Potential for Biomass Production and Remediation by Cultivation of the Marine Model Diatom Phaeodactylum tricornutum in Oil Field Produced Wastewater Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Maintenance and Cultivation
2.2. Media Preparation
2.3. Salinity and PW Cultivation Experiments
2.4. Cellular Growth and Photophysiology Measurements
2.5. Neutral Lipid Content Measurements
2.6. Chemical Analyses of McF-PW, EaS-PW, and Spent EaS-PW Medium
2.7. Statistical Analyses
3. Results
3.1. Growth Comparison of Phaeodactylum tricornutum Strains Pt1 and Pt4 at Various Salinities
3.2. Chemical Characterization of Two Oil Field Produced Water Sources
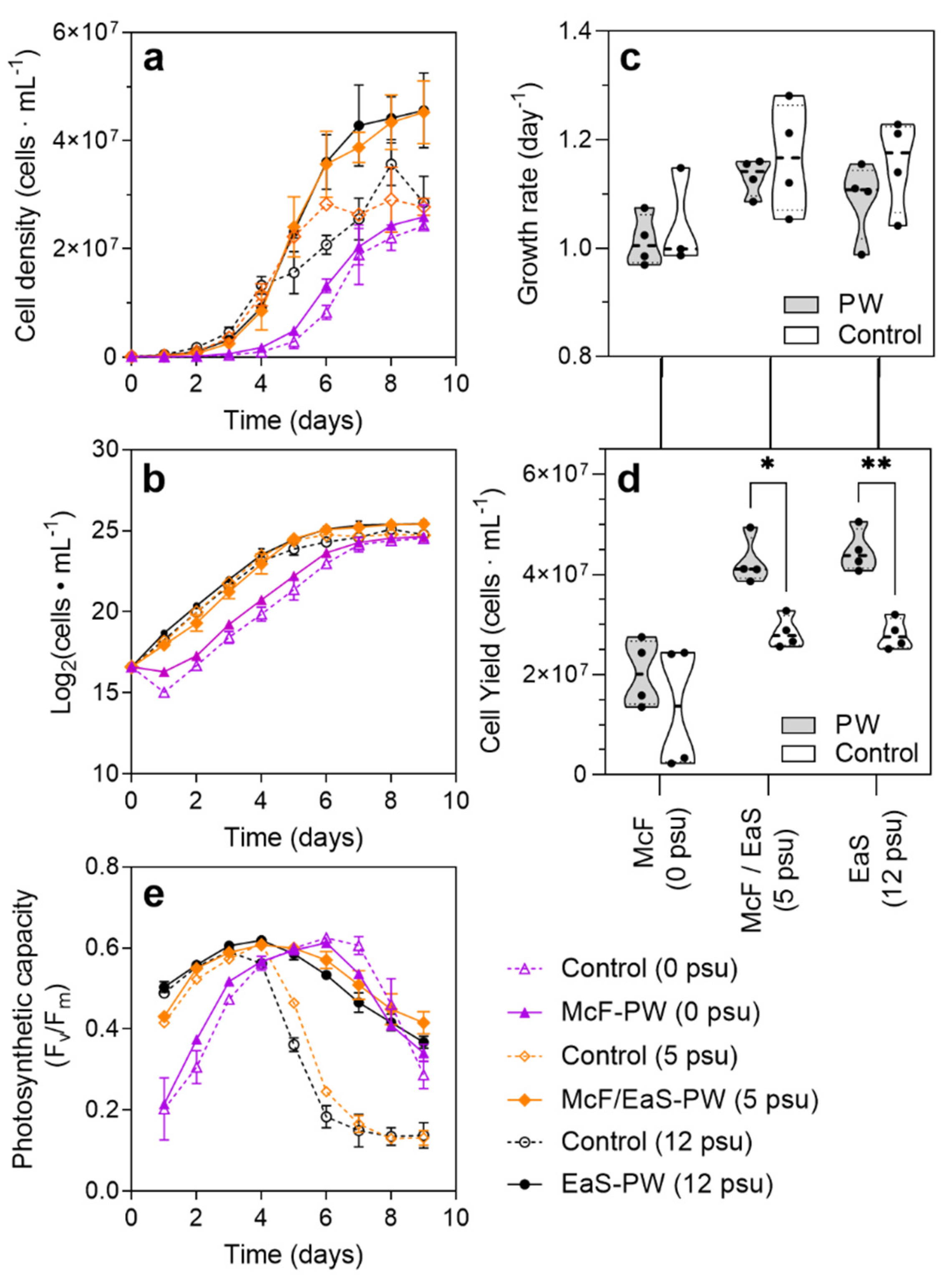
3.3. Growth Characterization and Lipid Accumulation of Pt1 in Three Distinct PW Media Formulations
3.4. Characterization of the EaS-PW Medium after P. tricornutum Cultivation
4. Discussion
4.1. Acclimation and Adaptation of Phaeodactylum tricornutum to Variation in Salinity
4.2. Cultivation of P. tricornutum in Oilfield PW
4.3. Remediation of Oilfield PW by P. tricornutum Cultivation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerra, K. Bureau of Reclamation, Oil and Gas Produced Water Management and Beneficial Use in the Western United States; Dundorf, S., Ed.; U.S. Department of the Interior Bureau of Reclamation: Denver, CO, USA, 2011. [Google Scholar]
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.; Nicot, J.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can we beneficially reuse produced water from oil and gas extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’Na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process. Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Murray-Gulde, C.; E Heatley, J.; Karanfil, T.; Rodgers, J.H.; E Myers, J. Performance of a hybrid reverse osmosis-constructed wetland treatment system for brackish oil field produced water. Water Res. 2003, 37, 705–713. [Google Scholar] [CrossRef]
- Mills, M.A.; Bonner, J.S.; McDonald, T.J.; Page, C.A.; Autenrieth, R.L. Intrinsic bioremediation of a petroleum-impacted wetland. Mar. Pollut. Bull. 2003, 46, 887–899. [Google Scholar] [CrossRef]
- Shutes, R. Artificial wetlands and water quality improvement. Environ. Int. 2001, 26, 441–447. [Google Scholar] [CrossRef]
- Pacheco, D.; Rocha, A.C.; Pereira, L.; Verdelhos, T. Microalgae Water Bioremediation: Trends and Hot Topics. Appl. Sci. 2020, 10, 1886. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Liu, J.; Danneels, B.; Vanormelingen, P.; Vyverman, W. Nutrient removal from horticultural wastewater by benthic filamentous algae Klebsormidium sp., Stigeoclonium spp. and their communities: From laboratory flask to outdoor Algal Turf Scrubber (ATS). Water Res. 2016, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef]
