Impact of Active Chlorines and •OH Radicals on Degradation of Quinoline Using the Bipolar Electro-Fenton Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Target Pollutant
2.2. Reagents and Instruments
2.3. Experimental Methods
2.3.1. Preparation of Quinoline Solution
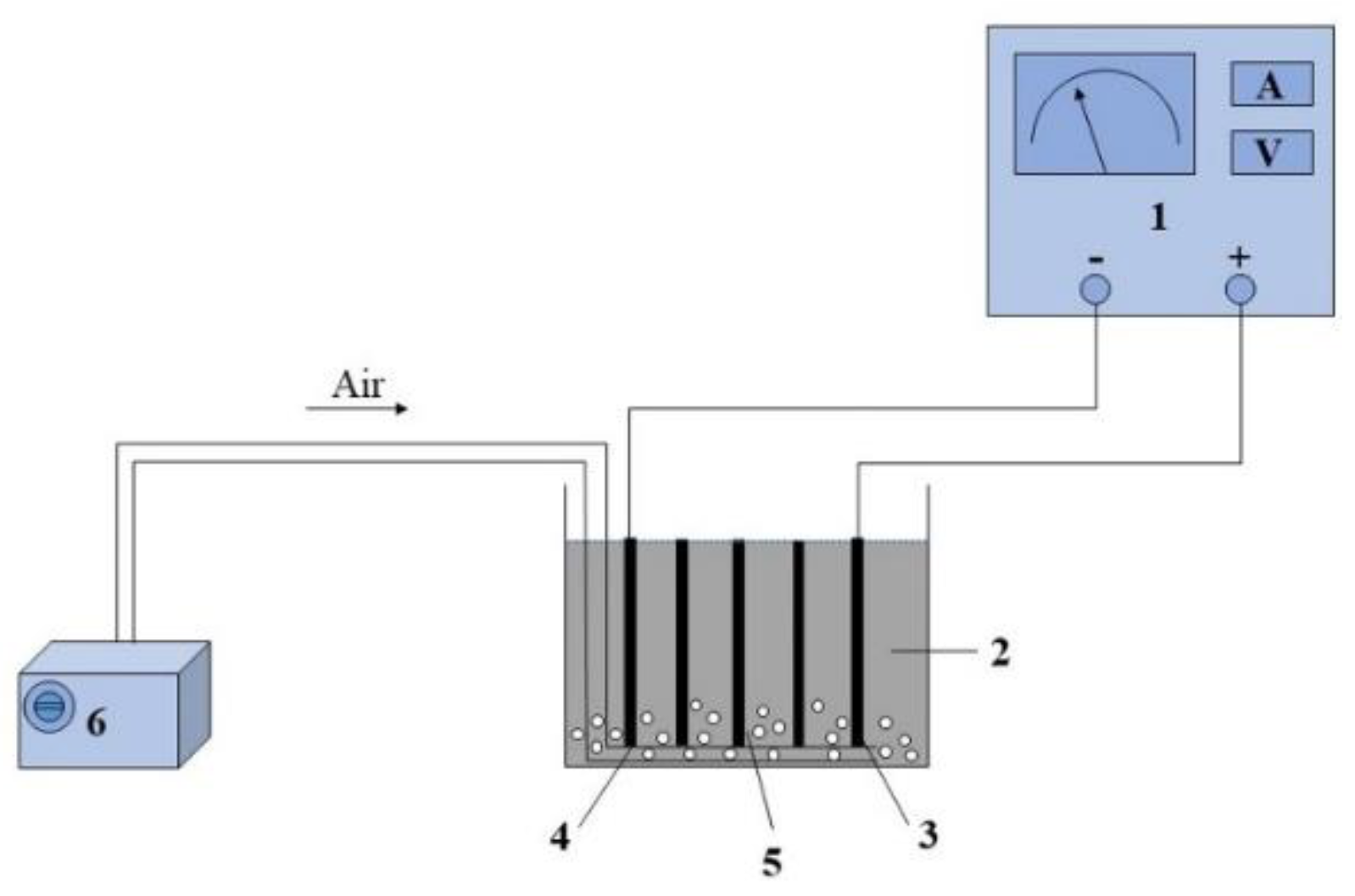
2.3.2. E-Fenton Experimental Apparatus
2.4. Analytical Test Methods
3. Results and Discussion
3.1. E-Fenton Single-Factor Experimental Results
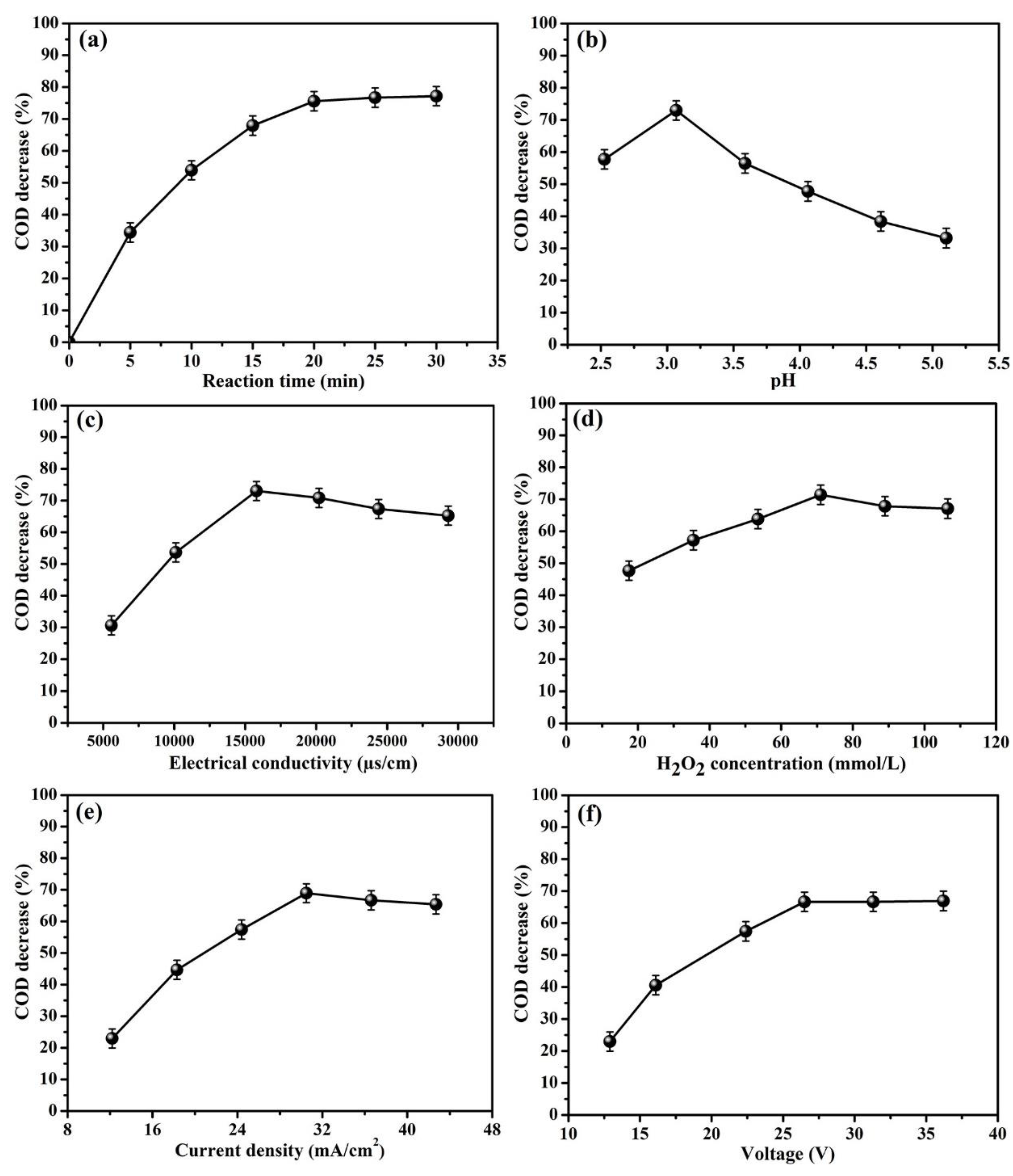
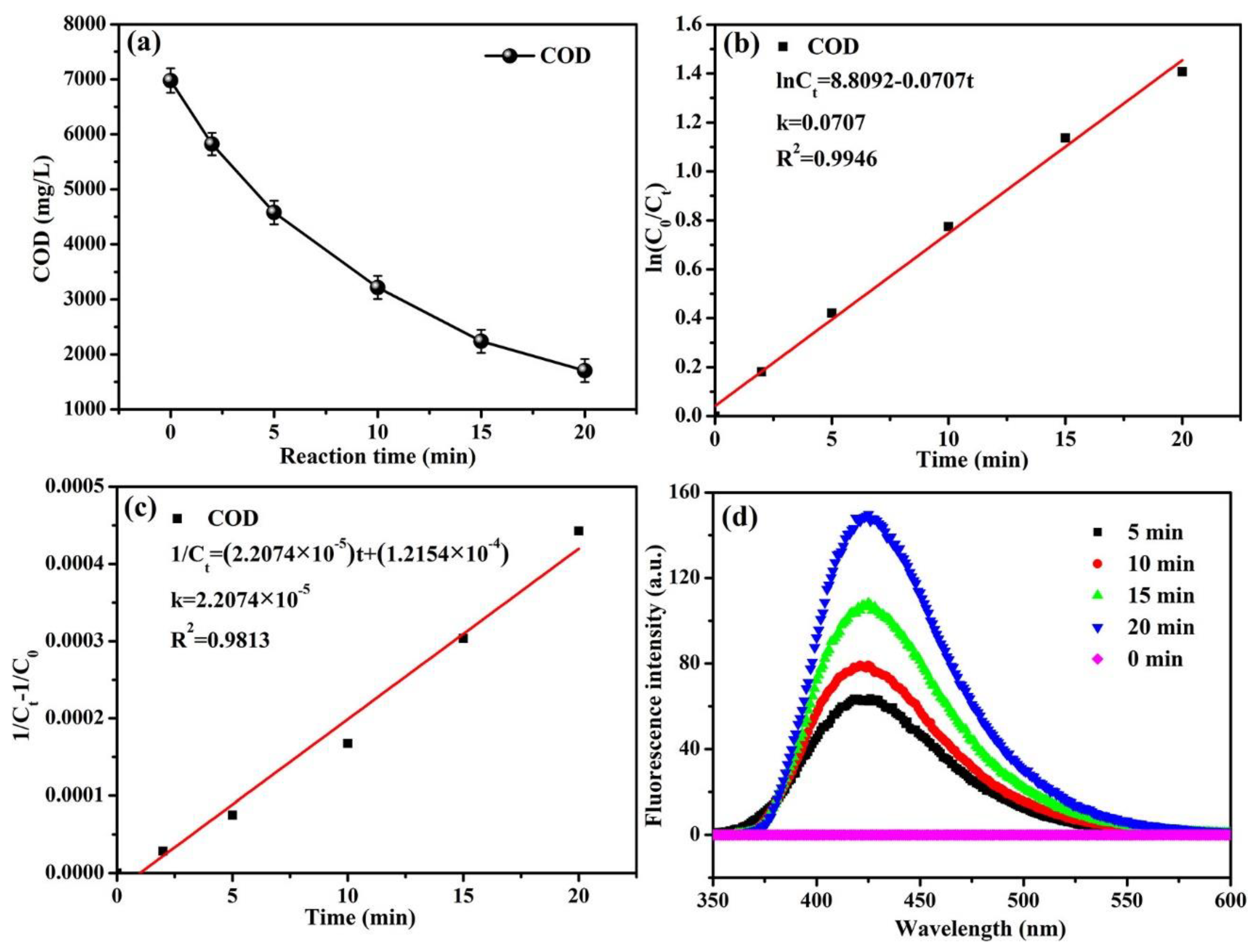
3.1.1. The Effect of Reaction Time on COD Decrease
3.1.2. The Effect of Initial pH on COD Decrease
3.1.3. The Effect of Conductivity on COD Decrease
3.1.4. The Effect of H2O2 Concentration on COD Decrease
3.1.5. The Effect of Current Density on COD Decrease
3.1.6. The Effect of Voltage on COD Decrease
3.2. Kinetics Analysis of COD Degradation
3.3. Measurement of Hydroxyl Radicals
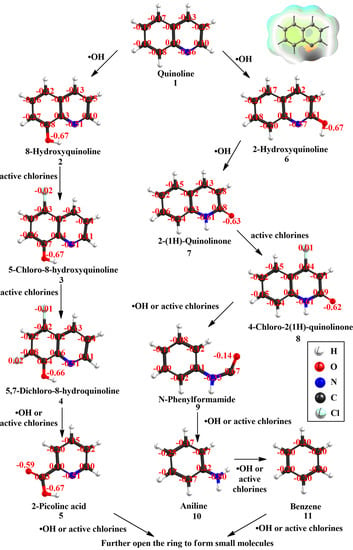
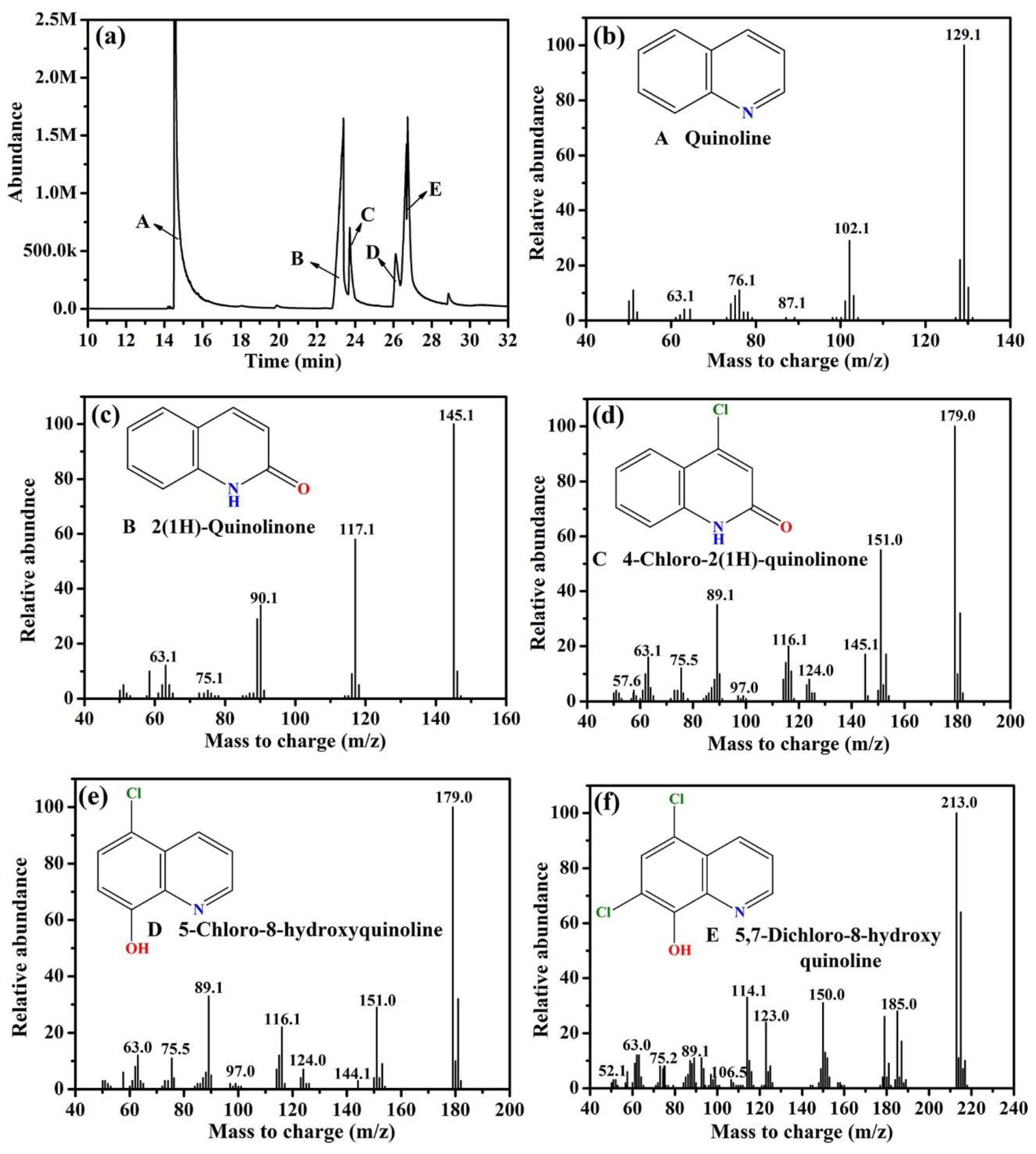
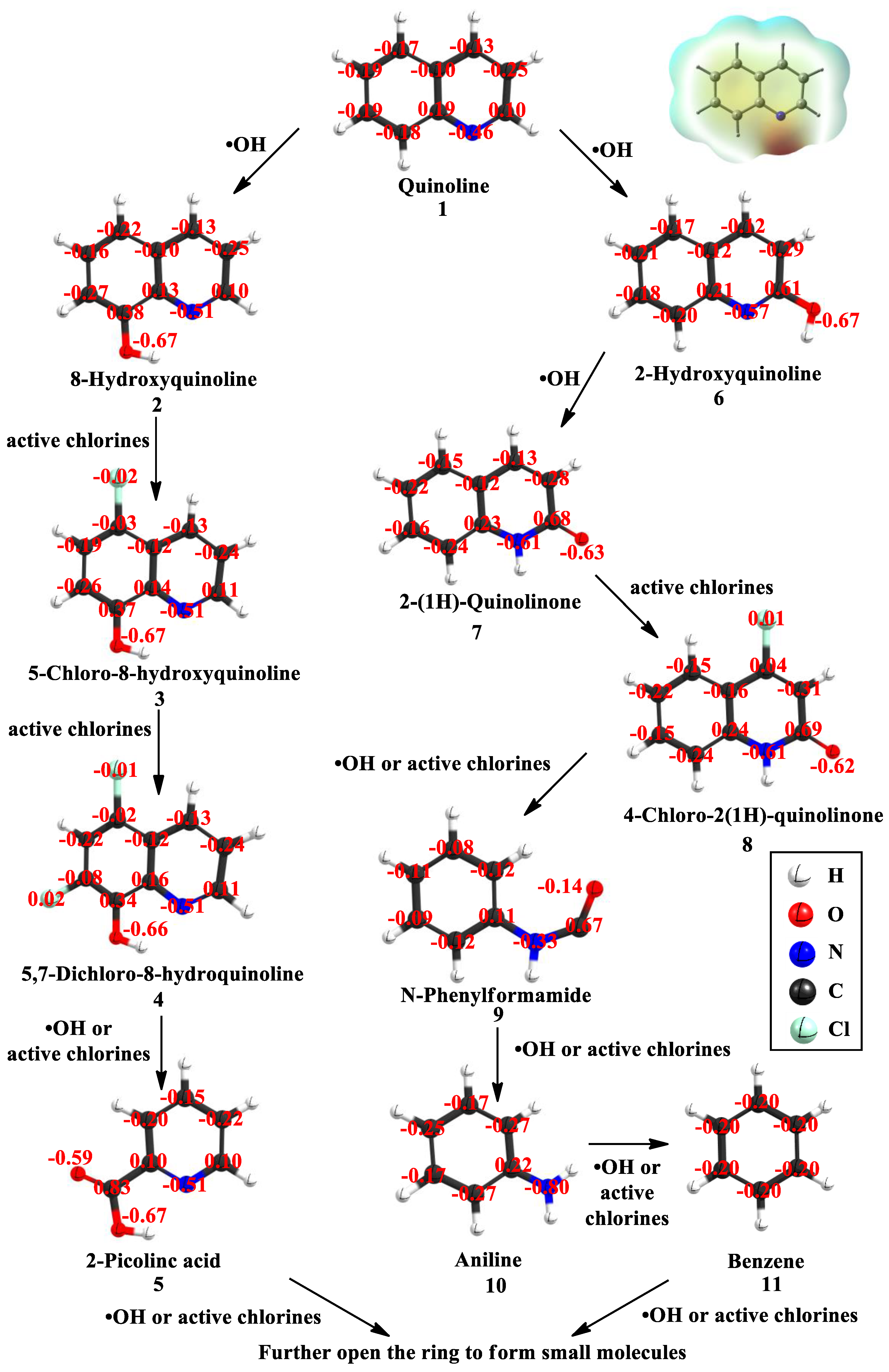
3.4. Degradation Pathways of Quinoline
