Identification of Natural and Anthropogenic Geochemical Processes Determining the Groundwater Quality in Port del Comte High Mountain Karst Aquifer (SE, Pyrenees)
Abstract
:1. Introduction
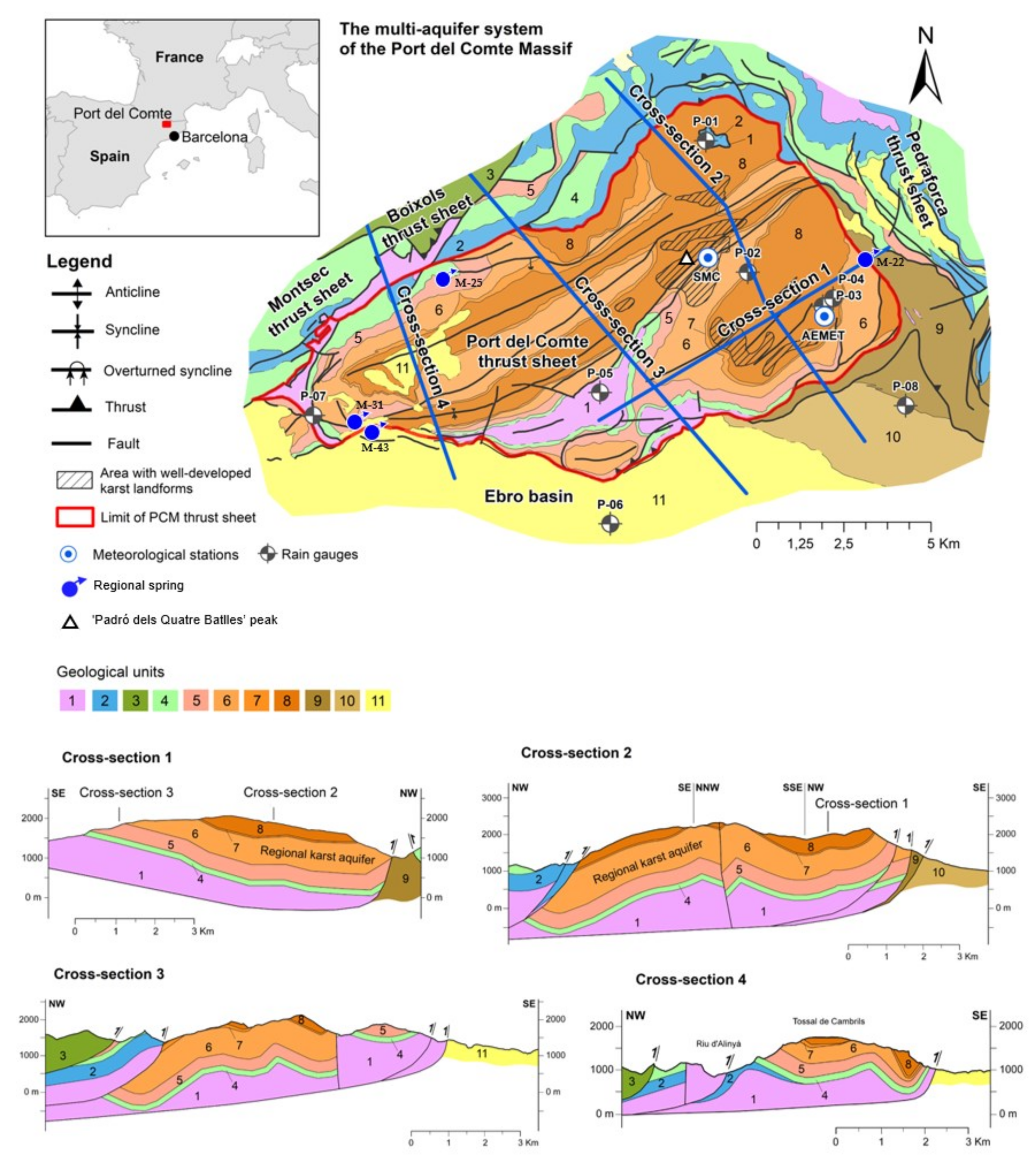
2. The Study Area
2.1. Geographical and Climatological Settings
2.2. Geology and Hydrogeology Setting
- (1)
- The hydrodynamic behavior of the system, simulating the system response with a set of semi-distributed rainfall-runoff HBV models [53,54], while taking into account the elevation dependences of both the hydrometeorological variables (e.g., precipitation and temperature) and the related processes (e.g., snow accumulation and ablation). The estimated groundwater storage capacity of the system is 35.2 hm3, and the mean annual groundwater discharge is 15.4 hm3; and
- (2)
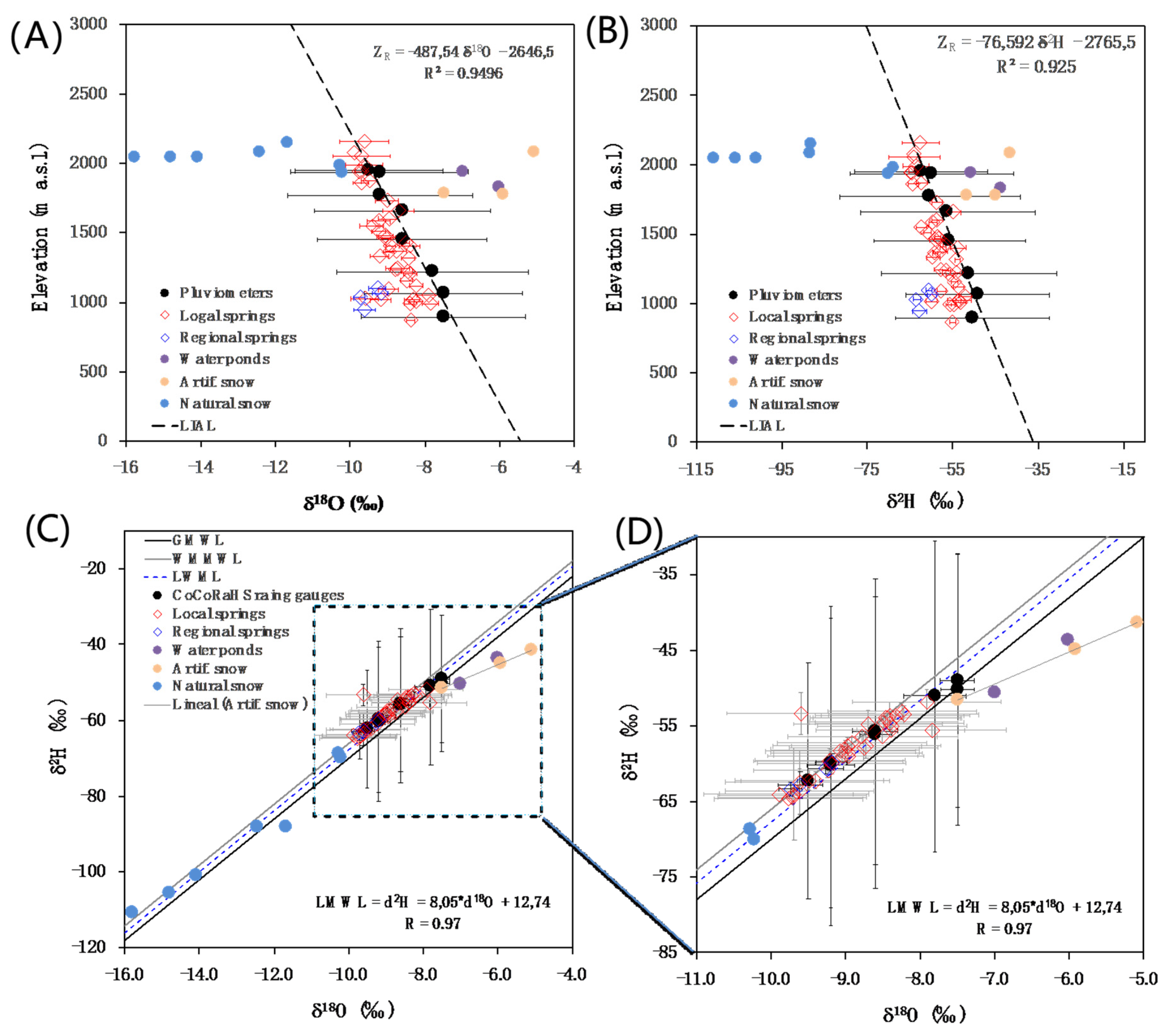
- The estimation of the mean transit times corresponding to the main springs draining the aquifer system. This is done by using a set of LPMs models [55] to simulate the environmental tracers’ content evolution in groundwater. The LPMs were implemented for the most important karst springs of the PCM systems, i.e., the four ‘regional springs’ named as M-22, M-25, M-31, and M-43 in Figure 1. The results indicate that the PCM karst system presents a relatively short mean transit time (~2.25 year). This result is relevant if the hydrological high conductive features existing in the karst system are taken into account, which may favor a fast contaminant migration from the recharge to the discharge areas in the case of eventual surface spills of contaminants.
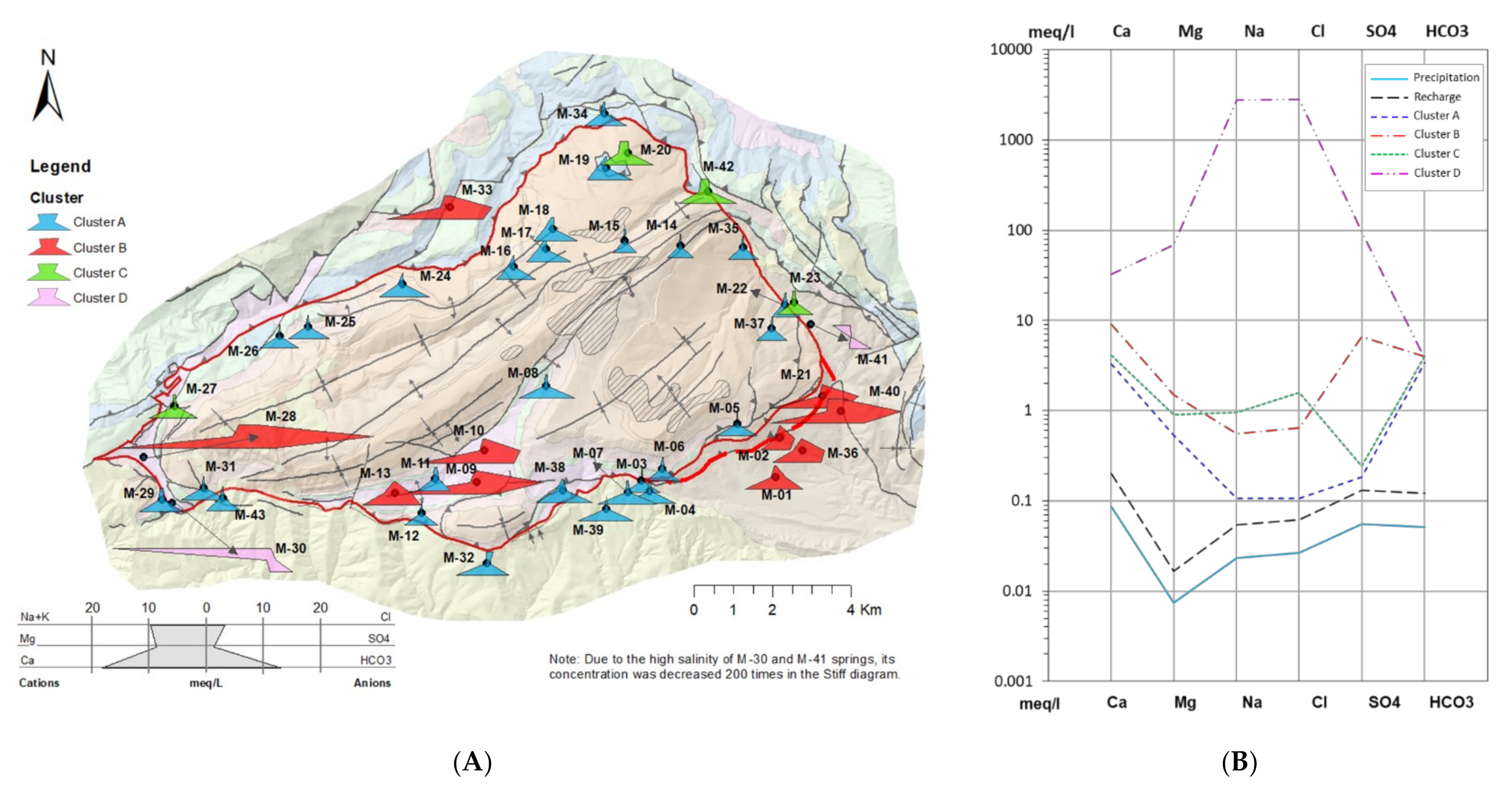
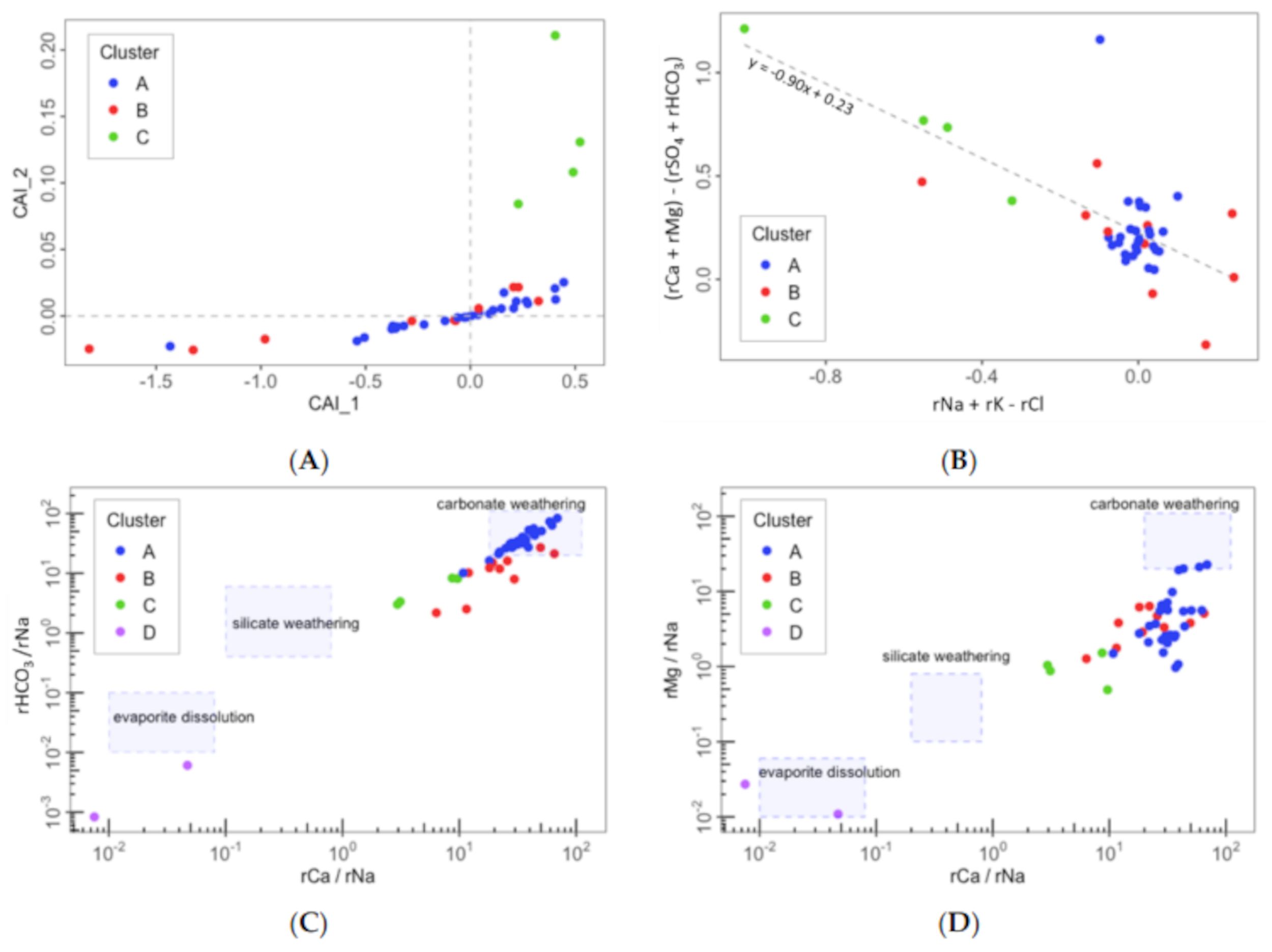
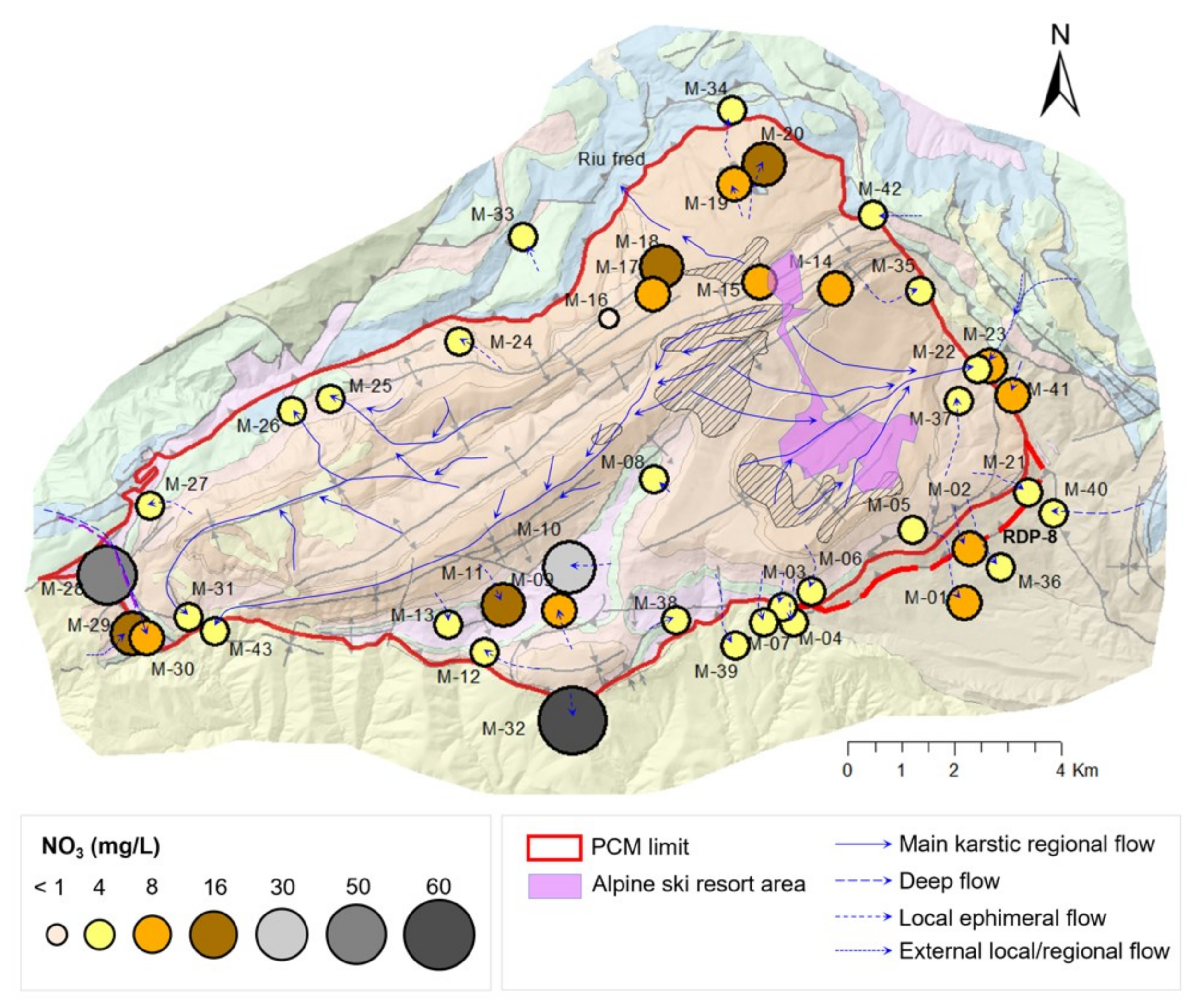
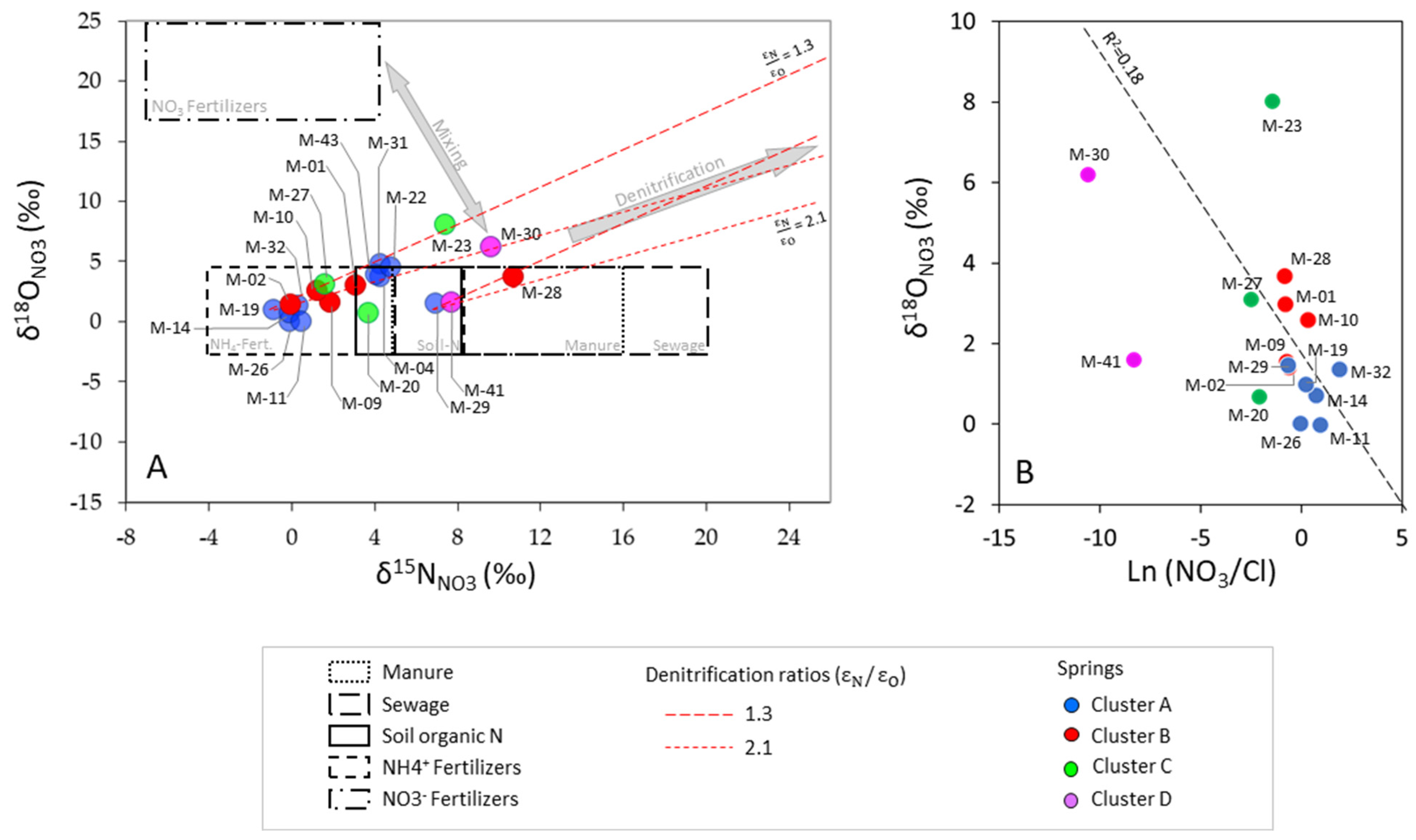
- Cluster A: 27 springs characterized by low mineralization and dominated by slightly alkaline Ca–HCO3 water type, which is associated with the Eocene carbonate materials conforming the main aquifer of PCM.
