Levels of Elements in Typical Mussels from the Southern Coast of Africa (Namibia, South Africa, Mozambique): Safety Aspect
Abstract
:1. Introduction
2. Materials and Methods
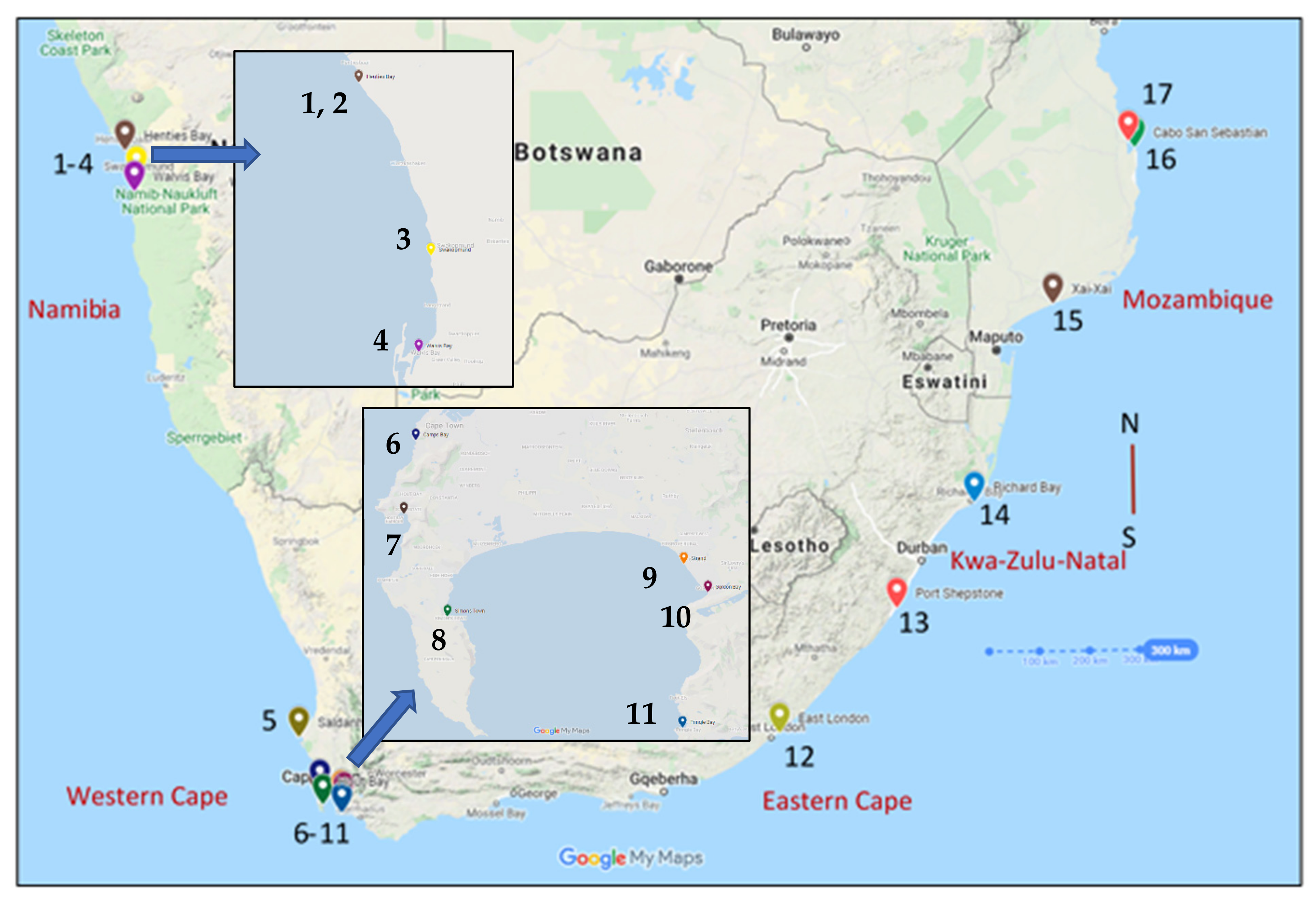
2.1. Sampling
2.2. Neutron Activation Analysis
2.3. Statistics
2.4. Risk Assessment
- The concentrations in soft tissues (wet weight basis) could be compared with maximum permissible limits (MPLs), which are established as safety guidelines for several elements (Cr, Ni, Zn, As, Se) in seafood;
- The assessment of differences between estimated weekly intakes (EWI) and PTWI by calculation of risk quotient (RQ) for elements Na, Al, Cr, Mn, Fe, Co, Ni, Zn, As, Se, Sr, Sb, I, U;
- The characterization of the amount of soft tissue (maximal provisional consumption rate MPCR, kg/week) that would need to be consumed per week by the average person to reach the provisional tolerable weekly intake (PTWI) established by the JECFA or related reference limits;
- Target hazardous quotient (THQ) and total hazardous index (HI) which corresponded to the sum of all quotients from elements with recommended RfDs ([29]: Mn, Fe, Co, Ni, Zn, Se, Sr, Sb, I, U) as its combinations for each station for the local coastal population
3. Results and Discussion
3.1. Concentrations of the Elements in Soft Tissues and Maximum Permissible Levels
- Cr content in mussels at st. 3 (Namibia), 12–15 (Eastern SA and Mozambique) reached MPL within the interquartile range. At st. 14 (Richards Bay) and 15 (Xai-Xai), the levels of Cr in all individuals were above MPL and significantly higher (p < 0.04) than at the other stations that can be explained by the matter of the terrigenous origin and features of suspended sediments from river runoff, which could be accumulated by mussels
- Zn content reached MPL only at st. 1 (Henties Bay, Namibia) and significantly higher (p < 0.001) than at other stations, which is probably associated with high anthropogenic inputs and its higher accumulation by Mytilus edulis in comparison with Mytilus galloprovincialis (almost the same site, st. 2)
- As content exceeded the MPL at the stations 1–3 (Namibia), 8–11 (Western Cape), 12, 13 (East of SA), and 15–17 (Mozambique) with the significantly higher levels at st. 16 and 17 (p < 0.01). The lower levels of As at st. 4–7 could be explained by local shifts of water masses during upwelling