- Delrue, F.; Álvarez-Díaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Judd, S.J.; Al Momani, F.A.O.; Znad, H.; Al Ketife, A.M.D. The cost benefit of algal technology for combined CO2 mitigation and nutrient abatement. Renew. Sustain. Energy Rev. 2017, 71, 379–387. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Patel, A.; Dixit, G.; Shah, E. Comprehensive evaluation of microalgal based dairy effluent treatment process for clean water generation and other value added products. Int. J. Phytoremediat. 2019, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef]
- Pate, R.; Klise, G.; Wu, B. Resource demand implications for US algae biofuels production scale-up. Appl. Energy 2011, 88, 3377–3388. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Wilson, M.H.; Groppo, J.; Placido, A.; Graham, S.; Morton, S.A.; Santillan-Jimenez, E.; Shea, A.; Crocker, M.; Crofcheck, C.; Andrews, R. CO2 recycling using microalgae for the production of fuels. Appl. Petrochem. Res. 2014, 4, 41–53. [Google Scholar] [CrossRef]
- Graham, E.J.S.; Dean, C.A.; Yoshida, T.M.; Twary, S.N.; Teshima, M.; Alvarez, M.A.; Zidenga, T.; Heikoop, J.; Perkins, G.; Rahn, T.A.; et al. Oil and gas produced water as a growth medium for microalgae cultivation: A review and feasibility analysis. Algal Res. 2017, 24, 492–504. [Google Scholar] [CrossRef]
- Hopkins, T.C.; Graham, E.J.S.; Schuler, A.J. Biomass and lipid productivity of Dunaliella tertiolecta in a produced water-based medium over a range of salinities. J. Appl. Phycol. 2019, 31, 3349–3358. [Google Scholar] [CrossRef]
- Hopkins, T.C.; Graham, E.J.S.; Schwilling, J.; Ingram, S.; Gómez, S.M.; Schuler, A.J. Effects of salinity and nitrogen source on growth and lipid production for a wild algal polyculture in produced water media. Algal Res. 2019, 38, 101406. [Google Scholar] [CrossRef]
- Rahman, A.; Pan, S.; Houston, C.; Selvaratnam, T. Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water. Water 2021, 13, 1183. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Isolation, screening and comprehensive characterization of candidate microalgae for biofuel feedstock production and dairy effluent treatment: A sustainable approach. Bioresour. Technol. 2019, 293, 121998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, S.; Hu, T.; Nugroho, Y.K.; Yin, Z.; Hu, D.; Chu, R.; Mo, F.; Liu, C.; Hiltunen, E. Effects of nitrogen source heterogeneity on nutrient removal and biodiesel production of mono- and mix-cultured microalgae. Energy Convers. Manag. 2019, 201, 112144. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiumparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2010, 7, 703–726. [Google Scholar] [CrossRef]
- Xue, J.; Balamurugan, S.; Li, T.; Cai, J.-X.; Chen, T.-T.; Wang, X.; Yang, W.-D.; Li, H.-Y. Biotechnological approaches to enhance biofuel producing potential of microalgae. Fuel 2021, 302, 121169. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels 2015, 8, 28. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Hildebrand, M.; Davis, A.K.; Smith, S.R.; Traller, J.C.; Abbriano, R. The place of diatoms in the biofuels industry. Biofuels 2012, 3, 221–240. [Google Scholar] [CrossRef]