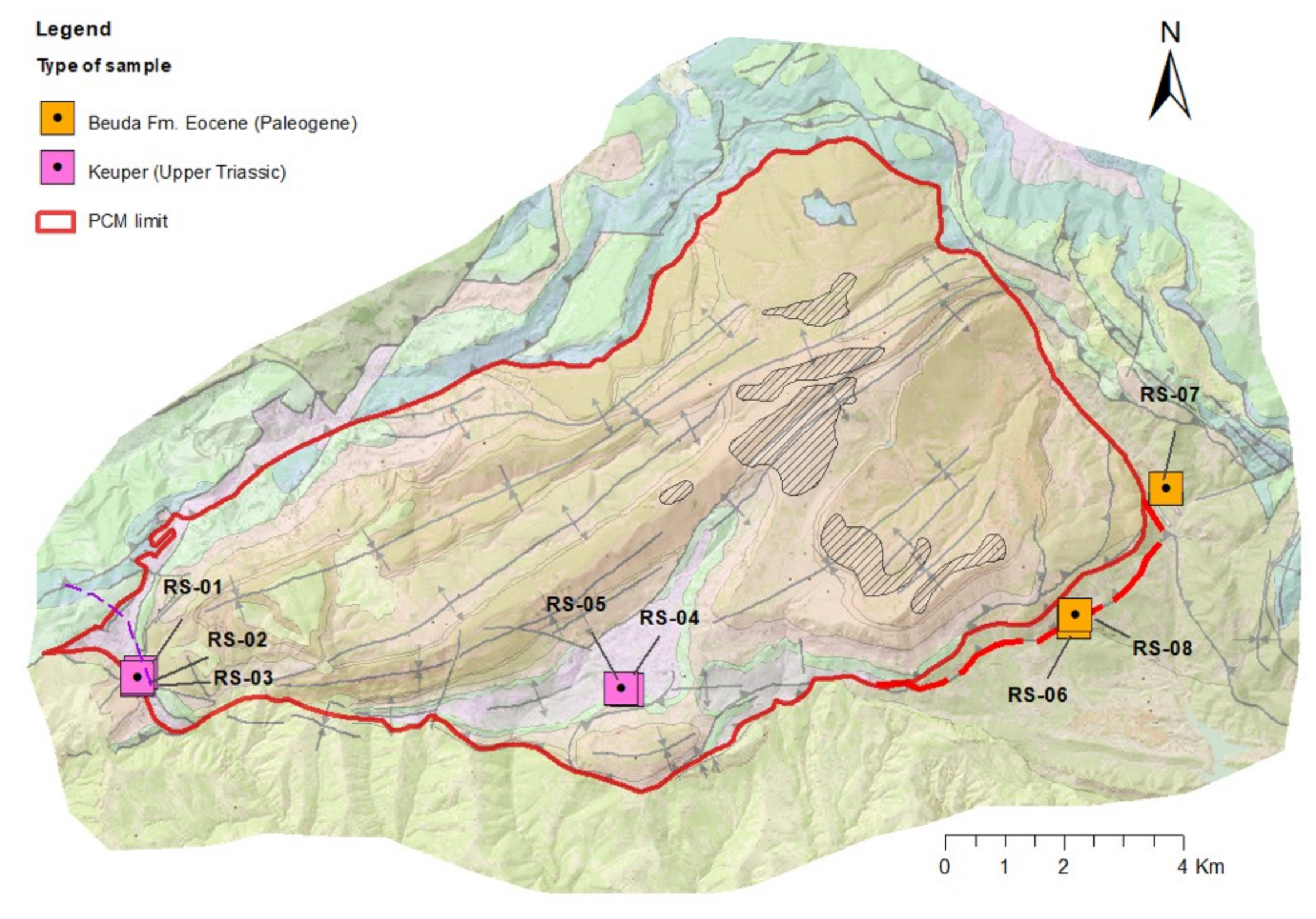
- Cluster B: 10 springs that include different types of water from Ca–HCO3 to Ca–HCO3–SO4, Ca–SO4–HCO3, and Ca–SO4, which are characterized by moderate mineralization. These springs are located both inside and outside the structural limits of the PCM trust sheet. The springs located inside the limits are mainly found in materials from the Cretaceous and Triassic (Keuper) that outcrop in the area. These materials underlie the main aquifer of the massif (the Eocene karst carbonate system). In the southeastern part of the study zone, there are five springs related to sediments with a high content of tertiary gypsum from the Eocene-Oligocene Beuda’s gypsum Formation, pinched out within the South Pyrenees thrust fault in the front SE of the PCM.
- Cluster C: 4 springs with water types of Ca–HCO3 and Ca–HCO3–Cl. Three of these springs are located at the boundaries of the PCM sheet.
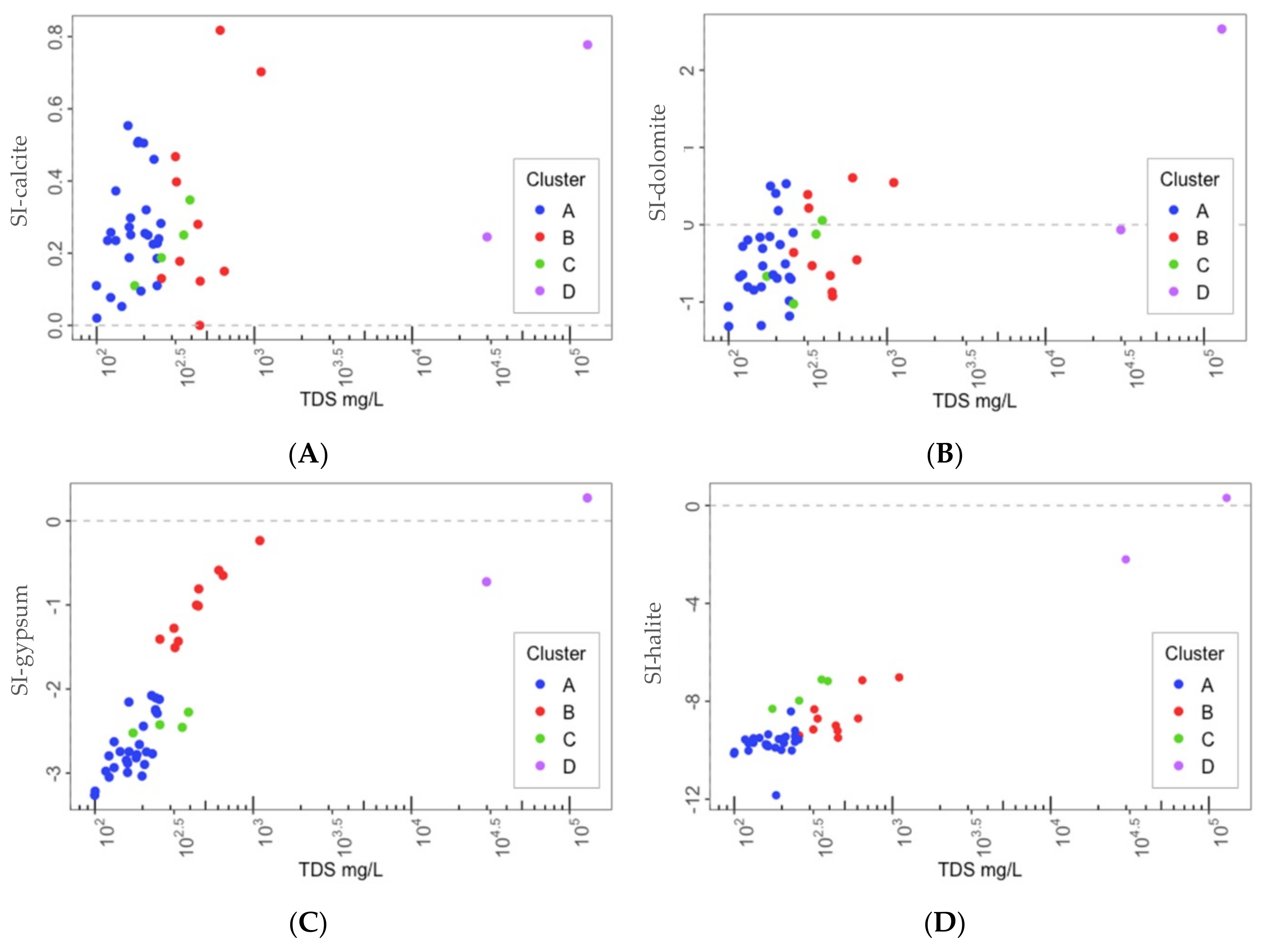
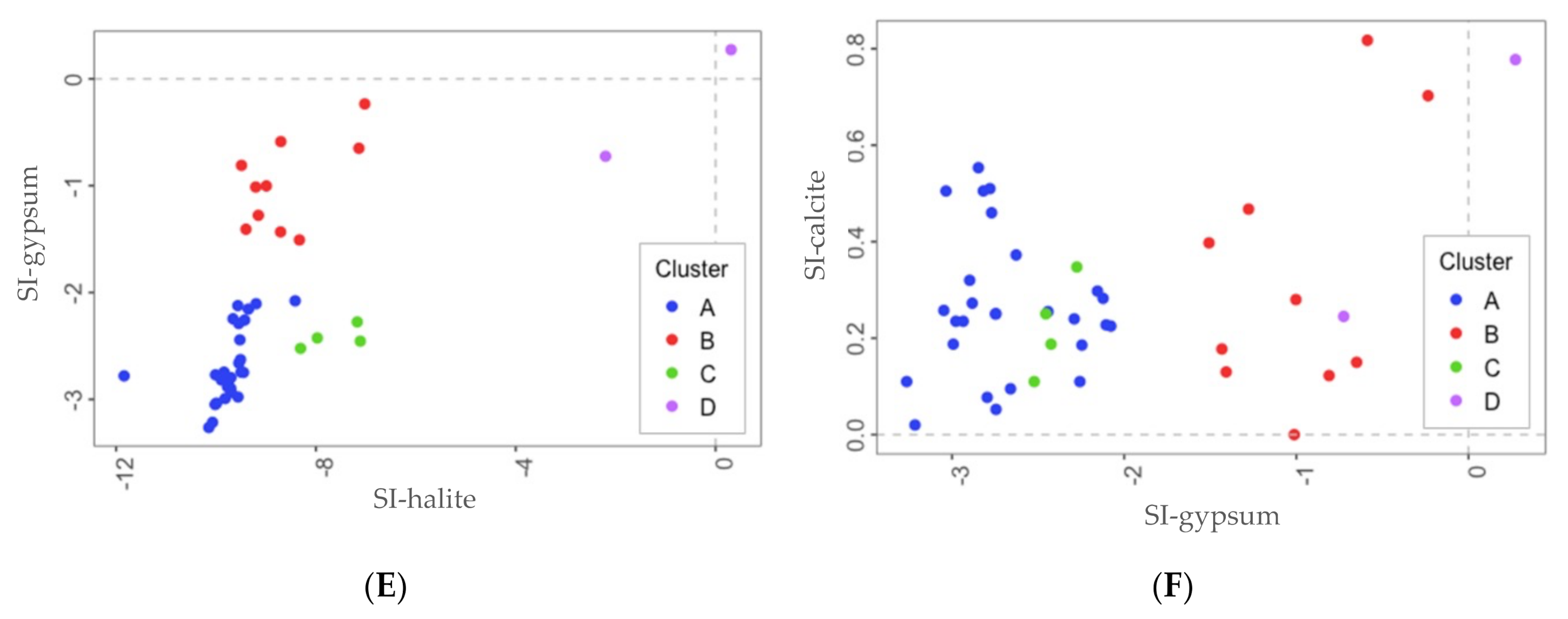
- Cluster D: corresponds to two salty springs with Na–Cl facies that are in the eastern and western limits of the PCM thrust sheet, respectively. They are characterized by remarkably high mineralization and are saturated relative to gypsum.
3. Materials and Methods
3.1. Field Measurements, Sampling, and Laboratory Analysis
3.2. Application of the Dual-Isotope Approach for δ34S and δ15N
3.3. Determination of Proportional Contributions of NO3 and SO4 Sources
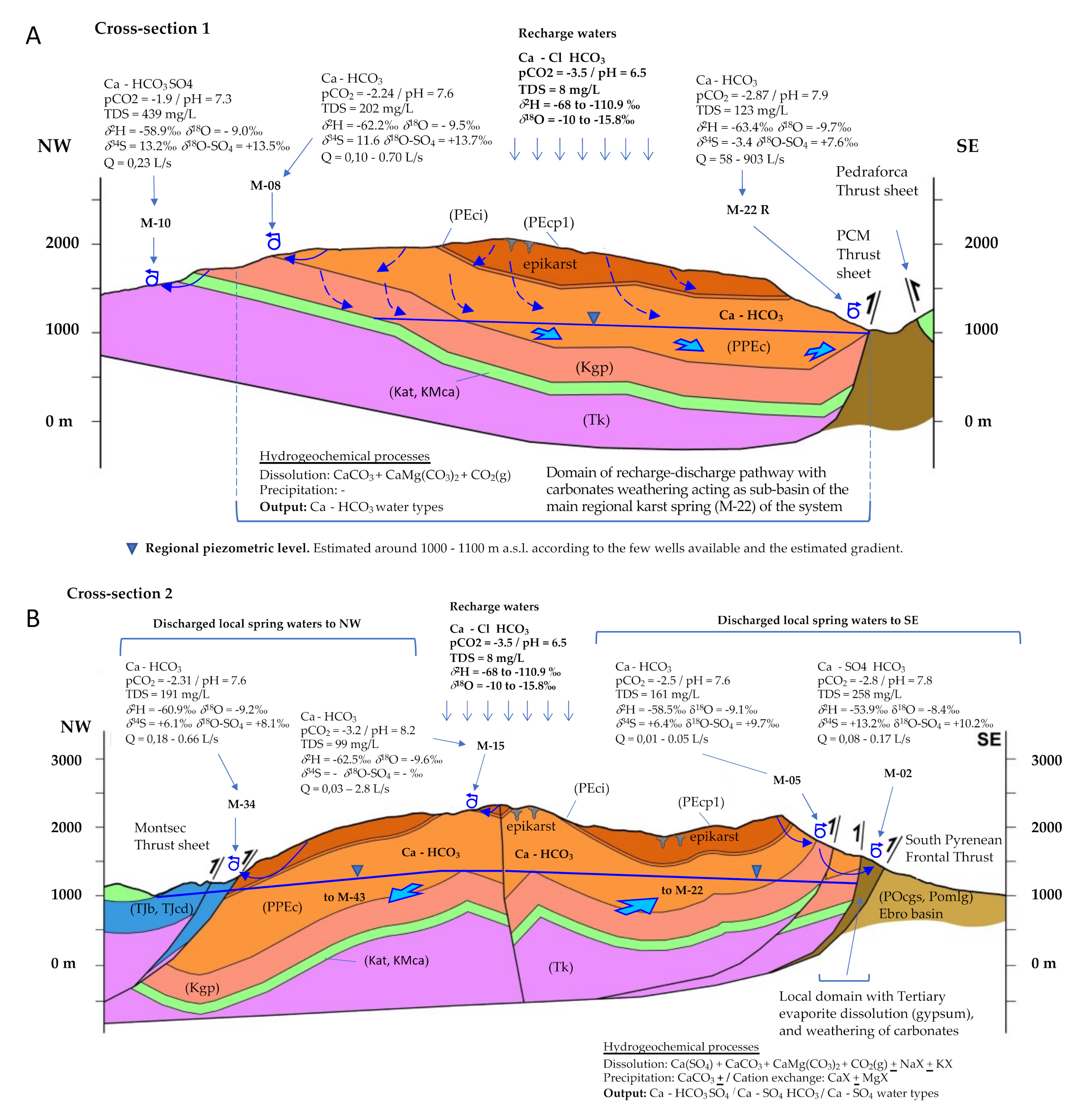
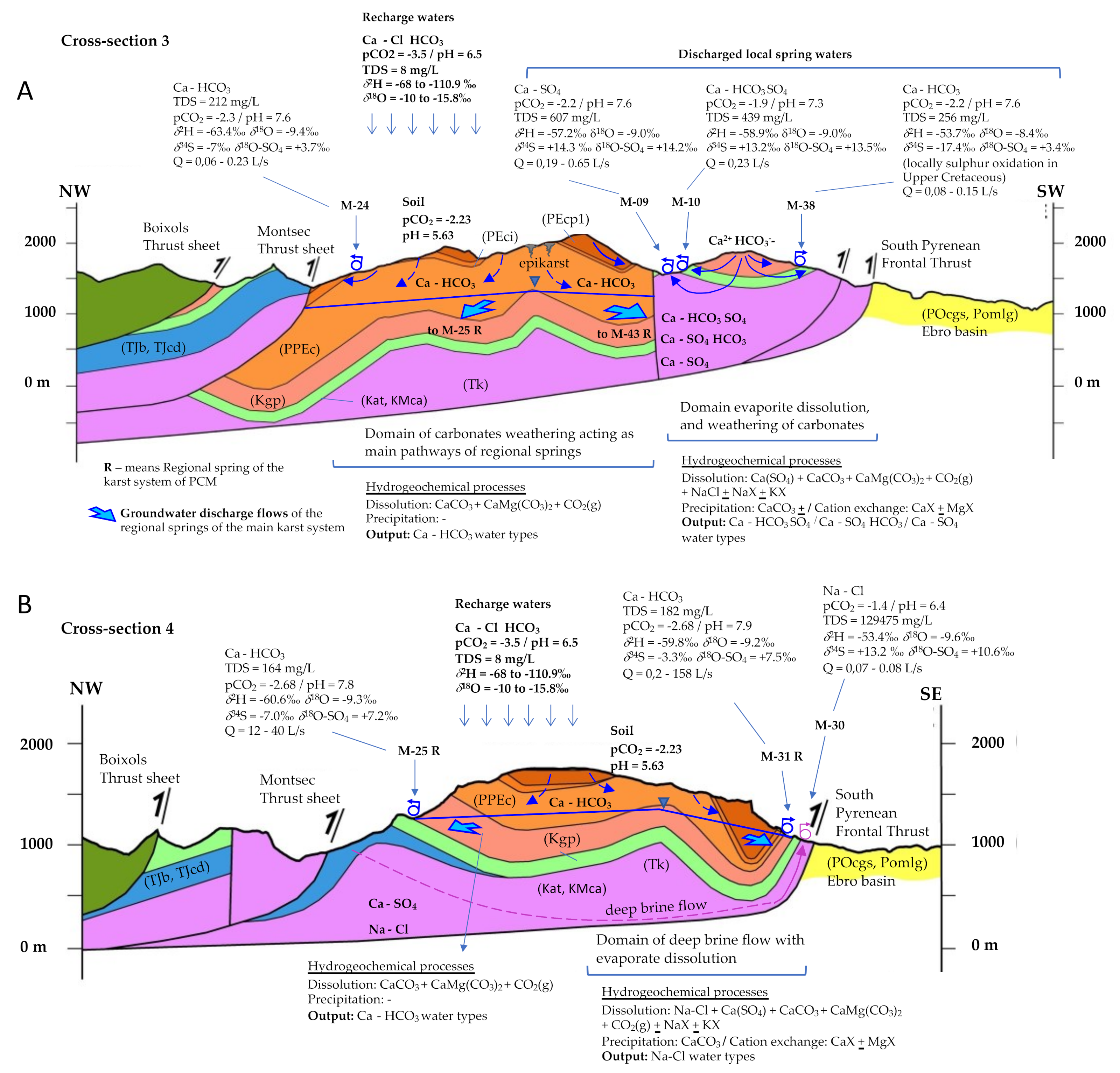
3.4. Delineation of the Main Recharge-Discharge Pathways
3.5. Inverse Hydrogeochemical Modeling for the Quantification of Chemical Processes
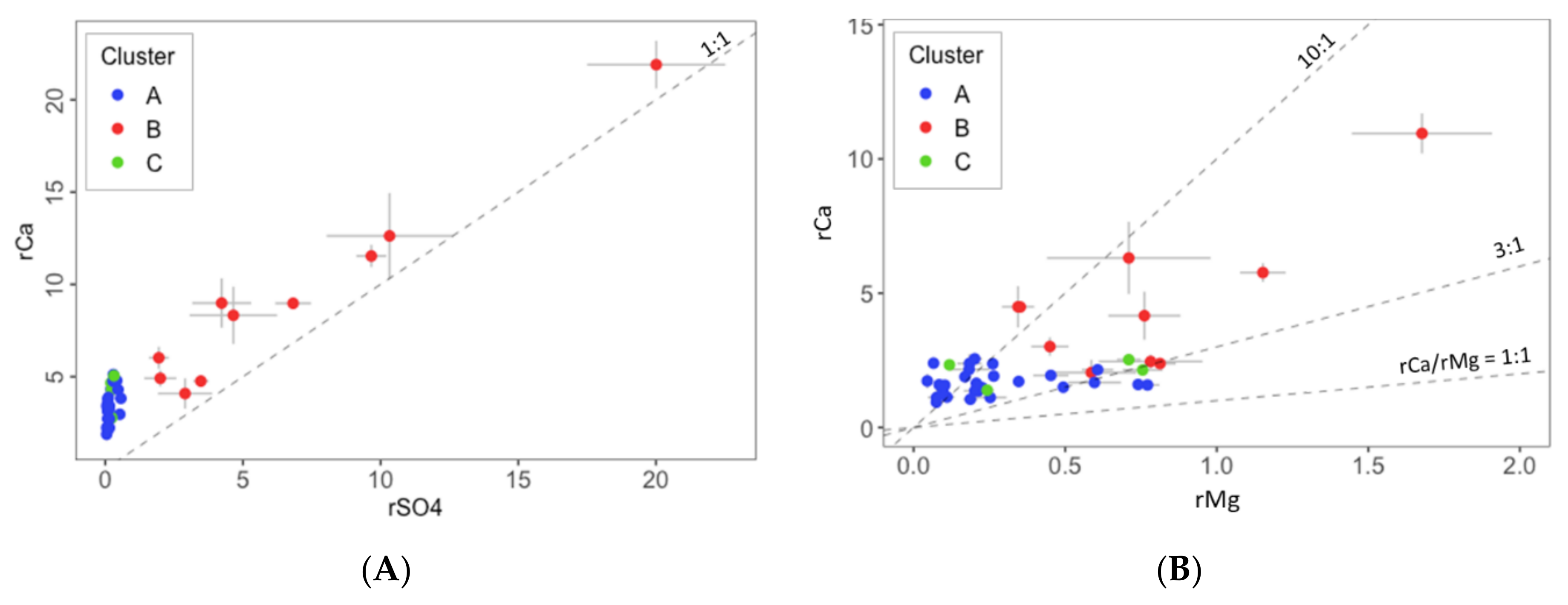
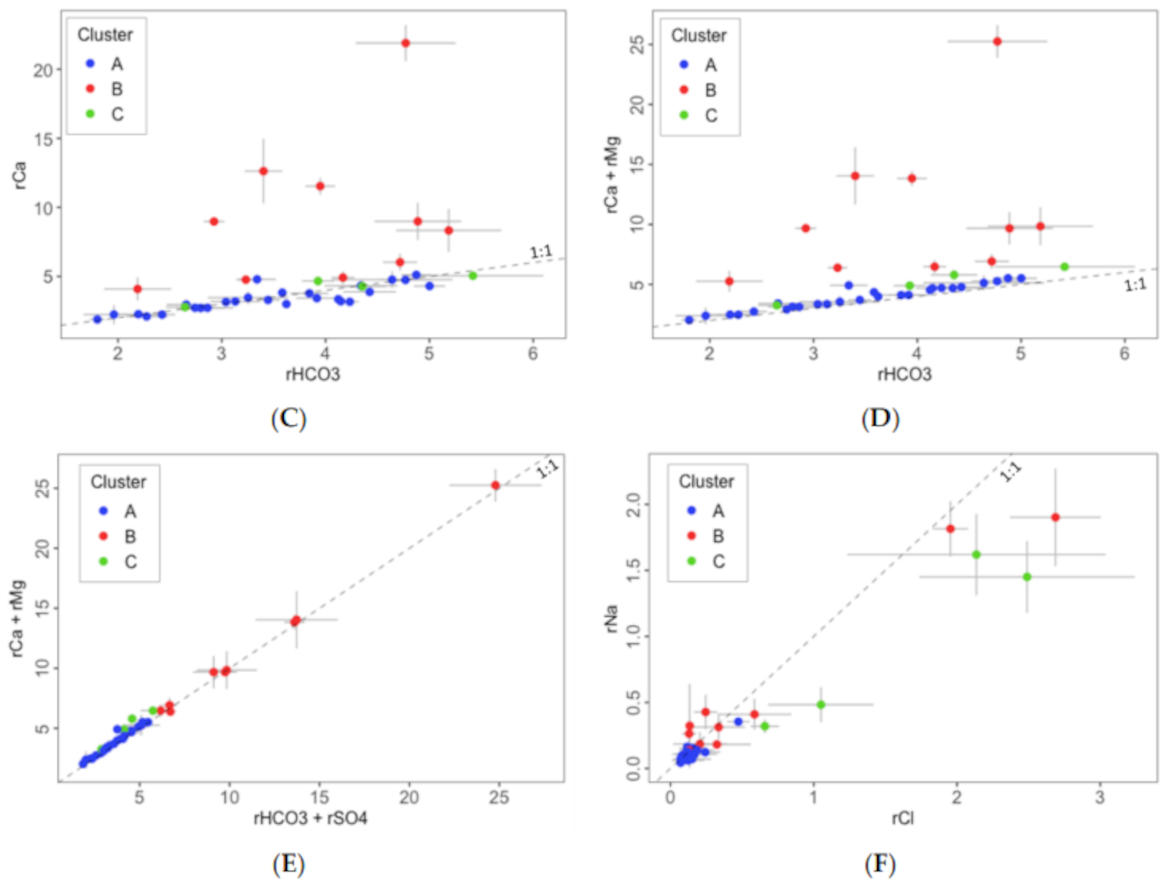
- Dissolution of carbonate minerals, such as calcite and dolomite, and precipitation of calcite according to Equations (5) and (6) [89]. Equation (7) is obtained as the sum of Equations (5) and (6), and it shows that the molar ratio Ca2+/Mg2+ is 3:1:
- Dissolution of evaporite minerals, such as gypsum and halite, according to Equations (8) and (9):
- Dedolomitization processes according to Equation (10) [71], which causes an increment of Ca2+ due to gypsum dissolution (as indicated in Equation (8)) and precipitation of calcite:
- Ion exchange reactions due to weathering reactions in marls, shales, and clays associated with Triassic and Cretaceous layers, according to the following Equations (11)–(14):
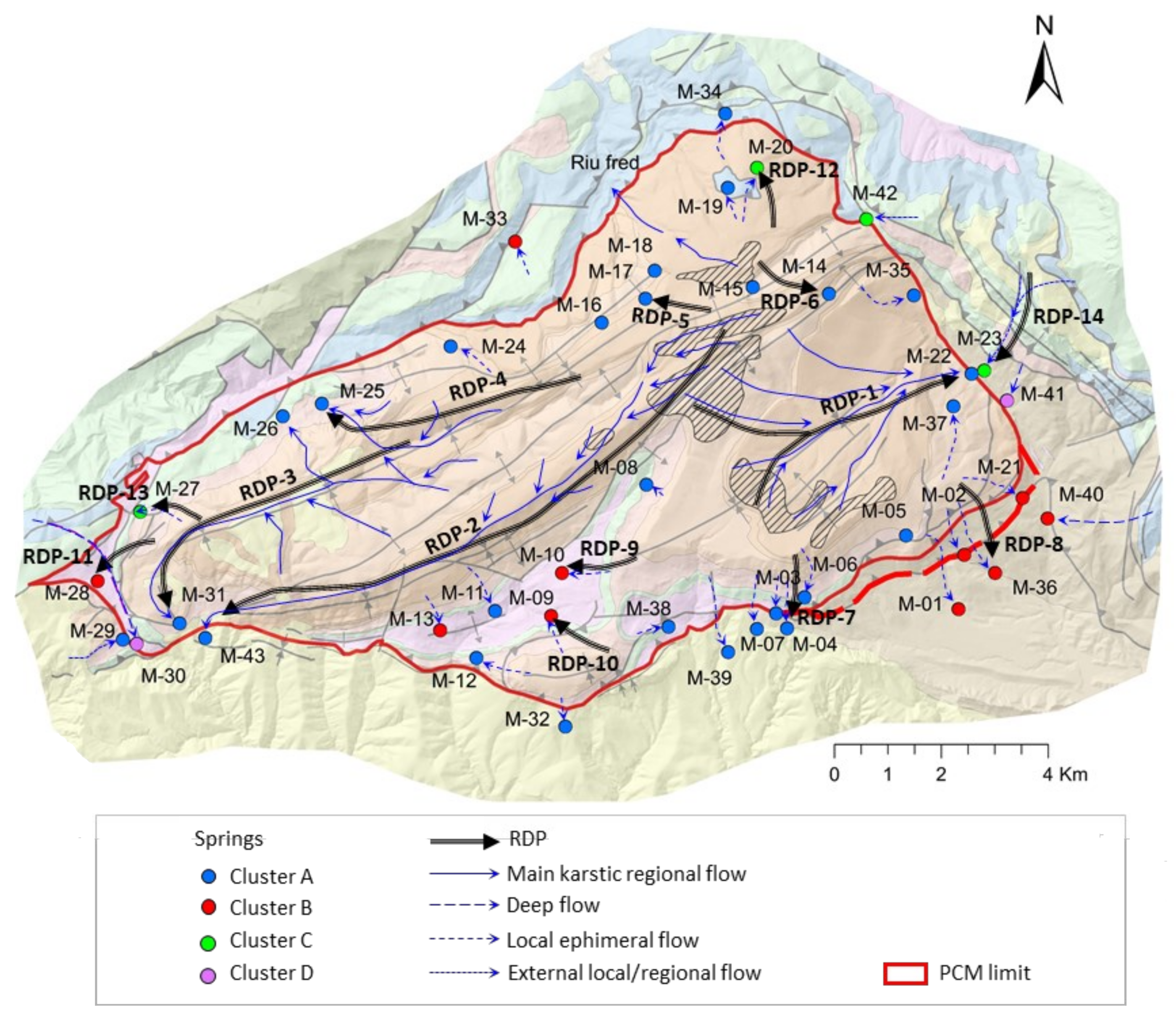
- RDP-Cluster A: RDP-1 to RDP-4 correspond to the four regionals springs (M-22, M-43, M-31, and M-25). RDP-05 is related to the springs located in the upper part of the PCM (M-16, M-17, M-18, and M-19) that discharge to the north and are oversaturated with respect to dolomite. On the contrary, RDP-06 refers to the other two springs located in the upper part of the PCM (M-14 and M-15) draining to the south, which are under-saturated with respect to dolomite. RDP-07 is related to the five local springs M-06, M-03, M-04, M-07, and M-39 of Cluster A that drain the southern part of the PCM through fractures that affect the PPEc unit or contact the underlying Kgp unit.
- RDP-Cluster B: RDP-08 represents the flow line associated with the four springs M-01, M-02, M-36, and M-21 that drain through the southeast part of the PCM while being affected by the presence of the Eocene-Oligocene Beuda’s gypsum Formation. RDP-09 and RDP-10 are associated with the local springs M-10 and M-09, respectively. According to the recharge elevation zone associated with these springs, the meteoric water enters the system through the Kgp unit. Then GW flows downstream through the Kat and KMca units and finally discharges through the Tk unit. RDP-11 is associated with M-28 spring, whose recharge zone is in the PPEc unit. Then, GW flows through the Cretaceous and discharges through the Tk unit.
- RDP-Cluster C: RDP-12 and RDP-13 correspond to local RDPs running through the Tk unit that contains halite. These two RDPs are located at the NW and W of the PCM, respectively. Finally, RDP-14 is a flow path out of the PCM boundaries and associated with spring M-23, a spring with a water-type like that of the neighboring spring M-22 (Figure 2A).
4. Results and Discussion
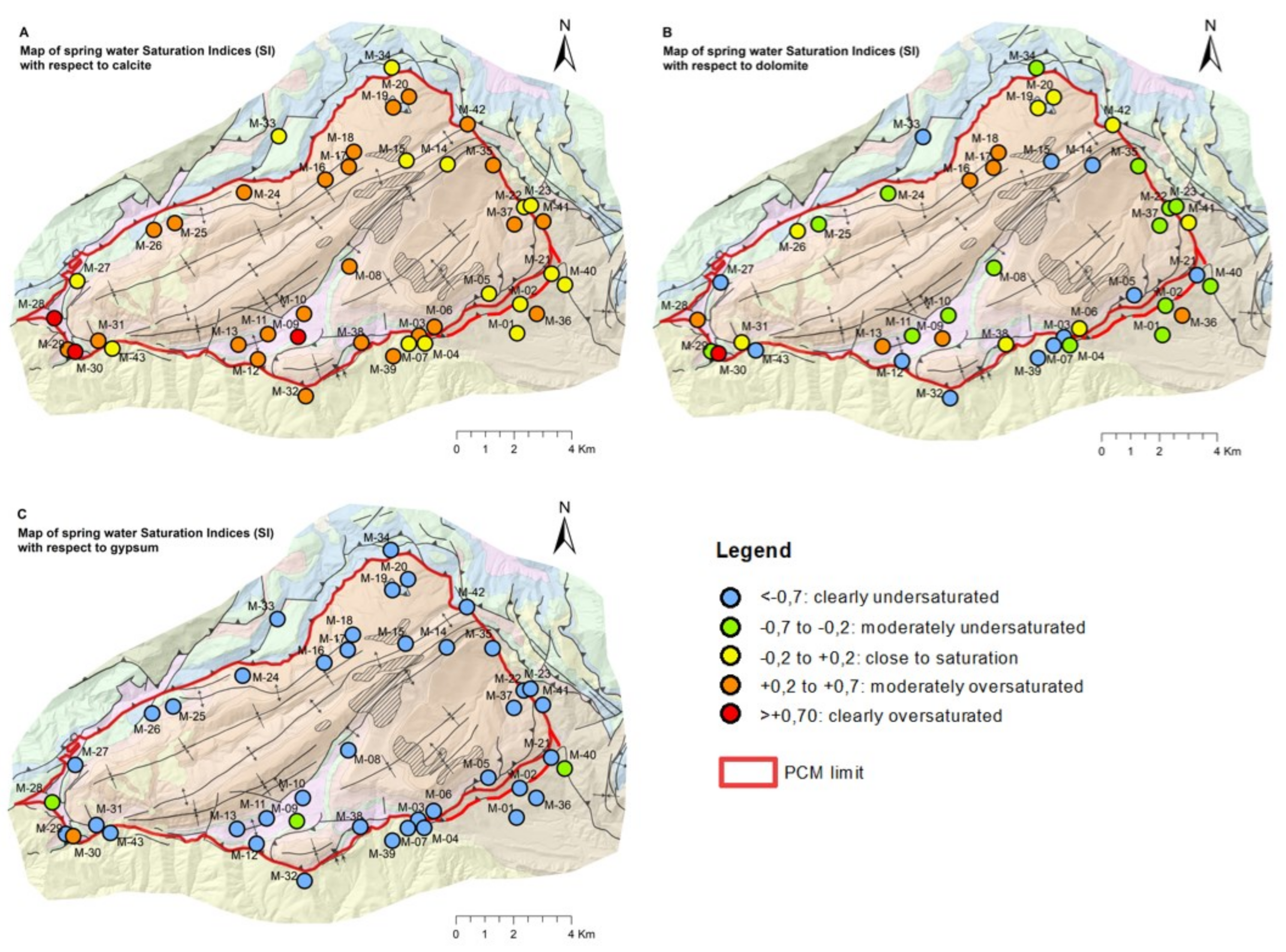
4.1. Saturation Indexes
4.2. Identification of Hydrogeochemical Processes Explaining the Spring Clusters
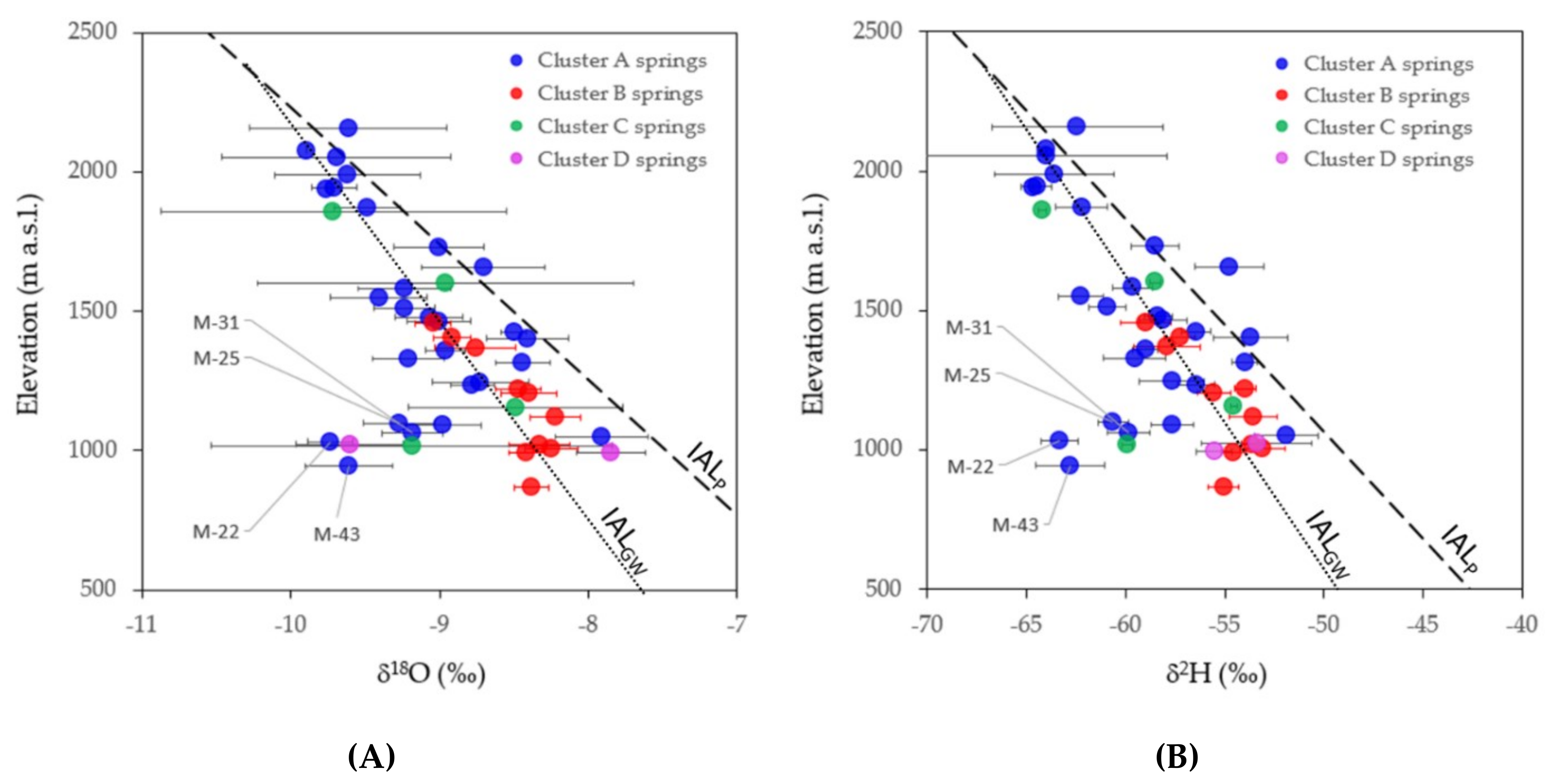
4.3. Aquifer Recharge Altitude Based on δ2H and δ18O in Precipitation and GW
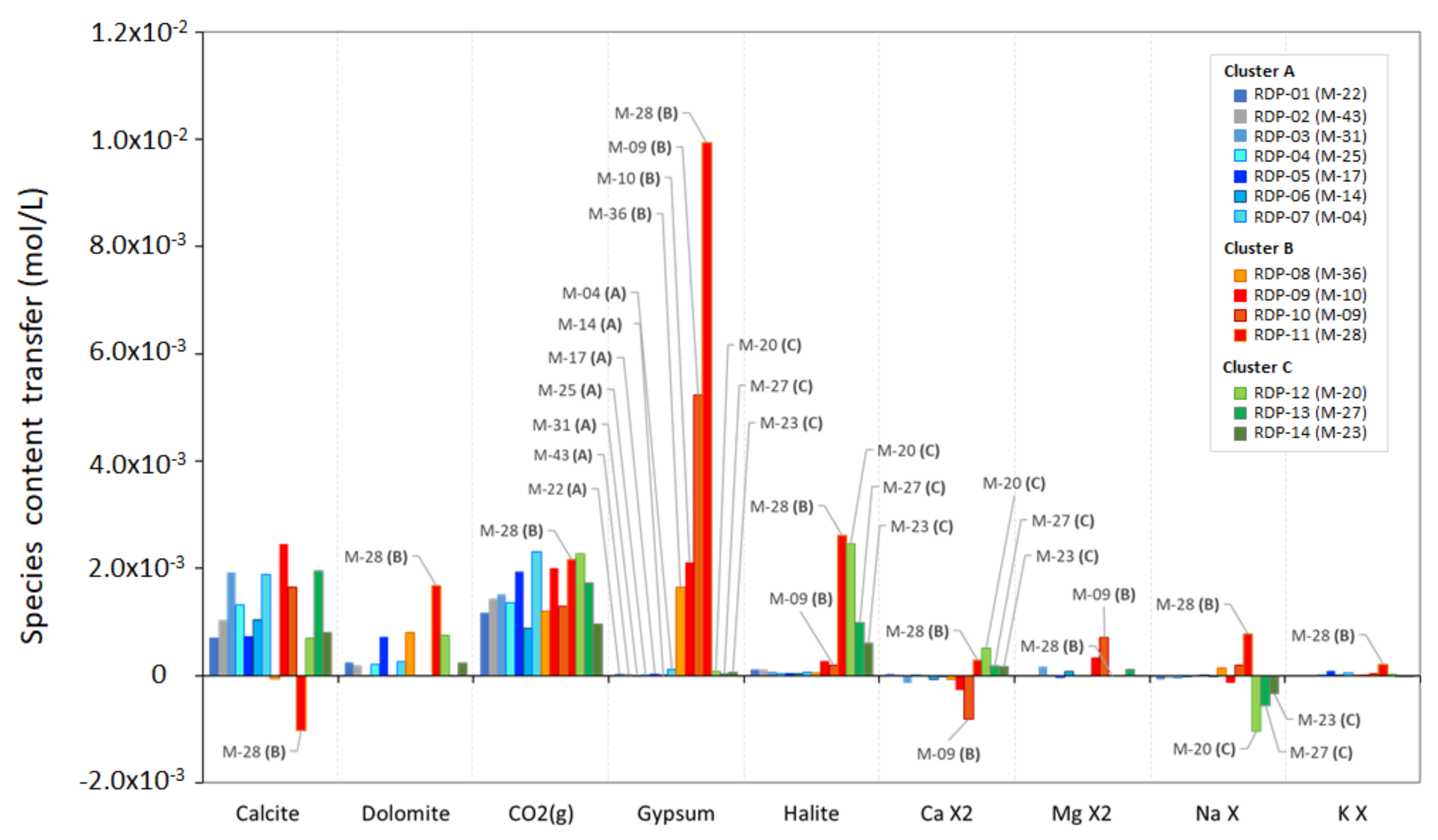
4.4. Quantification of Hydrogeochemical Processes along the Recharge-Discharge Pathways
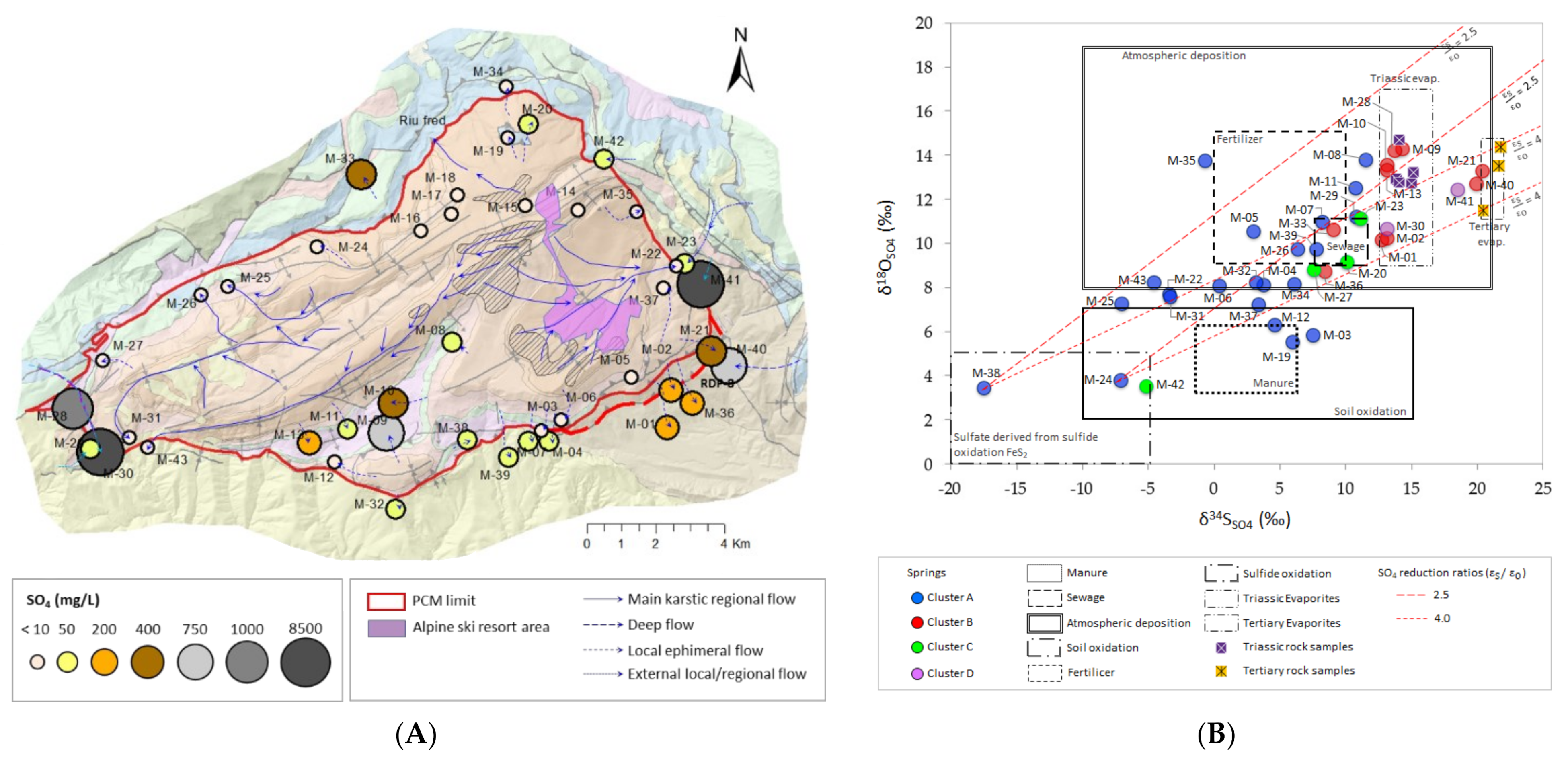
4.5. Identification of SO4 Sources in GW Based on Stable Isotopes
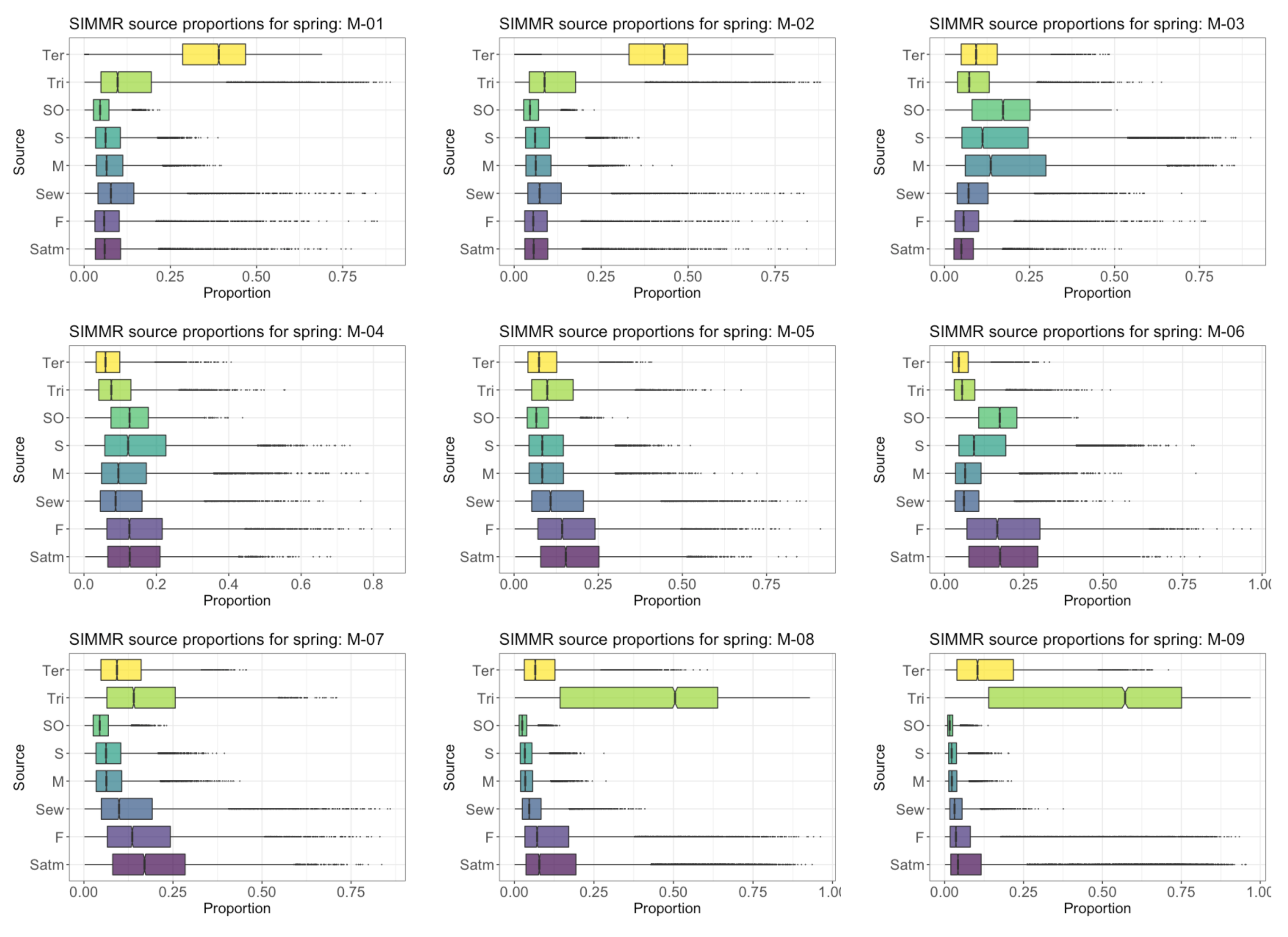
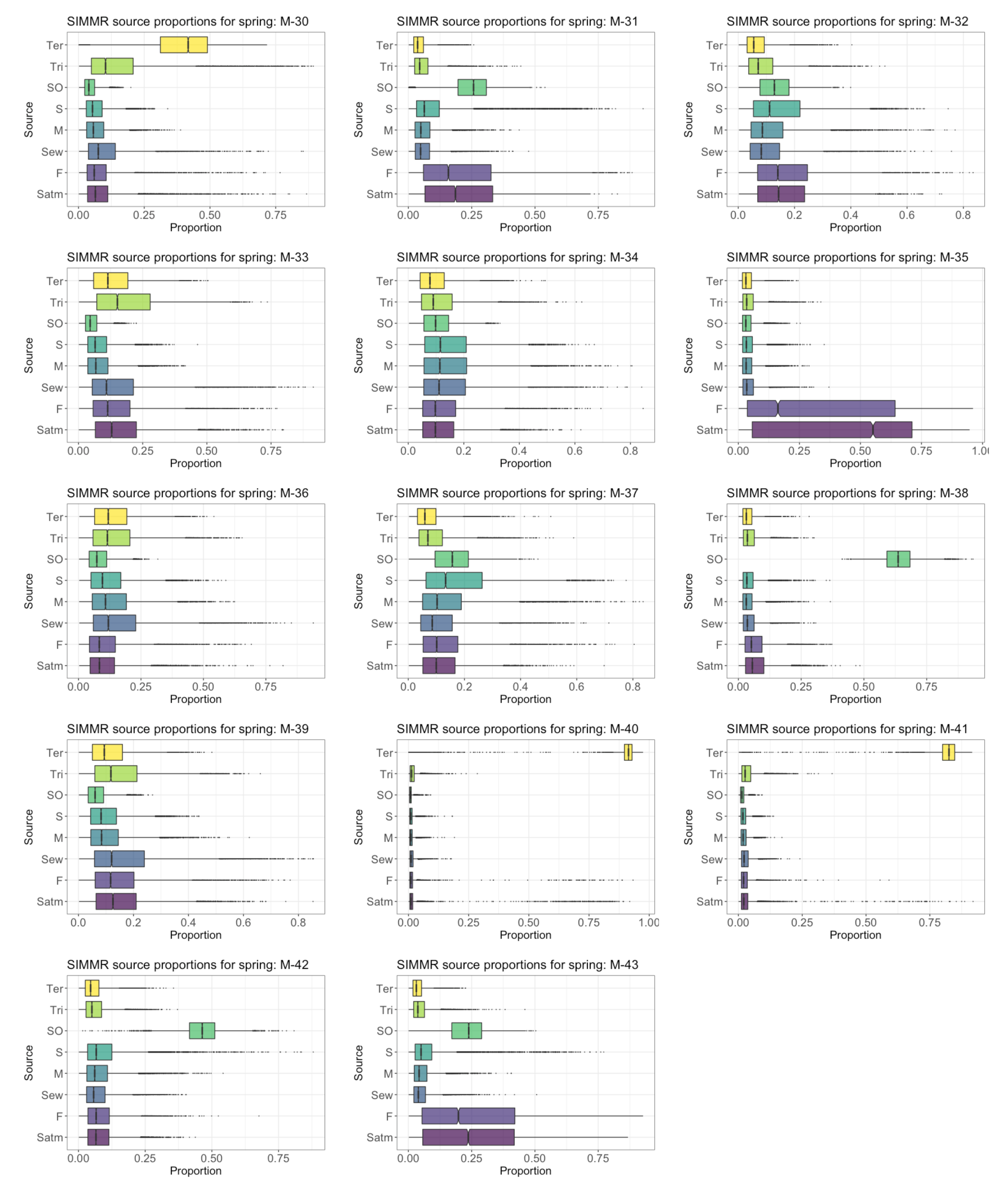
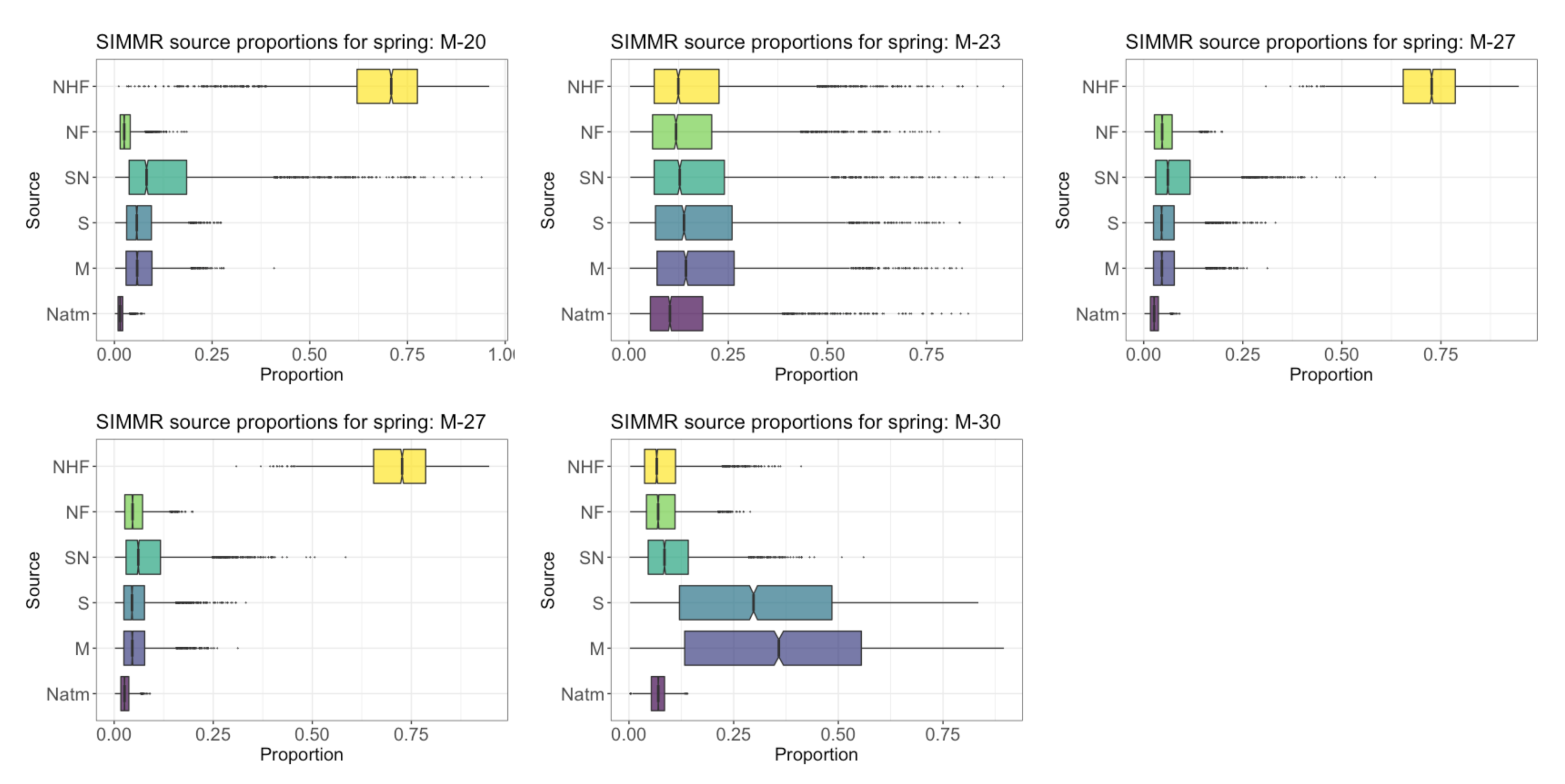
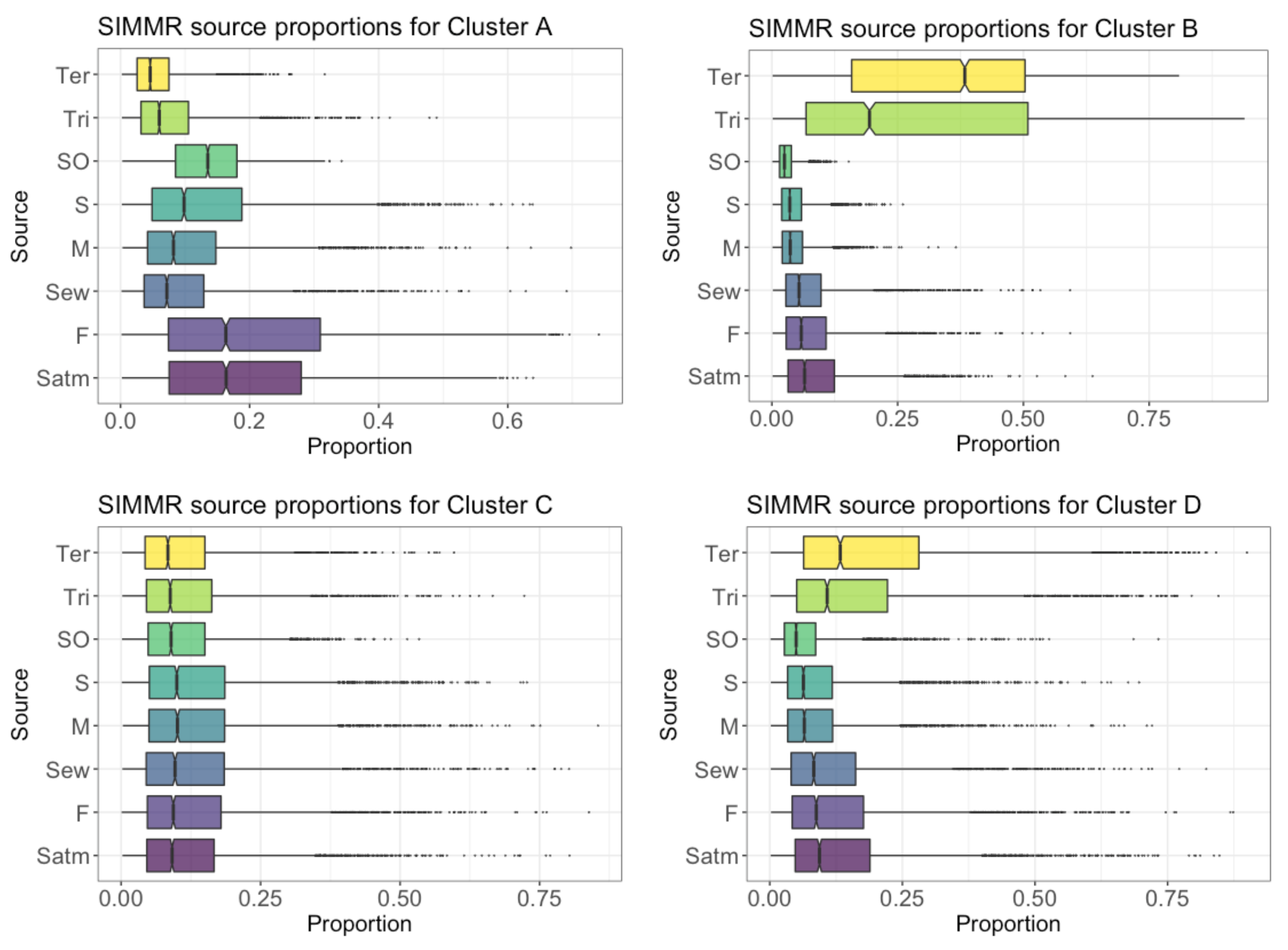
4.6. Proportional Contribution of SO4 Sources in GW in the PCM
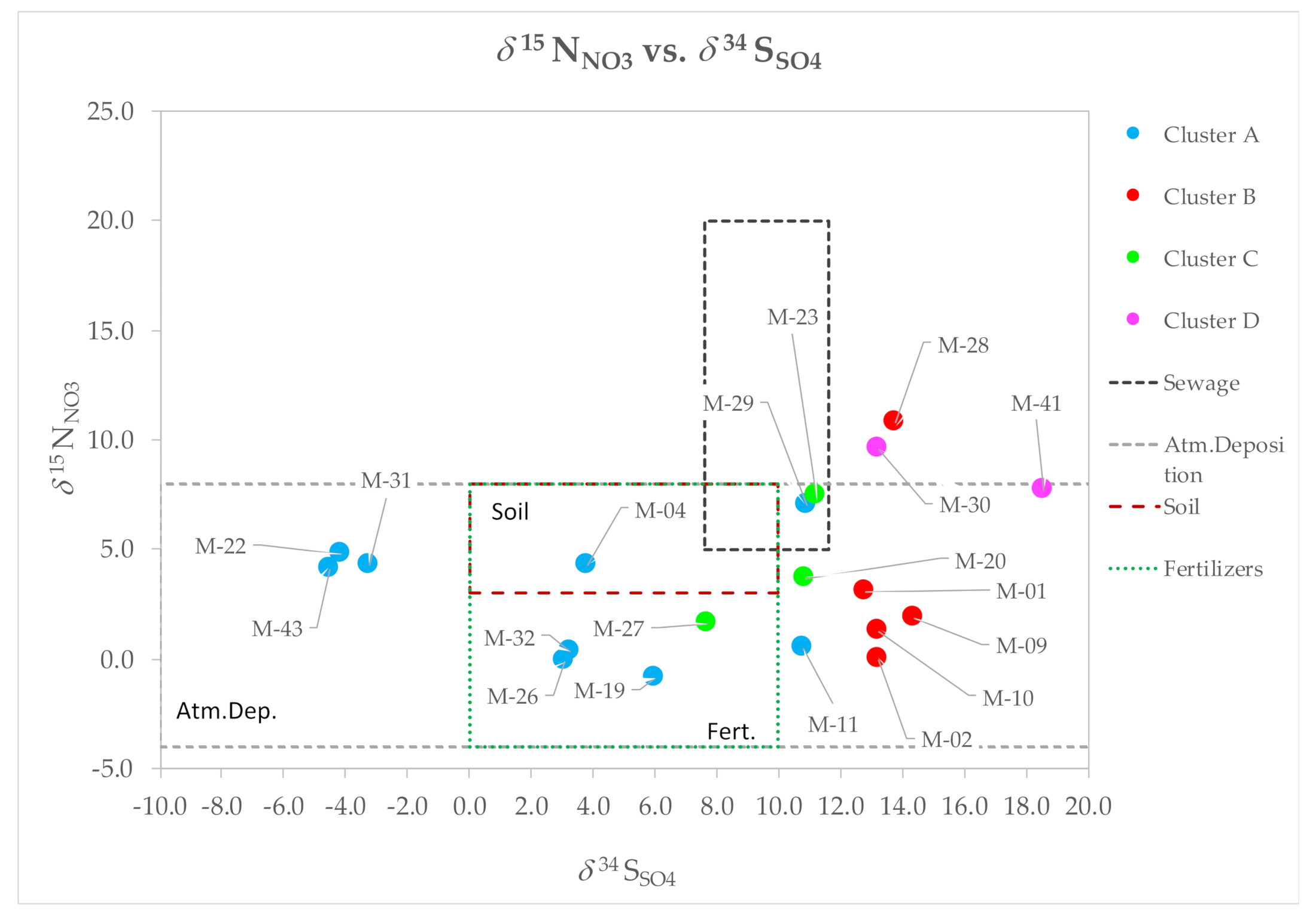
4.7. Identification of NO3 Sources in GW and Perspectives on Aquifer Vulnerability in PCM
4.8. Proportional Contribution of NO3 Sources in GW in PCM
4.9. Conceptual Model for Hydrogeochemical Evolution of GW in the PCM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
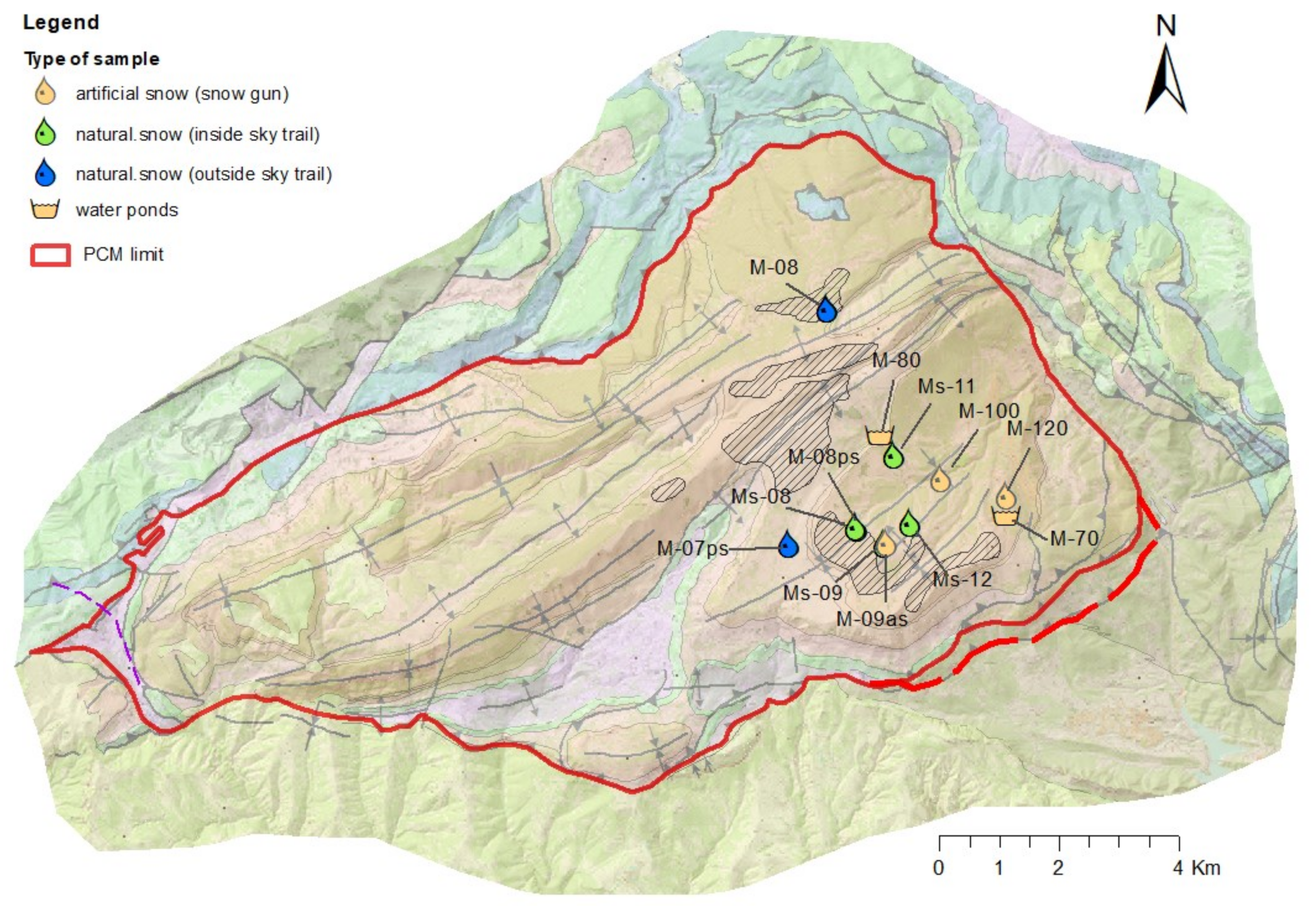
| Type of Sample | Number of Control Points | Total Field Campaigns | Total Number of Samples | Number of Analysis with Major Ions | Number of Analysis with Trace Metals | Total Number Analysis Stable Isotopes of δ2HH2O, δ18O | Total Number Analysis Stable Isotopes of δ34S, δ18OSO42- | Total Number Analysis Stable Isotopes of δ15N, δ18ONO3- |
|---|---|---|---|---|---|---|---|---|
| Pluviometers (quarterly) | 8 | 9 | 71 | - | - | 71 | - | - |
| Spring samples | - | - | 288 | 288 | 285 | 283 | 209 | 72 |
| Springs (biannually) | 40/43 | 4 | - | 138 | 136 | 134 | 88 | 42 |
| Spring (monthly) | 6 | 25 | - | 150 | 149 | 149 | 121 | 30 |
| Snow samples | 10 | 10 | 10 | 10 | 1 | - | ||
| Natural snow | 10 | - | - | 7 | 7 | 7 | - | - |
| Artificial snow | 3 | - | - | 3 | 3 | 3 | 1 | - |
| Water ponds (artificial snow production) | 2 | - | 2 | 2 | 2 | - | - | - |
| Total | 371 | |||||||
