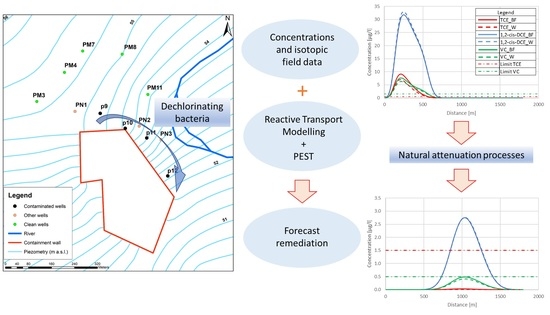
Evaluation of MNA in A Chlorinated Solvents-Contaminated Aquifer Using Reactive Transport Modeling Coupled with Isotopic Fractionation Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Case
2.2. Field Case
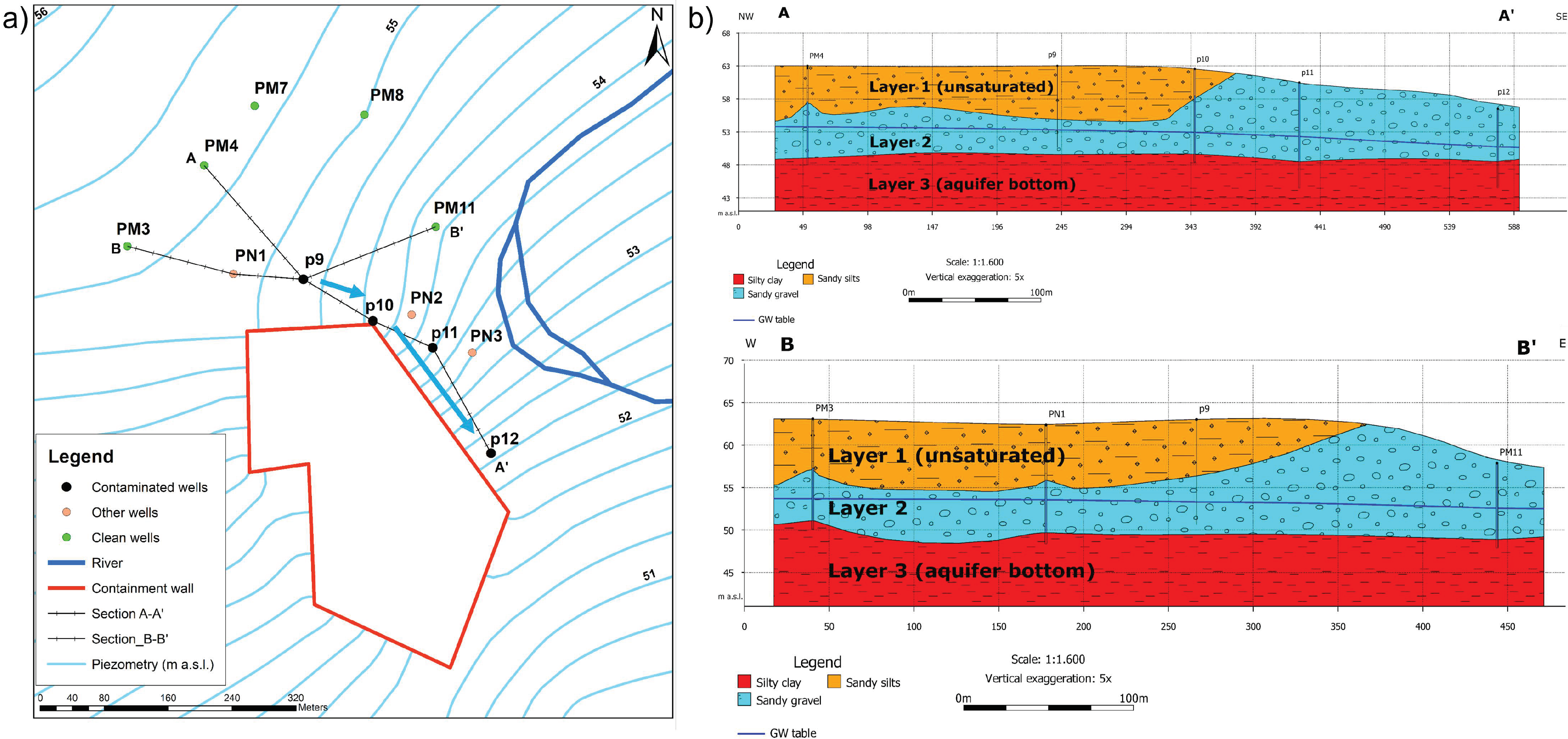
2.2.1. Hydrogeological Setting
- Sandy silts: consisting of recent Holocenic alluvial sediments, this layer is composed of sandy silts and silty fine sands, with an average thickness of 5 m. The hydraulic conductivity was derived from an in situ pumping test and estimated to be around 10−7 m/s. This layer is unsaturated.
- Sandy gravels: also consisting of recent Holocenic alluvial sediments, this layer is composed of polygenic gravels with sub-rounded clasts in sandy or silty sandy matrix. The hydraulic conductivity of the layer was derived from an in situ pumping test and estimated to be around 10−4 m/s. This layer hosts the phreatic aquifer that is characterized by a saturated thickness between 3 m and 5.30 m; the total thickness of the layer in the area is about 5–10 m.
- Silty clay: consisting of Pliocenic sediments, this layer reaches considerable thickness (greater than 50 m). This is considered the bottom of the aquifer (low permeability boundary), since is consisting of not permeable alluvial materials with a hydraulic conductivity estimated (from literature analyses) around 10−8–10−11 m/s.
