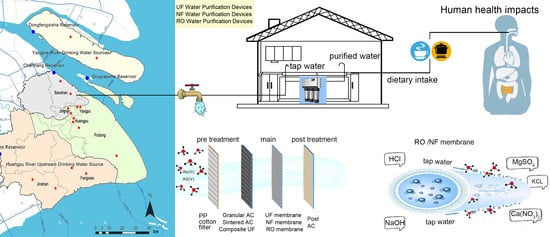
Reduction in Arsenic Exposure by Domestic Water Purification Devices in Shanghai Area and Related Health Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Quality Analysis of the End Water of Shanghai Tap Water Network
2.1.1. Sampling
2.1.2. Methods and Instruments
2.2. Simulation of Arsenic Contamination Experiments
2.2.1. Chemical Reagents and Instruments
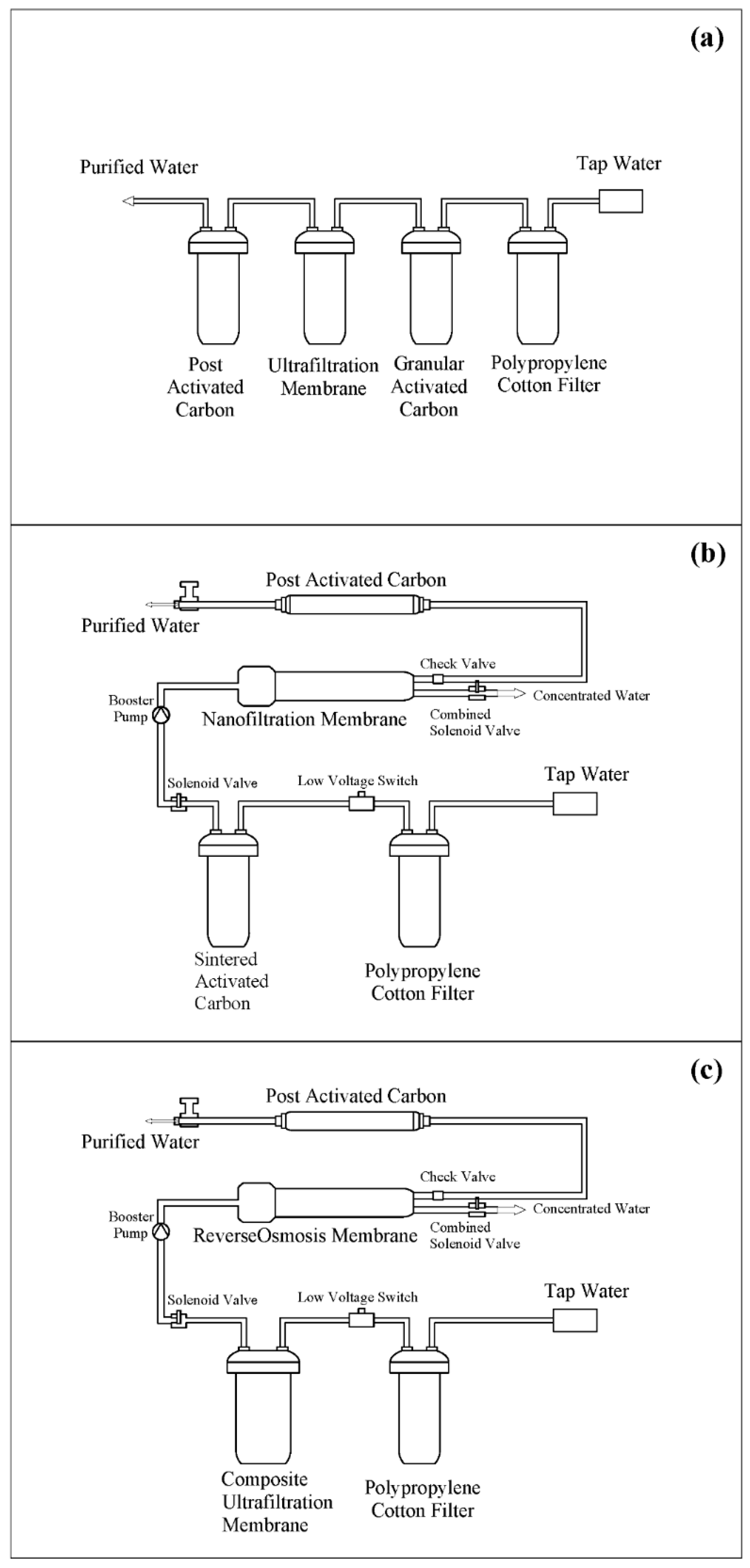
2.2.2. Experimental Method of Pollutants Removal
2.3. Health Risk Assessment
3. Results and Discussion
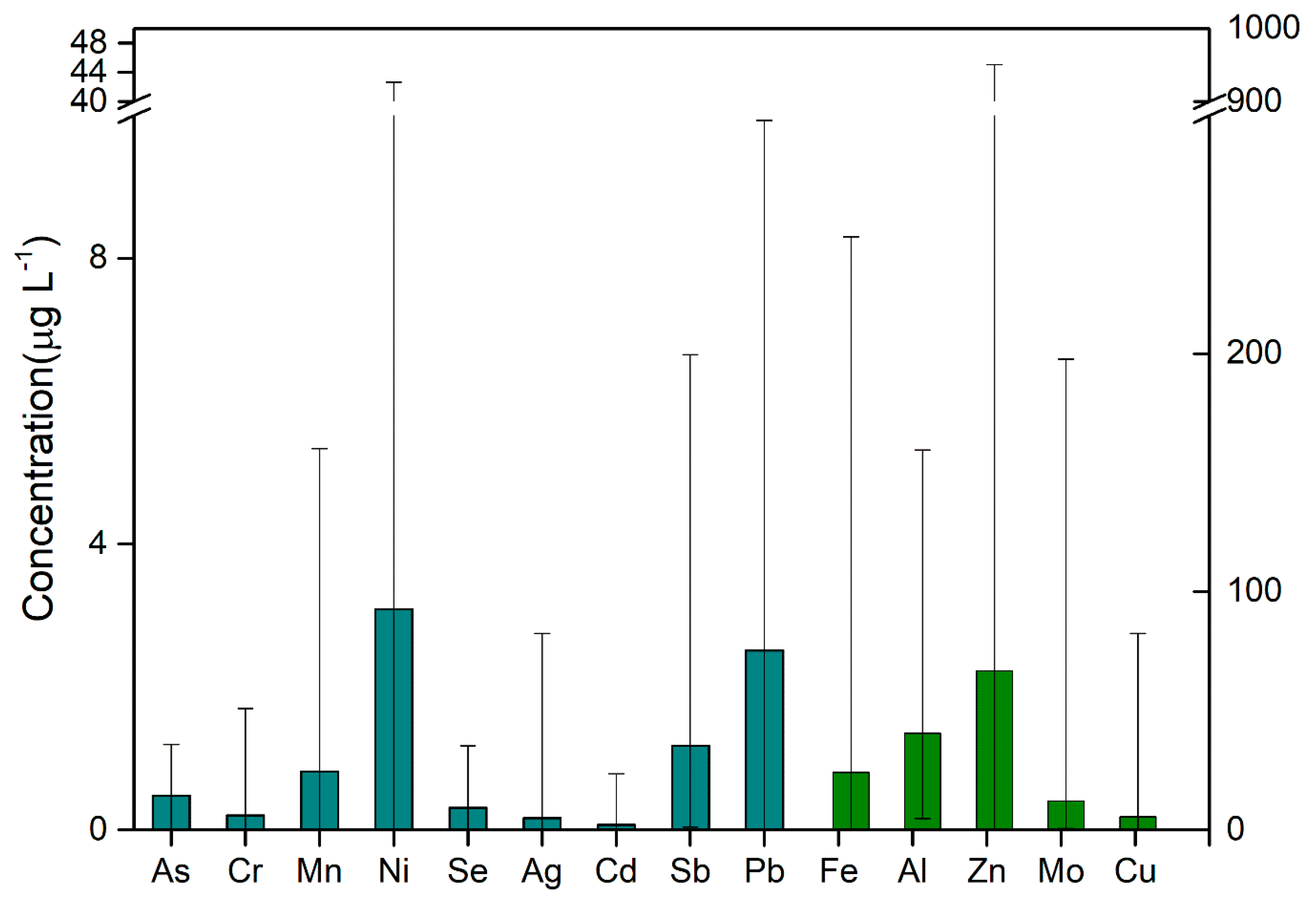
3.1. Distribution of Heavy Metals in Water at the End of Shanghai Water Pipeline Network
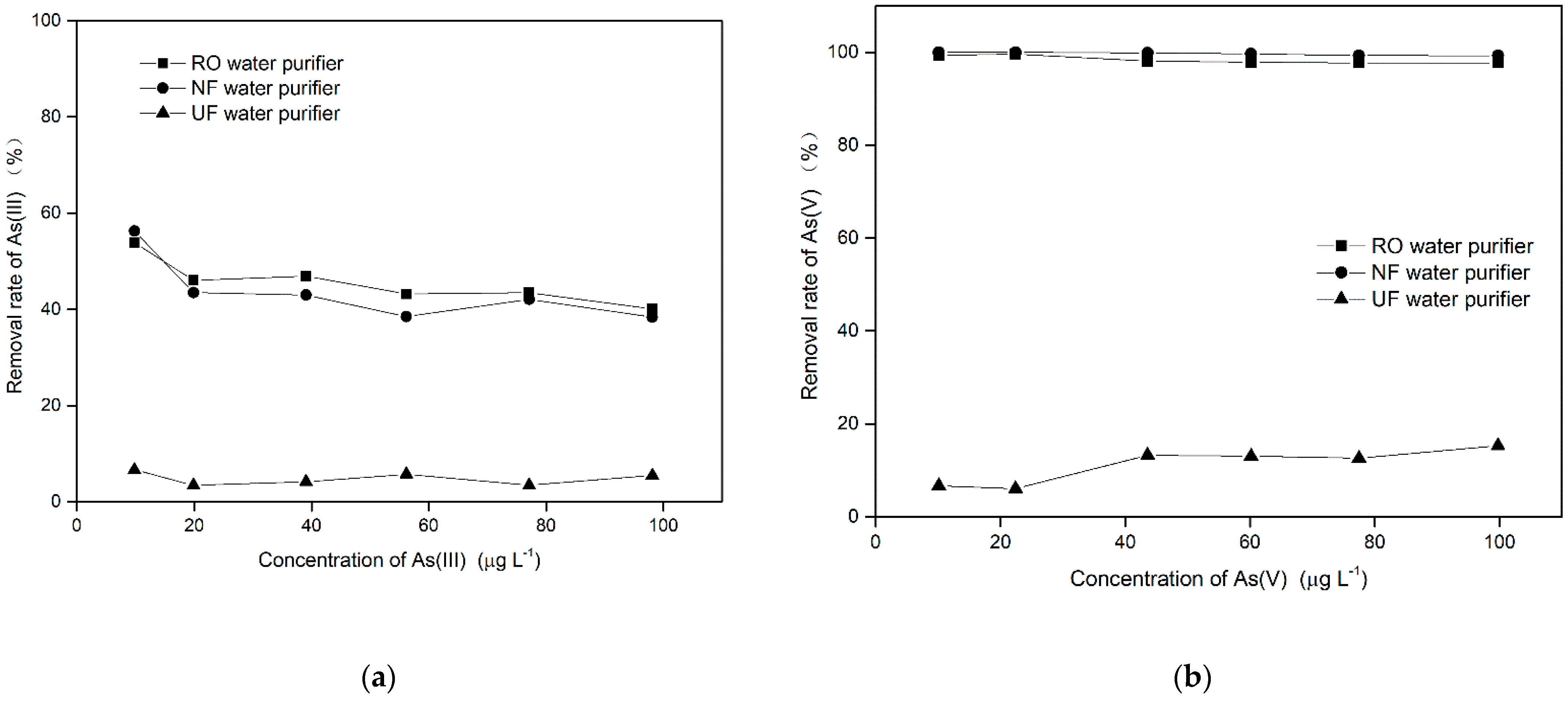
3.2. Evaluating the Effectiveness of Home Water Purifiers for the Removal of Arsenic Contamination
3.2.1. Evaluation of Water Purifiers for the Removal of Arsenic in Different Valence States
3.2.2. Evaluation of As(III) Removal Effect of Each Unit of Water Purifier
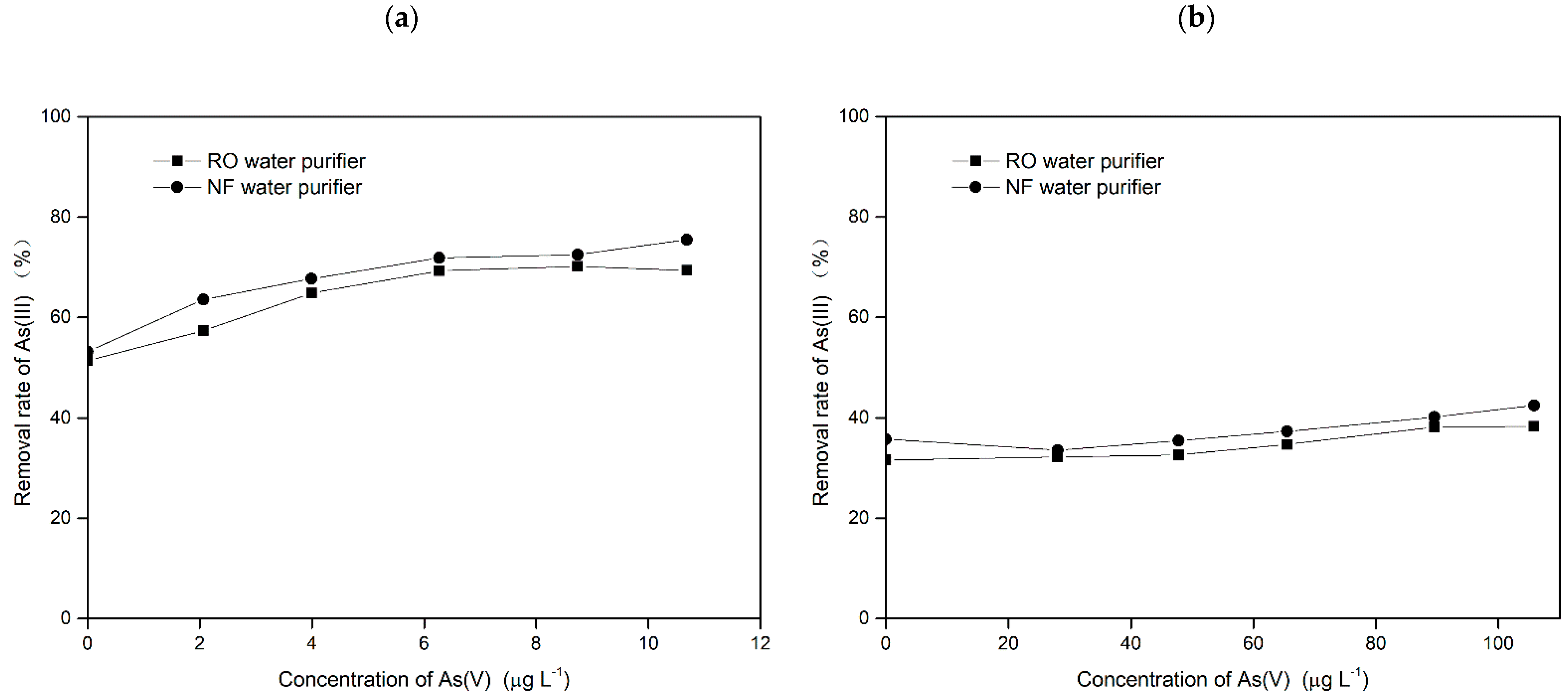
3.2.3. Evaluation of Water Purifiers for the Removal of Arsenic in Mixed Valence
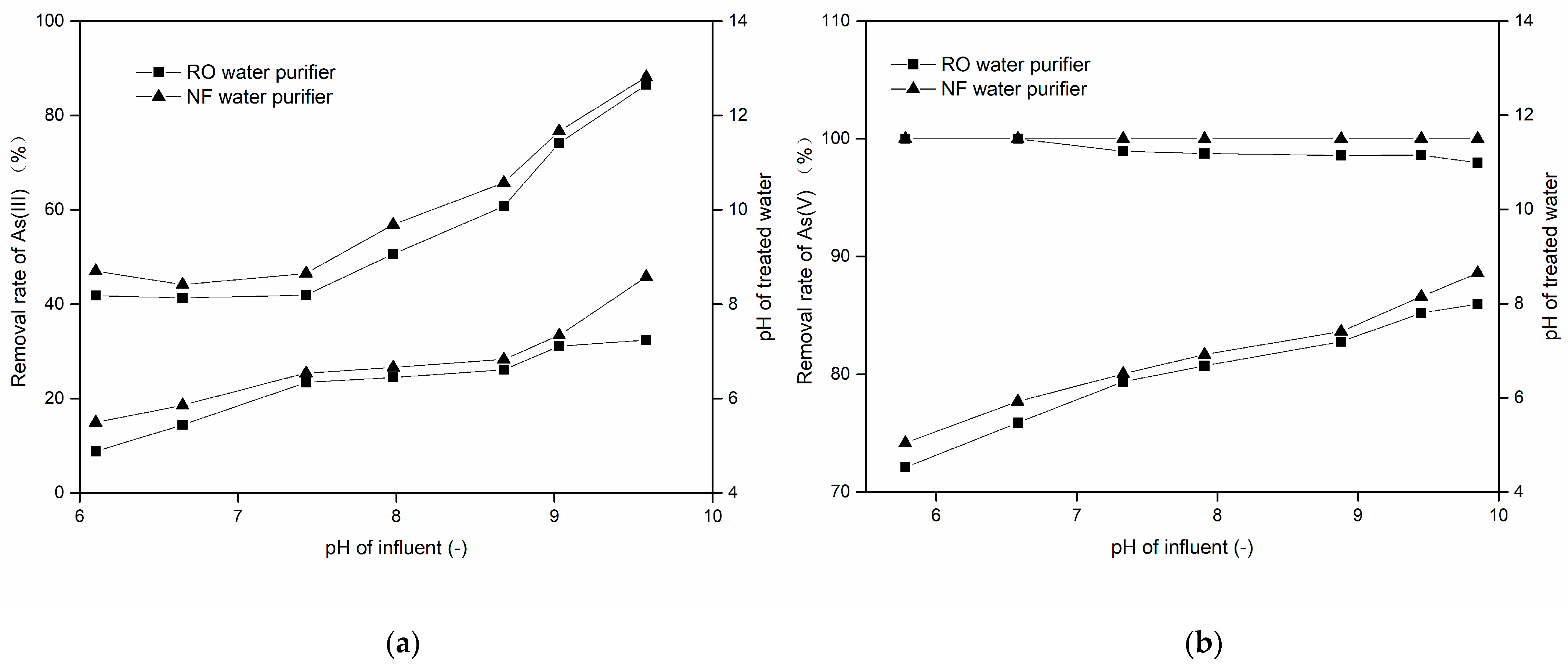
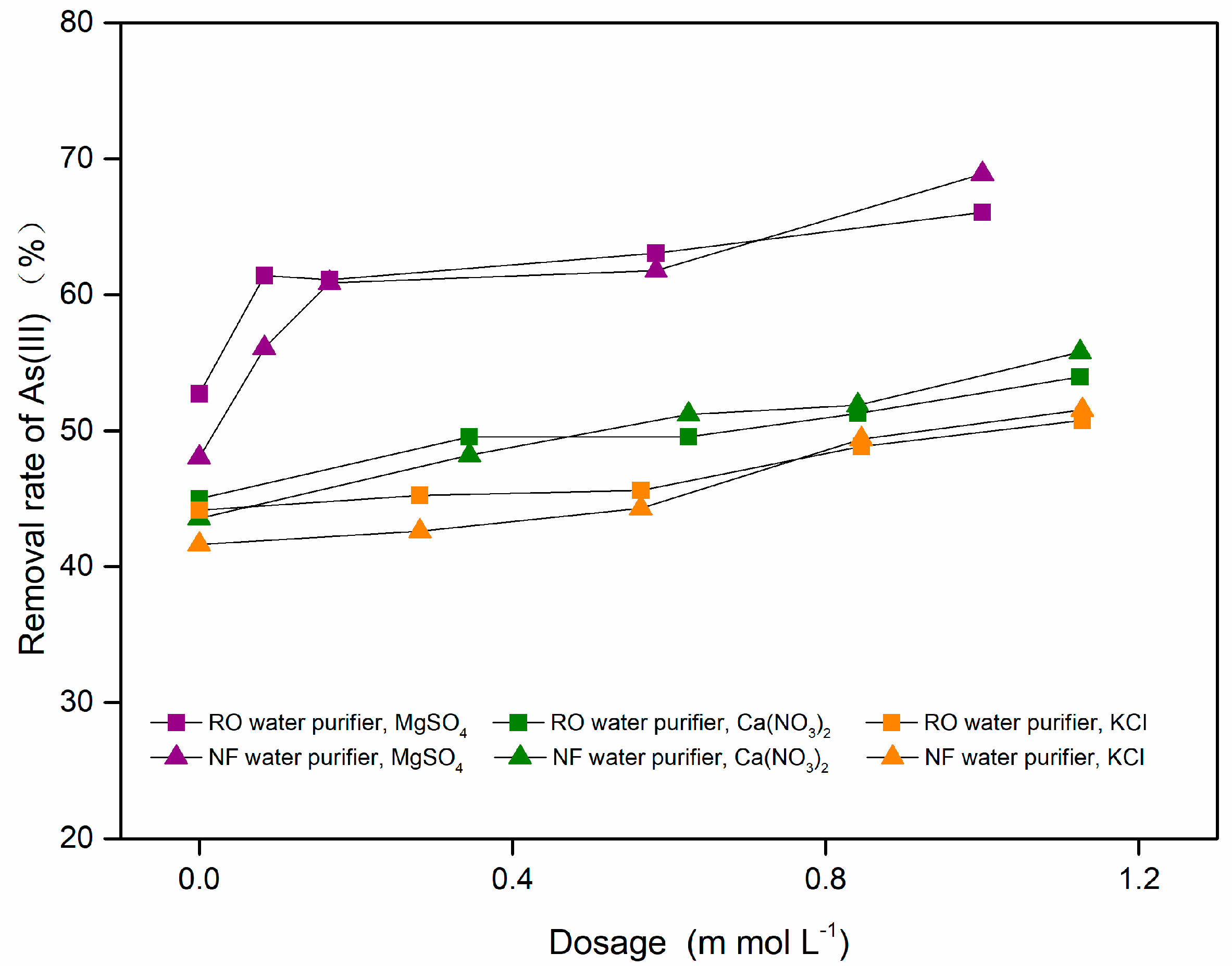
3.2.4. Effect of pH and Coexisting Ions
3.3. Health Risk Assessments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: First Addendum to Volume 1, Recommendations; World Health Organization: Geneva, Switzerland, 2006; Volume 1. [Google Scholar]