- Slattery, S.S.; Diamond, A.; Wang, H.; Therrien, J.A.; Lant, J.T.; Jazey, T.; Lee, K.; Klassen, Z.; Desgagné-Penix, I.; Karas, B.J.; et al. An Expanded Plasmid-Based Genetic Toolbox Enables Cas9 Genome Editing and Stable Maintenance of Synthetic Pathways in Phaeodactylum tricornutum. ACS Synth. Biol. 2018, 7, 328–338. [Google Scholar] [CrossRef]
- Karas, B.J.; Diner, R.E.; Lefebvre, S.C.; McQuaid, J.; Phillips, A.P.; Noddings, C.M.; Brunson, J.K.; Valas, R.E.; Deerinck, T.J.; Jablanovic, J.; et al. Designer diatom episomes delivered by bacterial conjugation. Nat. Commun. 2015, 6, 6925. [Google Scholar] [CrossRef]
- Wu, S.; Gu, W.; Huang, A.; Li, Y.; Kumar, M.; Lim, P.E.; Huan, L.; Gao, S.; Wang, G. Elevated CO2 improves both lipid accumulation and growth rate in the glucose-6-phosphate dehydrogenase engineered Phaeodactylum tricornutum. Microb. Cell Factories 2019, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Luo, L.; Jouhet, J.; Rébeillé, F.; Maréchal, E.; Hu, H.; Pan, Y.; Tan, X.; Chen, Z.; You, L.; et al. Enhanced triacylglycerol production in the diatom Phaeodactylum tricornutum by inactivation of a Hotdog-fold thioesterase gene using TALEN-based targeted mutagenesis. Biotechnol. Biofuels 2018, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, Y.; Bowler, C.; Zhang, L.; Hu, H. Knockdown of phosphoenolpyruvate carboxykinase increases carbon flux to lipid synthesis in Phaeodactylum tricornutum. Algal Res. 2016, 15, 50–58. [Google Scholar] [CrossRef]
- Daboussi, F.; LeDuc, S.; Maréchal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014, 5, 3831. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Huang, L.; Ji, P.-Y.; Chen, C.; Li, X.-S.; Gao, Y.-H.; Liang, J.-R. Using a mixture of wastewater and seawater as the growth medium for wastewater treatment and lipid production by the marine diatom Phaeodactylum tricornutum. Bioresour. Technol. 2019, 289, 121681. [Google Scholar] [CrossRef]
- Burch, A.R.; Yothers, C.W.; Salemi, M.R.; Phinney, B.S.; Pandey, P.; Franz, A.K. Quantitative label-free proteomics and biochemical analysis of Phaeodactylum tricornutum cultivation on dairy manure wastewater. J. Appl. Phycol. 2021, 33, 2105–2121. [Google Scholar] [CrossRef]
- Veronesiv, D.; D’Imporzano, G.; Salati, S.; Adani, F. Pre-treated digestate as culture media for producing algal biomass. Ecol. Eng. 2017, 105, 335–340. [Google Scholar] [CrossRef]
- Libralato, G.; Gentile, E.; Ghirardini, A.V. Wastewater effects on Phaeodactylum tricornutum (Bohlin): Setting up a classification system. Ecol. Indic. 2016, 60, 31–37. [Google Scholar] [CrossRef]
- Fetter, C.W. Applied Hydrogeology, 4th ed.; Waveland Press: Long Grove, IL, USA, 2018. [Google Scholar]
- Alley, B.; Beebe, A.; Rodgers, J.; Castle, J.W. Chemical and physical characterization of produced waters from conventional and unconventional fossil fuel resources. Chemosphere 2011, 85, 74–82. [Google Scholar] [CrossRef]