- Se content exceeded the MPL at stations 1–6 (Namibia and Saldanha bay), st. 7 (Hout Bay), st. 13 (Port Shepstone), st. 16 (San Sebastian, Mozambique) with significantly higher levels at st. 1 and 16 (p < 0.02). The distribution of Se could be explained by the local growth of the phytoplankton assemblages, which are used by mussels as a food
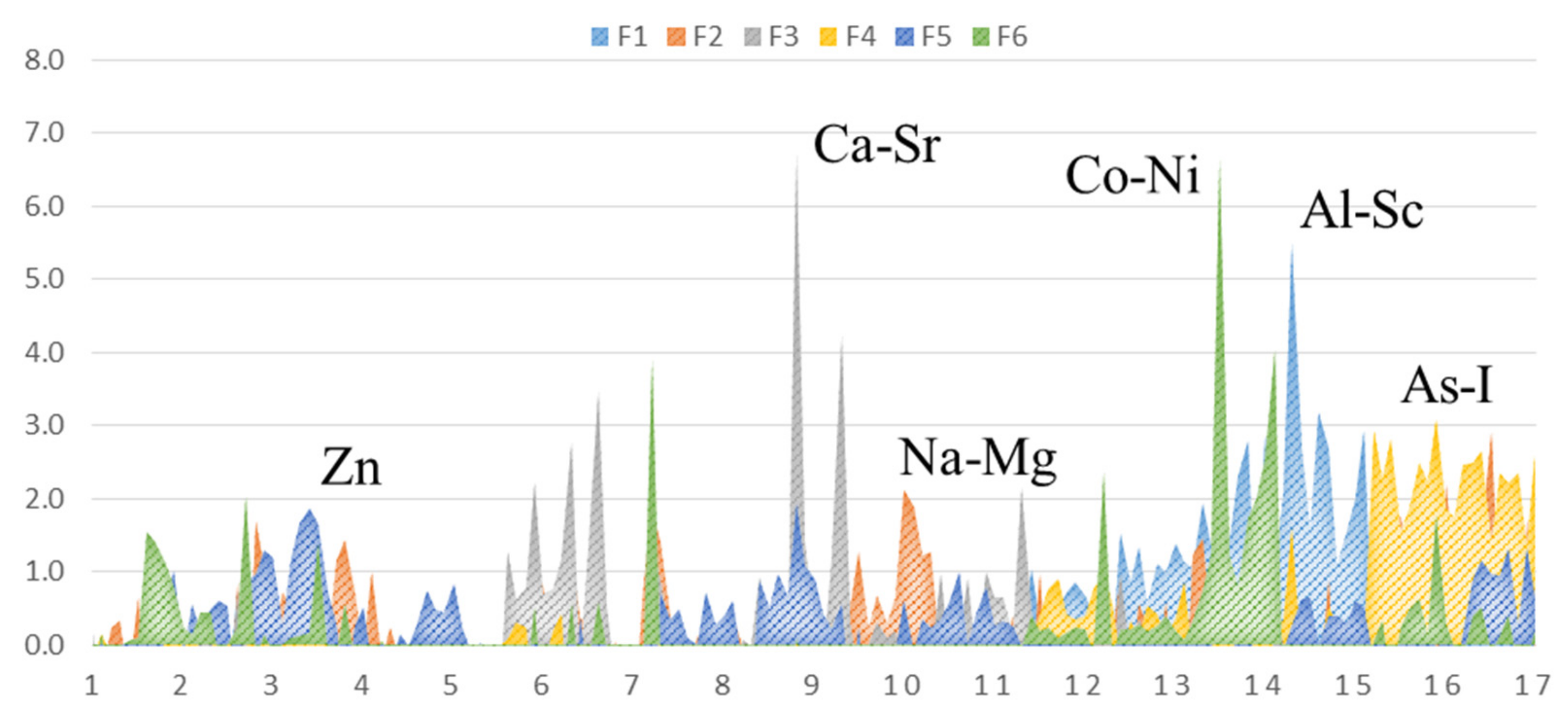
3.2. Groups of Elements
- F1 (29%, terrigenous): Al, Sc, V, Cr, Mn, Fe, Rb, Cs, Th.
- F2 (13%, salinity): Na, Mg, Cl
- F3 (9%, shell construction): Ca, Sr
- F4 (13%, mixed): As, Br, I
- F5 (6%, mixed): Zn, Se
- F6 (8%, anthropogenic): Co, Ni, Sb
- Terrigenous elements (Al, Sc, V, Cr, Mn, Fe, Rb, Cs, Th)—connected with suspended sediments and river runoff
- Anthropogenic elements (Co, Ni, Zn, As, Se, Br, I, Sb)
- Marine (Na, Mg, Cl)
- Others (K, Ca, Sr, U)
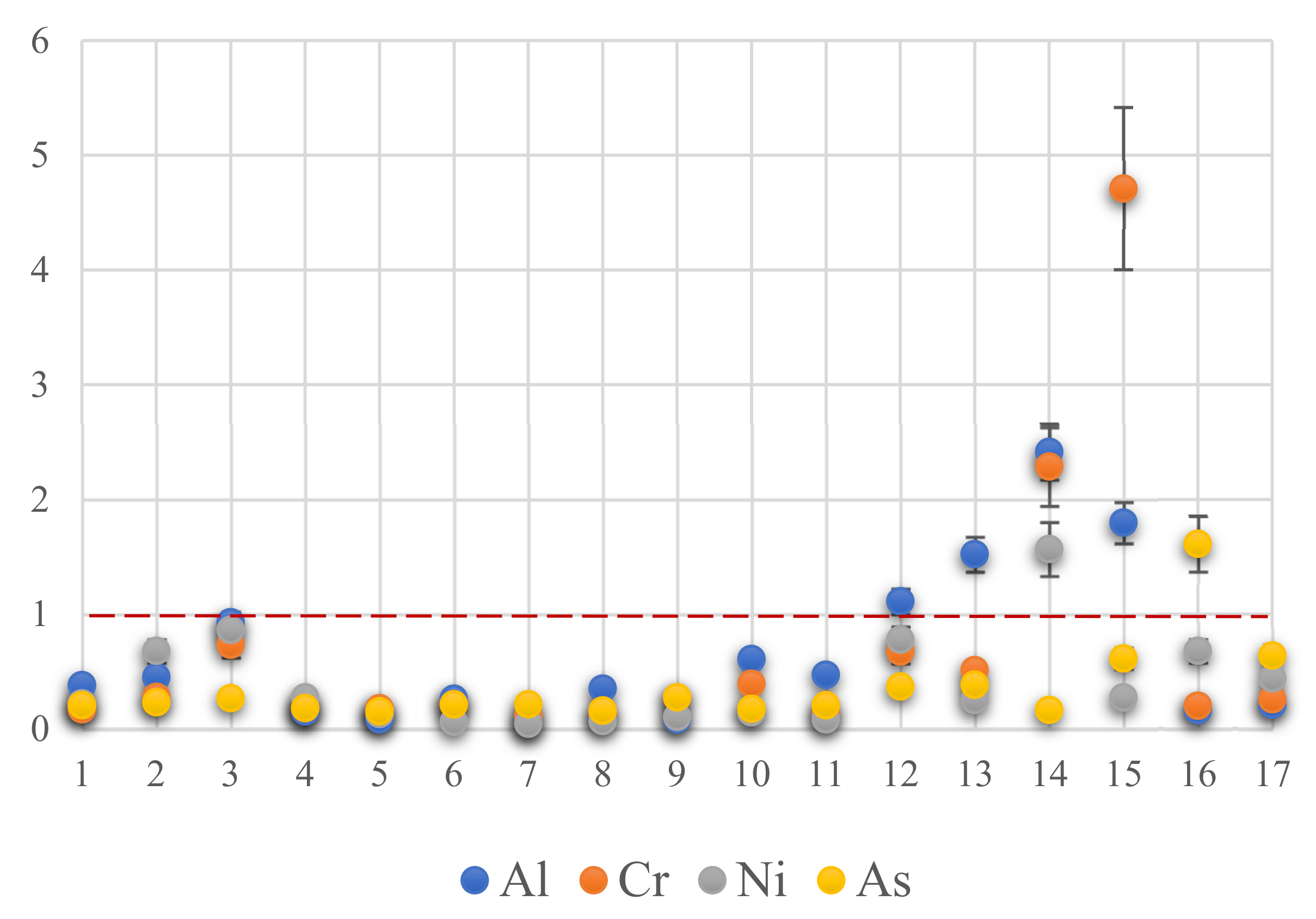
3.3. Risk Quotients and Maximum Provisional Consumption Rates among 14 Elements
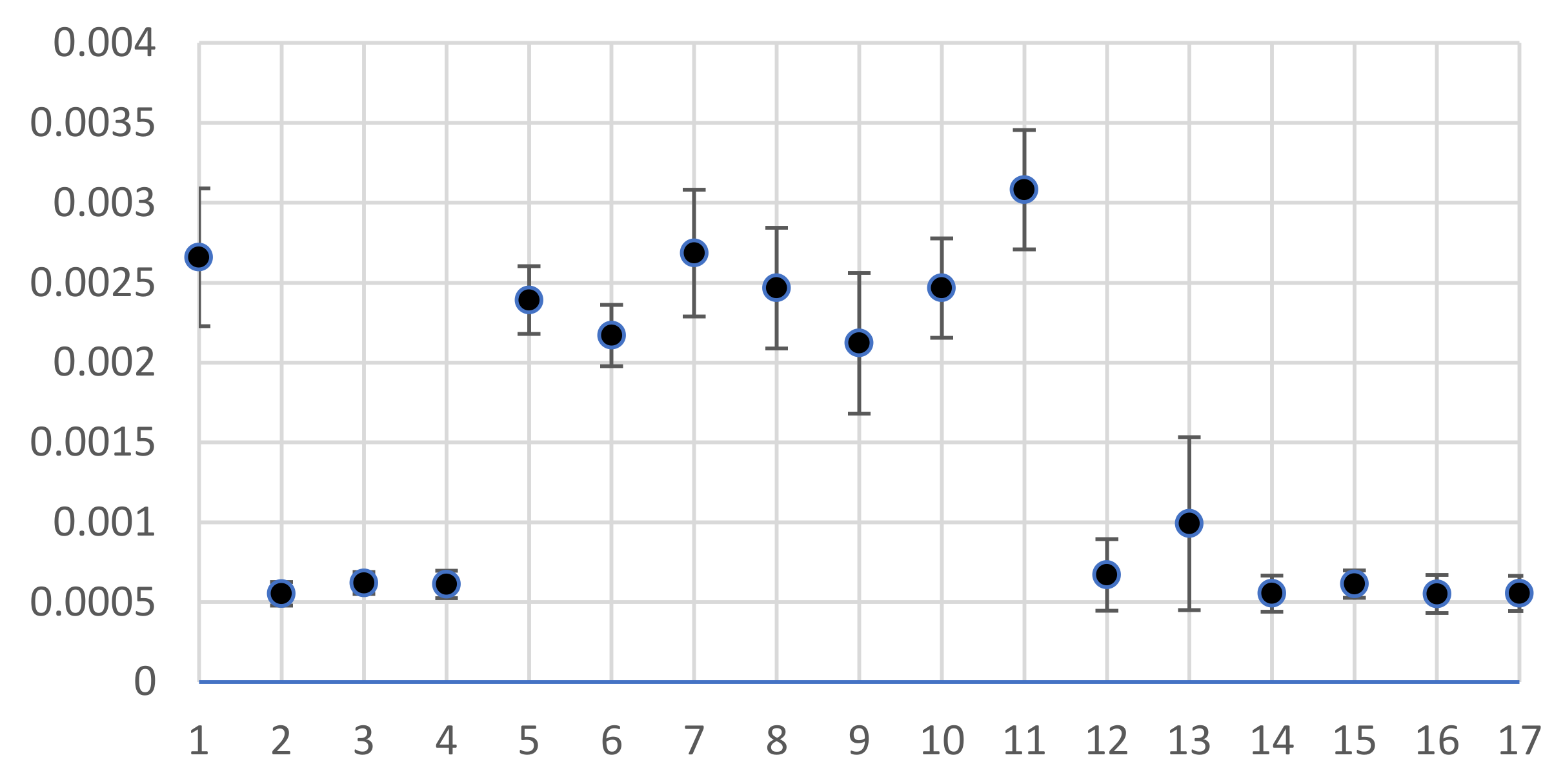
3.4. Target Hazard Quotients and Total Hazard Indices
3.5. Groups of Elements in Shells of Mussels
- Sc, Cr, Mn, Sb, Cs, Th reached close levels in comparison with soft tissues
- Ca and Sr were accumulated to 10 times higher levels than in soft tissues
- Cl and Zn were accumulated to 10–100 times lower concentrations than in soft tissues
- In polluted zones, V, Co, Ni, Br, U were accumulated at close levels in shells and in soft tissues
3.6. Elements with High Risks in Human Consumption
3.7. The Stations with High Risks in Consumption of Mussels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fru, W. Copper and Zinc in Water, Sediment and Gastropods in the Harbours of the Cape Town Metropole, South Africa. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2020. [Google Scholar]
- Sparks, C.; Odendaal, J.; Snyman, R. Metal concentrations in intertidal water and surface sediment along the west coast of the Cape Peninsula, Cape Town, South Africa. Water SA 2017, 43, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, Á.; Zumbado, M.; Henríquez-Hernández, L.A.; Boada, L.D.; Luzardo, O.P. Dietary Intake of Essential, Toxic, and Potentially Toxic Elements from Mussels (Mytilus spp.) in the Spanish Population: A Nutritional Assessment. Nutrients 2019, 11, 864. [Google Scholar] [CrossRef] [Green Version]
- Zuykov, M.; Pelletier, E.; Harper, D.A.T. Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere 2013, 93, 201–208. [Google Scholar] [CrossRef]