| ID | Num. Samples | Water Type | Cluster | GU | EC [μS/cm] | TDS [ppm] | pH | T [ºC] | Ca [ppm] | Mg [ppm] | Na [ppm] | K [ppm] | HCO3 [ppm] | Cl [ppm] | NO3 [ppm] | SO4 [ppm] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-03 | 4 | Ca-HCO3 | A | PEalb | 306.25 | 161.00 | 7.8 | 11.4 | 63.75 | 2.05 | 2.53 | 1.93 | 190.96 | 3.75 | 3.83 | 4.38 |
| M-04 | 25 | Ca-HCO3 | A | POcgs | 470.04 | 241.68 | 7.4 | 10.2 | 94.84 | 6.35 | 2.16 | 2.60 | 291.00 | 4.32 | 3.88 | 16.13 |
| M-05 | 4 | Ca-HCO3 | A | Qpe | 307.00 | 160.75 | 7.7 | 10.1 | 69.25 | 1.10 | 2.15 | 0.50 | 198.48 | 2.80 | 3.92 | 3.14 |
| M-06 | 4 | Ca-HCO3 | A | KMgp | 251.00 | 132.25 | 8.1 | 7.8 | 54.25 | 5.18 | 2.83 | 1.18 | 170.68 | 5.67 | 3.36 | 8.82 |
| M-07 | 4 | Ca-HCO3 | A | POcgs | 461.50 | 241.75 | 7.3 | 9.7 | 102.25 | 4.90 | 3.53 | 0.88 | 297.37 | 5.56 | 2.20 | 14.10 |
| M-08 | 4 | Ca-HCO3 | A | KMgp | 384.25 | 202.50 | 7.6 | 5.5 | 86.50 | 4.45 | 3.28 | 0.85 | 264.86 | 4.24 | 2.01 | 10.03 |
| M-11 | 4 | Ca-HCO3 | A | KMca | 312.75 | 164.00 | 8.0 | 10.7 | 59.25 | 5.50 | 3.78 | 0.65 | 162.11 | 4.16 | 10.84 | 25.35 |
| M-12 | 4 | Ca-HCO3 | A | KMgp | 252.00 | 132.25 | 7.9 | 8.5 | 54.50 | 2.35 | 1.70 | 0.58 | 167.31 | 4.29 | 1.88 | 4.19 |
| M-14 | 4 | Ca-HCO3 | A | PPEc | 190.75 | 100.25 | 8.0 | 5.8 | 44.75 | 1.85 | 1.43 | 0.48 | 119.61 | 2.55 | 5.48 | 2.62 |
| M-15 | 3 | Ca-HCO3 | A | PEci | 186.67 | 99.67 | 8.2 | 6.0 | 37.67 | 1.83 | 1.53 | 0.53 | 109.90 | 2.87 | 7.35 | 2.49 |
| M-16 | 1 | Ca-HCO3 | A | PEci | 306.00 | 184.00 | 8.0 | 13.4 | 60.00 | 12.00 | 1.00 | 0.80 | 221.00 | 2.50 | 0.50 | 6.30 |
| M-17 | 2 | Ca-HCO3 | A | PEci | 361.50 | 198.50 | 8.0 | 7.0 | 67.00 | 14.50 | 1.30 | 1.00 | 251.50 | 2.75 | 6.99 | 3.29 |
| M-18 | 1 | Ca-HCO3 | A | PEci | 385.00 | 231.00 | 7.9 | 11.9 | 64.00 | 18.00 | 1.70 | 3.80 | 253.00 | 2.50 | 8.30 | 6.40 |
| M-19 | 4 | Ca-HCO3 | A | TJcd | 392.00 | 206.00 | 7.8 | 7.4 | 63.25 | 18.75 | 1.85 | 0.73 | 258.30 | 4.43 | 5.62 | 4.60 |
| M-22 | 25 | Ca-HCO3 | A | Qvl | 241.04 | 122.71 | 7.9 | 7.4 | 44.64 | 6.14 | 1.63 | 0.49 | 147.94 | 5.35 | 3.19 | 7.14 |
| M-24 | 4 | Ca-HCO3 | A | PPEc | 402.50 | 211.50 | 7.6 | 8.2 | 77.50 | 11.00 | 3.18 | 1.08 | 269.94 | 6.59 | 3.00 | 5.45 |
| M-25 | 25 | Ca-HCO3 | A | KMgp | 323.76 | 164.32 | 7.8 | 8.0 | 65.84 | 5.03 | 1.75 | 0.59 | 210.24 | 3.36 | 2.54 | 5.87 |
| M-26 | 3 | Ca-HCO3 | A | KMca | 296.33 | 158.00 | 8.1 | 10.5 | 63.00 | 2.50 | 2.30 | 0.53 | 185.50 | 2.97 | 2.90 | 4.76 |
| M-29 | 4 | Ca-HCO3 | A | Qpe | 436.00 | 228.25 | 7.7 | 10.2 | 76.50 | 6.43 | 8.15 | 1.70 | 218.59 | 16.82 | 8.86 | 27.32 |
| M-31 | 25 | Ca-HCO3 | A | PPEc | 353.80 | 182.12 | 7.9 | 8.6 | 75.52 | 4.12 | 1.40 | 0.46 | 234.54 | 4.34 | 3.03 | 4.55 |
| M-32 | 4 | Ca-HCO3 | A | POmlg | 461.75 | 242.50 | 7.6 | 10.9 | 95.75 | 1.60 | 2.83 | 0.85 | 203.77 | 8.61 | 58.70 | 20.12 |
| M-34 | 4 | Ca-HCO3 | A | TJb | 331.75 | 191.00 | 7.6 | 7.6 | 68.50 | 8.43 | 2.90 | 0.75 | 238.90 | 4.22 | 1.94 | 7.15 |
| M-35 | 4 | Ca-HCO3 | A | PEcp1 | 232.00 | 123.00 | 8.1 | 12.6 | 42.00 | 4.55 | 1.53 | 0.48 | 138.85 | 3.27 | 3.48 | 4.12 |
| M-37 | 4 | Ca-HCO3 | A | Qvl | 223.75 | 117.25 | 8.1 | 8.2 | 45.25 | 2.68 | 2.40 | 0.40 | 133.98 | 3.99 | 3.81 | 4.43 |
| M-38 | 4 | Ca-HCO3 | A | KSCat | 486.50 | 255.50 | 7.6 | 8.8 | 86.00 | 14.75 | 2.85 | 1.08 | 305.16 | 3.92 | 1.65 | 22.39 |
| M-39 | 4 | Ca-HCO3 | A | POmlg | 472.25 | 247.50 | 7.5 | 11.2 | 95.25 | 4.48 | 2.48 | 0.55 | 283.15 | 5.95 | 1.78 | 13.75 |
| M-43 | 25 | Ca-HCO3 | A | POcgs | 283.76 | 144.36 | 7.7 | 9.0 | 54.48 | 4.90 | 2.51 | 0.43 | 174.64 | 5.47 | 2.88 | 6.95 |
| M-01 | 4 | Ca-HCO3 | B | PEm1 | 640.50 | 336.50 | 7.3 | 12.2 | 120.50 | 10.93 | 7.20 | 1.83 | 287.83 | 11.93 | 5.48 | 93.48 |
| M-02 | 4 | Ca-SO4 HCO3 | B | PEmb | 493.00 | 257.50 | 7.8 | 10.7 | 81.75 | 14.25 | 4.25 | 1.43 | 133.56 | 7.30 | 4.02 | 139.26 |
| M-09 | 4 | Ca-SO4 | B | Tk | 1155.50 | 606.75 | 7.9 | 9.0 | 252.50 | 17.25 | 9.83 | 2.28 | 207.56 | 8.70 | 4.29 | 495.92 |
| M-10 | 4 | Ca-SO4 HCO3 | B | Tk | 829.50 | 438.50 | 7.3 | 9.6 | 179.75 | 8.38 | 4.18 | 1.45 | 298.25 | 11.51 | 16.36 | 203.08 |
| M-13 | 4 | Ca-HCO3 SO4 | B | Tm | 574.25 | 320.75 | 7.7 | 11.2 | 98.25 | 19.00 | 9.43 | 1.65 | 254.25 | 20.83 | 1.94 | 96.03 |
| M-21 | 4 | Ca-SO4 | B | Qcoo | 867.25 | 453.25 | 7.4 | 11.8 | 179.50 | 8.55 | 3.18 | 1.13 | 178.47 | 4.63 | 3.43 | 327.72 |
| M-28 | 4 | Ca-SO4 | B | Tk | 2102.75 | 1102.75 | 7.5 | 12.9 | 438.00 | 40.75 | 43.75 | 9.03 | 291.14 | 95.40 | 42.70 | 961.12 |
| M-33 | 4 | Ca-SO4 HCO3 | B | Tk | 851.00 | 450.25 | 7.1 | 7.8 | 166.50 | 18.50 | 7.43 | 2.20 | 316.42 | 4.77 | 1.88 | 223.46 |
| M-36 | 4 | Ca-SO4 HCO3 | B | PEmb | 601.25 | 316.00 | 7.9 | 11.0 | 95.25 | 19.75 | 6.05 | 1.58 | 197.18 | 4.64 | 1.97 | 166.82 |
| M-40 | 4 | Ca-SO4 | B | Tk | 1234.13 | 644.88 | 7.2 | 12.3 | 230.75 | 28.00 | 41.75 | 2.40 | 240.88 | 69.38 | 2.72 | 464.13 |
| M-20 | 25 | Ca-HCO3 Cl | C | PEcp2 | 701.56 | 356.56 | 7.7 | 6.2 | 85.64 | 18.36 | 33.36 | 1.19 | 265.67 | 88.37 | 10.95 | 10.88 |
| M-23 | 4 | Ca-HCO3 | C | Qt0 | 332.25 | 174.25 | 7.8 | 8.3 | 55.50 | 5.90 | 7.35 | 0.63 | 161.46 | 23.39 | 5.37 | 11.25 |
| M-27 | 4 | Ca-HCO3 | C | KMca | 492.25 | 257.25 | 7.5 | 9.3 | 93.25 | 2.88 | 11.10 | 0.70 | 239.60 | 37.29 | 3.06 | 9.78 |
| M-42 | 4 | Ca-HCO3 | C | KMca | 747.00 | 390.25 | 7.5 | 10.6 | 101.00 | 17.25 | 37.25 | 1.08 | 330.65 | 75.82 | 2.43 | 15.16 |
| M-30 | 4 | Na-Cl | D | Tk | 247100 | 129475 | 6 | 15 | 744 | 1638 | 113946 | 3040 | 252 | 177879 | 4 | 8138 |
| M-41 | 4 | N -Cl | D | Tk | 57170 | 29855 | 7.3 | 12.3 | 546.00 | 76.75 | 13347 | 126.25 | 215.27 | 21196 | 5.11 | 1264.67 |
| ID | Num. Samples | Water Type | Cluster | Ca/Mg [mmol/L] | SI Calcite | SI Dolomite | SI Gypsum | SI Halite | SI pCO2g | ZR_Mean (m a.s.l.) | Zd (m a.s.l.) | ZR-Zd (m) | δ2HH20 (‰) | δ18OH20 (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-03 | 4 | Ca-HCO3 | A | 18.89 | 0.273 | −0.81 | −2.88 | −9.77 | −2.62 | 1865.14 | 1582 | 283.14 | −59.65 | −9.23 |
| M-04 | 25 | Ca-HCO3 | A | 9.08 | 0.186 | −0.68 | −2.25 | −9.66 | −2.07 | 1770.63 | 1464 | 306.63 | −58.11 | −9.01 |
| M-05 | 4 | Ca-HCO3 | A | 38.24 | 0.188 | −1.31 | −2.99 | −9.82 | −2.50 | 1751.76 | 1730 | 21.76 | −58.53 | −9.01 |
| M-06 | 4 | Ca-HCO3 | A | 6.37 | 0.373 | −0.20 | −2.63 | −9.51 | −2.97 | 1698.78 | 1657 | 41.78 | −54.75 | −8.70 |
| M-07 | 4 | Ca-HCO3 | A | 12.68 | 0.110 | −0.99 | −2.26 | −9.43 | −1.95 | 1803.03 | 1478 | 325.03 | −58.37 | −9.07 |