- Bretzler, A.; Johnson, C.A. The Geogenic Contamination Handbook: Addressing arsenic and fluoride in drinking water. Appl. Geochem. 2015, 63, 642–646. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Hull, E.A.; Barajas, M.; Burkart, K.A.; Fung, S.R.; Jackson, B.P.; Barrett, P.M.; Neumann, R.B.; Olden, J.D.; Gawel, J.E. Human health risk from consumption of aquatic species in arsenic-contaminated shallow urban lakes. Sci. Total Environ. 2021, 770, 145318. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Charlet, L. A review of arsenic presence in China drinking water. J. Hydrol. 2013, 492, 79–88. [Google Scholar] [CrossRef]
- Singh, P.; Borthakur, A.; Singh, R.; Bhadouria, R.; Singh, V.K.; Devi, P. A critical review on the research trends and emerging technologies for arsenic decontamination from water. Groundw. Sustain. Dev. 2021, 14, 100607. [Google Scholar] [CrossRef]
- Mondal, M.K.; Garg, R. A comprehensive review on removal of arsenic using activated carbon prepared from easily available waste materials. Environ. Sci. Pollut. Res. 2017, 24, 13295–13306. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Luettge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Bai, H.; Wang, W.; Zhang, Q. Enhanced arsenic removal from water and easy handling of the precipitate sludge by using FeSO4 with CaCO3 to Ca(OH)(2). Chemosphere 2019, 231, 134–139. [Google Scholar] [CrossRef]