- Erasmus, A.; Ikenaka, Y.; Nakayama, S.M.M.; Ishizuka, M.; Smit, N.J.; Wepener, V. Trophic transfer of pollutants within two intertidal rocky shore ecosystems in different biogeographic regions of South Africa. Mar. Pollut. Bull. 2020, 157, 111309. [Google Scholar] [CrossRef]
- Firth, D.C.; Salie, K.; O’Neill, B.; Hoffman, L.C. Monitoring of trace metal accumulation in two South African farmed mussel species, Mytilus galloprovincialis and Choromytilus meridionalis. Mar. Pollut. Bull. 2019, 141, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Wepener, V.; Degger, N. Monitoring Metals in South African Harbours between 2008 and 2009, Using Resident Mussels as Indicator Organisms. Afr. Zool. 2020, 55, 267–277. [Google Scholar] [CrossRef]
- Hofherr, J.; Martinsohn, J.; Cawthorn, D.; Rasco, B.; Naaum, A.M. Regulatory Frameworks for Seafood Authenticity and Traceability. In Seafood Authenticity and Traceability; Academic Press: Cambridge, MA, USA, 2016; pp. 47–82. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 7 October 2021).
- Storelli, M.M.; Marcotrigiano, G.O. Consumption of bivalve molluscs in italy: Estimated intake of cadmium and lead. Food Addit. Contam. 2001, 18, 303–307. [Google Scholar] [CrossRef]
- Ferrara, F.; Fabietti, F.; Delise, M.; Bocca, A.P.; Funari, E. Alkylphenolic compounds in edible molluscs of the Adriatic Sea (Italy). Environ. Sci. Technol. 2001, 35, 3109–3112. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, S.; Jovic, M. Health risks of heavy metals in the mediterranean mussels as seafood. Environ. Chem. Lett. 2012, 10, 119–130. [Google Scholar] [CrossRef]
- Jović, M.; Onjia, A.; Stanković, S. Toxic metal health risk by mussel consumption. Environ. Chem. Lett. 2012, 10, 69–77. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Leung, K.M.Y. Organotin contamination in seafood and its implication for human health risk in Hong Kong. Mar. Pollut. Bull. 2014, 85, 634–640. [Google Scholar] [CrossRef]
- Whyte, A.L.H.; Raumati Hook, G.; Greening, G.E.; Gibbs-Smith, E.; Gardner, J.P.A. Human dietary exposure to heavy metals via the consumption of greenshell mussels (Perna canaliculus Gmelin 1791) from the Bay of Islands, northern New Zealand. Sci. Total Environ. 2009, 407, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Nekhoroshkov, P.S.; Bezuidenhout, J.; Frontasyeva, M.V.; Zinicovscaia, I.I.; Yushin, N.S.; Vergel, K.N.; Petrik, L. Trace elements risk assessment for consumption of wild mussels along South Africa coastline. J. Food Compos. Anal. 2021, 98, 103825. [Google Scholar] [CrossRef]