| M-08 | 4 | Ca-HCO3 | A | 11.81 | 0.255 | −0.69 | −2.44 | −9.52 | −2.25 | 1935.20 | 1871 | 64.20 | −62.22 | −9.49 |
| M-11 | 4 | Ca-HCO3 | A | 6.54 | 0.298 | −0.31 | −2.16 | −9.35 | −2.85 | 1590.12 | 1245 | 345.12 | −57.65 | −8.73 |
| M-12 | 4 | Ca-HCO3 | A | 14.09 | 0.235 | −0.81 | −2.94 | −9.70 | −2.81 | 1679.61 | 1234 | 445.61 | −56.45 | −8.78 |
| M-14 | 4 | Ca-HCO3 | A | 14.69 | 0.020 | −1.32 | −3.22 | −10.07 | −3.04 | 2108.37 | 2053 | 55.37 | −64.01 | −9.69 |
| M-15 | 3 | Ca-HCO3 | A | 12.48 | 0.110 | −1.06 | −3.26 | −10.14 | −3.24 | 2228.43 | 2158 | 70.43 | −62.45 | −9.61 |
| M-16 | 1 | Ca-HCO3 | A | 3.04 | 0.510 | 0.50 | −2.78 | −11.84 | −2.74 | 2149.38 | 2077 | 72.38 | −64.03 | −9.90 |
| M-17 | 2 | Ca-HCO3 | A | 2.81 | 0.505 | 0.41 | −3.04 | −9.99 | −2.72 | 2073.96 | 1989 | 84.96 | −63.59 | −9.62 |
| M-18 | 1 | Ca-HCO3 | A | 2.16 | 0.460 | 0.53 | −2.77 | −10.01 | −2.59 | 2027.84 | 1940 | 87.84 | −64.64 | −9.76 |
| M-19 | 4 | Ca-HCO3 | A | 2.05 | 0.320 | 0.18 | −2.90 | −9.71 | −2.53 | 1995.60 | 1944 | 51.60 | −64.49 | −9.71 |
| M-22 | 25 | Ca-HCO3 | A | 4.41 | 0.077 | −0.65 | −2.80 | −9.70 | −2.87 | 2061.21 | 1032 | 1029.21 | −63.36 | −9.73 |
| M-24 | 4 | Ca-HCO3 | A | 4.28 | 0.250 | −0.26 | −2.75 | −9.45 | −2.32 | 1878.24 | 1550 | 328.24 | −62.26 | −9.41 |
| M-25 | 25 | Ca-HCO3 | A | 7.95 | 0.251 | −0.53 | −2.74 | −9.84 | −2.58 | 1850.63 | 1098 | 752.63 | −60.62 | −9.27 |
| M-26 | 3 | Ca-HCO3 | A | 15.31 | 0.553 | −0.16 | −2.85 | −9.76 | −2.96 | 1769.48 | 1091 | 678.48 | −57.65 | −8.98 |
| M-29 | 4 | Ca-HCO3 | A | 7.23 | 0.225 | −0.51 | −2.08 | −8.42 | −2.43 | 1259.67 | 1050 | 209.67 | −51.87 | −7.91 |
| M-31 | 25 | Ca-HCO3 | A | 11.14 | 0.505 | −0.15 | −2.82 | −9.89 | −2.68 | 1820.01 | 1062 | 758.01 | −59.85 | −9.18 |
| M-32 | 4 | Ca-HCO3 | A | 36.36 | 0.228 | −1.18 | −2.11 | −9.20 | −2.39 | 1483.70 | 1425 | 58.70 | −56.43 | −8.50 |
| M-34 | 4 | Ca-HCO3 | A | 4.94 | 0.095 | −0.65 | −2.66 | −9.54 | −2.31 | 1813.78 | 1511 | 302.78 | −60.91 | −9.24 |
| M-35 | 4 | Ca-HCO3 | A | 5.61 | 0.258 | −0.28 | −3.05 | −10.02 | −3.03 | 1852.12 | 1330 | 522.12 | −57.02 | −8.90 |
| M-37 | 4 | Ca-HCO3 | A | 10.28 | 0.235 | −0.68 | −2.98 | −9.56 | −3.09 | 1544.69 | 1315 | 229.69 | −54.00 | −8.44 |
| M-38 | 4 | Ca-HCO3 | A | 3.54 | 0.283 | −0.10 | −2.12 | −9.56 | −2.21 | 1534.22 | 1402 | 132.22 | −53.71 | −8.41 |
| M-39 | 4 | Ca-HCO3 | A | 12.93 | 0.240 | −0.71 | −2.29 | −9.55 | −2.13 | 1697.19 | 1360 | 337.19 | −59.01 | −8.96 |
| M-43 | 25 | Ca-HCO3 | A | 6.75 | 0.052 | −0.85 | −2.74 | −9.50 | −2.59 | 1996.16 | 944 | 1052.16 | −62.82 | −9.61 |
| M-01 | 4 | Ca-HCO3 | B | 6.70 | 0.178 | −0.53 | −1.43 | −8.71 | −1.98 | 1478.00 | 970 | 508.00 | −53.66 | −8.33 |
| M-02 | 4 | Ca-SO4 HCO3 | B | 3.49 | 0.130 | −0.36 | −1.41 | −9.40 | −2.80 | 1567.28 | 1220 | 347.28 | −53.97 | −8.47 |
| M-09 | 4 | Ca-SO4 | B | 8.89 | 0.818 | 0.61 | −0.59 | −8.70 | −2.75 | 1742.65 | 1404 | 338.65 | −57.26 | −8.92 |
| M-10 | 4 | Ca-SO4 HCO3 | B | 13.04 | 0.280 | −0.66 | −1.00 | −8.99 | −1.99 | 1758.76 | 1456 | 302.76 | −58.98 | −9.04 |
| M-13 | 4 | Ca-HCO3 SO4 | B | 3.14 | 0.398 | 0.22 | −1.51 | −8.33 | −2.41 | 1449.55 | 1205 | 244.55 | −55.55 | −8.40 |
| M-21 | 4 | Ca-SO4 | B | 12.75 | 0.123 | −0.93 | −0.81 | −9.49 | −2.25 | 1501.83 | 992 | 509.83 | −54.57 | −8.41 |
| M-28 | 4 | Ca-SO4 | B | 6.53 | 0.703 | 0.55 | −0.24 | −7.02 | −2.21 | 1409.40 | 1119 | 290.40 | −53.57 | −8.22 |
| M-33 | 4 | Ca-SO4 HCO3 | B | 5.47 | 0.000 | −0.87 | −1.01 | −9.21 | −1.74 | 1603.08 | 1369 | 234.08 | −57.92 | −8.76 |
| M-36 | 4 | Ca-SO4 HCO3 | B | 2.93 | 0.468 | 0.39 | −1.28 | −9.15 | −2.74 | 1446.25 | 1005 | 441.25 | −53.11 | −8.25 |
| M-40 | 4 | Ca-SO4 | B | 5.01 | 0.150 | −0.46 | −0.65 | −7.14 | −1.97 | 1455.97 | 867 | 588.97 | −55.05 | −8.38 |
| M-20 | 25 | Ca-HCO3 Cl | C | 2.83 | 0.250 | −0.12 | −2.46 | −7.11 | −2.37 | 2010.28 | 1858 | 152.28 | −64.15 | −9.71 |
| M-23 | 4 | Ca-HCO3 | C | 5.71 | 0.110 | −0.67 | −2.52 | −8.31 | −2.73 | 1817.54 | 1017 | 800.54 | −59.94 | −9.18 |
| M-27 | 4 | Ca-HCO3 | C | 19.70 | 0.188 | −1.03 | −2.43 | −7.97 | −2.26 | 1557.56 | 1156 | 401.56 | −54.54 | −8.49 |
| M-42 | 4 | Ca-HCO3 | C | 3.56 | 0.348 | 0.06 | −2.28 | −7.17 | −2.13 | 1720.97 | 1601 | 119.97 | −58.55 | −8.96 |
| M-30 | 4 | Na-Cl | D | 0.28 | 0.770 | 2.53 | 0.27 | 0.31 | −1.82 | 1324.16 | 1023 | 301.16 | −53.39 | −9.60 |
| M-41 | 4 | Na-Cl | D | 4.32 | 0.133 | −0.36 | −0.69 | −2.20 | −2.26 | 1061.74 | 993 | 68.74 | −55.52 | −7.85 |
| ID | Date | Water Type | Sample Type | EC [μS/cm] | TDS [ppm] | pH | T [ºC] | Ca [ppm] | Mg [ppm] | Na [ppm] | K [ppm] | HCO3 [ppm] | CO3 [ppm] | Cl [ppm] | NO3 [ppm] | SO4 [ppm] | δ2HH20 (‰) | δ18OH20 (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-09as | 09/12/13 | Ca-HCO3 Cl | T-1 | 49 | 24 | 10.3 | - | 5.6 | 0.5 | 1.3 | 1.2 | 11.6 | 2.9 | 4.9 | 1.6 | 1.6 | −41.4 | −5.1 |
| M-120 | 07/12/14 | Ca-HCO3 | T-1 | 142 | 71 | 10.0 | - | 18.0 | 4.0 | 6.3 | 1.2 | 55.0 | 12.0 | 9.3 | 1.3 | 5.0 | −45.0 | −5.9 |
| M-100 | 07/12/14 | Ca-HCO3 | T-1 | 51 | 26 | 9.6 | - | 8.9 | 1.0 | 1.2 | 0.2 | 24.0 | <2.4 | 2.5 | <1 | 1.1 | −51.6 | −7.5 |
| M-08ps | 09/12/13 | Ca Na-Cl | T-2 | 25 | 13 | 7.0 | - | <2 | <0.4 | 1.4 | 1.3 | 3.0 | <2.4 | 4.0 | 0.2 | <0.7 | −110.9 | −15.8 |
| Ms-11 | 09/03/14 | Ca-Cl HCO3 | T-2 | 21 | 11 | 6.7 | - | <2 | <0.4 | 1.0 | 0.9 | 3.5 | <2.4 | <2.5 | 2.9 | <0.7 | −70.0 | −10.2 |
| Ms-09 | 09/03/14 | Ca-Cl HCO3 | T-2 | 16 | 8 | 6.2 | - | <2 | <0.4 | 1.0 | 0.4 | 2.7 | <2.4 | <2.5 | 2.3 | <0.7 | −88.3 | −12.4 |
| Ms-08 | 09/03/14 | Ca-Cl | T-2 | 21 | 10 | 5.6 | - | <2 | <0.4 | 1.0 | 0.7 | 1.2 | <2.4 | <2.5 | 3.5 | <0.7 | −101.0 | −14.1 |
| Ms-12 | 09/03/14 | Ca-Cl HCO3 | T-2 | 13 | 6 | 5.7 | - | <2 | <0.4 | 1.0 | 0.4 | 2.5 | <2.4 | <2.5 | 1.9 | <0.7 | −68.7 | −10.3 |
| M-07ps | 07/12/13 | Ca-Cl HCO3 | T-3 | 5 | 2 | 6.7 | - | <2 | <0.4 | 1.0 | 0.3 | 2.7 | <2.4 | 2.5 | 0.3 | <0.7 | −105.8 | −14.8 |
| M-10ps | 12/01/14 | Ca-Cl HCO3 | T-3 | 20 | 9 | 5.5 | - | <2 | <0.4 | 1.0 | 0.2 | 3.7 | <2.4 | 4.2 | <1 | 1.4 | −88.1 | −11.7 |
| M-80 | 23710/14 | Ca-HCO3 | T-4 | 142 | 71 | 8.3 | 10.6 | 28.0 | 1.8 | 1.6 | 0.7 | 83.0 | <2.4 | 4.2 | <1 | 3.3 | −50.7 | −7.0 |
| M-70 | 23/10/14 | Ca-HCO3 | T-4 | 177 | 88 | 8.2 | 10.2 | 27.0 | 3.5 | 4.8 | 1.0 | 74.0 | <2.4 | 19.9 | 1.7 | 5.3 | −43.6 | −6.0 |
| Precipitation and Recharge Water Chemistry | HCO3 [ppm] | Ca [ppm] | Cl [ppm] | K [ppm] | Mg [ppm] | Na [ppm] | SO4 [ppm] | NO3 [ppm] |