- De Martino, A.; Meichenin, A.; Shi, J.; Pan, K.; Bowler, C. Genetic and phenotypic characterization ofPhaeodactylum tricornutum(Bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Young, E.F.; Holt, J.T. Prediction and analysis of long-term variability of temperature and salinity in the Irish Sea. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Kniebusch, M.; Meier, H.M.; Radtke, H. Changing Salinity Gradients in the Baltic Sea as a Consequence of Altered Freshwater Budgets. Geophys. Res. Lett. 2019, 46, 9739–9747. [Google Scholar] [CrossRef]
- Rastogi, A.; Vieira, F.R.J.; Deton-Cabanillas, A.-F.; Veluchamy, A.; Cantrel, C.; Wang, G.; Vanormelingen, P.; Bowler, C.; Piganeau, G.; Hu, H.; et al. A genomics approach reveals the global genetic polymorphism, structure, and functional diversity of ten accessions of the marine model diatom Phaeodactylum tricornutum. ISME J. 2020, 14, 347–363. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Stock, W.; Blommaert, L.; Daveloose, I.; Vyverman, W.; Sabbe, K. Assessing the suitability of Imaging-PAM fluorometry for monitoring growth of benthic diatoms. J. Exp. Mar. Biol. Ecol. 2019, 513, 35–41. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques, 1st ed.; Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Ignatia, L.; Franz, A.K.; Wong, D.M.; Faulina, S.A.; Tsui, M.; Kanti, A.; Boundy-Mills, K. An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. J. Microbiol. Methods 2012, 91, 321–328. [Google Scholar] [CrossRef]
- van Tol, H.M.; Armbrust, E.V. Genome-scale metabolic model of the diatom Thalassiosira pseudonana highlights the importance of nitrogen and sulfur metabolism in redox balance. PLoS ONE 2021, 16, e0241960. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, R.C.; Syrett, P.J. Uptake of Nitrate by the DiatomPhaeodactylum tricornutum. J. Exp. Bot. 1981, 32, 19–25. [Google Scholar] [CrossRef]
- Kuenzler, E.J.; Ketchum, B.H. Rate of phosphorus uptake by phaeodactylum tricornutum. Biol. Bull. 1962, 123, 134–145. [Google Scholar] [CrossRef]
- De Martino, A.; Bartual, A.; Willis, A.; Meichenin, A.; Villazán, B.; Maheswari, U.; Bowler, C. Physiological and Molecular Evidence that Environmental Changes Elicit Morphological Interconversion in the Model Diatom Phaeodactylum tricornutum. Protist 2011, 162, 462–481. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Volcani, B.E. The polymorphic diatomphaeodactylum tricornutum: Ultrastructure of its morphotypes1,2. J. Phycol. 1978, 14, 10–21. [Google Scholar] [CrossRef]
- Przeslawski, R. Combined effects of solar radiation and desiccation on the mortality and development of encapsulated embryos of rocky shore gastropods. Mar. Ecol. Prog. Ser. 2005, 298, 169–177. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Chaney, M.L.; Boomhower, J.P.; Tyburczy, W.R.; Connor, K.; Somero, G.N. Rhythms of Gene Expression in a Fluctuating Intertidal Environment. Curr. Biol. 2008, 18, 1501–1507. [Google Scholar] [CrossRef]