3.5. Mass Balance and Cost Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Ma, W.C.; Han, H.J.; Han, Y.X.; Ma, W.W. Catalytic ozonation of quinoline using nano-MgO: Efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Y.M.; Gan, L.; Xu, H.; Yan, N.; Liu, R.; Rittmann, B.E. Internal loop photo-biodegradation reactor used for accelerated quinoline degradation and mineralization. Biodegradation 2014, 25, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Abussaud, B.A.; Ulkem, N.; Berk, D.; Kubes, G.J. Wet air oxidation of benzene. Ind. Eng. Chem. Res. 2008, 47, 4325–4331. [Google Scholar] [CrossRef] [Green Version]
- Kritzer, P.; Dinjus, E. An assessment of supercritical water oxidation (SCWO): Existing problems, possible solutions and new reactor concepts. Chem. Eng. J. 2001, 83, 207–214. [Google Scholar] [CrossRef]
- Sarmento, A.P.; Borges, A.C.; Matos, A.T.D.; Romualdo, L.L. Sulfamethoxazole and trimethoprim degradation by Fenton and Fenton-like processes. Water 2020, 12, 1655. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Bedolla-Guzman, A.; Feria-Reyes, R.; Gutierrez-Granados, S.; Peralta-Hernández, J.M. Decolorization and degradation of reactive yellow HF aqueous solutions by electrochemical advanced oxidation processes. Environ. Sci. Pollut. Res. 2016, 24, 1–9. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A.; Yusoff, M.S. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag. 2010, 30, 2113–2121. [Google Scholar] [CrossRef]