- Walker, M.; Seiler, R.L.; Meinert, M. Effectiveness of household reverse-osmosis systems in a Western U.S. region with high arsenic in groundwater. Sci. Total Environ. 2008, 389, 245–252. [Google Scholar] [CrossRef]
- Uddin, M.T.; Mozumder, M.S.I.; Islam, M.A.; Deowan, S.A.; Hoinkis, J. Nanofiltration Membrane Process for the Removal of Arsenic from Drinking Water. Chem. Eng. Technol. 2007, 30, 1248–1254. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manage. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, J.; Li, W.; Beecham, S.; Mulcahy, D. Aqueous arsenite removal by simultaneous ultraviolet photocatalytic oxidation-coagulation of titanium sulfate. J. Hazard. Mater. 2016, 303, 162–170. [Google Scholar] [CrossRef]
- Slotnick, M.J.; Meliker, J.R.; Nriagu, J.O. Effects of time and point-of-use devices on arsenic levels in Southeastern Michigan drinking water, USA. Sci. Total Environ. 2006, 369, 42–50. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.d.S.; Amaral, M.C.S. Dead-end ultrafiltration as a cost-effective strategy for improving arsenic removal from high turbidity waters in conventional drinking water facilities. Chem. Eng. J. 2021, 417, 128132. [Google Scholar] [CrossRef]