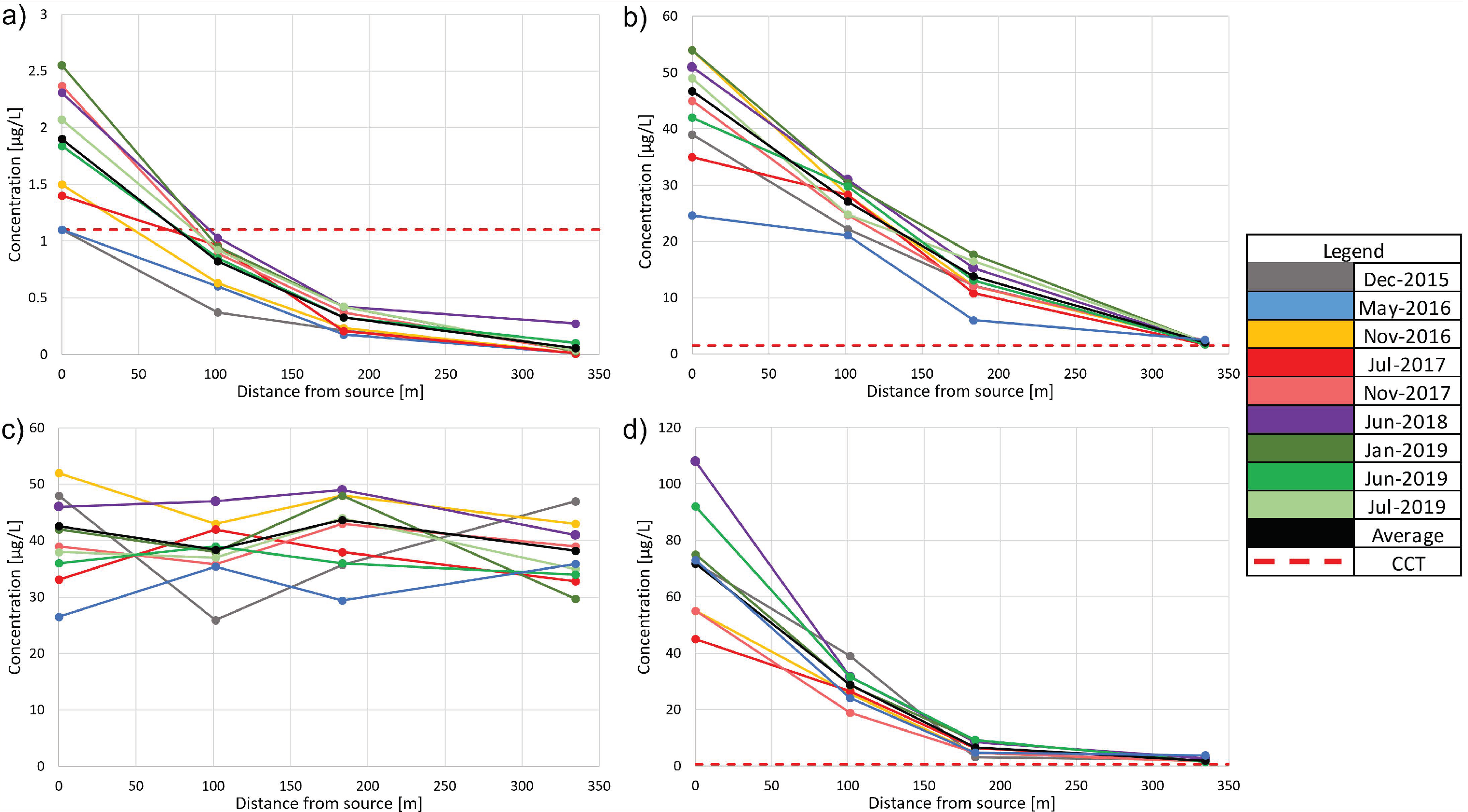
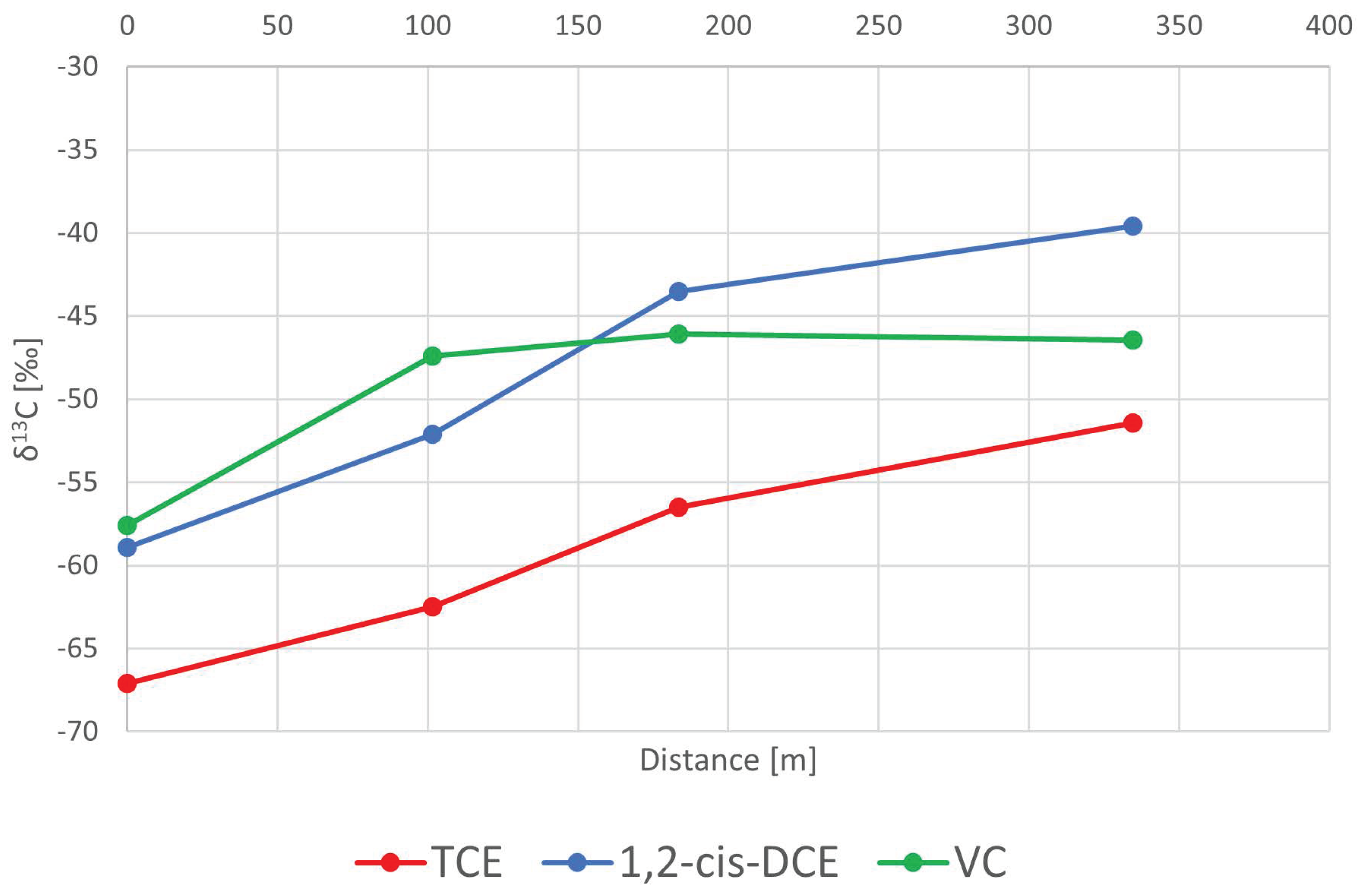
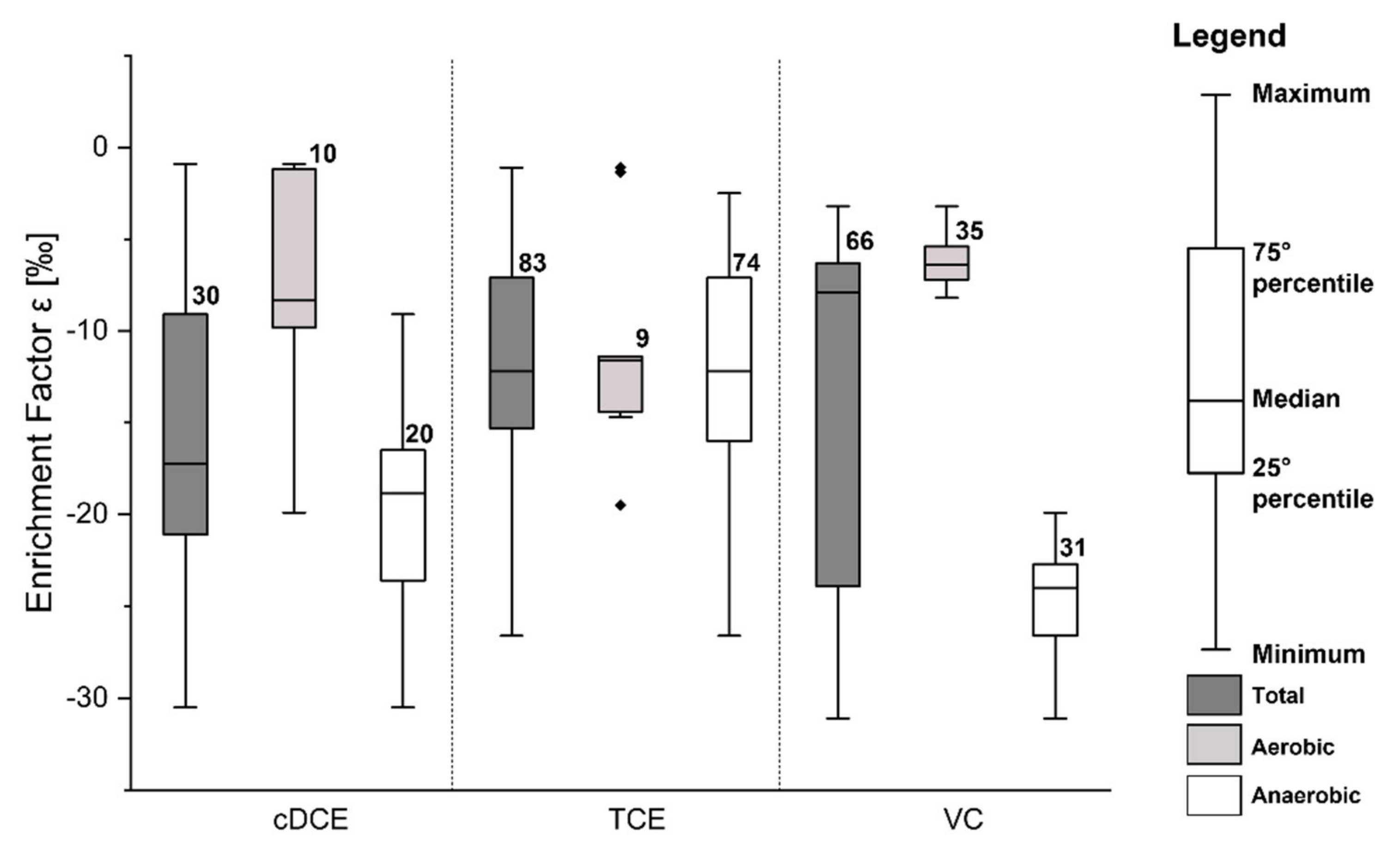
2.2.2. Analysis of Historical Concentrations and Isotopic Composition
2.2.3. Conceptual Site Model Setup
2.2.4. Analytical and Numerical Model Setup
- 1D simulations, due to the simple hydrogeological setting
- homogeneous aquifer properties
- the degradation follows a 1st order kinetic with a 1:1 molar stoichiometry
- degradation rate constants and enrichment factors are set constant
- the only degradation process is the reductive dechlorination, with a simplified reaction chain, i.e., only TCE, 1,2-cis-DCE, and VC are produced and consumed
- the degradation takes action only in the dissolved phase
- the source is located in proximity to well p9 and contains TCE, 1,2-cis-DCE, and VC.
2.2.5. Model Calibration
2.2.6. Predictive Model
3. Results
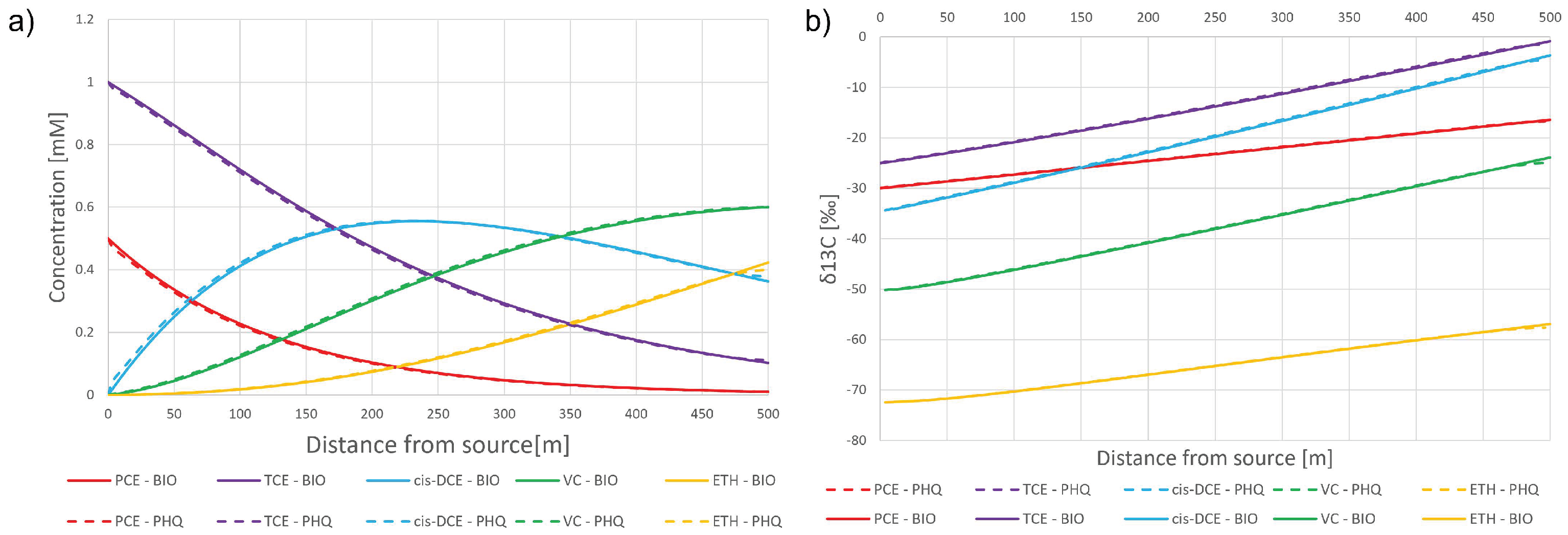
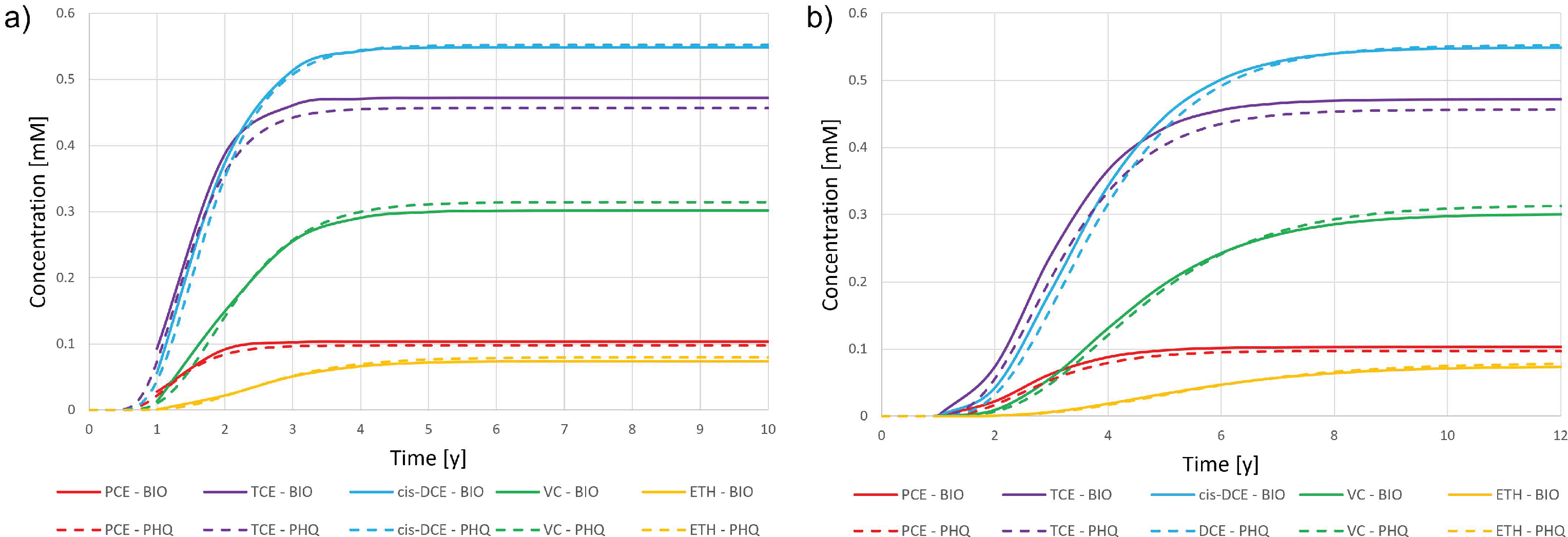
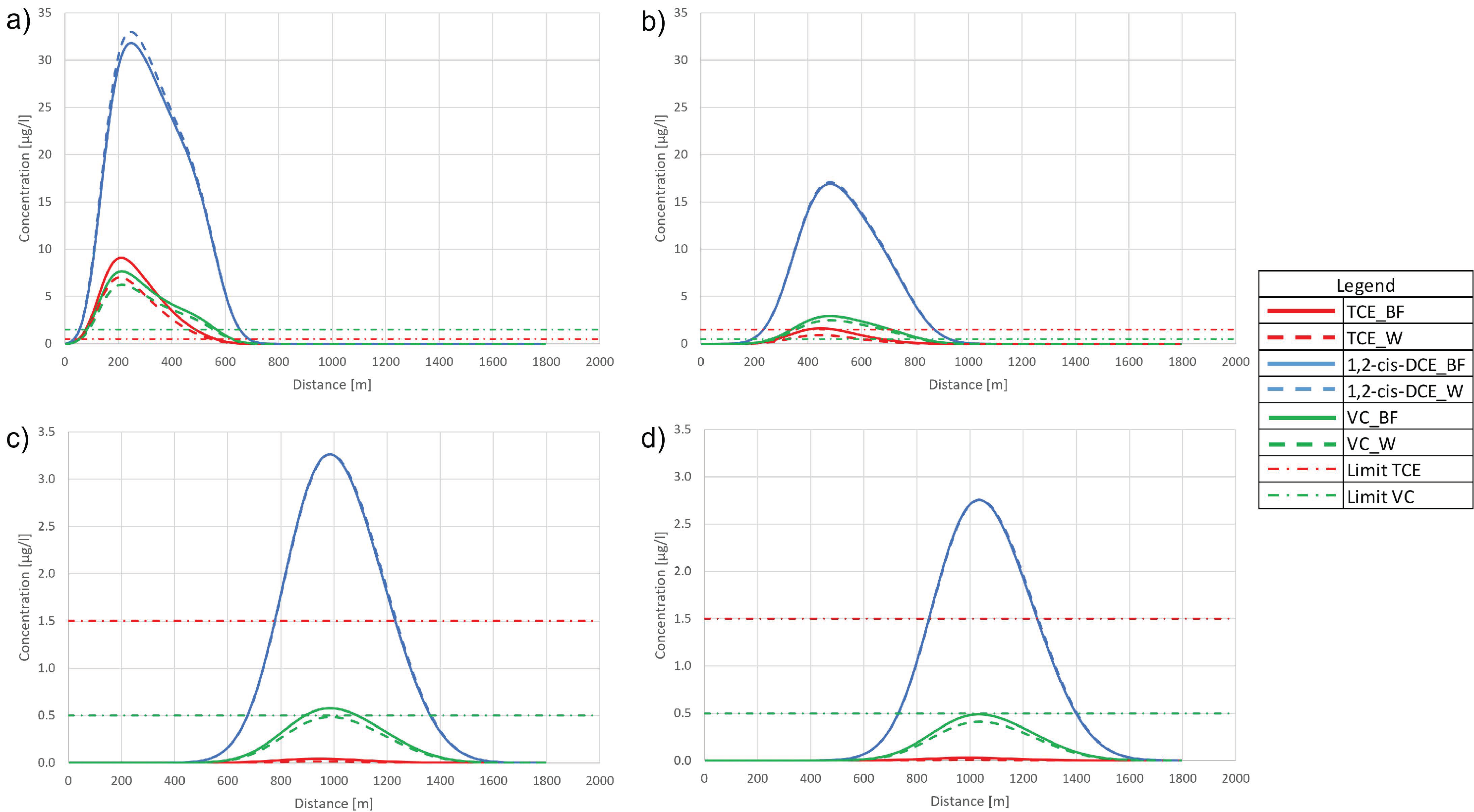
3.1. Synthetic Case
3.1.1. Case 1
3.1.2. Case 2
3.2. Real Case
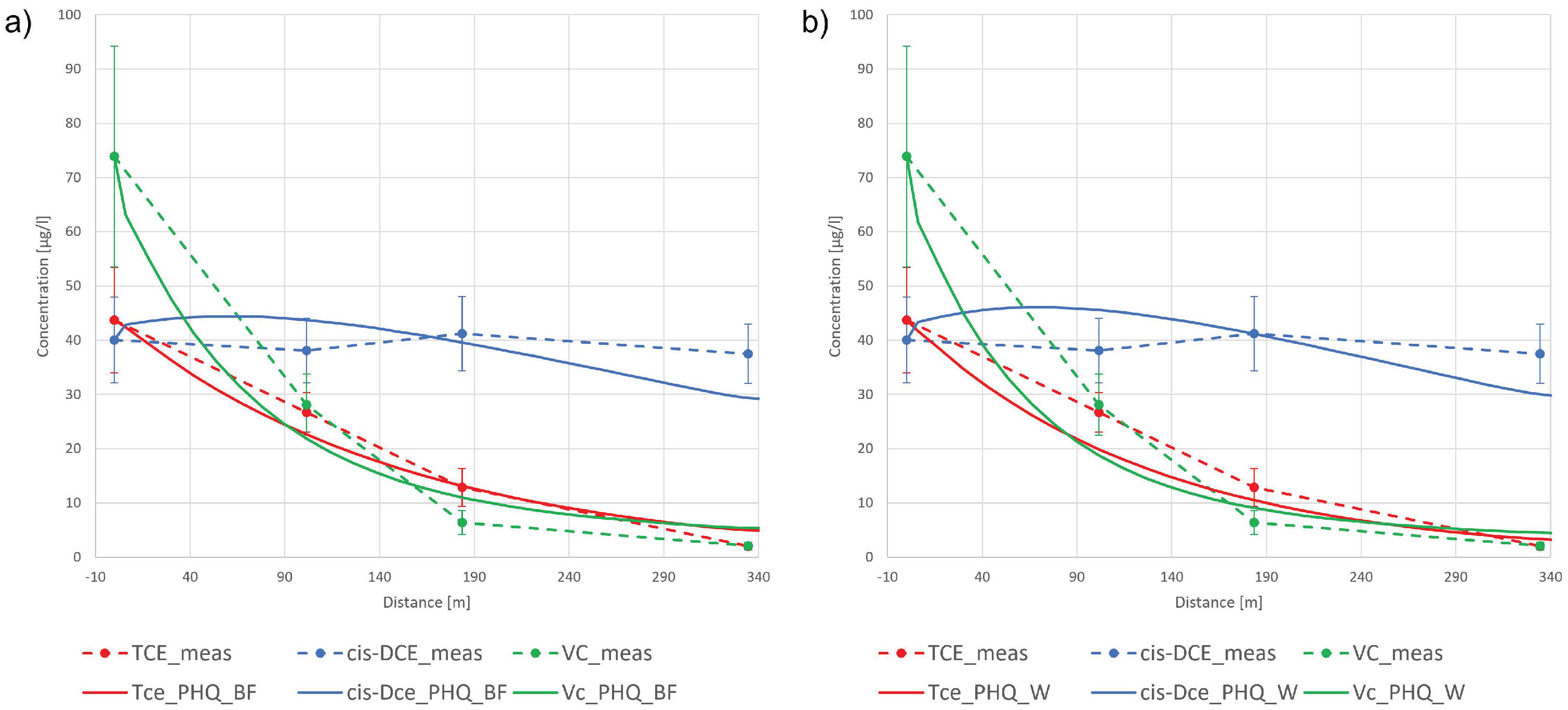
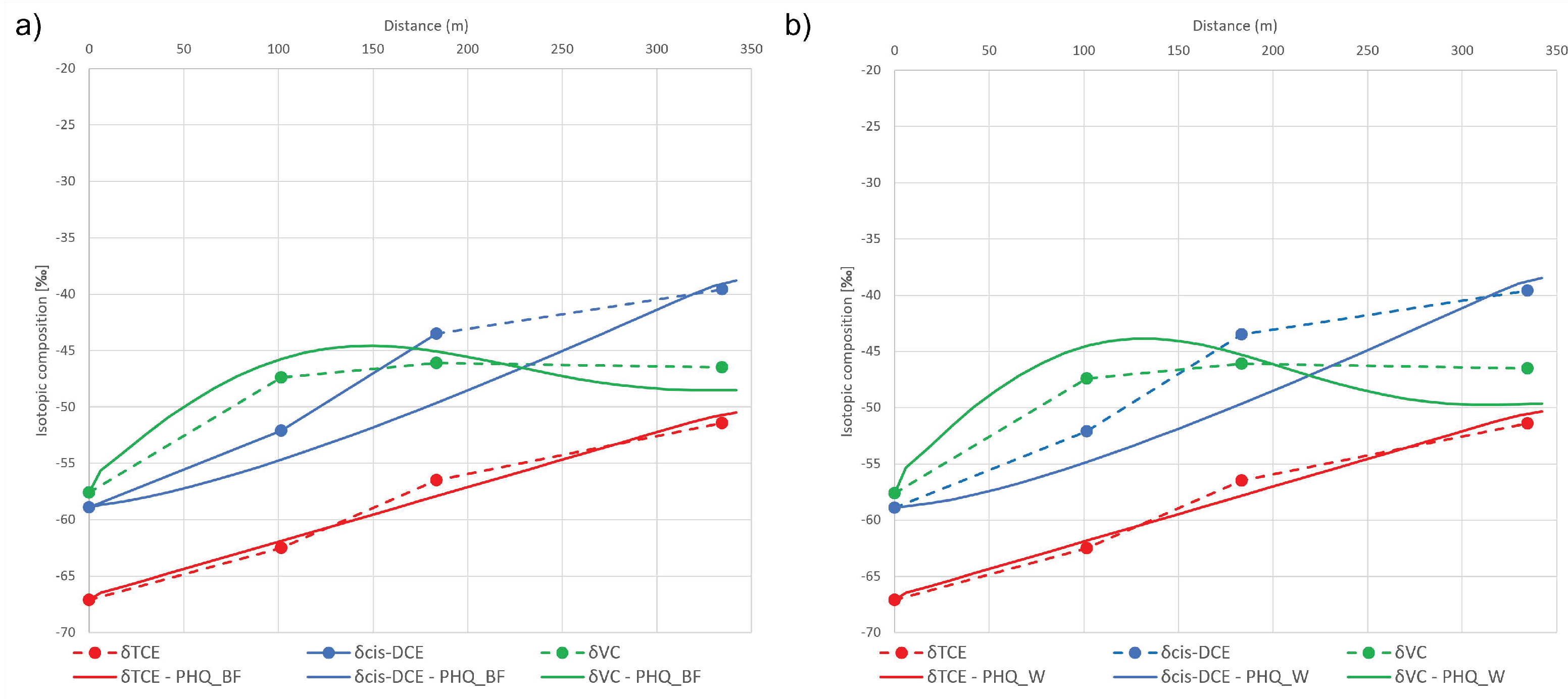
3.2.1. Interpretative Model
3.2.2. Predictive Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Doherty, R.E. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 2—Trichloroethylene and 1,1,1-Trichloroethane. Environ. Forensics 2000, 1, 83–93. [Google Scholar] [CrossRef]
- Doherty, R.E. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1—Historical Background; Carbon Tetrachloride and Tetrachloroethylene. Environ. Forensics 2000, 1, 69–81. [Google Scholar] [CrossRef]