|---|---|---|---|---|---|---|---|---|
| Precipitation water from the meteorological station of La Molina (42°20’30’’ N, 1°57’14” E, altitude 1704 m a.s.l.) | 3.14 | 1.73 | 0.94 | 0.35 | 0.09 | 0.54 | 2.66 | 1.31 |
| Estimated average recharge (evapo-concentrated water chemistry in the PMC applying a reduced concentration factor). | 7.35 | 4.06 | 2.19 | 0.82 | 0.20 | 1.25 | 6.23 | 3.07 |
| Spring | Cluster | Num. Samples | GU (BG50M) | SO4 [ppm] | Water Type | δ34SSO4 (‰) | δ18OSO4 (‰) |
|---|---|---|---|---|---|---|---|
| M-03 | A | 2 | PEalb | 4.44 | Ca-HCO3 | +7.6 | +5.8 |
| M-04 | A | 21 | POcgs | 16.29 | Ca-HCO3 | +3.9 | +8.1 |
| M-05 | A | 1 | Qpe | 3.39 | Ca-HCO3 | +6.4 | +9.7 |
| M-06 | A | 2 | Kgp | 9.82 | Ca-HCO3 | +0.4 | +8.0 |
| M-07 | A | 3 | POcgs | 14.14 | Ca-HCO3 | +8.3 | +10.9 |
| M-08 | A | 2 | Kgp | 8.72 | Ca-HCO3 | +11.6 | +13.7 |
| M-11 | A | 3 | KMca | 25.13 | Ca-HCO3 | +10.8 | +12.5 |
| M-12 | A | 1 | Kgp | 4.03 | Ca-HCO3 | +4.7 | +6.3 |
| M-19 | A | 2 | TJcd | 4.52 | Ca-HCO3 | +6.0 | +5.5 |
| M-22 | A | 21 | Qvl | 7.07 | Ca-HCO3 | −3.3 | +7.6 |
| M-24 | A | 2 | PPEc | 5.44 | Ca-HCO3 | −7.0 | +3.7 |
| M-25 | A | 20 | Kgp | Kgp | Ca-HCO3 | −7.0 | +7.2 |
| M-26 | A | 1 | KMca | 4.94 | Ca-HCO3 | +3.1 | +10.5 |
| M-29 | A | 3 | Qpe | 28.42 | Ca-HCO3 | +10.9 | +11.1 |
| M-31 | A | 19 | PPEc | 4.45 | Ca-HCO3 | −3.3 | +7.5 |
| M-32 | A | 2 | POmlg | 19.72 | Ca-HCO3 | +3.2 | +8.2 |
| M-34 | A | 3 | TJb | 6.93 | Ca-HCO3 | +6.1 | +8.1 |
| M-35 | A | 1 | PEcp1 | 4.49 | Ca-HCO3 | −0.6 | +13.7 |
| M-37 | A | 2 | Qvl | 3.89 | Ca-HCO3 | +3.5 | +7.2 |
| M-38 | A | 3 | Kat | 21.52 | Ca-HCO3 | −17.5 | +3.4 |
| M-39 | A | 2 | POmlg | 14.69 | Ca-HCO3 | +7.8 | +9.7 |
| M-43 | A | 21 | POcgs | 6.66 | Ca-HCO3 | −4.5 | +8.2 |
| M-01 | B | 2 | PEm1 | 106.52 | Ca-HCO3 | +12.8 | +10.1 |
| M-02 | B | 4 | PEmb | 139.26 | Ca-SO4 HCO3 | +13.2 | +10.2 |
| M-09 | B | 4 | Tk | 495.92 | Ca-SO4 | +14.4 | +14.2 |
| M-10 | B | 4 | Tk | 203.08 | Ca-SO4 HCO3 | +13.2 | +13.5 |
| M-13 | B | 4 | Tm | 96.03 | Ca-HCO3 SO4 | +13.2 | +13.3 |
| M-21 | B | 4 | Qcoo | 327.72 | Ca-SO4 | +20.4 | +13.2 |
| M-28 | B | 4 | Tk | 961.12 | Ca-SO4 | +13.8 | +14.1 |
| M-33 | B | 4 | Tk | 223.46 | Ca-SO4 HCO3 | +9.1 | +10.6 |
| M-36 | B | 4 | PEmb | 166.82 | Ca-SO4 HCO3 | +8.5 | +8.7 |
| M-40 | B | 4 | Tk | 464.13 | Ca-SO4 | +20.0 | +12.7 |
| M-20 | C | 22 | PEcp2 | 10.8 | Ca-HCO3 Cl | +10.2 | +9.1 |
| M-23 | C | 2 | Qt0 | 12.2 | Ca-HCO3 | +11.2 | +11.1 |
| M-27 | C | 2 | KMca | 9.7 | Ca-HCO3 | +7.7 | +8.8 |
| M-42 | C | 3 | KMca | 14.9 | Ca-HCO3 | −5.1 | +3.5 |
| M-30 | D | 4 | Tk | 8138.20 | Na-Cl | +13.2 | +10.6 |
| M-41 | D | 4 | Tk | 1264.67 | Na-Cl | +18.6 | +12.4 |
| Rock Sample ID | Lithology | Geology | Geological Unit (BG50M) | δ34SSO4 (‰) | δ18OSO4 (‰) |
|---|---|---|---|---|---|
| RS-01 | massive nodular gypsum with shales | Keuper (Upper Triassic) | Tk | +14.2 | +14.8 |
| RS-02 | massive nodular gypsum with shales | Keuper (Upper Triassic) | Tk | +14.3 | +12.9 |
| RS-03 | massive nodular gypsum with shales | Keuper (Upper Triassic) | Tk | +14.1 | +13.0 |
| RS-04 | massive nodular gypsum with shales | Keuper (Upper Triassic) | Tk | +15.3 | +13.3 |
| RS-05 | massive nodular gypsum with shales | Keuper (Upper Triassic) | Tk | +15.1 | +12.8 |
| RS-06 | laminated gypsum and marls | Beuda Fm. Eocene (Paleogene) | Pexb | +22.0 | +14.5 |
| RS-07 | laminated gypsum and marls | Beuda Fm. Eocene (Paleogene) | Pexb | +20.7 | +11.6 |
| RS-08 | laminated gypsum and marls | Beuda Fm. Eocene (Paleogene) | Pexb | +21.9 | +13.6 |
| Spring | Clust | Num. Samples | GU (BG50M) | NO3 [ppm] | Water Type | δ15NNO3 (‰) | δ18ONO3 (‰) |
|---|---|---|---|---|---|---|---|
| M-04 | A | 11 | POcgs | 4.15 | Ca-HCO3 | 4.3 | 3.7 |
| M-11 | A | 2 | KMca | 10.76 | Ca-HCO3 | 0.5 | 0.0 |
| M-14 | A | 1 | PPEc | 11.69 | Ca-HCO3 | −0.1 | 0.7 |
| M-19 | A | 3 | TJcd | 6.06 | Ca-HCO3 | −0.9 | 1.0 |
| M-22 | A | 4 | Qvl | 5.62 | Ca-HCO3 | 4.8 | 4.5 |
| M-26 | A | 1 | KMca | 2.54 | Ca-HCO3 | −0.1 | 0.0 |
| M-29 | A | 3 | Qpe | 8.78 | Ca-HCO3 | 7.0 | 1.5 |
| M-31 | A | 4 | PPEc | 3.73 | Ca-HCO3 | 4.3 | 4.8 |
| M-32 | A | 3 | POmlg | 57.27 | Ca-HCO3 | 0.3 | 1.4 |
| M-43 | A | 2 | POcgs | 3.64 | Ca-HCO3 | 4.1 | 3.9 |
| M-01 | B | 2 | PEm1 | 6.16 | Ca-HCO3 | 3.1 | 3.0 |
| M-02 | B | 2 | PEmb | 4.58 | Ca-SO4 HCO3 | −0.1 | 1.4 |
| M-09 | B | 2 | Tk | 4.90 | Ca-SO4 | 1.9 | 1.5 |
| M-10 | B | 3 | Tk | 18.14 | Ca-SO4 HCO3 | 1.2 | 2.6 |
| M-28 | B | 3 | Tk | 38.60 | Ca-SO4 | 10.7 | 3.7 |
| M-20 | C | 21 | PEcp2 | 11.3 | Ca-HCO3 Cl | 3.7 | 0.7 |
| M-23 | C | 2 | Qt0 | 4.4 | Ca-HCO3 | 7.4 | 8.0 |
| M-27 | C | 1 | KMca | 2.1 | Ca-HCO3 | 1.6 | 3.1 |
| M-30 | D | 1 | Tk | 4.49 | Na-Cl | 9.6 | 6.2 |
| M-41 | D | 1 | Tk | 6.75 | Na-Cl | 7.7 | 1.6 |
| Springs | Water Type | Clust | RDP-1 | n | sm | Ca/Mg | Cal | Dol | CO2(g) | Gyp | Hal | CaΧ2 | MgΧ2 | NaΧ | KΧ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-22R | Ca-HCO3 | A | RDP-01 | 8 | Model 4 | 4.41 | 7.10 × 10−4 | 2.48 × 10−4 | 1.17 × 10−3 | 3.59 × 10−5 | 1.13 × 10−4 | 3.80 × 10−5 | - | −7.60 × 10−5 | - |
| Model 6 | 7.86 × 10−4 | 2.10 × 10−4 | 1.17 × 10−3 | 3.59 × 10−5 | 1.13 × 10−4 | - | 3.80 × 10−5 | −7.60 × 10−5 | - | ||||||
| M-43R | Ca-HCO3 | A | RDP-02 | 4 | Model 2 | 6.75 | 1.03 × 10−3 | 1.96 × 10−4 | 1.44 × 10−3 | 2.93 × 10−5 | 1.11 × 10−4 | 2.15 × 10−5 | - | −4.00 × 10−5 | −3.00 × 10−6 |
| Model 3 | 1.08 × 10−3 | 1.75 × 10−4 | 1.44 × 10−3 | 2.93 × 10−5 | 1.11 × 10−4 | - | 2.15 × 10−5 | −4.00 × 10−5 | −3.00 × 10−6 | ||||||
| M-31R | Ca-HCO3 | A | RDP-03 | 8 | Model 7 | 11.14 | 1.93 × 10−3 | - | 1.51 × 10−3 | 2.31 × 10−6 | 7.70 × 10−5 | −1.35 × 10−4 | 1.64 × 10−4 | −5.60 × 10−5 | −3.00 × 10−6 |
| - | - | - | - | - | - | - | - | - | - | ||||||
| M-25R | Ca-HCO3 | A | RDP-04 | 4 | Model 2 | 7.95 | 1.31 × 10−3 | 2.03 × 10−4 | 1.36 × 10−3 | 6.69 × 10−6 | 3.86 × 10−5 | 2.66 × 10−6 | - | −7.00 × 10−6 | 1.67 × 10−6 |
| Model 3 | 1.32 × 10−3 | 2.00 × 10−4 | 1.36 × 10−3 | 6.69 × 10−6 | 3.86 × 10−5 | - | 2.66 × 10−6 | −7.00 × 10−6 | 1.67 × 10−6 | ||||||
| M-17 | Ca-HCO3 | A | RDP-05 | 8 | Model 5 | 2.81 | 7.33 × 10−4 | 7.26 × 10−4 | 1.93 × 10−3 | 3.41 × 10−5 | 4.40 × 10−5 | - | −4.35 × 10−5 | 9.99 × 10−7 | 8.60 × 10−5 |
| Model 6 | 7.33 × 10−4 | 7.26 × 10−4 | 1.93 × 10−3 | 3.41 × 10−5 | 4.50 × 10−5 | - | −4.30 × 10−5 | - | 8.60 × 10−5 | ||||||