- Dingle, R.V.; Nelson, G. Sea-bottom temperature, salinity and dissolved oxygen on the continental margin off south-western Africa. S. Afr. J. Mar. Sci. 2010, 13, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Painter, S.C. The biogeochemistry and oceanography of the East African Coastal Current. Prog. Oceanogr. 2020, 186, 102374. [Google Scholar] [CrossRef]
- Trott, C.B.; Subrahmanyam, B.; Washburn, C.E. Investigating the response of temperature and salinity in the agulhas current region to enso events. Remote Sens. 2021, 13, 1829. [Google Scholar] [CrossRef]
- Vellemu, E.C.; Omoregie, E. Lead Pollution: A Growing Concern Along the Namibian Coastal Waters. Int. Sci. Technol. J. Namib. 2014, 3, 21–34. [Google Scholar]
- Skov, H.; Bloch, R.; Stuer-Lauridsen, F.; Uushona, D. Strategic Environmental Assessment (SEA) for the coastal areas of the Erongo and Kunene Regions. Namib. Coast Conserv. Manag. Proj. 2008, 111, 172. [Google Scholar]
- Greenberg, R.R.; Bode, P.; De Nadai Fernandes, E.A. Neutron activation analysis: A primary method of measurement. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 193–241. [Google Scholar] [CrossRef]
- Dmitriev, A.Y.; Pavlov, S.S. Automation of the quantitative determination of elemental content in samples using neutron activation analysis on the IBR-2 reactor at the frank laboratory for neutron physics, joint institute for nuclear research. Phys. Part. Nucl. Lett. 2013, 10, 33–36. [Google Scholar] [CrossRef]
- Pavlov, S.S.; Dmitriev, A.Y.; Frontasyeva, M.V. Automation system for neutron activation analysis at the reactor IBR-2, Frank Laboratory of Neutron Physics, Joint Institute for Nuclear Research, Dubna, Russia. J. Radioanal. Nucl. Chem. 2016, 309, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test. Corsini Encycl. Psychol. 2010, 1. [Google Scholar] [CrossRef]
- Korkmaz, C.; Ay, Ö.; Çolakfakioğlu, C.; Cicik, B.; Erdem, C. Heavy Metal Levels in Muscle Tissues of Solea solea, Mullus barbatus, and Sardina pilchardus Marketed for Consumption in Mersin, Turkey. Water Air Soil Pollut. 2017, 228, 315. [Google Scholar] [CrossRef]
- Yap, C.K.; Cheng, W.H.; Karami, A.; Ismail, A. Health risk assessments of heavy metal exposure via consumption of marine mussels collected from anthropogenic sites. Sci. Total Environ. 2016, 553, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Integrated Risk Information System | US EPA. Available online: https://www.epa.gov/iris (accessed on 9 November 2021).