- Jiang, C.C.; Zhang, J.F. Progress and prospect in electro-Fenton process for wastewater treatment. J. Zhejiang Univ. Sci. A 2007, 8, 1118–1125. [Google Scholar]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Mitadera, M.; Spataru, N.; Fujishima, A. Electrochemical oxidation of aniline at boron-doped diamond electrodes. J. Appl. Electrochem. 2004, 34, 249–254. [Google Scholar] [CrossRef]
- Soares, I.C.D.C.; Silva, D.R.D.; Nascimento, J.H.O.D.; Garcia-Segura, S.; Martínez-Huitle, C.A. Functional group influences on the reactive azo dye decolorization performance by electrochemical oxidation and electro-Fenton technologies. Environ. Sci. Pollut. Res. 2017, 24, 24167–24176. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E.; Oturan, N.; Oturan, M.A. Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene. Appl. Catal. B 2007, 72, 382–394. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, J.X.; Olajuyin, A.M.; Sharshar, M.M.; Mu, T.Z.; Yang, M.H.; Xing, J.M. Effective degradation of rhodamine B by electro-Fenton process, using ferromagnetic nanoparticles loaded on modified graphite felt electrode as reusable catalyst: In neutral pH condition and without external aeration. Environ. Sci. Pollut. Res. 2016, 23, 15471–15482. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ko, Y.J.; Lee, S.; Hong, S.W.; Lee, W.S.; Choi, J.W. Degradation of organic compounds in actual wastewater by electro-Fenton process and evaluation of energy consumption. Water Air Soil Pollut. 2018, 229, 335. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Nematollahi, D.; Azarian, G.; Godini, K.; Berizi, Z. Activated sludge treatment by electro-Fenton process: Parameter optimization and degradation mechanism. Korean J. Chem. Eng. 2015, 32, 1570–1577. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Cheng, X.W.; Cheng, Q.F.; Deng, X.Y.; Wang, P.; Liu, H.L. Construction of TiO2 nano-tubes arrays coupled with Ag2S nano-crystallites photoelectrode and its enhanced visible light photocatalytic performance and mechanism. Electrochim. Acta 2015, 184, 264–275. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.H.; Tan, Q.Q.; Wang, Q.; Jiao, Y.L.; Oturan, N.; Oturan, M.A. Degradation of organics in reverse osmosis concentrate by electro-Fenton process. J. Hazard. Mater. 2012, 215–216, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.L.; Li, Y.Y.; Wang, A. Biodegradability enhancement of wastewater containing cefalexin by means of the electro-Fenton oxidation process. J. Hazard. Mater. 2012, 227–228, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.L.; Han, H.J.; Jia, S.Y.; Zhuang, H.F.; Xu, P.; Wang, D. Heterogeneous electro-Fenton oxidation of catechol catalyzed by nano-Fe3O4: Kinetics with the Fermi’s equation. J. Taiwan Inst. Chem. Eng. 2015, 56, 138–147. [Google Scholar] [CrossRef]
- Mogyoródy, F. Electrochemical degradation of thiocarbamates in NaCl solutions. J. Appl. Electrochem. 2006, 36, 773–781. [Google Scholar] [CrossRef]
- Mogyoródy, F. Reaction pathways in the electrochemical degradation of thiocarbamate herbicides in NaCl solution. J. Appl. Electrochem. 2006, 36, 635–642. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Kaur, P.; Sangal, V.K.; Kushwaha, J.P. Parametric study of electro-Fenton treatment for real textile wastewater, disposal study and its cost analysis. Int. J. Environ. Sci. Technol. 2018, 16, 801–810. [Google Scholar] [CrossRef]
- Yu, F.K.; Zhou, M.H.; Yu, X.M. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim. Acta 2015, 163, 182–189. [Google Scholar] [CrossRef]