- Mccarty, P.L. Groundwater Contamination by Chlorinated Solvents: History, Remediation Technologies and Strategies; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–28. [Google Scholar]
- Stroo, H.F.; Ward, C.H. In Situ Remediation of Chlorinated Solvent Plumes; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 1441914013. [Google Scholar]
- Wiedemeier, T.; Swanson, M.; Moutoux, D.; Gordon, E.; Wilson, J.; Wilson, B.; Kampbell, D.; Haas, P.; Miller, R.; Hansen, J.; et al. Technical Protocol for Evaluating Natural Attenuation of Chlorinated Solvents in Ground Water; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Zanini, A.; Ghirardi, M.; Emiliani, R. A multidisciplinary approach to evaluate the effectiveness of natural attenuation at a contaminated site. Hydrology 2021, 8, 101. [Google Scholar] [CrossRef]
- Maymó-Gatell, X.; Chien, Y.T.; Gossett, J.M.; Zinder, S.H. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 1997, 276, 1568–1571. [Google Scholar] [CrossRef]
- He, J.; Ritalahti, K.M.; Aiello, M.R.; Löffler, F.E. Complete detoxification of vinyl chloride by an anaerobic enrichment culture and identification of the reductively dechlorinating population as a Dehalococcoides species. Appl. Environ. Microbiol. 2003, 69, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Němeček, J.; Marková, K.; Špánek, R.; Antoš, V.; Kozubek, P.; Lhotský, O.; Černík, M. Hydrochemical Conditions for Aerobic/Anaerobic Biodegradation of Chlorinated Ethenes—A Multi-Site Assessment. Water 2020, 12, 322. [Google Scholar] [CrossRef] [Green Version]