- Evaluation of Certain Food Additives: Eighty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/iris/handle/10665/330612 (accessed on 9 November 2021).
- Chiesa, L.M.; Ceriani, F.; Caligara, M.; Di Candia, D.; Malandra, R.; Panseri, S.; Arioli, F. Mussels and clams from the italian fish market. is there a human exposition risk to metals and arsenic? Chemosphere 2018, 194, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.K.; Mok, C.S. Metallic contamination in oyster and other seafood in Hong Kong. Food Addit. Contam. 1991, 8, 333–342. [Google Scholar] [CrossRef]
- Lin, H.T.; Wong, S.S.; Li, G.C. Heavy metal content of rice and shellfish in Taiwan. J. Food Drug Anal. 2004, 12, 167–174. [Google Scholar] [CrossRef]
- National Department of Health (NDoH); Statistics South Africa (Stats SA); South African Medical Research Council (SAMRC); National Department of Health (NDoH) South African Medical Research Council (SAMRC); ICF. South Africa Demographic and Health Survey 2016; NdoH: Rockville, MD, USA; Stats SA: Pretoria, South Africa, 2019.
- World Health Organization. Evaluation of Certain Food Additives: Eighty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series; No. 1020, 2019. Available online: https://www.who.int/publications/i/item/9789241210294 (accessed on 9 November 2021).
- Konz, J.J.; Lisi, K.; Friebele, E.; Dixon, D.A. Exposure Factors Handbook; Versar, Inc.: Springfield, VA, USA, 1989. [Google Scholar]
- DOH (Department of Health). Foodstuffs, Cosmetics and Disinfectants Act: Regulations: Maximum Levels of Metals in Foodstuffs (English/Setswana); Government Printing Works: Pretoria, South Africa, 2018.
- Nauen, C.E. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Products. FAO Fish. Circ. 1983, 764, 1983. [Google Scholar]
- Richir, J. Trace Elements in Marine Environments: Occurrence, Threats and Monitoring with Special Focus on the Coastal Mediterranean. J. Environ. Anal. Toxicol. 2016, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Nessim, R.B.; Tadros, H.R.Z.; Abou Taleb, A.E.A.; Moawad, M.N. Chemistry of the Egyptian Mediterranean coastal waters. Egypt. J. Aquat. Res. 2015, 41, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.L.; Robinson, T.B.; Lange, L.; Mead, A. Marine biodiversity in South Africa: An evaluation of current states of knowledge. PLoS ONE 2010, 5, e12008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, W.-X. Linking trace element variations with macronutrients and major cations in marine mussels Mytilus edulis and Perna viridis. Environ. Toxicol. Chem. 2015, 34, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Quijano, M.A.; Moreno, P.; Gutiérrez, A.M.; Pérez-Conde, M.C.; Cámara, C. Selenium speciation in animal tissues after enzymatic digestion by high-performance liquid chromatography coupled to plasma mass spectrometry. J. Mass Spectrom. 2000, 35, 878–884. [Google Scholar] [CrossRef]
- Özdemir, A. Relationships of Formation, Migration, and Trapping Between Petroleum and Iodine. Rev. Artic. 2018, 3, 110–153. [Google Scholar]
- Mirlean, N.; Andrus, V.E.; Baisch, P.; Griep, G.; Casartelli, M.R. Arsenic pollution in Patos Lagoon estuarine sediments, Brazil. Mar. Pollut. Bull. 2003, 46, 1480–1484. [Google Scholar] [CrossRef]
- Peycheva, K.; Panayotova, V.; Stancheva, R.; Makedonski, L.; Merdzhanova, A.; Cicero, N.; Parrino, V.; Fazio, F. Trace elements and omega-3 fatty acids of wild and farmed mussels (Mytilus galloprovincialis) consumed in Bulgaria: Human health risks. Int. J. Environ. Res. Public Health 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Vander Putten, E.; Dehairs, F.; Keppens, E.; Baeyens, W. High resolution distribution of trace elements in the calcite shell layer of modern Mytilus edulis: Environmental and biological controls. Geochim. Cosmochim. Acta 2000, 64, 997–1011. [Google Scholar] [CrossRef]