- Asma, S.; Said, A.; Fatima, S.; Brahim, E.I.; Souad, E.I.; Ait, A.E.H.; Ali, A. Electro-Fenton degradation of trimellitic and pyromellitic acids: Kinetics and mechanism. Electrocatalysis 2018, 9, 716–724. [Google Scholar]
- Oturan, N.; Aravindakumar, C.T.; Olvera-Vargas, H.; Sunil Paul, M.M.; Oturan, M.A. Electro-Fenton oxidation of para-aminosalicylic acid: Degradation kinetics and mineralization pathway using Pt/carbon-felt and BDD/carbon-felt cells. Environ. Sci. Pollut. Res. 2017, 25, 20363–20373. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Zhou, M.H. A comparative study of azo dye decolorization by electro-Fenton in two common electrolytes. J. Chem. Technol. Biotechnol. 2010, 84, 1544–1549. [Google Scholar] [CrossRef]
- Hou, B.L.; Ren, B.Z.; Deng, R.J.; Zhu, G.C.; Wang, Z.H.; Li, Z. Three-dimensional electro-Fenton oxidation of N-heterocyclic compounds with a novel catalytic particle electrode: High activity, wide pH range and catalytic mechanism. RSC Adv. 2017, 7, 15455–15462. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.L.; Chang, F.M.; Wang, C.P.; Xu, H.; Wu, J.; Zuo, J.E.; Wang, K.J. Degradation of the nitrogenous heterocyclic compound quinoline by O3/UV. Environ. Sci. 2016, 37, 3884–3890. [Google Scholar]