| M-14 | Ca-HCO3 | A | RDP-06 | 18 | Model 8 | 14.69 | 1.04 × 10−3 | - | 8.75 × 10−4 | 4.00 × 10−9 | 3.70 × 10−5 | −6.99 × 10−5 | 6.74 × 10−5 | 2.00 × 10−6 | 3.00 × 10−6 |
| - | - | - | - | - | - | - | - | - | - | ||||||
| M-04 | Ca-HCO3 | A | RDP-07 | 4 | Model 2 | 9.08 | 1.88 × 10−3 | 2.53 × 10−4 | 2.31 × 10−3 | 1.08 × 10−4 | 6.50 × 10−5 | −1.20 × 10−5 | - | −2.30 × 10−5 | 4.70 × 10−5 |
| Model 3 | 1.86 × 10−3 | 2.65 × 10−4 | 2.31 × 10−3 | 1.08 × 10−4 | 6.50 × 10−5 | - | −1.20 × 10−5 | −2.30 × 10−5 | 4.70 × 10−5 | ||||||
| M-36 | Ca-SO4 HCO3 | B | RDP-08 | 4 | Model 2 | 2.93 | −6.42 × 10−5 | 8.02 × 10−4 | 1.20 × 10−3 | 1.64 × 10−3 | 5.13 × 10−5 | −7.64 × 10−5 | - | 1.40 × 10−4 | 1.30 × 10−5 |
| Model 3 | −2.17 × 10−4 | 8.79 × 10−4 | 1.20 × 10−3 | 1.64 × 10−3 | 5.13 × 10−5 | - | −7.64 × 10−5 | 1.40 × 10−4 | 1.30 × 10−5 | ||||||
| M-10 | Ca-SO4 HCO3 | B | RDP-09 | 4 | Model 4 | 13.04 | 2.45 × 10−3 | - | 2.01 × 10−3 | 2.11 × 10−3 | 2.67 × 10−4 | −2.74 × 10−4 | 3.35 × 10−4 | −1.38 × 10−4 | 1.64 × 10−5 |
| - | - | - | - | - | - | - | - | - | - | ||||||
| M-09 | Ca-SO4 | B | RDP-10 | 5 | Model 5 | 8.89 | 1.65 × 10−3 | - | 1.29 × 10−3 | 5.23 × 10−3 | 1.86 × 10−4 | −8.15 × 10−4 | 7.03 × 10−4 | 1.86 × 10−4 | 3.76 × 10−5 |
| - | - | - | - | - | - | - | - | - | - | ||||||
| M-28 | Ca-SO4 | B | RDP-11 | 4 | Model 2 | 6.53 | −1.03 × 10−3 | 1.67 × 10−3 | 2.16 × 10−3 | 9.94 × 10−3 | 2.61 × 10−3 | 2.88 × 10−4 | - | 7.80 × 10−4 | 2.03 × 10−4 |
| Model 3 | −4.50 × 10−4 | 1.38 × 10−3 | 2.16 × 10−3 | 9.94 × 10−3 | 2.61 × 10−3 | - | 2.88 × 10−4 | −7.80 × 10−4 | 2.03 × 10−4 | ||||||
| M-20 | Ca-HCO3 Cl | C | RDP-12 | 4 | Model 2 | 2.83 | 6.92 × 10−4 | 7.50 × 10−4 | 2.27 × 10−3 | 7.06 × 10−5 | 2.45 × 10−3 | 5.09 × 10−4 | - | −1.04 × 10−3 | 1.70 × 10−5 |
| - | - | - | - | - | - | - | - | - | - | ||||||
| M-27 | Ca-HCO3 | C | RDP-13 | 3 | Model 3 | 19.7 | 1.94 × 10−3 | - | 1.72 × 10−3 | 2.77 × 10−5 | 9.82 × 10−4 | 1.77 × 10−4 | 1.08 × 10−4 | −5.64 × 10−4 | −7.00 × 10−6 |
| - | - | - | - | - | - | - | - | - | - | ||||||
| M-23 | Ca-HCO3 | C | RDP-14 | 4 | Model 2 | 5.71 | 8.01 × 10−4 | 2.35 × 10−4 | 9.55 × 10−4 | 6.11 × 10−5 | 6.04 × 10−4 | 1.68 × 10−4 | - | −3.33 × 10−4 | −3.00 × 10−6 |
| Model 3 | 1.14 × 10−3 | 6.70 × 10−5 | 9.55 × 10−4 | 6.11 × 10−5 | 6.04 × 10−4 | - | 1.68 × 10−4 | −3.33 × 10−4 | −3.00 × 10−6 |


References
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef] [Green Version]
- Parise, M.; Closson, D.; Gutiérrez, F.; Stevanović, Z. Anticipating and managing engineering problems in the complex karst environment. Environ. Earth Sci. 2015, 74, 7823–7835. [Google Scholar] [CrossRef]
- Hartmann, A.; Jasechko, S.; Gleeson, T.; Wada, Y.; Andreo, B.; Barberá, J.A.; Brielmann, H.; Bouchaou, L.; Charlier, J.-B.; Darling, W.G.; et al. Risk of groundwater contamination widely underestimated because of fast flow into aquifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2024492118. [Google Scholar] [CrossRef] [PubMed]
- Hock, R.; Rasul, G.; Adler, C.; Cáceres, B.; Gruber, S.; Hirabayashi, Y.; Jackson, M.; Kääb, A.; Kang, S.; Kutuzov, S.; et al. 2019: High mountain areas. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC—Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; pp. 131–202. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-414230. (accessed on 1 June 2021).
- Jódar, J.; Lambán, L.J.; González, A.; Martos, S.; Custodio, E. High Mountain Karst Aquifer Vulnerability to Climate Change and Groundwater Transit Times. In Proceedings of the EGU General Assembly 2020, Online, 4–8 May 2020. [Google Scholar] [CrossRef]
- Jódar, J.; Herms, I.; Lambán, L.J.; Martos-Rosillo, S.; Herrera-Lameli, C.; Urrutia, J.; Soler, A.; Custodio, E. Isotopic content in high mountain karst aquifers as a proxy for climate change impact in Mediterranean zones: The Port del Comte karst aquifer (SE Pyrenees, Catalonia, Spain). Sci. Total Environ. 2021, 790, 148036. [Google Scholar] [CrossRef]
- Lambán, L.J.; Jódar, J.; Custodio, E.; Soler, A.; Sapriza, G.; Soto, R. Isotopic and hydrogeochemical characterization of high-altitude karst aquifers in complex geological settings. The Ordesa and Monte Perdido National Park (Northern Spain) case study. Sci. Total Environ. 2015, 506–507, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Sheikhy Narany, T.; Bittner, D.; Disse, M.; Chiogna, G. Spatial and temporal variability in hydrochemistry of a small-scale dolomite karst environment. Environ. Earth Sci. 2019, 78, 273. [Google Scholar] [CrossRef]
- Mudarra, M.; Andreo, B.; Mudry, J. Monitoring groundwater in the discharge area of a complex karst aquifer to assess the role of the saturated and unsaturated zones. Environ. Earth Sci. 2012, 65, 2321–2336. [Google Scholar] [CrossRef]
- Montalván, F.J.; Heredia, J.; Ruiz, J.M.; Pardo-Igúzquiza, E.; García de Domingo, A.; Elorza, F.J. Hydrochemical and isotopes studies in a hypersaline wetland to define the hydrogeological conceptual model: Fuente de Piedra Lake (Málaga, Spain). Sci. Total Environ. 2017, 576, 335–346. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Bloise, L.; Calabrese, E.; Marini, L.; Vespasiano, G.; Muto, F. Geochemical modeling of water-rock interaction processes in the Pollino National Park. Geofluids 2021, 2021, 6655711. [Google Scholar] [CrossRef]
- Goldscheider, N. Overview of methods applied in karst hydrogeology. In Karst Aquifers—Characterization and Engineering; Professional Practice in Earth Sciences Series; Stevanović, Z., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 127–145. ISBN 978-3-319-12849-8. [Google Scholar]
- Simsek, C.; Elci, A.; Gunduz, O.; Erdogan, B. Hydrogeological and hydrogeochemical characterization of a karstic mountain region. Environ. Geol. 2008, 54, 291–308. [Google Scholar] [CrossRef]
- Mustafa, O.; Merkel, B.; Weise, S. Assessment of Hydrogeochemistry and environmental isotopes in karst springs of Makook anticline, Kurdistan Region, Iraq. Hydrology 2015, 2, 48–68. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhang, C.; Xiao, Q.; Bu, H. Hydrogeochemical characteristics of a closed karst groundwater basin in North China. J. Radioanal. Nucl. Chem. 2020, 325, 365–379. [Google Scholar] [CrossRef]
- De la Torre, B.; Mudarra, M.; Andreo, B. Investigating karst aquifers in tectonically complex alpine areas coupling geological and hydrogeological methods. J. Hydrol. X 2020, 6, 100047. [Google Scholar] [CrossRef]