- Majola, N.; Mzimela, H.M.; Izegaegbe, J.I. Metal bioaccumulation and energy biomarkers in tissues of two populations of Chiromantes eulimene from Richards Bay Harbour, South Africa. Sci. Afr. 2020, 10, e00558. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mukherjee, A.; Ahmed, K.M. A Review of Groundwater Arsenic in the Bengal Basin, Bangladesh and India: From Source to Sink. Curr. Pollut. Rep. 2015, 1, 220–247. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, G.; Jolley, D.; Morrison, J. Evolution of chemical contaminant and toxicology studies, part 2—Case studies of Selenium and Arsenic. S. Pac. J. Nat. Appl. Sci. 2003, 21, 6. [Google Scholar] [CrossRef]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Ibrahim, E.M. 59 Effect of parenteral supplementation of vitamin E plus selenium on nutrient digestibility, productive performance and some serum biochemical indicators of lambs. Egypt. J. Sheep Goat Sci. 2017, 12, 59–70. [Google Scholar]
- Leung, A.M.; Braverman, L.E. Iodine-induced thyroid dysfunction. Curr. Opin. Endocrinol. Diabetes. Obes. 2012, 19, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolmykova, L.I.; Korobova, E.M.; Baranchukov, V.S.; Kurnosova, I.V.; Silenok, A.V.; Makarova, E.M. Chemical composition of groundwater used for drinking in conditions of natural deficiency of iodine and selenium and evaluation of its health effect: The case of Bryansk region (Russia). Environ. Geochem. Health 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirokawa, T. Iodine and Iodine Species in Seawater: Speciation, Distribution, and Dynamics. In Comprehensive Handbook of Iodine–Nutritional, Biochemical, Pathological and Therapeutic Aspects; Academic Press: Burlington, UK, 2009; pp. 83–91. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Chance, R.J.; Sherwen, T.; Adams, T.J.; Ball, S.M.; Evans, M.J.; Hepach, H.; Hollis, L.D.J.; Hughes, C.; Jickells, T.D.; et al. Marine iodine emissions in a changing world. Proc. R. Soc. A Math. Phys. Eng. Sci. 2021, 477, 20200824. [Google Scholar] [CrossRef]
- Omoregie, E.; Vellemu, E.C.; Nashima, F.; Mudumbi, S.B.; Liswaniso, G.; Shimooshili, K. Assessment of copper levels along the Namibian marine coastline. GSC Biol. Pharm. Sci. 2019, 7, 48–55. [Google Scholar] [CrossRef]

| Elements | SRMs | Uncertainties % | Recovery Rate, % | MDC, ppm | |
|---|---|---|---|---|---|
| Determined | Certified | ||||
| Na | 2709a | 8.3 | 2.5 | 100.5 | 22 |
| Mg | 1566b | 10.5 | 2.1 | 102 | 271 |
| Al | 1573a | 5.6 | 2 | 99.7 | 12 |
| Cl | 1632c | 8.4 | 3.6 | 100.7 | 141 |
| K | 1632c | 10 | 3 | 103.1 | 523 |
| Ca | 1549 | 27.8 | 3.8 | 97.1 | 238 |
| Sc | 667 | 5.5 | 5.1 | 98.4 | 0.005 |
| V | 1547 | 11.6 | 8.1 | 101.9 | 0.1 |
| Cr | FFA1 | 9.4 | 5.1 | 102 | 1.2 |
| Mn | 1573a | 8 | 3.3 | 98.6 | 0.4 |
| Fe | 2709a | 5.5 | 2.1 | 99.3 | 22 |
| Co | 667 | 4.2 | 5.6 | 101.4 | 0.02 |
| Ni | FFA1 | 10 | 5.9 | 95.2 | 0.7 |
| Zn | 1632c | 9.1 | 10.7 | 98.4 | 0.4 |
| As | 1632c | 5.7 | 4.4 | 97.2 | 0.07 |
| Se | 667 | 44.2 | 5 | 106.9 | 0.15 |
| Br | OBTL5 | 4.3 | 6.2 | 108.4 | 0.12 |
| Rb | 2709a | 16.6 | 3 | 93.8 | 0.06 |
| Sr | 667 | 8.6 | 30 | 102.8 | 1.9 |
| Sb | FFA1 | 8.9 | 14.2 | 98 | 0.002 |
| I | 1547 | 33.2 | 30 | 94.3 | 0.7 |
| Cs | 1632c | 5.4 | 1.7 | 95.5 | 0.005 |
| Th | 667 | 4.5 | 5 | 103.5 | 0.007 |
| U | 1632c | 3.9 | 2.3 | 95.5 | 0.03 |
| Zones | Namibia | Saldanha Bay | Cape Town | False Bay | Eastern SA | Mozambique |