- Chen, A.S.C.; Wang, L.; Sorg, T.J.; Lytle, D.A. Removing arsenic and co-occurring contaminants from drinking water by full-scale ion exchange and point-of-use/point-of-entry reverse osmosis systems. Water Res. 2020, 172, 115455. [Google Scholar] [CrossRef] [PubMed]
- Doré, E.; Formal, C.; Muhlen, C.; Williams, D.; Harmon, S.; Pham, M.; Triantafyllidou, S.; Lytle, D.A. Effectiveness of point-of-use and pitcher filters at removing lead phosphate nanoparticles from drinking water. Water Res. 2021, 201, 117285. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Ahmad, D.; Balkundae, P.; Kausley, S.; Malhotra, C. Development of low cost point-of-use (POU) interventions for instant decontamination of drinking water in developing countries. J. Water Process Eng. 2020, 37, 101435. [Google Scholar] [CrossRef]
- Trajano Gomes da Silva, D.; Ebdon, J.; Okotto-Okotto, J.; Ade, F.; Mito, O.; Wanza, P.; Kwoba, E.; Mwangi, T.; Yu, W.; Wright, J.A. A longitudinal study of the association between domestic contact with livestock and contamination of household point-of-use stored drinking water in rural Siaya County (Kenya). Int. J. Hyg. Environ. Health 2020, 230, 113602. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yan, X.; Gong, W.; Liu, X.; Wang, L.; Gao, L. Multi-objective based scheduling algorithm for sudden drinking water contamination incident. Swarm Evol. Comput. 2020, 55, 100674. [Google Scholar] [CrossRef]
