Degradation of Trimethoprim Using the UV/Free Chlorine Process: Influencing Factors and Optimal Operating Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.1.1. Experimental Materials
2.1.2. UV Reactor
2.1.3. Experimental Procedures
2.1.4. Analysis Method
2.2. Equilibrium Calculation
2.2.1. Free Chlorine Equilibrium
2.2.2. TMP Equilibrium
2.3. Kinetic Model Development
3. Results and Discussion
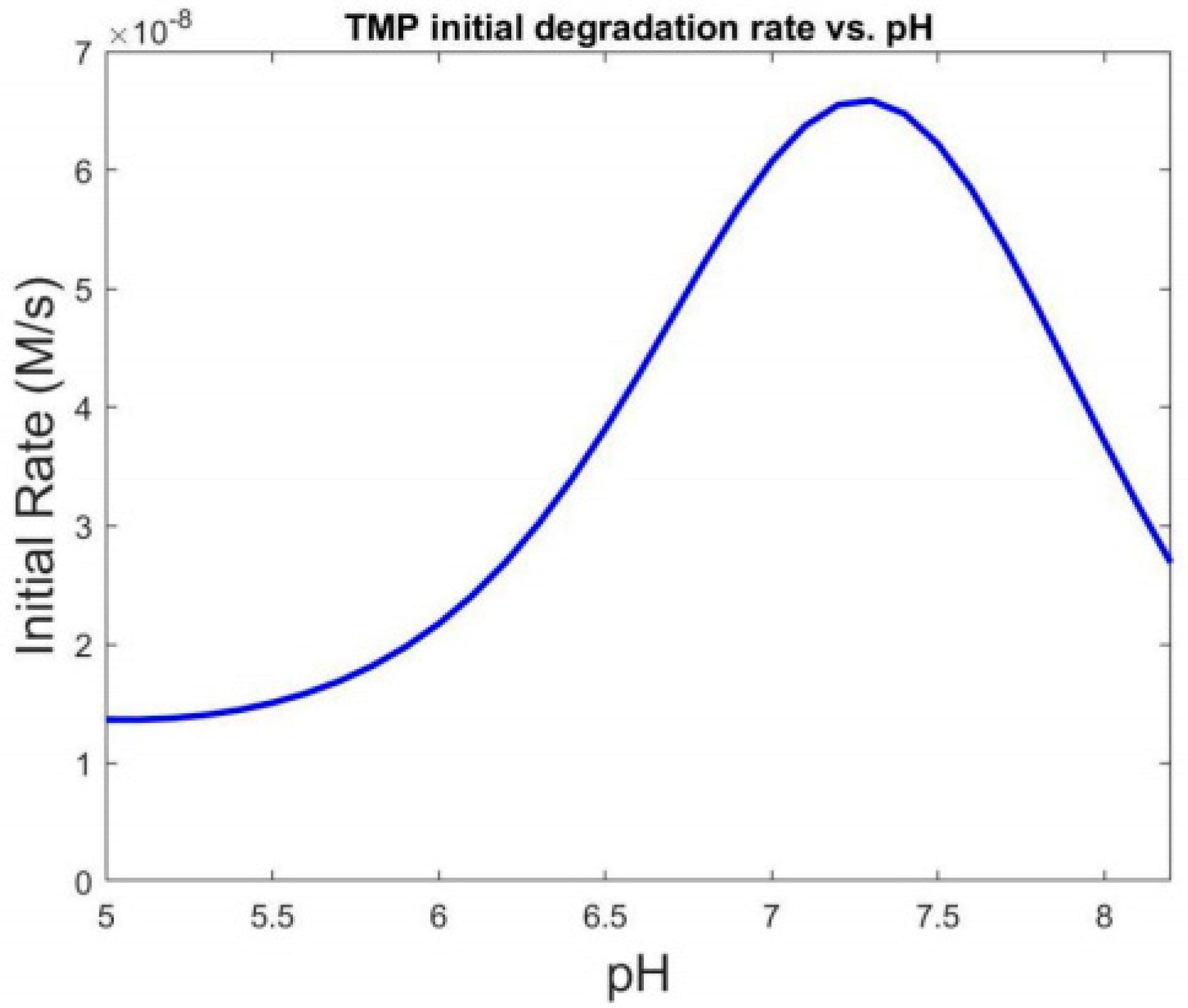