- Raven, J.A.; Geider, R.J. Adaptation, acclimation and regulation in algal photosynthesis. In Photosynthesis in Algae; Advances in Photosynthesis and Respiration; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 14, pp. 385–412. [Google Scholar]
- Bulankova, P.; Sekulić, M.; Jallet, D.; Nef, C.; van Oosterhout, C.; Delmont, T.O.; Vercauteren, I.; Osuna-Cruz, C.M.; Vancaester, E.; Mock, T.; et al. Mitotic recombination between homologous chromosomes drives genomic diversity in diatoms. Curr. Biol. 2021. [Google Scholar] [CrossRef]
- Alipanah, L.; Rohloff, J.; Winge, P.; Bones, A.M.; Brembu, T. Whole-cell response to nitrogen deprivation in the diatomPhaeodactylum tricornutum. J. Exp. Bot. 2015, 66, 6281–6296. [Google Scholar] [CrossRef]
- Li, W.; Gao, K.; Beardall, J. Interactive Effects of Ocean Acidification and Nitrogen-Limitation on the Diatom Phaeodactylum tricornutum. PLoS ONE 2012, 7, e51590. [Google Scholar] [CrossRef]
- Liu, N.; Beardall, J.; Gao, K. Elevated CO2 and associated seawater chemistry do not benefit a model diatom grown with increased availability of light. Aquat. Microb. Ecol. 2017, 79, 137–147. [Google Scholar] [CrossRef][Green Version]
- Geider, R.J.; Roche, J.; Greene, R.M.; Olaizola, M. Response of the photosynthetic apparatus of phaeodactylum tricornutum (bacillariophyceae) to nitrate, phosphate, or iron starvation. J. Phycol. 1993, 29, 755–766. [Google Scholar] [CrossRef]
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 1981, 17, 374–384. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Triacylglycerol accumulation and change in fatty acid content of four marine oleaginous microalgae under nutrient limitation and at different culture ages. J. Basic Microbiol. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Burrows, E.H.; Bennette, N.B.; Carrieri, D.; Dixon, J.L.; Brinker, A.; Frada, M.; Baldassano, S.N.; Falkowski, P.G.; Dismukes, G.C. Dynamics of Lipid Biosynthesis and Redistribution in the Marine Diatom Phaeodactylum tricornutum Under Nitrate Deprivation. BioEnergy Res. 2012, 5, 876–885. [Google Scholar] [CrossRef]
- Remmers, I.M.; D’Adamo, S.; Martens, D.E.; de Vos, R.C.; Mumm, R.; America, A.H.; Cordewener, J.H.; Bakker, L.V.; Peters, S.A.; Wijffels, R.H.; et al. Orchestration of transcriptome, proteome and metabolome in the diatom Phaeodactylum tricornutum during nitrogen limitation. Algal Res. 2018, 35, 33–49. [Google Scholar] [CrossRef]
- Yang, Z.-K.; Ma, Y.-H.; Zheng, J.-W.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Proteomics to reveal metabolic network shifts towards lipid accumulation following nitrogen deprivation in the diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2014, 26, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A Critical Review of the Literature on Hydrogen Sulfide Toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, B.; Chen, F.; Pan, K. How marine diatoms cope with metal challenge: Insights from the morphotype-dependent metal tolerance in Phaeodactylum tricornutum. Ecotoxicol. Environ. Saf. 2021, 208, 111715. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, J.; Chen, F.; Zou, Y.; Wei, Y.; Zhong, H.; Pan, K. Mechanisms underlying silicon-dependent metal tolerance in the marine diatom Phaeodactylum tricornutum. Environ. Pollut. 2020, 262, 114331. [Google Scholar] [CrossRef]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef]