- Dempster, H.S.; Lollar, B.S.; Feenstra, S. Tracing organic contaminants in groundwater: A new methodology using compound-specific isotopic analysis. Environ. Sci. Technol. 1998, 31, 3193–3197. [Google Scholar] [CrossRef]
- Hunkeler, D.; Aravena, R. Determination of compound-specific carbon isotope ratios of chlorinated methanes, ethanes, and ethenes in aqueous samples. Environ. Sci. Technol. 2000, 34, 2839–2844. [Google Scholar] [CrossRef] [Green Version]
- Hunkeler, D.; Aravena, R.; Butler, B.J. Monitoring microbial dechlorination of tetrachloroethene (PCE) in groundwater using compound-specific stable carbon isotope ratios: Microcosm and field studies. Environ. Sci. Technol. 1999, 33, 2733–2738. [Google Scholar] [CrossRef] [Green Version]
- Hunkeler, D.; Aravena, R.; Parker, B.L.; Cherry, J.A.; Diao, X. Monitoring oxidation of chlorinated ethenes by permanganate in groundwater using stable isotopes: Laboratory and field studies. Environ. Sci. Technol. 2003, 37, 798–804. [Google Scholar] [CrossRef] [Green Version]
- Aelion, C.M.; Höhener, P.; Hunkeler, D.; Aravena, R. Environmental Isotopes in Biodegradation and Bioremediation; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420012613. [Google Scholar]
- Looney, B.; Heitkamp, M.M.; Wein, G.; Bagwell, C.C.; Vangelas, K.K.; Adams, K.K.-M.; Gilmore, T.; Cutshall, N.; Major, D.; Truex, M.; et al. Characterization and Monitoring of Natural Attenuation of Chlorinated Solvents in Groundwater: A Systems Approach; Pubinfo: Aiken, SC, USA, 2006. [Google Scholar]