- Curnin, S.; Brooks, B. Making waves: How do we prepare for the next drinking water disaster? Water Res. 2020, 185, 116277. [Google Scholar] [CrossRef]
- EPA. U.S. Integrated Risk Information System (Iris) Chemical Assessment Summary Arsenic, Inorganic; Casrn 7440-38-2; U.S. Environmental Protection Agency, National Center for Environmental Assessment: Washington, DC, USA, 2002.
- Exposure Factors Handbook of Chinese Population; Ministry of Ecology and Environment of People′s Republic of China: Beijing, China, 2013.
- Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; Coutinho de Paula, E.; Amaral, M.C.S. Arsenic contamination, effects and remediation techniques: A special look onto membrane separation processes. Process Saf. Environ. Prot. 2021, 148, 604–623. [Google Scholar] [CrossRef]
- Rajendran; Marlar, R.; Garg, S.; Bajpai, S. Chapter 17—Study of Transport Models for Arsenic Removal Using Nanofiltration Process: Recent Perspectives. In Emerging Technologies in Environmental Bioremediation; Shah, M.P., Rodriguez-Couto, S., Sevinç Şengör, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 391–405. [Google Scholar]
- Shemirani, F.; Baghdadi, M.; Ramezani, M. Preconcentration and determination of ultra trace amounts of arsenic(III) and arsenic(V) in tap water and total arsenic in biological samples by cloud point extraction and electrothermal atomic absorption spectrometry. Talanta 2005, 65, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Kappeler, A.; Lindauer, U.; Kistler, D.; Berg, M.; Sigg, L. Arsenite and Arsenate Binding to Dissolved Humic Acids: Influence of pH, Type of Humic Acid, and Aluminum. Environ. Sci. Technol. 2006, 40, 6015–6020. [Google Scholar] [CrossRef] [Green Version]
- Freger, V.; Arnot, T.C.; Howell, J.A. Separation of concentrated organic/inorganic salt mixtures by nanofiltration. J. Membr. Sci. 2000, 178, 185–193. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Melse, J. Quantifying Public Health Risk in the Who Guidelines for Drinking-Water Quality: A Burden of Disease Approach; World Health Organization: Bilthoven, The Netherlands, 2003. [Google Scholar]
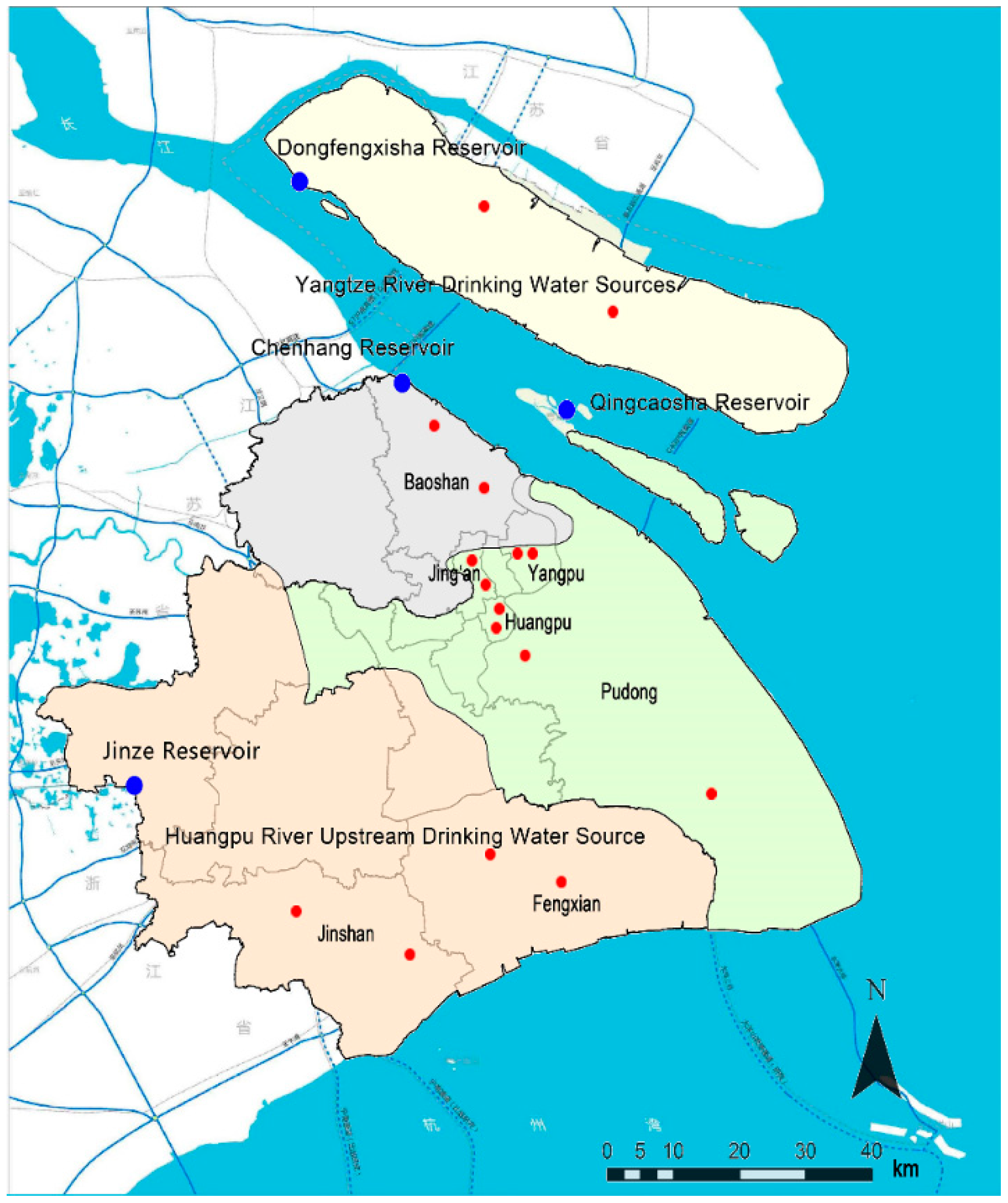
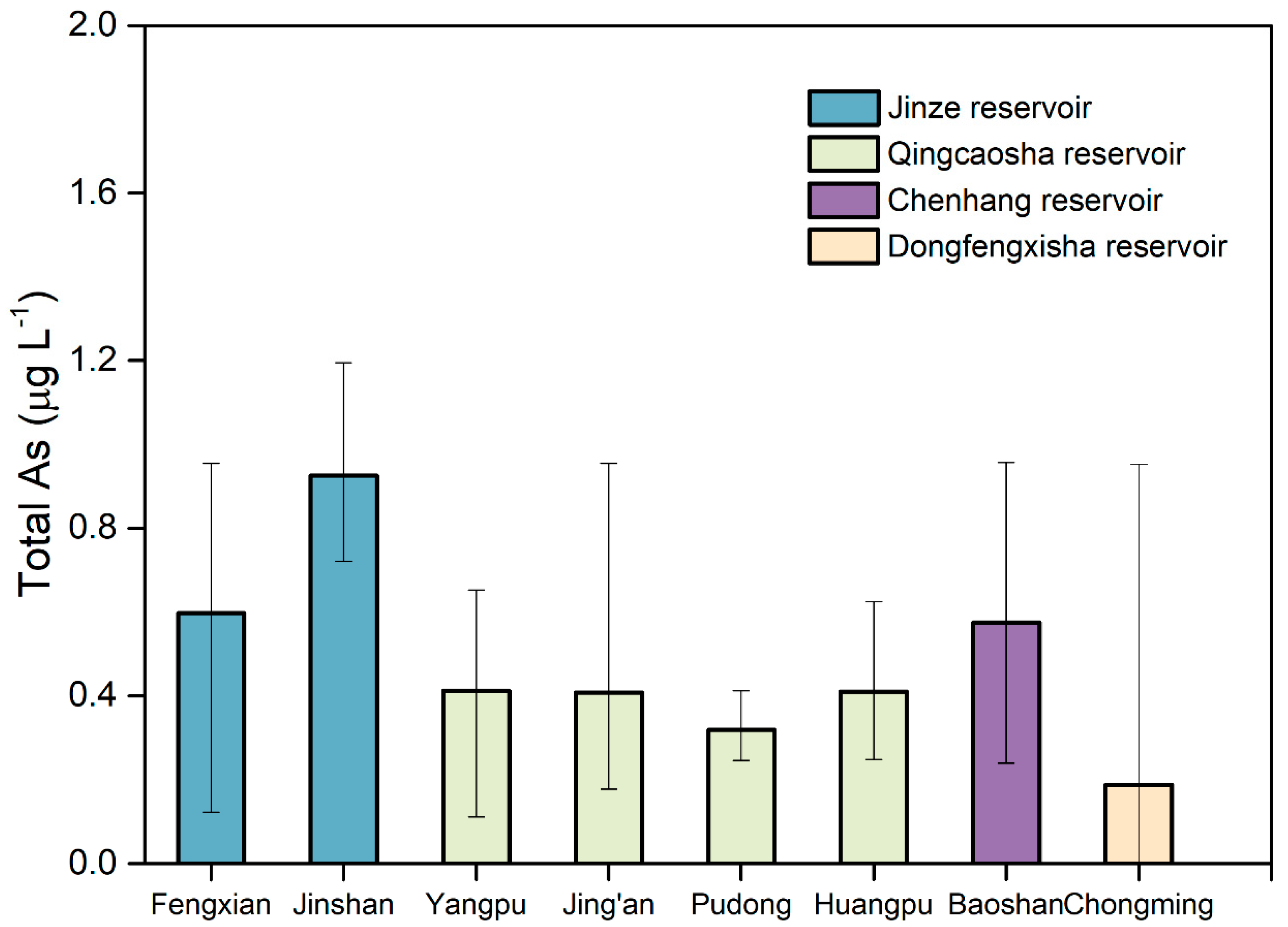
| Drinking Water Source | District | Sampling |
|---|---|---|
| Jinze Reservoir | Jinshan | Tap water in a resident, tap water in industrial park |
| Fengxian | Tap water in two residents | |
| Qingcaosha | Yangpu | Tap water in a university, tap water in a resident |
| Jing’an | Tap water in a resident, tap water in industrial park | |
| Pudong | Tap water in two residents | |
| Huangpu | Tap water in two residents | |
| Chenhang Reservoir | Baoshan | Tap water in two residents |
| Dongfengxisha Reservoir | Chongming | Tap water in two residents |
| Water Purifier Process | Unit | Concentration of As(III) | ||