- Krishna, K.; Arup, G.; Prince, V.; Kalaiyarasan, T.; Bhuvnesh, K. Effects of Fluoride on Respiration and Photosynthesis in Plants: An Overview. Ann. Environ. Sci. Toxicol. 2018, 2, 043–047. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Lutz, A.; Berry, K.A.; Yang, W. Recommendations for fluoride limits in drinking water based on estimated daily fluoride intake in the Upper East Region, Ghana. Sci. Total. Environ. 2015, 532, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Redmon, J.H.; Kondash, A.J.; Womack, D.; Lillys, T.; Feinstein, L.; Cabrales, L.; Weinthal, E.; Vengosh, A. Is Food Irrigated with Oilfield-Produced Water in the California Central Valley Safe to Eat? A Probabilistic Human Health Risk Assessment Evaluating Trace Metals Exposure. Risk Anal. 2020, 41, 1463–1477. [Google Scholar] [CrossRef]
- Antia, N.J.; Klut, M.E. Fluoride Addition Effects on Euryhaline Phytoplankter Growth in Nutrient-Enriched Seawater at an Estuarine Level of Salinity. Bot. Mar. 1981, 24, 147–152. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B. Toxic effects of fluoranthene and copper on marine diatom Phaeodactylum tricornutum. J. Environ. Sci. 2008, 20, 1363–1372. [Google Scholar] [CrossRef]
- Davis, A.K.; Hildebrand, M.; Palenik, B. Gene Expression Induced by Copper Stress in the Diatom Thalassiosira pseudonana. Eukaryot. Cell 2006, 5, 1157–1168. [Google Scholar] [CrossRef]
- Stuart, R.K.; Dupont, C.L.; Johnson, D.A.; Paulsen, I.T.; Palenik, B. Coastal Strains of Marine Synechococcus Species Exhibit Increased Tolerance to Copper Shock and a Distinctive Transcriptional Response Relative to Those of Open-Ocean Strains. Appl. Environ. Microbiol. 2009, 75, 5047–5057. [Google Scholar] [CrossRef]
- Smyth, D.A.; Dugger, W.M. Cellular changes during boron-deficient culture of the diatom Cylindrotheca fusiformis. Physiol. Plant. 1981, 51, 111–117. [Google Scholar] [CrossRef]
- Lewin, J. Boron as a growth requirement for diatoms. J. Phycol. 1966, 2, 160–163. [Google Scholar] [CrossRef]
- Truu, J.; Truu, M.; Espenberg, M.; Nõlvak, H.; Juhanson, J. Phytoremediation and Plant-Assisted Bioremediation in Soil and Treatment Wetlands: A Review. Open Biotechnol. J. 2015, 9, 85–92. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, M.; Kraay, G.; Timmermans, K. Cell death in phytoplankton: Correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. Eur. J. Phycol. 2001, 36, 167–177. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Wang, G.; Dai, S.; He, H.; Xiang, W. A comparative analysis of fatty acid composition and fucoxanthin content in six Phaeodactylum tricornutum strains from diff erent origins. Chin. J. Oceanol. Limnol. 2016, 34, 391–398. [Google Scholar] [CrossRef]