- Declercq, I.; Cappuyns, V.; Duclos, Y. Monitored natural attenuation (MNA) of contaminated soils: State of the art in Europe—A critical evaluation. Sci. Total Environ. 2012, 426, 393–405. [Google Scholar] [CrossRef]
- Rügner, H.; Finkel, M.; Kaschl, A.; Bittens, M. Application of monitored natural attenuation in contaminated land management-A review and recommended approach for Europe. Environ. Sci. Policy 2006, 9, 568–576. [Google Scholar] [CrossRef]
- Clement, T.P.; Truex, M.J.; Lee, P. A case study for demonstrating the application of U.S. EPA’s monitored natural attenuation screening protocol at a hazardous waste site. J. Contam. Hydrol. 2002, 59, 133–162. [Google Scholar] [CrossRef]
- Aziz, C.E.; Newell, C.J.; Gonzales, J.R.; Haas, P.; Clement, T.P.; Sun, Y.; Jewett, D.G. BIOCHLOR Natural Attenuation Decision Support System; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Colombani, N.; Gargini, A.; Mastrocicco, M. The use of monitored natural attenuation as a cost-effetive technique for groundwater restoration: The NIT site (ITALY). In Proceedings of the 1st International Workshop on Aquifer Vulnerability and Risk, Mexico City, Mexico, 28–30 May 2003. [Google Scholar]
- McGuire, T.M.; Newell, C.J.; Looney, B.B.; Vangelas, K.M. Historical and Retrospective Survey of Monitored Natural Attenuation: A Line of Inquiry Supporting Monitored Natural Attenuation and Enhanced Passive Remediation of Chlorinated Solvents; Savannah River Site, Funding Organisation, US Department of Energy: Washington, DC, USA, 2003.