- Guateque-Londoo, J.F.; Serna-Galvis, E.A.; Vila-Torres, Y.; Torres-Palma, R.A. Degradation of losartan in fresh urine by sonochemical and photochemical advanced oxidation processes. Water 2020, 12, 3398. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R. Fenton-based electrochemical degradation of metolachlor in aqueous solution by means of BDD and Pt electrodes: Influencing factors and reaction pathways. Environ. Sci. Pollut. Res. 2019, 26, 2580–2591. [Google Scholar] [CrossRef]
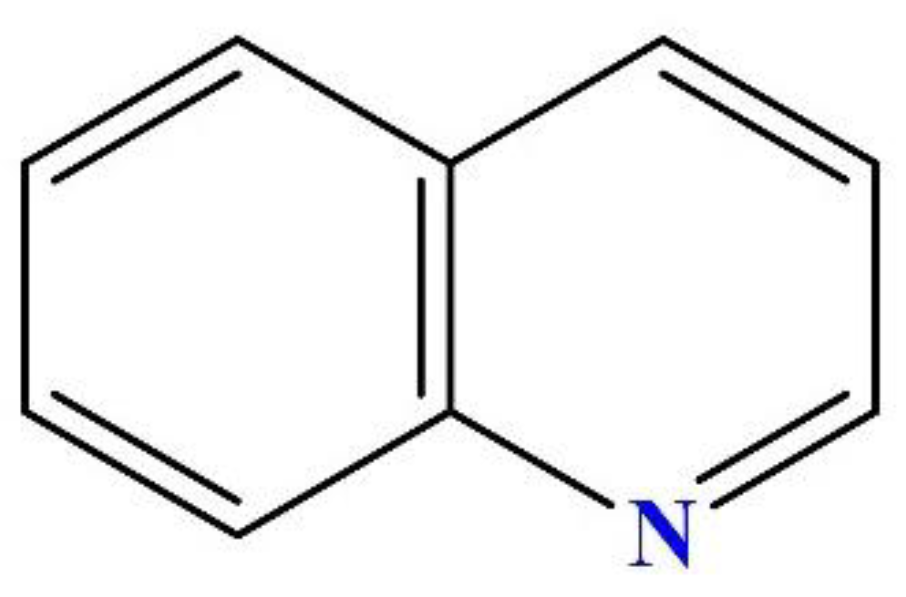
| Structure and Isosurface | Atoms | Fukui Function Indices | ||
|---|---|---|---|---|
| f + | f − | f ave | ||
 (a) 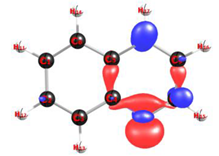 (b) | C1 | 0.08230 | 0.08188 | 0.08209 |
| C2 | 0.08467 | 0.08017 | 0.08242 | |
| C3 | 0.08359 | 0.09483 | 0.08921 | |
| C4 | 0.07142 | 0.08377 | 0.07759 | |
| C5 | 0.07145 | 0.07644 | 0.07394 | |
| C6 | 0.08744 | 0.08955 | 0.08849 | |
| N7 | 0.09924 | 0.19795 | 0.14859 | |
| C8 | 0.10085 | 0.07889 | 0.08987 | |
| C9 | 0.09294 | 0.08442 | 0.08868 | |
| C10 | 0.11795 | 0.06248 | 0.09021 | |
| H11 | 0.01491 | 0.00823 | 0.01157 | |
| H12 | 0.01469 | 0.00854 | 0.01161 | |