- Ladygina, N.; Dedyukhina, E.G.; Vainshtein, M.B. A review on microbial synthesis of hydrocarbons. Process. Biochem. 2006, 41, 1001–1014. [Google Scholar] [CrossRef]


| Analyte | Units | McF-PW Source a | EaS-PW Source a | EaS-PW Medium after | RC (%) |
|---|---|---|---|---|---|
| Total Dissolved Solids | mg/L | 1160 | 12,600 | 13,000 | 3 |
| Conductivity | mmhos/cm | 1.8 | 20 | 21 | 5 |
| Salinity | psu | 0 | 12 | 12 | 0 |
| pH | pH units | 7.90 | 8.56 | 9.91 | 16 |
| Total Alkalinity | mg/L | 100 | 630 | 530 | −16 |
| HCO3− | mg/L | 100 | 490 | 0 | −100 |
| CO32− | mg/L | <10 | 140 | 470 | 236 |
| OH− | mg/L | <10 | <10 | 61 | 510 |
| Total Hydrocarbon | mg/L | 1.13 | 6.64 | 8.70 | 31 |
| C6–10 range | mg/L | <0.05 | <0.05 | <0.05 | 0 |
| C10–28 range | mg/L | <0.05 | 2.59 | 3.39 | 31 |
| C28–35 range | mg/L | 1.13 | 4.1 | 5.31 | 30 |
| NO3− | mg/L | <0.50 (55) | 20.50 (55) | 6.00 | −92 |
| PO43− | mg/L | <0.30 (3.4) | 4.6 (3.4) | 1.9 | −76 |
| SO42− | mg/L | 180 (0.3) | 390 (0.3) | 310 | −21 |
| SiO2 | mg/L | 31 | 100 | <40 | −60 |
| B | mg/L | 1.6 | 34 | 40 | 8 |
| Ba | mg/L | <0.10 | 0.96 | <1.00 | 4 |
| Ca | mg/L | 19 | 34 | 37 | 8 |
| Cl | mg/L | 440 | 7100 | 6600 | −7 |
| Cu | mg/L | <0.05 | 0.06 | <0.05 | −18 |
| F | mg/L | 0.77 | 4.60 | 1.30 | −72 |
| Fe | mg/L | <0.10 (0.7) | <0.10 (0.7) | <0.10 | −85 |
| K | mg/L | 5.1 | 65.0 | 59.0 | −9 |
| Mg | mg/L | 0.43 | 54.00 | 24.00 | −56 |
| Na | mg/L | 380 (21) | 4400 (21) | 5000 | 13 |
| Sr | mg/L | 0.33 | 2.80 | 2.80 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillard, J.T.F.; Hernandez, A.L.; Contreras, J.A.; Francis, I.M.; Cabrales, L. Potential for Biomass Production and Remediation by Cultivation of the Marine Model Diatom Phaeodactylum tricornutum in Oil Field Produced Wastewater Media. Water 2021, 13, 2700. https://doi.org/10.3390/w13192700
Gillard JTF, Hernandez AL, Contreras JA, Francis IM, Cabrales L. Potential for Biomass Production and Remediation by Cultivation of the Marine Model Diatom Phaeodactylum tricornutum in Oil Field Produced Wastewater Media. Water. 2021; 13(19):2700. https://doi.org/10.3390/w13192700
Chicago/Turabian StyleGillard, Jeroen T. F., Alexander L. Hernandez, Javier A. Contreras, Isolde M. Francis, and Luis Cabrales. 2021. "Potential for Biomass Production and Remediation by Cultivation of the Marine Model Diatom Phaeodactylum tricornutum in Oil Field Produced Wastewater Media" Water 13, no. 19: 2700. https://doi.org/10.3390/w13192700
APA StyleGillard, J. T. F., Hernandez, A. L., Contreras, J. A., Francis, I. M., & Cabrales, L. (2021). Potential for Biomass Production and Remediation by Cultivation of the Marine Model Diatom Phaeodactylum tricornutum in Oil Field Produced Wastewater Media. Water, 13(19), 2700. https://doi.org/10.3390/w13192700