|---|---|---|---|---|---|---|
| Stations | St. 1–4 | St. 5 | St. 6–7 | St. 8–11 | St. 12–14 | St. 15–17 |
| Samples | n = 36 | n = 10 | n = 18 | n = 40 | n = 28 | n = 29 |
| Elements | ||||||
| Na | 33,110 ± 4970 | 25,080 ± 4110 | 37,950 ± 5600 | 35,280 ± 3850 | 31,450 ± 5970 | 39,870 ± 8960 |
| Mg | 6020 ± 1010 | 4350 ± 860 | 6760 ± 700 | 6720 ± 750 | 6020 ± 730 | 7530 ± 1130 |
| Al | 560 ± 270 | 100 ± 50 | 440 ± 340 | 290 ± 110 | 2880 ± 610 | 1200 ± 240 |
| Cl | 53,920 ± 7850 | 41,960 ± 6610 | 57,300 ± 8450 | 56,000 ± 7800 | 54,320 ± 10,530 | 62,210 ± 14,880 |
| K | 8510 ± 1520 | 7720 ± 1530 | 5570 ± 720 | 6820 ± 990 | 4170 ± 630 | 5030 ± 1580 |
| Ca | 4710 ± 1320 | 3000 ± 560 | 8570 ± 4490 | 8370 ± 3860 | 5700 ± 1120 | 7140 ± 1810 |
| Sc | 0.13 ± 0.08 | 0.02 ± 0.01 | 0.09 ± 0.03 | 0.05 ± 0.02 | 0.62 ± 0.17 | 0.34 ± 0.1 |
| V | 1.3 ± 0.5 | 0.8 ± 0.2 | 0.8 ± 0.3 | 1 ± 0.3 | 6.3 ± 1.4 | 6.6 ± 2.7 |
| Cr | 2.9 ± 1.9 | 2.4 ± 0.7 | 3.3 ± 1.2 | 2.4 ± 0.9 | 12.9 ± 5.3 | 15.8 ± 9.8 |
| Mn | 9 ± 4 | 3 ± 2 | 7 ± 3 | 7 ± 2 | 34 ± 7 | 19 ± 8 |
| Fe | 550 ± 240 | 150 ± 70 | 330 ± 90 | 250 ± 70 | 1980 ± 470 | 1280 ± 380 |
| Co | 1.37 ± 0.97 | 0.64 ± 0.17 | 0.31 ± 0.06 | 0.26 ± 0.07 | 4.68 ± 2.64 | 1.52 ± 0.32 |
| Ni | 9.4 ± 4.9 | 3.1 ± 0.9 | 2.5 ± 0.7 | 1.6 ± 0.6 | 15.1 ± 7.6 | 9.4 ± 3.8 |
| Zn | 290 ± 170 | 200 ± 60 | 240 ± 90 | 180 ± 60 | 90 ± 20 | 100 ± 20 |
| As | 6.9 ± 1.3 | 4.4 ± 1 | 5.8 ± 0.5 | 7.2 ± 1.1 | 9.4 ± 1.7 | 20.9 ± 7.2 |
| Se | 6 ± 1.3 | 3.9 ± 1 | 3 ± 0.8 | 2.2 ± 0.5 | 3.6 ± 0.6 | 3.8 ± 0.6 |
| Br | 314 ± 48 | 203 ± 28 | 336 ± 40 | 292 ± 24 | 326 ± 58 | 559 ± 164 |
| Rb | 3.9 ± 0.7 | 3.7 ± 0.4 | 3.7 ± 1.3 | 3.6 ± 0.4 | 4.4 ± 1 | 3 ± 0.5 |
| Sr | 48 ± 11 | 30 ± 6 | 95 ± 32 | 88 ± 38 | 60 ± 11 | 85 ± 12 |
| Sb | 0.03 ± 0.01 | 0.01 ± 0 | 0.09 ± 0.11 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.06 ± 0.01 |
| I | 16 ± 6 | 5 ± 2 | 16 ± 2 | 12 ± 3 | 26 ± 4 | 57 ± 13 |
| Cs | 0.07 ± 0.03 | 0.02 ± 0.01 | 0.13 ± 0.1 | 0.06 ± 0.03 | 0.15 ± 0.04 | 0.05 ± 0.02 |
| Th | 0.21 ± 0.19 | 0.01 ± 0.01 | 0.15 ± 0.14 | 0.07 ± 0.03 | 0.79 ± 0.25 | 0.4 ± 0.23 |
| U | 0.19 ± 0.08 | 0.1 ± 0.04 | 0.31 ± 0.09 | 0.19 ± 0.06 | 0.26 ± 0.09 | 0.35 ± 0.12 |
| Regions | Species | St. | Sampling Sites | Na | Al | Cr | Mn | Fe | Co | Ni | Zn | As | Se | Sr | Sb | I | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Namibia | ME | 1 | Henties Bay | 138 | 0.5 | 1.2 | 32 | 1.7 | 61 | 0.9 | 2 | 1 | 0.7 | 13 | 14 | 1.3 | 10 |
| MG | 2 | Henties Bay | 173 | 0.4 | 0.7 | 21 | 2.1 | 37 | 0.3 | 4 | 0.9 | 0.9 | 20 | 22 | 0.7 | 26 | |
| MG | 3 | Swakopmund | 120 | 0.2 | 0.3 | 7 | 0.8 | 25 | 0.2 | 8 | 0.8 | 1.3 | 12 | 13 | 0.6 | 7 | |
| MG | 4 | Walvis Bay | 111 | 1.3 | 1.1 | 22 | 1.2 | 163 | 0.7 | 7 | 1.1 | 1.2 | 12 | 9 | 1 | 35 | |
| Western Cape | MG | 5 | Saldanha outer bay | 165 | 2.4 | 1.1 | 37 | 4.2 | 166 | 1.5 | 5 | 1.3 | 1.4 | 22 | 38 | 3.3 | 21 |
| MG | 6 | Camps Bay | 112 | 0.3 | 0.5 | 18 | 2 | 333 | 1.4 | 3 | 1.1 | 3.7 | 6 | 5 | 0.9 | 9 | |
| MG | 7 | Hout Bay | 117 | 0.6 | 1.6 | 17 | 3.3 | 425 | 2.9 | 6 | 1.3 | 1.3 | 5 | 1 | 2.7 | 9 | |
| MG | 8 | Simonstown | 128 | 4.1 | 1.8 | 32 | 3.6 | 613 | 4.5 | 5 | 0.9 | 2.5 | 11 | 12 | 1.8 | 16 | |
| MG | 9 | Strand | 137 | 2.4 | 0.7 | 32 | 3.4 | 326 | 2 | 5 | 0.7 | 3.1 | 2 | 14 | 2.2 | 12 | |
| MG | 10 | Gordon Bay | 122 | 0.8 | 0.9 | 9 | 3.2 | 479 | 3.3 | 4 | 0.9 | 2.9 | 10 | 13 | 0.9 | 20 | |
| MG | 11 | Pringle Bay | 134 | 0.4 | 1.6 | 36 | 2.9 | 393 | 2.4 | 10 | 1 | 3.2 | 6 | 7 | 1.8 | 14 | |
| Kwa-Zulu-Natal | MG | 12 | East London | 115 | 0.1 | 0.3 | 6 | 0.6 | 19 | 0.3 | 14 | 0.5 | 2 | 14 | 7 | 0.7 | 8 |