|---|---|---|---|---|
| 10 µg L−1 | 50 µg L−1 | 100 µg L−1 | ||
| Pre-treatment | Synthetic fiber | 3.48–5.42% | 1.42–3.85% | 0.83–1.93% |
| Sintered activated carbon | 7.51–8.62% | 4.51–7.77% | 4.28–7.96% | |
| Granular activated carbon | 4.82–8.59% | 6.73–9.74% | 4.15–6.69% | |
| Main | Composite ultrafiltration membrane | 12.34–14.55% | 9.98–15.48% | 10.94–13.04% |
| Ultrafiltration membrane | 9.40–11.17% | 8.62–12.59% | 9.92–11.94% | |
| Reverse osmosis membrane | 57.48–86.25% | 50.71–79.04% | 47.55–65.00% | |
| Nanofiltration membrane | 53.12–85.65% | 49.9–79.39% | 48.39–66.20% | |
| Post-treatment | Post Activated carbon | 3.73–4.90% | 4.04–6.20% | 2.07–3.01% |
| Membrane | Pore Size | Category | Propulsion | Mechanisms | Interception Molecular Weight |
|---|---|---|---|---|---|
| Ultrafiltration membrane | 5 nm–0.1μm | Composite | 0.1–1.0 MPa | Screening, adsorption | 1000–300,000 |
| Nanofiltration membrane | 1–5 nm | Composite | 0.2–1.5 MPa | Spatial barrier effect, Donnan effect, adsorption/solubilization | 100–1000 |
| Reverse osmosis membrane | <1 nm | Composite | 0.1–10 MPa | Diffusion | All ions |
| District | Tap Water | RO | NF | UF |
|---|---|---|---|---|
| Fengxian | 4.65 × 10−6 | 1.61 × 10−6 | 1.39 × 10−6 | 3.86 × 10−6 |
| Jinshan | 6.74 × 10−6 | 2.18 × 10−6 | 1.83 × 10−6 | 5.10 × 10−6 |
| Yangpu | 3.68 × 10−6 | 1.09 × 10−6 | 7.05 × 10−7 | 2.72 × 10−6 |