- Newell, C.J.; Cowie, I.; McGuire, T.M.; McNab, W.W. Multiyear Temporal Changes in Chlorinated Solvent Concentrations at 23 Monitored Natural Attenuation Sites. J. Environ. Eng. 2006, 132, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, D.; Herrero, J.; Parker, B.L.; Carmona, J.M. Natural attenuation of pools and plumes of carbon tetrachloride and chloroform in the transition zone to bottom aquitards and the microorganisms involved in their degradation. Sci. Total Environ. 2020, 712, 135679. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Chen, R.; Deng, S.; Yu, R.; Long, T. Assessment of Biodegradation in Natural Attenuation Process of Chlorinated Hydrocarbons Contaminated Site: An Anaerobic Microcosm Study. Soil Sediment Contam. 2020, 29, 165–179. [Google Scholar] [CrossRef]
- Ferrey, M.F.M.; Wilson, J.T. Complete Natural Attenuation of PCE and TCE without Vinyl Chloride and Ethene Accumulation. Available online: https://www.researchgate.net/publication/259366445_Complete_Natural_Attenuation_of_PCE_and_TCE_without_Vinyl_Chloride_and_Ethene_Accumulation (accessed on 15 September 2020).
- Kawabe, Y.; Komai, T. A Case Study of Natural Attenuation of Chlorinated Solvents Under Unstable Groundwater Conditions in Takahata, Japan. Bull. Environ. Contam. Toxicol. 2019, 102, 280–286. [Google Scholar] [CrossRef]
- Schiefler, A.A.; Tobler, D.J.; Overheu, N.D.; Tuxen, N. Extent of natural attenuation of chlorinated ethenes at a contaminated site in Denmark. Energy Procedia 2018, 146, 188–193. [Google Scholar] [CrossRef]
- Kao, C.M.; Wang, Y.S. Field investigation of the natural attenuation and intrinsic biodegradation rates at an underground storage tank site. Environ. Geol. 2001, 40, 622–631. [Google Scholar] [CrossRef]
- Kao, C.M.; Huang, W.Y.; Chang, L.J.; Chen, T.Y.; Chien, H.Y.; Hou, F. Application of monitored natural attenuation to remediate a petroleum-hydrocarbon spill site. Water Sci. Technol. 2006, 53, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, E.F.; Ripp, J.A.; Azzolina, N.A.; Madsen, E.L.; Mauro, D.M.; Taylor, T. Monitored Natural Attenuation of Manufactured Gas Plant Tar Mono- and Polycyclic Aromatic Hydrocarbons in Ground Water: A 14-Year Field Study. Ground Water Monit. Remediat. 2009, 29, 66–76. [Google Scholar] [CrossRef]
- Brown, R.A.; Wilson, J.T.; Ferrey, M. Monitored natural attenuation forum: The case for abiotic MNA. Remediat. J. 2007, 17, 127–137. [Google Scholar] [CrossRef]
- Steefel, C.I.; Appelo, C.A.J.; Arora, B.; Jacques, D.; Kalbacher, T.; Kolditz, O.; Lagneau, V.; Lichtner, P.C.; Mayer, K.U.; Meeussen, J.C.L.; et al. Reactive transport codes for subsurface environmental simulation. Comput. Geosci. 2015, 19, 445–478. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Arora, B.; Şengör, S.S.; Druhan, J.L.; Wanner, C.; van Breukelen, B.M.; Steefel, C.I. Microbially mediated kinetic sulfur isotope fractionation: Reactive transport modeling benchmark. Comput. Geosci. 2020, 25, 1–13. [Google Scholar] [CrossRef]
- Younes, A.; Fahs, M.; Ackerer, P. Modeling of Flow and Transport in Saturated and Unsaturated Porous Media. Water 2021, 13, 1088. [Google Scholar] [CrossRef]
- Xu, T.; Ye, Y.; Zhang, Y.; Xie, Y. Recent advances in experimental studies of steady-state dilution and reactive mixing in saturated porous media. Water 2018, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Newell, C.J.; Mcleod, R.K.; Gonzales, J.R.; Wilson, J.T. BIOSCREEN Natural Attenuation Decision Support System; U.S. Environmental Protection Agency: Washington, DC, USA, 1996.
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, And Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1999.