| H13 | 0.01184 | 0.01110 | 0.01147 | |
| H14 | 0.01550 | 0.01045 | 0.01297 | |
| H15 | 0.01495 | 0.01341 | 0.01418 | |
| H16 | 0.01615 | 0.00989 | 0.01302 | |
| H17 | 0.02015 | 0.00804 | 0.01409 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, J.; Wang, J.; Cui, C.-X.; Wang, B.; Zhang, Y. Impact of Active Chlorines and •OH Radicals on Degradation of Quinoline Using the Bipolar Electro-Fenton Process. Water 2021, 13, 128. https://doi.org/10.3390/w13020128
Zhang W, Chen J, Wang J, Cui C-X, Wang B, Zhang Y. Impact of Active Chlorines and •OH Radicals on Degradation of Quinoline Using the Bipolar Electro-Fenton Process. Water. 2021; 13(2):128. https://doi.org/10.3390/w13020128
Chicago/Turabian StyleZhang, Wenlong, Jun Chen, Jichao Wang, Cheng-Xing Cui, Bingxing Wang, and Yuping Zhang. 2021. "Impact of Active Chlorines and •OH Radicals on Degradation of Quinoline Using the Bipolar Electro-Fenton Process" Water 13, no. 2: 128. https://doi.org/10.3390/w13020128
APA StyleZhang, W., Chen, J., Wang, J., Cui, C.-X., Wang, B., & Zhang, Y. (2021). Impact of Active Chlorines and •OH Radicals on Degradation of Quinoline Using the Bipolar Electro-Fenton Process. Water, 13(2), 128. https://doi.org/10.3390/w13020128