- Farlin, J.; Małoszewski, P. On using lumped parameter models and temperature cycles in heterogeneous aquifers. Groundwater 2018, 56, 969–977. [Google Scholar] [CrossRef]
- Jódar, J.; Custodio, E.; Lambán, L.J.; Martos-Rosillo, S.; Herrera-Lameli, C.; Sapriza-Azuri, G. Vertical variation in the amplitude of the seasonal isotopic content of rainfall as a tool to jointly estimate the groundwater recharge zone and transit times in the Ordesa and Monte Perdido National Park Aquifer System, North-Eastern Spain. Sci. Total Environ. 2016, 573, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Jódar, J.; González-Ramón, A.; Martos-Rosillo, S.; Heredia, J.; Herrera, C.; Urrutia, J.; Caballero, Y.; Zabaleta, A.; Antigüedad, I.; Custodio, E.; et al. Snowmelt as a determinant factor in the hydrogeological behaviour of high mountain karst aquifers: The Garcés Karst System, Central Pyrenees (Spain). Sci. Total Environ. 2020, 748, 141363. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Puga, M.A.; Vallejos, A.; Sola, F.; Daniele, L.; Molina, L.; Pulido-Bosch, A. Groundwater flow and residence time in a karst aquifer using ion and isotope characterization. Int. J. Environ. Sci. Technol. 2016, 13, 2579–2596. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Xu, X.; Wang, Q.; Wang, M.; Feng, J.; Fu, T. Temporal variations of spring water in karst areas: A case study of Jinan Spring Area, Northern China. Water 2020, 12, 1009. [Google Scholar] [CrossRef] [Green Version]
- Safari, M.; Hezarkhani, A.; Mashhadi, S.R. Hydrogeochemical characteristics and water quality of Aji-Chay River, Eastern Catchment of Lake Urmia, Iran. J. Earth Syst. Sci. 2020, 129, 199. [Google Scholar] [CrossRef]
- Chalikakis, K.; Plagnes, V.; Guerin, R.; Valois, R.; Bosch, F.P. Contribution of geophysical methods to karst-system exploration: An overview. Hydrogeol. J. 2011, 19, 1169–1180. [Google Scholar] [CrossRef]
- Jia, Z.; Zang, H.; Hobbs, P.; Zheng, X.; Xu, Y.; Wang, K. Application of inverse modeling in a study of the hydrogeochemical evolution of karst groundwater in the Jinci Spring Region, Northern China. Environ. Earth Sci. 2017, 76, 312. [Google Scholar] [CrossRef]
- Moran-Ramírez, J.; Ramos-Leal, J.A.; Mahlknecht, J.; Santacruz-DeLeón, G.; Martín-Romero, F.; Fuentes Rivas, R.; Mora, A. Modeling of groundwater processes in a karstic aquifer of Sierra Madre Oriental, Mexico. Appl. Geochem. 2018, 95, 97–109. [Google Scholar] [CrossRef]
- Zheng, X.; Zang, H.; Zhang, Y.; Chen, J.; Zhang, F.; Shen, Y. A study of hydrogeochemical processes on karst groundwater using a mass balance model in the Liulin Spring Area, North China. Water 2018, 10, 903. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ceballos, R.; Canul-Macario, C.; Pacheco-Castro, R.; Pacheco-Ávila, J.; Euán-Ávila, J.; Merino-Ibarra, M. Regional hydrogeochemical evolution of groundwater in the ring of cenotes, Yucatán (Mexico): An Inverse Modelling Approach. Water 2021, 13, 614. [Google Scholar] [CrossRef]
- Chen, Z.; Goldscheider, N. Modeling spatially and temporally varied hydraulic behavior of a folded karst system with dominant conduit drainage at catchment scale, Hochifen–Gottesacker, Alps. J. Hydrol. 2014, 514, 41–52. [Google Scholar] [CrossRef]
- Herms, I.; Jódar, J.; Soler, A.; Vadillo, I.; Lambán, L.J.; Martos-Rosillo, S.; Núñez, J.A.; Arnó, G.; Jorge, J. Contribution of isotopic research techniques to characterize high-mountain-Mediterranean karst aquifers: The Port del Comte (Eastern Pyrenees) aquifer. Sci. Total Environ. 2019, 656, 209–230. [Google Scholar] [CrossRef]
- Betzler, C. A Carbonate complex in an active foreland basin: The Paleogene of the Sierra de Port del Comte and the Sierra del Cadi (Southern Pyrenees). Geodin. Acta 1989, 3, 207–220. [Google Scholar] [CrossRef]
- Vergès i Masip, J. Estudi Geològic del Vessant Sud del Pirineu Oriental i Central. Evolució Cinemàtica en 3D; Universitat de Barcelona UB: Barcelona, Spain, 1993. [Google Scholar]
- Barcelona City Council. Evolution of Water Consumption in the City of Barcelona; Barcelona City Council: Barcelona, Spain, 2008.
- Herms, I. 3D geological modelling as a tool for supporting spring catchment delineation in high-mountain karst aquifers: The case study of the Port Del Comte (Eastern Pyrenees) Aquifer. In Proceedings of the 5th European Meeting on 3D Geological Modelling, Bern, Switzerland, 21–24 May 2019. [Google Scholar]
- Arnó, G.; Conesa, A.; Carreras, X.; Camps, V.; Fraile, J.; Herms, I.; Iglesias, M. Mapa de Vulnerabilitat Intrínseca a la Contaminació dels Aqüífers de Catalunya (MVIAC) 2020, Escala 1:100.000. Barcelona. Institut Cartogràfic i Geològic de Catalunya (ICGC) i Agència Catalana de l’Aigua (ACA). Available online: https://www.icgc.cat/ca/Administracio-i-empresa/Eines/Visualitzadors-Geoindex/Geoindex-Vulnerabilitat-intrinseca-a-la-contaminacio-dels-aqueifers (accessed on 15 January 2021).
- DARP. Proyecto de Prospección e Investigación Hidrogeológica (Solsonès–Lleida); DARP (Departament d’Agricultura, Ramaderia i Pesca, Generalitat de Catalunya): Barcelona, Spain, 1990; pp. 20–117.
- Gil, R.; Núñez, I. Estudio Hidrogeológico de la Sierra de Odén–Port del Comte (Solsonès–Lleida); CIHS: Barcelona, Spain, 2003; p. 85. [Google Scholar]
- Núñez, I.; Gil, R.; Vázquez, E. Estudio hidrogeológico de la cabecera de la Ribera Salada, (Lleida). In Proceedings of the VIII Simposio de Hidrogeología, Zaragoza, Spain, 18–22 October 2004; pp. 107–120. [Google Scholar]
- Chen, Z.; Hartmann, A.; Wagener, T.; Goldscheider, N. Dynamics of water fluxes and storages in an alpine karst catchment under current and potential future climate conditions. Hydrol. Earth Syst. Sci. 2018, 22, 3807–3823. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Igúzquiza, E.; Collados-Lara, A.J.; Pulido-Velàzquez, D. Potential future impact of climate change on recharge in the Sierra de Las Nieves (Southern Spain) high-relief karst aquifer using regional climate models and statistical corrections. Environ. Earth Sci. 2019, 78, 598. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- ICGC. Full Alt Urgell. Mapa Geològic Comarcal de Catalunya 1:50.000. 2007. Available online: https://www.icgc.cat/en/Public-Administration-and-Enterprises/Downloads/Geological-and-geothematic-cartography/Geological-cartography/Geological-map-1-50-000/Regional-geological-map-of-Catalonia-1-50-000 (accessed on 10 January 2021).