- Prommer, H.; Davis, G.B.; Barry, D.A. PHT3D—A Three-Dimensional Biogeochemical Transport Model for Modelling Natural and Enhanced Remediation; Centre for Groundwater Studies, CSIRO: Canberra, Australia, 1999; pp. 21–25. [Google Scholar]
- Prommer, H.; Barry, D.A.; Chiang, W.H.; Zheng, C. PHT3D-A MODFLOW/MTBDMS-based reactive multi ‘component transport model. Groundwater 2001, 41, 477–483. [Google Scholar]
- Prommer, H.; Barry, D.A.; Zheng, C. MODFLOW/MT3DMS-based reactive multicomponent transport modeling. Ground Water 2003, 41, 247–257. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Rolle, M. PHT3D: A reactive multicomponent transport model for saturated porous media. Ground Water 2010, 48, 627–632. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, P. MT3DMS: A Modular Three-Dimensional Multispeces Transport Model for Simulation of Advection, Dispersion, and Chemical Reactions of Contaminants in Groundwater Systems; Technical Report; Waterways Experiment Station, US Army Corps of Engineers: Washington, DC, USA, 1999. [Google Scholar]
- Harbaugh, A.W. MODFLOW-2005, The U.S. Geological Survey Modular Ground-Water Model-the Ground-Water Flow Process; USG: Reston, VA, USA, 2005. [Google Scholar]
- Clement, T.P.; Sun, Y.; Hooker, B.S.; Petersen, J.N. Modeling multispecies reactive transport in ground water. Gr. Water Monit. Remediat. 1998, 18, 79–92. [Google Scholar] [CrossRef]
- Kolditz, O.; Bauer, S.; Bilke, L.; Böttcher, N.; Delfs, J.O.; Fischer, T.; Görke, U.J.; Kalbacher, T.; Kosakowski, G.; McDermott, C.I.; et al. OpenGeoSys: An open-source initiative for numerical simulation of thermo-hydro-mechanical/chemical (THM/C) processes in porous media. Environ. Earth Sci. 2012, 67, 589–599. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Chen, Z.; Shao, H.; Kuschk, P.; Kolditz, O. Multi-Component Reactive Transport Modeling of Pce Degradation at a Pilot Scale Constructed Wetland Bitterfeld; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2012; pp. 1–10. [Google Scholar]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOURGHREACT: A Simulation Program for Non-isothermal Multiphase Reactive Geochemical Transport in Variably Saturated Geologic Media. Comput. Geosci. 2004, 32, 1–203. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.U. A Numerical Model for Multicomponent Reactive Transport in Variably Saturated Porous Media; University of Waterloo: Waterloo, ON, Canada, 1999. [Google Scholar]
- Mayer, K.U.; Frind, E.O.; Blowes, D.W. Multicomponent reactive transport modeling in variably saturated porous media using a generalized formulation for kinetically controlled reactions. Water Resour. Res. 2002, 38, 1–21. [Google Scholar] [CrossRef]
- Henderson, T.H.; Mayer, K.U.; Parker, B.L.; Al, T.A. Three-dimensional density-dependent flow and multicomponent reactive transport modeling of chlorinated solvent oxidation by potassium permanganate. J. Contam. Hydrol. 2009, 106, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Höhener, P.; Elsner, M.; Eisenmann, H.; Atteia, O. Improved constraints on in situ rates and on quantification of complete chloroethene degradation from stable carbon isotope mass balances in groundwater plumes. J. Contam. Hydrol. 2015, 182, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Höhener, P. Simulating stable carbon and chlorine isotope ratios in dissolved chlorinated groundwater pollutants with BIOCHLOR-ISO. J. Contam. Hydrol. 2016, 195, 52–61. [Google Scholar] [CrossRef]
- Wexler, E.J. Analytical Solutions for One-, Two-, and Three-dimensional Solute Transport in Ground-Water Systems with Uniform Flow; USGS: Reston, VA, USA, 1992.
- Höhener, P.; Atteia, O. Multidimensional analytical models for isotope ratios in groundwater pollutant plumes of organic contaminants undergoing different biodegradation kinetics. Adv. Water Resour. 2010, 33, 740–751. [Google Scholar] [CrossRef]
- Höhener, P.; Atteia, O. Rayleigh equation for evolution of stable isotope ratios in contaminant decay chains. Geochim. Cosmochim. Acta 2014, 126, 70–77. [Google Scholar] [CrossRef]
- Van Breukelen, B.M.; Hunkeler, D.; Volkering, F. Quantification of sequential chlorinated ethene degradation by use of a reactive transport model incorporating isotope fractionation. Environ. Sci. Technol. 2005, 39, 4189–4197. [Google Scholar] [CrossRef] [Green Version]
- Van Breukelen, B.M.; Thouement, H.A.A.; Stack, P.E.; Vanderford, M.; Philp, P.; Kuder, T. Modeling 3D-CSIA data: Carbon, chlorine, and hydrogen isotope fractionation during reductive dechlorination of TCE to ethene. J. Contam. Hydrol. 2017, 204, 79–89. [Google Scholar] [CrossRef]
- Appelo, C.; de Vet, W. Modeling in situ iron removal from groundwater with trace elements such as As. In Arsenic in Ground Water; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; pp. 381–401. [Google Scholar]
- Appelo, C.A.J.; van der Weiden, M.J.J.; Tournassat, C.; Charlet, L. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ. Sci. Technol. 2002, 36, 3096–3103. [Google Scholar] [CrossRef]
- Sracek, O.; Bhattacharya, P.; Jacks, G.; Gustafsson, J.P.; von Brömssen, M. Behavior of arsenic and geochemical modeling of arsenic enrichment in aqueous environments. Appl. Geochem. 2004, 19, 169–180. [Google Scholar] [CrossRef]
- Stollenwerk, K.G.; Breit, G.N.; Welch, A.H.; Yount, J.C.; Whitney, J.W.; Foster, A.L.; Uddin, M.N.; Majumder, R.K.; Ahmed, N. Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci. Total Environ. 2007, 379, 133–150. [Google Scholar] [CrossRef]
- Charlet, L.; Chakraborty, S.; Appelo, C.A.J.; Roman-Ross, G.; Nath, B.; Ansari, A.A.; Lanson, M.; Chatterjee, D.; Mallik, S.B. Chemodynamics of an arsenic “hotspot” in a West Bengal aquifer: A field and reactive transport modeling study. Appl. Geochem. 2007, 22, 1273–1292. [Google Scholar] [CrossRef]
- Liu, Y.; Goor, J.; Robinson, C.E. Behaviour of soluble reactive phosphorus within field-scale bioretention systems. J. Hydrol. 2021, 601, 126597. [Google Scholar] [CrossRef]
- Zamane, S.; Gori, D.; Höhener, P. Multistep partitioning causes significant stable carbon and hydrogen isotope effects during volatilization of toluene and propan-2-ol from unsaturated sandy aquifer sediment. Chemosphere 2020, 251, 126345. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, C.; Long, Y.; Yang, Q.; Zhou, S.; Wu, P.; Jiang, J.; Zhou, W.; Hu, X. Interaction analysis of hydrochemical factors and dissolved heavy metals in the karst Caohai Wetland based on PHREEQC, cooccurrence network and redundancy analyses. Sci. Total Environ. 2021, 770, 145361. [Google Scholar] [CrossRef]
- Sprocati, R.; Masi, M.; Muniruzzaman, M.; Rolle, M. Modeling electrokinetic transport and biogeochemical reactions in porous media: A multidimensional Nernst-Planck-Poisson approach with PHREEQC coupling. Adv. Water Resour. 2019, 127, 134–147. [Google Scholar] [CrossRef]
- Aeppli, C.; Hofstetter, T.B.; Amaral, H.I.F.; Kipfer, R.; Schwarzenbach, R.P.; Berg, M. Quantifying in situ transformation rates of chlorinated ethenes by combining compound-specific stable isotope analysis, groundwater dating, and carbon isotope mass balances. Environ. Sci. Technol. 2010, 44, 3705–3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antelmi, M.; Renoldi, F.; Alberti, L. Analytical and Numerical Methods for a Preliminary Assessment of the Remediation Time of Pump and Treat Systems. Water 2020, 12, 2850. [Google Scholar] [CrossRef]