| Jing’an | 6.59 × 10−6 | 1.69 × 10−6 | 1.17 × 10−6 | 5.24 × 10−6 |
| Pudong | 1.78 × 10−6 | 2.53 × 10−7 | 2.54 × 10−7 | 1.40 × 10−6 |
| Huangpu | 1.40 × 10−6 | 2.54 × 10−6 | 2.54 × 10−7 | 8.68 × 10−7 |
| Baoshan | 4.96 × 10−6 | 1.40 × 10−6 | 1.17 × 10−6 | 3.45 × 10−6 |
| Chongming | 2.54 × 10−7 | 2.54 × 10−7 | 2.54 × 10−7 | 2.54 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.; Lu, H.; Zhu, Z.; Sui, M.; Qiu, Y.; Yin, D. Reduction in Arsenic Exposure by Domestic Water Purification Devices in Shanghai Area and Related Health Risk Assessment. Water 2021, 13, 2916. https://doi.org/10.3390/w13202916
Qin Q, Lu H, Zhu Z, Sui M, Qiu Y, Yin D. Reduction in Arsenic Exposure by Domestic Water Purification Devices in Shanghai Area and Related Health Risk Assessment. Water. 2021; 13(20):2916. https://doi.org/10.3390/w13202916
Chicago/Turabian StyleQin, Qin, Hongtao Lu, Zhiliang Zhu, Minghao Sui, Yanling Qiu, and Daqiang Yin. 2021. "Reduction in Arsenic Exposure by Domestic Water Purification Devices in Shanghai Area and Related Health Risk Assessment" Water 13, no. 20: 2916. https://doi.org/10.3390/w13202916
APA StyleQin, Q., Lu, H., Zhu, Z., Sui, M., Qiu, Y., & Yin, D. (2021). Reduction in Arsenic Exposure by Domestic Water Purification Devices in Shanghai Area and Related Health Risk Assessment. Water, 13(20), 2916. https://doi.org/10.3390/w13202916