- Lauber, U.; Kotyla, P.; Morche, D.; Goldscheider, N. Hydrogeology of an alpine rockfall aquifer system and its role in flood attenuation and maintaining baseflow. Hydrol. Earth Syst. Sci. 2014, 18, 4437–4452. [Google Scholar] [CrossRef] [Green Version]
- Hood, J.L.; Hayashi, M. Assessing the Application of a laser rangefinder for determining snow depth in inaccessible alpine terrain. Hydrol. Earth Syst. Sci. 2010, 14, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Goldscheider, N. Alpine hydrogeologie. Grundwasser 2011, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Bakalowicz, M. Karst groundwater: A challenge for new resources. Hydrogeol. J. 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Goldscheider, N.; Drew, D. Methods in karst hydrogeology. In International Contributions to Hydrogeology 26; Taylor&Francis: London, UK, 2007; ISBN 978-0-415-42873-6. [Google Scholar]
- Wetzel, K.-F. On the hydrology of the partnach area in the Wetterstein Mountains (Bavarian Alps). Erdkunde 2004, 58, 172–186. [Google Scholar] [CrossRef]
- Malard, A.; Sinreich, M.; Jeannin, P.-Y. A novel approach for estimating karst groundwater recharge in mountainous regions and its application in Switzerland: Karst groundwater recharge: Approach and application. Hydrol. Process. 2016, 30, 2153–2166. [Google Scholar] [CrossRef]
- Epting, J.; Page, R.M.; Auckenthaler, A.; Huggenberger, P. Process-based monitoring and modeling of karst springs–linking intrinsic to specific vulnerability. Sci. Total Environ. 2018, 625, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Jódar, J.; Custodio, E.; Liotta, M.; Lambán, L.J.; Herrera, C.; Martos-Rosillo, S.; Sapriza, G.; Rigo, T. Correlation of the seasonal isotopic amplitude of precipitation with annual evaporation and altitude in alpine regions. Sci. Total Environ. 2016, 550, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Beven, K.J. Rainfall-Runoff Modelling, The Primer, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2012; ISBN 978-0-470-71459-1. [Google Scholar]
- Andreo, B.; Liñán, C.; Carrasco, F.; Jiménez de Cisneros, C.; Caballero, F.; Mudry, J. Influence of rainfall quantity on the isotopic composition (18O and 2H) of water in mountainous areas. application for groundwater research in the Yunquera-Nieves Karst Aquifers (S Spain). Appl. Geochem. 2004, 19, 561–574. [Google Scholar] [CrossRef]
- Bergström, S. Development and Application of a Conceptual Runoff Model for Scandinavian Catchments; SMHI: Norrköping, Sweden, 1976; p. 134. [Google Scholar]
- Seibert, J. HBV Light Version 2. User’s Manual; Stockholm University: Uppsala, Sweden, 2005. [Google Scholar]
- Małoszewski, P.; Zuber, A. Lumped Parameter Models for the Interpretation of Environmental Tracer Data. Manual on Mathematical Models in Isotope Hydrology; IAEA: Vienna, Austria, 1996. [Google Scholar]
- Maréchal, J.C.; Bailly-Comte, V.; Hickey, C.; Maurice, L.; Stroj, A.; Bunting, S.Y.; Charlier, J.B.; Elster, D.; Hakoun, V.; Herms, I.; et al. GeoERA Resources of Groundwater Harmonized at Cross-Border and Pan-European Scale (RESOURCE) Project’. Deliverable 5.3 Karst and Chalk Aquifers Classification and Management Recommendations; BRGM: Toulousse, France, 2021; p. 114. [Google Scholar]
- Mangin, A. Contribution à L’étude Hydrodynamique des Aquifères Karstiques; Université de Dijon: Dijon, France, 1975. [Google Scholar]
- Freixes, A. Els Aqüífers Càrstics dels Pirineus de Catalunya. Interès Estratègic i Sostenibilitat; Universitat de Barcelona UB: Barcelona, Spain, 2014. [Google Scholar]
- Herms, I.; Jódar, J.; Soler, A.; Lambán, L.J.; Custodio, E.; Núñez, J.A.; Arnó, G.; Ortego, M.I.; Parcerisa, D.; Jorge, J. Evaluation of natural background levels of high mountain karst aquifers in complex hydrogeological settings. A gaussian mixture model approach in the Port del Comte (SE, Pyrenees) case study. Sci. Total Environ. 2021, 756, 143864. [Google Scholar] [CrossRef] [PubMed]
- Fraley, C.; Raftery, A.E. Model-based clustering, discriminant analysis, and density estimation. J. Am. Stat. Assoc. 2002, 97, 611–631. [Google Scholar] [CrossRef]
- Bouveyron, C.; Brunet-Saumard, C. Model-based clustering of high-dimensional data: A review. Comput. Stat. Data Anal. 2014, 71, 52–78. [Google Scholar] [CrossRef] [Green Version]
- Camarero, L.; Catalan, J. Chemistry of bulk precipitation in the Central and Eastern Pyrenees, Northeast Spain. Atmos. Environ. Part Gen. Top. 1993, 27, 83–94. [Google Scholar] [CrossRef]
- Bergström, S. The HBV Model–Its Structure and Applications; Reports Hydrology; SMHI: Norrkoping, Sweden, 1992. [Google Scholar]
- Alcalá, F.J.; Custodio, E. Atmospheric chloride deposition in continental Spain. Hydrol. Process. 2008, 22, 3636–3650. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results: Guidelines and recommended terms for expressing stable isotope results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, L.I.; Ahmad, M.; Aggarwal, P.; Duren, M.; Pöltenstein, L.; Araguas, L.; Kurttas, T. Worldwide proficiency test for routine analysis of δ2H and δ 18O in water by isotope-ratio mass spectrometry and laser absorption spectroscopy: Proficiency Test for δ 2H and δ 18O in natural waters. Rapid Commun. Mass Spectrom. 2012, 26, 1641–1648. [Google Scholar] [CrossRef]
- Epstein, S.; Mayeda, T. Variation of O18 content of waters from natural sources. Geochim. Cosmochim. Acta 1953, 4, 213–224. [Google Scholar] [CrossRef]
- McIlvin, M.R.; Altabet, M.A. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal. Chem. 2005, 77, 5589–5595. [Google Scholar] [CrossRef]
- Dogramaci, S.S.; Herczeg, A.L.; Schi, S.L.; Bone, Y. Controls on δ 34S and δ18O of dissolved sulfate in aquifers of the Murray basin, Australia and their use as indicators of flow processes. Appl. Geochem. 2001, 14, 475–488. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods: Reston, VA, USA, 2013; Book 6, Chapter A43; p. 497.
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press: London, UK, 2005. [Google Scholar]
- Puig, R.; Folch, A.; Menció, A.; Soler, A.; Mas-Pla, J. Multi-isotopic study (15N, 34S, 18O, 13C) to identify processes affecting nitrate and sulfate in response to local and regional groundwater mixing in a large-scale flow system. Appl. Geochem. 2013, 32, 129–141. [Google Scholar] [CrossRef]
- Puig, R.; Soler, A.; Widory, D.; Mas-Pla, J.; Domènech, C.; Otero, N. Characterizing sources and natural attenuation of nitrate contamination in the Baix Ter Aquifer System (NE Spain) using a multi-isotope approach. Sci. Total Environ. 2017, 580, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; McDonnell, J.J. Tracing nitrogen sources and cycling in catchments. In Isotope Tracers in Catchment Hydrology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 519–576. [Google Scholar] [CrossRef]
- Xue, D.; De Baets, B.; Van Cleemput, O.; Hennessy, C.; Berglund, M.; Boeckx, P. Use of a Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ. Pollut. 2012, 161, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Phillips, D.L.; Bearhop, S.; Semmens, B.X.; Ward, E.J.; Moore, J.W.; Jackson, A.L.; Grey, J.; Kelly, D.J.; Inger, R. Bayesian stable isotope mixing models. Environmetrics 2013, 24, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef]
- Kazakis, N.; Matiatos, I.; Ntona, M.-M.; Bannenberg, M.; Kalaitzidou, K.; Kaprara, E.; Mitrakas, M.; Ioannidou, A.; Vargemezis, G.; Voudouris, K. Origin, implications and management strategies for nitrate pollution in surface and ground waters of anthemountas basin based on a δ15N-NO3− and δ18O-NO3−isotope approach. Sci. Total Environ. 2020, 724, 138211. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yun, S.-T.; Mayer, B.; Lee, J.-H.; Kim, T.-S.; Kim, H.-K. Quantification of nitrate sources in groundwater using hydrochemical and dual isotopic data combined with a bayesian mixing model. Agric. Ecosyst. Environ. 2015, 199, 369–381. [Google Scholar] [CrossRef]
- Matiatos, I. Nitrate Source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: A case study of Asopos Basin (Central Greece). Sci. Total Environ. 2016, 541, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, B.; Wang, J.; Zhang, H.; Guo, L.; Lin, H. Using dual isotopes and a bayesian isotope mixing model to evaluate nitrate sources of surface water in a drinking water source watershed, East China. Water 2016, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Xu, W.; Cai, Y.; Tan, Q.; Xu, Y. Estimating the proportional contributions of multiple nitrate sources in shallow groundwater with a Bayesian isotope mixing model. Int. J. Environ. Sci. Dev. 2016, 7, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zheng, T.; Zheng, X.; Hao, Y.; Yuan, R. Nitrate source apportionment in groundwater using bayesian isotope mixing model based on nitrogen isotope fractionation. Sci. Total Environ. 2020, 718, 137242. [Google Scholar] [CrossRef]
- Paredes, I. Agricultural and urban delivered nitrate pollution input to Mediterranean temporary freshwaters. Agric. Ecosyst. Environ. 2020, 294, 106859. [Google Scholar] [CrossRef]
- El Gaouzi, F.-Z.J.; Sebilo, M.; Ribstein, P.; Plagnes, V.; Boeckx, P.; Xue, D.; Derenne, S.; Zakeossian, M. Using δ15N and δ18O values to identify sources of nitrate in karstic springs in the Paris Basin (France). Appl. Geochem. 2013, 35, 230–243. [Google Scholar] [CrossRef]
- Ming, X.; Groves, C.; Wu, X.; Chang, L.; Zheng, Y.; Yang, P. Nitrate migration and transformations in groundwater quantified by dual nitrate isotopes and hydrochemistry in a karst world heritage site. Sci. Total Environ. 2020, 735, 138907. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Halas, S.; Bottrell, S.H. Sources and impact of sulphate on groundwaters of Triassic carbonate aquifers, upper Silesia, Poland. J. Hydrol. 2013, 486, 136–150. [Google Scholar] [CrossRef]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.K.; Ornelas-Soto, N.; Mahlknecht, J. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef] [PubMed]
- Szramek, K.; Walter, L.M.; Kanduč, T.; Ogrinc, N. Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquat. Geochem. 2011, 17, 357–396. [Google Scholar] [CrossRef]
- Brook, G.A.; Folkoff, M.E.; Box, E.O. A World model of soil carbon Dioxide. Earth Surf. Process. Landf. 1983, 8, 79–88. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigations and Developemnt; UNESCO: Paris, France, 1965; pp. 54–83. [Google Scholar]
- Kumar, P.; Mahajan, A.K.; Kumar, A. Groundwater geochemical facie: Implications of rock-water interaction at the Chamba City (HP), Northwest Himalaya, India. Environ. Sci. Pollut. Res. 2020, 27, 9012–9026. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Wang, J.; Lu, N.; Fu, B. Inter-comparison of stable isotope mixing models for determining plant water source partitioning. Sci. Total Environ. 2019, 666, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; Tang, Z.; Shahab, A.; Siddique, J.; Faheem, M.; Fatima, M. Hydrogeochemical characterization and suitability assessment of groundwater: A case study in Central Sindh, Pakistan. Int. J. Environ. Res. Public Health 2019, 16, 886. [Google Scholar] [CrossRef] [Green Version]
- Custodio, E.; Jódar, J. Simple solutions for steady–state diffuse recharge evaluation in sloping homogeneous unconfined aquifers by means of atmospheric tracers. J. Hydrol. 2016, 540, 287–305. [Google Scholar] [CrossRef]
- Municio, G. Estudi Geològic del Marge Merididonal de la Làmina del Port del Comte (Pirineu Oriental): Desxifrant-ne L’estructura i Estratigrafía. Bachelor’s Thesis, Universitat Autònoma de Barcelona, Catalonia, Spain, 2017. [Google Scholar]
- Blum, A.E.; Stillings, L.L. Chapter 7. Feldspar dissolution kinetics. In Chemical Weathering Rates of Silicate Minerals; White, A.F., Brantley, S.L., Eds.; De Gruyter: Berlin, Germany, 1995; pp. 291–352. ISBN 978-1-5015-0965-0. [Google Scholar]
- Van Stempvoort, D.R.; Krouse, H.R. Controls of δ 18 O in Sulfate: Review of experimental data and application to specific environments. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 550, pp. 446–480. ISBN 978-0-8412-2772-9. [Google Scholar]
- Cravotta, C.A. Use of Stable Isotopes of Carbon, Nitrogen, and Sulfur to Identify Sources of Nitrogen in Surface Waters in the Lower Susquehanna River Basin, Pennsylvania; Water-Supply Paper; U.S. Geological Survey: New Cumberland, PA, USA, 1997.
- Krouse, H.R.; Mayer, B. Sulphur and oxygen isotopes in Sulphate. In Environmental Tracers in Subsurface Hydrology; Cook, P.G., Herczeg, A.L., Eds.; Springer: Boston, MA, USA, 2000; pp. 195–231. ISBN 978-1-4613-7057-4. [Google Scholar]
- Mayer, B. Assessing sources and transformations of sulphate and nitrate in the hydrosphere using isotope techniques. In Isotopes in the Water Cycle; Aggarwal, P.K., Gat, J.R., Froehlich, K.F.O., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 67–89. ISBN 978-1-4020-3010-9. [Google Scholar]
- Vitòria, L.; Otero, N.; Soler, A.; Canals, À. Fertilizer characterization: Isotopic data (N, S, O, C, and Sr). Environ. Sci. Technol. 2004, 38, 3254–3262. [Google Scholar] [CrossRef]
- Otero, N.; Soler, A.; Canals, À. Controls of δ34S and δ18O in dissolved sulphate: Learning from a detailed survey in the Llobregat River (Spain). Appl. Geochem. 2008, 23, 1166–1185. [Google Scholar] [CrossRef]
- Ortí, F.; Pérez-López, A.; García-Veigas, J.; Rosell, L.; Cendón, D.I.; Pérez-Valera, F. Sulfate Isotope Compositions (δ34S, δ18O) and Strontium Isotopic Ratios (87sr/86sr) of Triassic Evaporites in the Betic Cordillera (se Spain); SGE: Salamanca, Spain, 2014; Volume 13. [Google Scholar]
- Utrilla, R.; Pierre, C.; Orti, F.; Pueyo, J.J. Oxygen and sulphur isotope compositions as indicators of the origin of Mesozoic and Cenozoic Evaporites from Spain. Chem. Geol. 1992, 102, 229–244. [Google Scholar] [CrossRef]
- Mizutani, Y.; Rafter, T.A. Isotopic behaviour of sulphate oxygen in the bacterial reduction of sulphate. Geochem. J. 1973, 6, 183–191. [Google Scholar] [CrossRef]
- Otero, N.; Vitòria, L.; Soler, A.; Canals, A. Fertiliser characterisation: Major, trace and rare earth elements. Appl. Geochem. 2005, 20, 1473–1488. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kelley, C.J.; Keller, C.K.; Evans, R.D.; Orr, C.H.; Smith, J.L.; Harlow, B.A. Nitrate–nitrogen and oxygen isotope ratios for identification of nitrate sources and dominant nitrogen cycle processes in a tile-drained dryland agricultural field. Soil Biol. Biochem. 2013, 57, 731–738. [Google Scholar] [CrossRef]
- Mengis, M.; Walther, U.; Bernasconi, S.M.; Wehrli, B. Limitations of using δ18O for the source identification of nitrate in agricultural soils. Environ. Sci. Technol. 2001, 35, 1840–1844. [Google Scholar] [CrossRef]
- Cabello, P.; Roldán, M.D.; Moreno-Vivián, C. Nitrate reduction and the nitrogen cycle in Archaea. Microbiology 2004, 150, 3527–3546. [Google Scholar] [CrossRef] [PubMed]
- Kroopnick, P.; Craig, H. Atmospheric oxygen: Isotopic composition and solubility fractionation. Science 1972, 175, 54–55. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. In Stable Isotopes in Ecology and Environmental Science; Michener, R., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 375–449. ISBN 978-0-470-69185-4. [Google Scholar]
- Vitòria, L.; Soler, A.; Canals, À.; Otero, N. Environmental isotopes (N, S, C, O, D) to determine natural attenuation processes in nitrate contaminated waters: Example of Osona (NE Spain). Appl. Geochem. 2008, 23, 3597–3611. [Google Scholar] [CrossRef]
- Aravena, R.; Mayer, B. Isotopes and processes in the nitrogen and sulfur cycles. In Environmental Isotopes in Biodegradation and Bioremediation; Aelion, C.M., Höhener, P., Hunkeler, D., Aravena, R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 203–246. ISBN 978-1-56670-661-2. [Google Scholar]
- Fukada, T.; Hiscock, K.M.; Dennis, P.F.; Grischek, T. A Dual isotope approach to identify denitrification in groundwater at a River-Bank infiltration site. Water Res. 2003, 37, 3070–3078. [Google Scholar] [CrossRef]
- Böttcher, J.; Strebel, O.; Voerkelius, S.; Schmidt, H.-L. Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer. J. Hydrol. 1990, 114, 413–424. [Google Scholar] [CrossRef]
| Recharge Elevation (m a.s.l.) | |||||
|---|---|---|---|---|---|
| Average | Min | Max | δ18O | δ2H | |
| Cluster A | 1823 | 1259 | 2228 | −1.5 | −9.8 |
| Cluster B | 1541 | 1409 | 1758 | −1.5 | −9.7 |
| Cluster C | 1776 | 1557 | 2010 | −1.2 | −8.0 |
| Cluster D | 1193 | 1061 | 1324 | −1.2 | −9.3 |
| Cluster | Total Mass Dissolved (mol/L) | Calcite (%) | Dolomite (%) | CO2(g) (%) | Gypsum (%) | Halite (%) | CaΧ2 (%) | MgΧ2 (%) | NaΧ (%) | KΧ (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| RDP-A (a) | 3.25 × 10−3 | 37.02 | 9.87 | 48.25 | 1.11 | 2.20 | - | 0.75 | - | 0.81 |
| RDP-B (b) | 9.73 × 10−3 | 4.00 | 12.15 | 17.12 | 52.23 | 9.89 | - | 3.21 | 0.56 | 0.83 |
| RDP-C (c) | 4.61 × 10−3 | 24.81 | 7.61 | 32.03 | 1.20 | 25.16 | 6.18 | 2.99 | - | 0.02 |
| Cluster A | Cluster B | Cluster C | Cluster D | |
|---|---|---|---|---|
| δ34SSO4 (‰) | +3.6 | +13.2 | +8.9 | +15.9 |
| δ18OSO4 (‰) | +8.1 | +12.9 | +8.9 | +11.5 |
| SO4 (mg/L) | 6.9 | 213.3 | 11.5 | 4701 |
| δ15NNO3 (‰) | +3.3 | +3.8 | +3.9 | +8.6 |
| δ18ONO3 (‰) | +2.9 | +2.6 | +1.4 | +3.9 |
| NO3 (mg/L) | 10.1 | 16.8 | 10.4 | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herms, I.; Jódar, J.; Soler, A.; Lambán, L.J.; Custodio, E.; Núñez, J.A.; Arnó, G.; Parcerisa, D.; Jorge-Sánchez, J. Identification of Natural and Anthropogenic Geochemical Processes Determining the Groundwater Quality in Port del Comte High Mountain Karst Aquifer (SE, Pyrenees). Water 2021, 13, 2891. https://doi.org/10.3390/w13202891
Herms I, Jódar J, Soler A, Lambán LJ, Custodio E, Núñez JA, Arnó G, Parcerisa D, Jorge-Sánchez J. Identification of Natural and Anthropogenic Geochemical Processes Determining the Groundwater Quality in Port del Comte High Mountain Karst Aquifer (SE, Pyrenees). Water. 2021; 13(20):2891. https://doi.org/10.3390/w13202891
Chicago/Turabian StyleHerms, Ignasi, Jorge Jódar, Albert Soler, Luís Javier Lambán, Emilio Custodio, Joan Agustí Núñez, Georgina Arnó, David Parcerisa, and Joan Jorge-Sánchez. 2021. "Identification of Natural and Anthropogenic Geochemical Processes Determining the Groundwater Quality in Port del Comte High Mountain Karst Aquifer (SE, Pyrenees)" Water 13, no. 20: 2891. https://doi.org/10.3390/w13202891
APA StyleHerms, I., Jódar, J., Soler, A., Lambán, L. J., Custodio, E., Núñez, J. A., Arnó, G., Parcerisa, D., & Jorge-Sánchez, J. (2021). Identification of Natural and Anthropogenic Geochemical Processes Determining the Groundwater Quality in Port del Comte High Mountain Karst Aquifer (SE, Pyrenees). Water, 13(20), 2891. https://doi.org/10.3390/w13202891