- Filippini, M.; Nijenhuis, I.; Kümmel, S.; Chiarini, V.; Crosta, G.; Richnow, H.H.; Gargini, A. Multi-element compound specific stable isotope analysis of chlorinated aliphatic contaminants derived from chlorinated pitches. Sci. Total Environ. 2018, 640–641, 153–162. [Google Scholar] [CrossRef]
- Slater, G.F.; Ahad, J.M.E.; Sherwood Lollar, B.; Allen-King, R.; Sleep, B. Carbon isotope effects resulting from equilibrium sorption of dissolved VOCs. Anal. Chem. 2000, 72, 5669–5672. [Google Scholar] [CrossRef]
- Schüth, C.; Taubald, H.; Bolaño, N.; Maciejczyk, K. Carbon and hydrogen isotope effects during sorption of organic contaminants on carbonaceous materials. J. Contam. Hydrol. 2003, 64, 269–281. [Google Scholar] [CrossRef]
- Druhan, J.L.; Winnick, M.J. Reactive transport of stable isotopes. Elements 2019, 15, 107–110. [Google Scholar] [CrossRef]
- Mattavelli, L.; Novelli, L. Geochemistry and habitat of natural gases in Italy. Org. Geochem. 1988, 13, 1–13. [Google Scholar] [CrossRef]
- Nijenhuis, I.; Schmidt, M.; Pellegatti, E.; Paramatti, E.; Richnow, H.H.; Gargini, A. A stable isotope approach for source apportionment of chlorinated ethene plumes at a complex multi-contamination events urban site. J. Contam. Hydrol. 2013, 153, 92–105. [Google Scholar] [CrossRef]
- Buscheck, T.E.; Alcantar, C.M. Regression Techniques and Analytical Solutions to Demonstrate Intrinsic Bioremediation; Battelle Press: Columbus, OH, USA, 1995; ISBN 1-57477-002-0. [Google Scholar]
- Tonkin, M.; Doherty, J. Calibration-constrained Monte Carlo analysis of highly parameterized models using subspace techniques. Water Resour. Res. 2009, 45, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Doherty, J. PEST Model-Independent Parameter Estimation; Watermark Numerical Computing: Brisbane, Australia, 2004. [Google Scholar]
- Van Breukelen, B.M.; Griffioen, J.; Röling, W.F.M.; van Verseveld, H.W. Reactive transport modelling of biogeochemical processes and carbon isotope geochemistry inside a landfill leachate plume. J. Contam. Hydrol. 2004, 70, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Yabusaki, S.B.; Fang, Y.; Long, P.E.; Resch, C.T.; Peacock, A.D.; Komlos, J.; Jaffe, P.R.; Morrison, S.J.; Dayvault, R.D.; White, D.C.; et al. Uranium removal from groundwater via in situ biostimulation: Field-scale modeling of transport and biological processes. J. Contam. Hydrol. 2007, 93, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Prommer, H.; Aziz, L.H.; Bolaño, N.; Taubald, H.; Schüth, C. Modelling of geochemical and isotopic changes in a column experiment for degradation of TCE by zero-valent iron. J. Contam. Hydrol. 2008, 97, 13–26. [Google Scholar] [CrossRef]
- Antoniou, E.A.; Stuyfzand, P.J.; van Breukelen, B.M. Reactive transport modeling of an aquifer storage and recovery (ASR) pilot to assess long-term water quality improvements and potential solutions. Appl. Geochem. 2013, 35, 173–186. [Google Scholar] [CrossRef]
- Kavousi, A.; Reimann, T.; Liedl, R.; Raeisi, E. Karst aquifer characterization by inverse application of MODFLOW-2005 CFPv2 discrete-continuum flow and transport model. J. Hydrol. 2020, 587, 124922. [Google Scholar] [CrossRef]
- Kruisdijk, E.; van Breukelen, B.M. Reactive transport modelling of push-pull tests: A versatile approach to quantify aquifer reactivity. Appl. Geochem. 2021, 131, 104998. [Google Scholar] [CrossRef]
- Colombo, L.; Alberti, L.; Mazzon, P.; Antelmi, M. Null-Space Monte Carlo Particle Backtracking to Identify Groundwater Tetrachloroethylene Sources. Front. Environ. Sci. 2020, 8, 142. [Google Scholar] [CrossRef]
- Suarez, M.P.; Rifai, H.S. Biodegradation rates for fuel Hydrocarbons and Chlorinated Solvents in groundwater. Bioremediat. J. 1999, 3, 337–362. [Google Scholar] [CrossRef]
- Buscheck, T.E.; Alcantar, C.M. Regression Techniques and Analytical Solutions to Demonstrate; International Nuclear Information System (INIS): Vienna, Austria, 1995. [Google Scholar]
| Case | RTM 1 | Sorption | IF 2 |
|---|---|---|---|
| Case 1 | ✓ | ✗ | ✓ |
| Case 2 | ✓ | ✓ | ✗ |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Domain length | L | 334 | m |
| Total simulation time | - | 50 | y |
| Average pore water velocity | m/s | ||
| Hydraulic conductivity | m/s | ||
| Hydraulic gradient | 0.003 | - | |
| Effective porosity | θ | 0.2 | - |
| Longitudinal dispersivity | 10 | m | |
| Transversal and vertical dispersivity | 0 | m | |
| Bulk density | 1.7 | g/cm3 | |
| Fraction of organic carbon 1 | 0.0034 | - |
| Parameter | Interpretative Model | Forecast Model | Unit |
|---|---|---|---|
| Value | |||
| Cell number | 29 | 150 | - |
| Shift number 1 | 350 | 79 | - |
| Time step length | 4,444,444 | 4,444,444 | s |
| Flow direction | Forward | forward | - |
| Boundary Conditions (BCs) | constant—flux | constant—flux | - |
| Cell lengths | 12 | 12 | m |
| Stress Period | Period | Duration (Days) | Initial Conditions |
|---|---|---|---|
| SP1 | Pre-source removal | 204 | Steady-state concentrations |
| SP2 | During source removal | 204 | Half of the steady-state concentrations |
| SP3 | Post-source removal | variable 1 | Null |
| Compound | Pre-Calibration | Best Fit | Weighted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| λ [1/y] | λ/R [1/y] | RMSE [µg/L] | λ [1/y] | λ/R [1/y] | RMSE [µg/L] | λ [1/y] | λ/R [1/y] | RMSE [µg/L] | |
| TCE | 3.20 | 0.86 | 5.61 | 2.22 | 0.59 | 2.53 | 2.64 | 0.71 | 1.45 |
| 1,2-cis-DCE | 0.045 | 0.012 | 25.01 | 1.040 | 0.28 | 4.52 | 1.02 | 0.27 | 3.32 |
| VC | 3.85 | 1.03 | 3.11 | 4.80 | 1.28 | 4.01 | 5.43 | 1.45 | 2.58 |
| Compound | Pre-Calibration | Best Fit | Weighted | |||
|---|---|---|---|---|---|---|
| ε [‰] | RMSE [‰] | ε [‰] | RMSE [‰] | ε [‰] | RMSE [‰] | |
| TCE | −22.9 | 28.88 | −8.2 | 1.32 | −7.1 | 1.35 |
| 1,2-cis-DCE | −30.5 | 16.89 | −30.5 | 3.50 | −30.5 | 3.26 |
| VC | −31.2 | 43.34 | −15.9 | 0.66 | −16.0 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antelmi, M.; Mazzon, P.; Höhener, P.; Marchesi, M.; Alberti, L. Evaluation of MNA in A Chlorinated Solvents-Contaminated Aquifer Using Reactive Transport Modeling Coupled with Isotopic Fractionation Analysis. Water 2021, 13, 2945. https://doi.org/10.3390/w13212945
Antelmi M, Mazzon P, Höhener P, Marchesi M, Alberti L. Evaluation of MNA in A Chlorinated Solvents-Contaminated Aquifer Using Reactive Transport Modeling Coupled with Isotopic Fractionation Analysis. Water. 2021; 13(21):2945. https://doi.org/10.3390/w13212945
Chicago/Turabian StyleAntelmi, Matteo, Pietro Mazzon, Patrick Höhener, Massimo Marchesi, and Luca Alberti. 2021. "Evaluation of MNA in A Chlorinated Solvents-Contaminated Aquifer Using Reactive Transport Modeling Coupled with Isotopic Fractionation Analysis" Water 13, no. 21: 2945. https://doi.org/10.3390/w13212945
APA StyleAntelmi, M., Mazzon, P., Höhener, P., Marchesi, M., & Alberti, L. (2021). Evaluation of MNA in A Chlorinated Solvents-Contaminated Aquifer Using Reactive Transport Modeling Coupled with Isotopic Fractionation Analysis. Water, 13(21), 2945. https://doi.org/10.3390/w13212945