| MG | 13 | Port Shepston | 130 | 0.1 | 0.4 | 5 | 0.5 | 59 | 0.8 | 13 | 0.5 | 1.5 | 8 | 5 | 0.6 | 11 | |
| MG | 14 | Richards bay | 146 | 0.1 | 0.2 | 4 | 0.4 | 7 | 0.1 | 15 | 1.2 | 1.8 | 16 | 3 | 1.3 | 14 | |
| Mozambique | MG | 15 | Xai Xai | 125 | 0.1 | 0.1 | 2 | 0.2 | 39 | 0.7 | 10 | 0.7 | 3.6 | 14 | 13 | 1.2 | 9 |
| Ms | 16 | Cabo San Sebastian | 85 | 1.2 | 1 | 50 | 3.3 | 88 | 0.3 | 13 | 0.2 | 1 | 6 | 7 | 0.3 | 9 | |
| Ms | 17 | Vilanculos | 88 | 1 | 0.8 | 22 | 3.1 | 140 | 0.5 | 13 | 0.3 | 3.2 | 7 | 7 | 0.2 | 6 | |
| PTWI [mg/kg bw/week] | 24500 | 2 | 0.02 | 0.98 | 5.6 | 0.7 | 35 | 7 | 0.036 | 0.035 | 4.2 | 0.003 | 0.12 | 0.02 | |||
| Stations | Mn | Fe | Co | Ni | Zn | Se | Sr | Sb | I | U | HI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.08 | 0.35 | 0.01 | 0.21 | 0.22 | 0.01 | 0.01 | 0.09 | 0.01 | 1 |
| 2 | 0.01 | 0.06 | 0.36 | 0.04 | 0.05 | 0.12 | 0.01 | 0 | 0.08 | 0 | 0.72 |
| 3 | 0.02 | 0.15 | 0.48 | 0.03 | 0.03 | 0.08 | 0.01 | 0.01 | 0.15 | 0.01 | 0.95 |
| 4 | 0 | 0.05 | 0.25 | 0.02 | 0.06 | 0.12 | 0.01 | 0.01 | 0.07 | 0 | 0.58 |
| 5 | 0 | 0.02 | 0.23 | 0.01 | 0.08 | 0.09 | 0.01 | 0 | 0.03 | 0 | 0.48 |
| 6 | 0.01 | 0.07 | 0.15 | 0.01 | 0.11 | 0.03 | 0.02 | 0.02 | 0.17 | 0.01 | 0.6 |
| 7 | 0 | 0.04 | 0.09 | 0 | 0.05 | 0.1 | 0.01 | 0.01 | 0.03 | 0.01 | 0.35 |
| 8 | 0 | 0.02 | 0.08 | 0 | 0.06 | 0.05 | 0.01 | 0.01 | 0.06 | 0.01 | 0.31 |
| 9 | 0 | 0.03 | 0.09 | 0 | 0.05 | 0.04 | 0.02 | 0 | 0.05 | 0.01 | 0.3 |
| 10 | 0.01 | 0.05 | 0.1 | 0 | 0.1 | 0.05 | 0.01 | 0.01 | 0.11 | 0.01 | 0.46 |
| 11 | 0 | 0.05 | 0.1 | 0 | 0.05 | 0.05 | 0.02 | 0.02 | 0.08 | 0.01 | 0.37 |
| 12 | 0.02 | 0.24 | 0.92 | 0.02 | 0.03 | 0.08 | 0.01 | 0.02 | 0.19 | 0.01 | 1.55 |
| 13 | 0.03 | 0.34 | 0.8 | 0.02 | 0.03 | 0.1 | 0.01 | 0.02 | 0.22 | 0.01 | 1.59 |
| 14 | 0.04 | 0.36 | 2.64 | 0.05 | 0.03 | 0.06 | 0.01 | 0.03 | 0.11 | 0 | 3.33 |
| 15 | 0.03 | 0.48 | 1 | 0.02 | 0.05 | 0.04 | 0.01 | 0.01 | 0.1 | 0.01 | 1.76 |
| 16 | 0 | 0.05 | 0.36 | 0.02 | 0.04 | 0.16 | 0.02 | 0.02 | 0.45 | 0.01 | 1.12 |
| 17 | 0 | 0.05 | 0.26 | 0.02 | 0.03 | 0.05 | 0.02 | 0.02 | 0.55 | 0.02 | 1.03 |
| RfD | 140 | 700 | 0.3 | 40 | 300 | 5 | 600 | 0.4 | 17 | 3 |
| Namibia | Western Cape | East of SA | Mozambique | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| MPL | ■■■ | ■■ | ■■■ | ■ | ■ | ■ | ■ | ■ | ■■ | ■■ | ■ | ■■ | ■■■ | ■ | ■■ | ■■ | ■ |
| MPCR (<200 g/week) | ■■ | ■ | ■ | ■■■ | ■■■ | ■ | ■ | ||||||||||
| RQ>1 | ■ | ■ | ■■■ | ■■■ | ■ | ■ | |||||||||||
| THQ>1 | ■ | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekhoroshkov, P.; Bezuidenhout, J.; Zinicovscaia, I.; Yushin, N.; Vergel, K.; Frontasyeva, M. Levels of Elements in Typical Mussels from the Southern Coast of Africa (Namibia, South Africa, Mozambique): Safety Aspect. Water 2021, 13, 3238. https://doi.org/10.3390/w13223238
Nekhoroshkov P, Bezuidenhout J, Zinicovscaia I, Yushin N, Vergel K, Frontasyeva M. Levels of Elements in Typical Mussels from the Southern Coast of Africa (Namibia, South Africa, Mozambique): Safety Aspect. Water. 2021; 13(22):3238. https://doi.org/10.3390/w13223238
Chicago/Turabian StyleNekhoroshkov, Pavel, Jacques Bezuidenhout, Inga Zinicovscaia, Nikita Yushin, Konstantin Vergel, and Marina Frontasyeva. 2021. "Levels of Elements in Typical Mussels from the Southern Coast of Africa (Namibia, South Africa, Mozambique): Safety Aspect" Water 13, no. 22: 3238. https://doi.org/10.3390/w13223238
APA StyleNekhoroshkov, P., Bezuidenhout, J., Zinicovscaia, I., Yushin, N., Vergel, K., & Frontasyeva, M. (2021). Levels of Elements in Typical Mussels from the Southern Coast of Africa (Namibia, South Africa, Mozambique): Safety Aspect. Water, 13(22), 3238. https://doi.org/10.3390/w13223238








