. Chem. Technol. Biotechnol. 2001, 76, 593–597. [Google Scholar] [CrossRef]
- El-Saharty, A.; Mahmoud, S.; Manjood, A.H.; Nassar, A.A.H.; Ahmed, A.M. Effect of apricot stone activated carbon adsorbent on the removal of toxic heavy metals ions from aqueous solutions. Int. J. Ecotoxicol. Ecobiol. 2018, 3, 51–62. [Google Scholar] [CrossRef]
- Ahirwar Tech, B.M.; Scholar, R.; Kumar Sharma Professor, A.; Sharma Professor, S.; Bharti Ahirwar Tech, C.M.; Ahirwar, B.; Kumar Sharma, A.; Sharma, S. Impact Factor: 5.2 IJAR. IJAR 2017, 3, 459–462. [Google Scholar]
- Sun, Y.; Yang, G.; Zhang, L. Biosorption of heavy metals: A case study using potato peel waste. Desalin. Water Treat. 2017, 83, 159–167. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.; Issa, A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett–Burman design. J. Chem. 2019. [Google Scholar] [CrossRef] [Green Version]
- Ali, A. Removal of Mn (II) from water using chemically modified banana peels as efficient adsorbent. Environ. Nanotechnol. Monit. Manag. 2017, 7, 57–63. [Google Scholar] [CrossRef]
- Mondal, N.K.; Samanta, A.; Chakraborty, S.; Shaikh, W.A. Enhanced chromium(VI) removal using banana peel dust: Isotherms, kinetics and thermodynamics study. Sustain. Water Resour. Manag. 2017, 4, 489–497. [Google Scholar] [CrossRef]
- Sahmoune, M.N. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ. Chem. Lett. 2019, 17, 697–704. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Zhao, W.; Huang, S. Four different kinds of peels as adsorbents for the removal of Cd (II) from aqueous solution: Kinetics, isotherm and mechanism. J. Taiwan Inst. Chem. Eng. 2018, 88, 146–151. [Google Scholar] [CrossRef]
- Shrestha, S.L. A Comparative Study of Fe (II) Ion Adsorption onto Chemically Activated Banana Peel and Sawdust Bio-adsorbent. Int. J. Appl. Sci. Biotechnol. 2018, 6, 137–141. [Google Scholar] [CrossRef]
- Sharma, H.K.; Sofi, I.R.; Wani, K.A. Low cost absorbents, techniques, and heavy metal removal efficiency. In Biostimulation Remediation Technologies for Groundwater Contaminants; IGI Global: Gwalior, India, 2018. [Google Scholar]
- Regkouzas, P.; Diamadopoulos, E. Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Yahia, I.S.; Shkir, M.; Keshk, S.M.A.S. Physicochemical properties of a nanocomposite (graphene oxide-hydroxyapatite-cellulose) immobilized by Ag nanoparticles for biomedical applications. Results Phys. 2020, 16, 102990. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Naggar, M.E.; Malhat, F.M.; El Sharkawi, H.M. Efficient removal of pesticides and heavy metals from wastewater and the antimicrobial activity of f-MWCNTs/PVA nanocomposite film. J. Clean. Prod. 2019, 206, 315–325. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Rehman, R.; Farooq, S.; Mahmud, T. Use of agro-waste Musa acuminata and Solanum tuberosum peels for economical sorptive removal of emerald green dye in ecofriendly way. J. Clean. Prod. 2019, 206, 819–826. [Google Scholar] [CrossRef]
- Sakthivel, T.; Ramachandran, R.; Kirubakaran, K. Photocatalytic properties of copper-two dimensional graphitic carbon nitride hybrid film synthesized by pyrolysis method. J. Environ. Chem. Eng. 2018, 6, 2636–2642. [Google Scholar] [CrossRef]
- Deshmukh, K.K.; Hase, G.J.; Gaje, T.R.; Phatangare, N.D.; Shilpa, G.; Ashwini, V. Titanium oxide nanoparticles and degradation of dye by nanoparticles. Int. J. Mater. Sci. 2018, 13, 23–30. [Google Scholar]
- Dey, A.; Nangare, V.; More, P.V.; Khan, M.A.S.; Khanna, P.K.; Sikder, A.K.; Chattopadhyay, S. A graphene titanium dioxide nanocomposite (GTNC): One pot green synthesis and its application in a solid rocket propellant. RSC Adv. 2015, 5, 63777–63785. [Google Scholar] [CrossRef]
- Chougala, L.S.; Yatnatti, M.S.; Linganagoudar, R.K.; Kamble, R.R.; Kadadevarmath, J.S. A simple approach on synthesis of TiO2 nanoparticles and its application in dye sensitized solar cells. J. Nano Electron. Phys. 2017, 9. [Google Scholar] [CrossRef]
- Liang, S.; Han, Y.; Wei, L.; McDonald, A.G. Production and characterization of bio-oil and bio-char from pyrolysis of potato peel wastes. Biomass Convers. Biorefin. 2015, 5, 237–246. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef]
- Ali, A.; Saeed, K.; Mabood, F. Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alex. Eng. J. 2016, 55, 2933–2942. [Google Scholar] [CrossRef] [Green Version]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B.I. The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J. Hazard. Mater. 2006, 134, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Bueno, B.Y.M.; Torem, M.L.; Molina, F.; Mesquita, L.M.S. de Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: Equilibrium and kinetic studies. Miner. Eng. 2008, 1, 65–75. [Google Scholar] [CrossRef]
- Azouaou, N.; Sadaoui, Z.; Mokaddem, H. Removal of cadmium from aqueous solution by adsorption on vegetable wastes. J. Appl. Sci. 2008, 8, 4638–4643. [Google Scholar] [CrossRef] [Green Version]
- Osman1a, H.E.; Badwy, R.K.; Ahmad, H.F. Usage of some agricultural by-products in the removal of some heavy metals from industrial wastewater. J. Phytol. 2010, 2, 51–62. [Google Scholar]
- Jacques, R.A.; Lima, E.C.; Dias, S.L.P.; Mazzocato, A.C.; Pavan, F.A. Yellow passion-fruit shell as biosorbent to remove Cr (III) and Pb (II) from aqueous solution. Sep. Purif. Technol. 2007, 57, 193–198. [Google Scholar] [CrossRef]
- Aksu, Z.; İşoğlu, İ.A. Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process. Biochem. 2005, 40, 3031–3044. [Google Scholar] [CrossRef]
- Pehlivan, E.; Cetin, S.; Yanık, B.H. Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash. J. Hazard. Mater. 2006, 135, 193–199. [Google Scholar] [CrossRef]
- Eslamzadeh, T.; Nasernezhad, B.; Esmaeil, B.M. Removal of heavy metals from aqeous solution by carrot residues. Iran. J. Sci. Technol. Trans. A-Sci. 2004, 28, 161–167. [Google Scholar]
- Schiewer, S.; Patil, S.B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903. [Google Scholar] [CrossRef]
- Arslanoglu, H.; Altundogan, H.S.; Tumen, F. Preparation of cation exchanger from lemon and sorption of divalent heavy metals. Bioresour. Technol. 2008, 99, 2699–2705. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gouanvé, F.; Marais, S.; Bessadok, A.; Langevin, D.; Morvan, C.; Métayer, M. Study of water sorption in modified flax fibers. J. Appl. Polym. Sci. 2006, 101, 4281–4289. [Google Scholar] [CrossRef]
- Huang, Y.; Shih, M. Effect of linearized expressions of Langmuir equations on the prediction of the adsorption of methylene blue on rice husk. Int. J. Sci. Res. Publ. 2016, 6, 549–554. [Google Scholar]
- Chung, H.K.; Kim, W.H.; Park, J.; Cho, J.; Jeong, T.Y.; Park, P.K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]




| Adsorbents | Heavy Metals | Maximum Adsorption Capacity (m mol g−1) |
|---|---|---|
| Olive waste | Cd | 0.0655 [46] |
| Orange peel | Cd | 0.335 [42] |
| Orange bark | Cd | 0.3101 [46] |
| Rice hull | Ni, Cd, Cu, Zn, Pb | 0.085, 0.125, 0.93, 0.17, 0.2 [47] |
| Yellow passion-fruit shell | Cr | 0.732–1.637 [48] |
| Sugar beet pulp (Beta vulgaris) | Cu, Zn | 0.309, 0.356 [49,50] |
| Carrot residues | Cu | 0.937 [51] |
| Grapefruit peel | Cd | 0.24 [52] |
| Lemon peel | Cd, Mn, Pb | 0.46, 0.43, 0.869 [53] |
| Lemon resin | Mn, Pb | 0.429, 0.869 [53] |
| Adsorbate | Adsorbents | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|---|
| qe | K1ad | R2 | qexp | qe | K2ad | R2 | ||
| (mg/g) | (min−1) | (mg/g) | (mg/g) | (mg/gmin) | ||||
| Lead | Biomass | 14.561 | −0.004 | 0.963 | 51.671 | 56.497 | 0.003 | 0.9863 |
| Biochar | 32.055 | −0.0053 | 0.847 | 68.237 | 70.921 | 4.426 | 0.9878 | |
| TiO2 NC | 21.892 | −0.005 | 0.961 | 76.751 | 82.644 | 0.0025 | 0.9949 | |
| Adsorbate | Adsorbents | Langmuir Model | Experimental Value | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Xm | KL | R2 | qe (mg/g) | 1/n | KF (mg/g) | R2 | |||
| (mg/g) | (L/mg) | ||||||||
| Lead | Biomass | 20.96 | 0.02 | 0.90 | 14.66 | 7.84 | 0.53 | −2.22 | 0.97 |
| Biochar | 67.11 | 0.009 | 0.51 | 31.21 | 13.48 | 0.71 | −2.26 | 0.95 | |
| TiO2 NC | 200 | 0.007 | 0.66 | 63.85 | 47.72 | 0.87 | 0.69 | 0.95 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashfaq, A.; Nadeem, R.; Bibi, S.; Rashid, U.; Hanif, M.A.; Jahan, N.; Ashfaq, Z.; Ahmed, Z.; Adil, M.; Naz, M. Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels). Water 2021, 13, 3344. https://doi.org/10.3390/w13233344
Ashfaq A, Nadeem R, Bibi S, Rashid U, Hanif MA, Jahan N, Ashfaq Z, Ahmed Z, Adil M, Naz M. Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels). Water. 2021; 13(23):3344. https://doi.org/10.3390/w13233344
Chicago/Turabian StyleAshfaq, Aamna, Razyia Nadeem, Shamsa Bibi, Umer Rashid, Muhammad Asif Hanif, Nazish Jahan, Zeeshan Ashfaq, Zubair Ahmed, Muhammad Adil, and Maleeha Naz. 2021. "Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels)" Water 13, no. 23: 3344. https://doi.org/10.3390/w13233344
APA StyleAshfaq, A., Nadeem, R., Bibi, S., Rashid, U., Hanif, M. A., Jahan, N., Ashfaq, Z., Ahmed, Z., Adil, M., & Naz, M. (2021). Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels). Water, 13(23), 3344. https://doi.org/10.3390/w13233344