3.1. Effect of pH
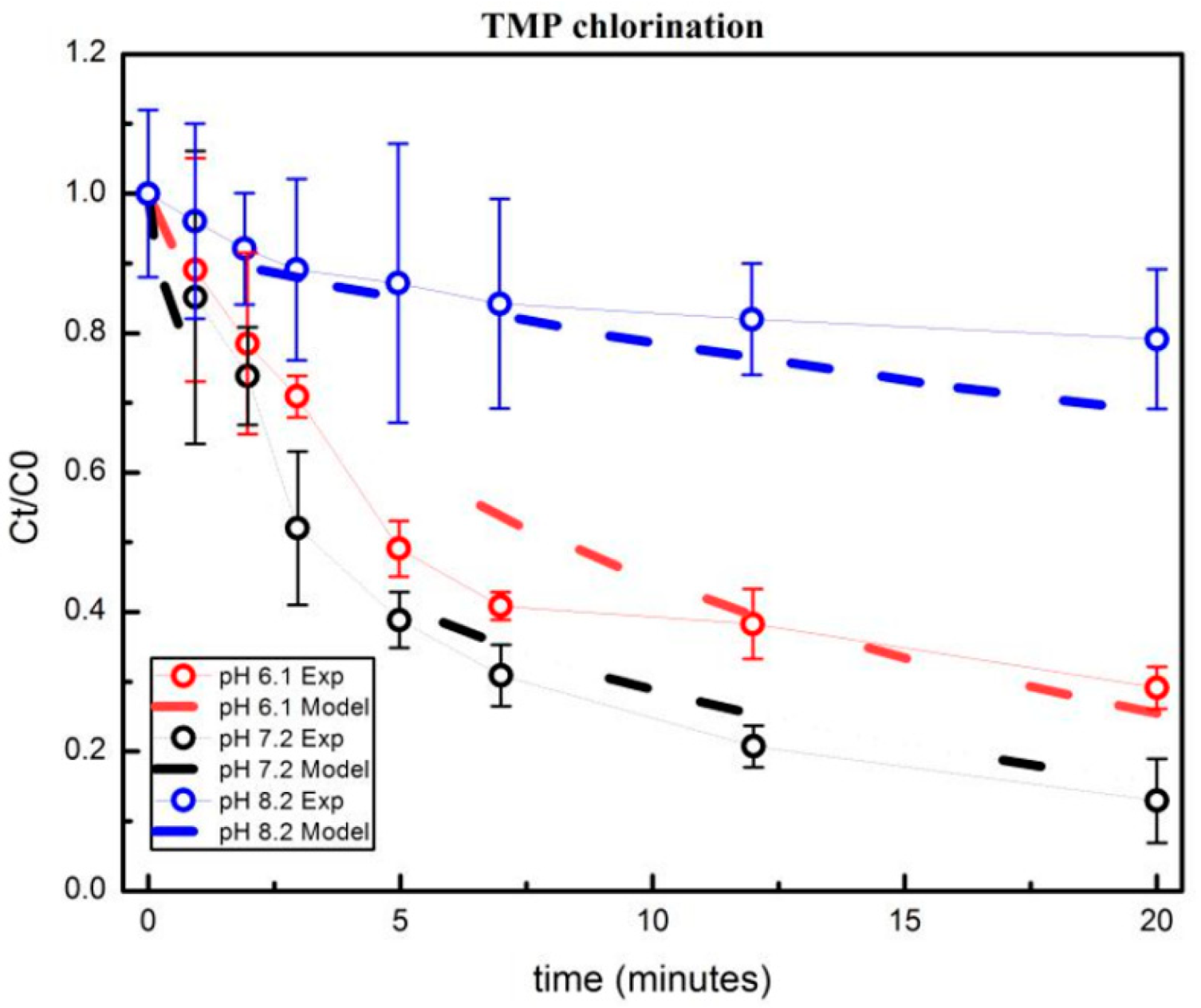
3.1.1. Effect of pH on the Oxidation of the Free Chlorine
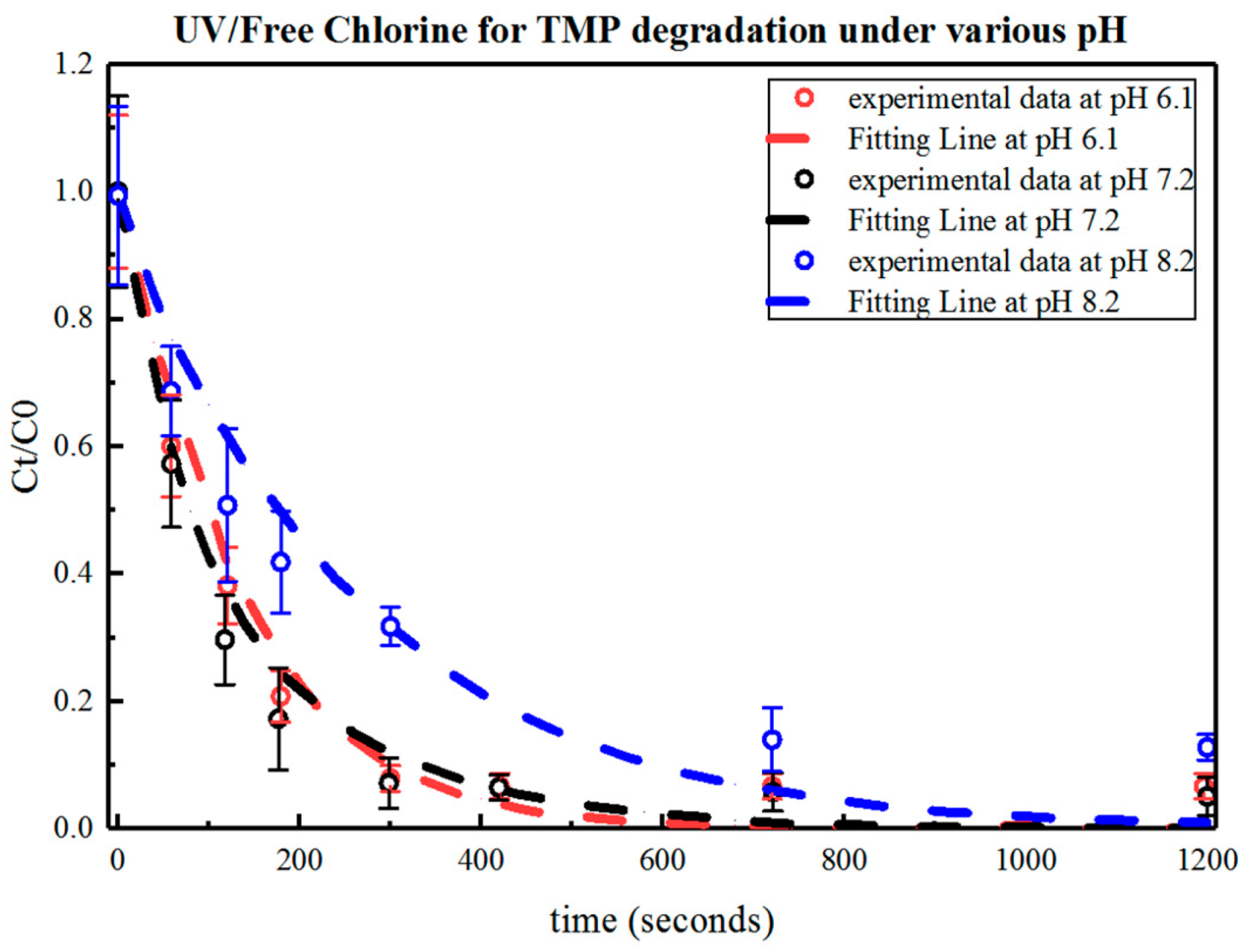
3.1.2. Effect of pH on the UV/Free Chlorine Process
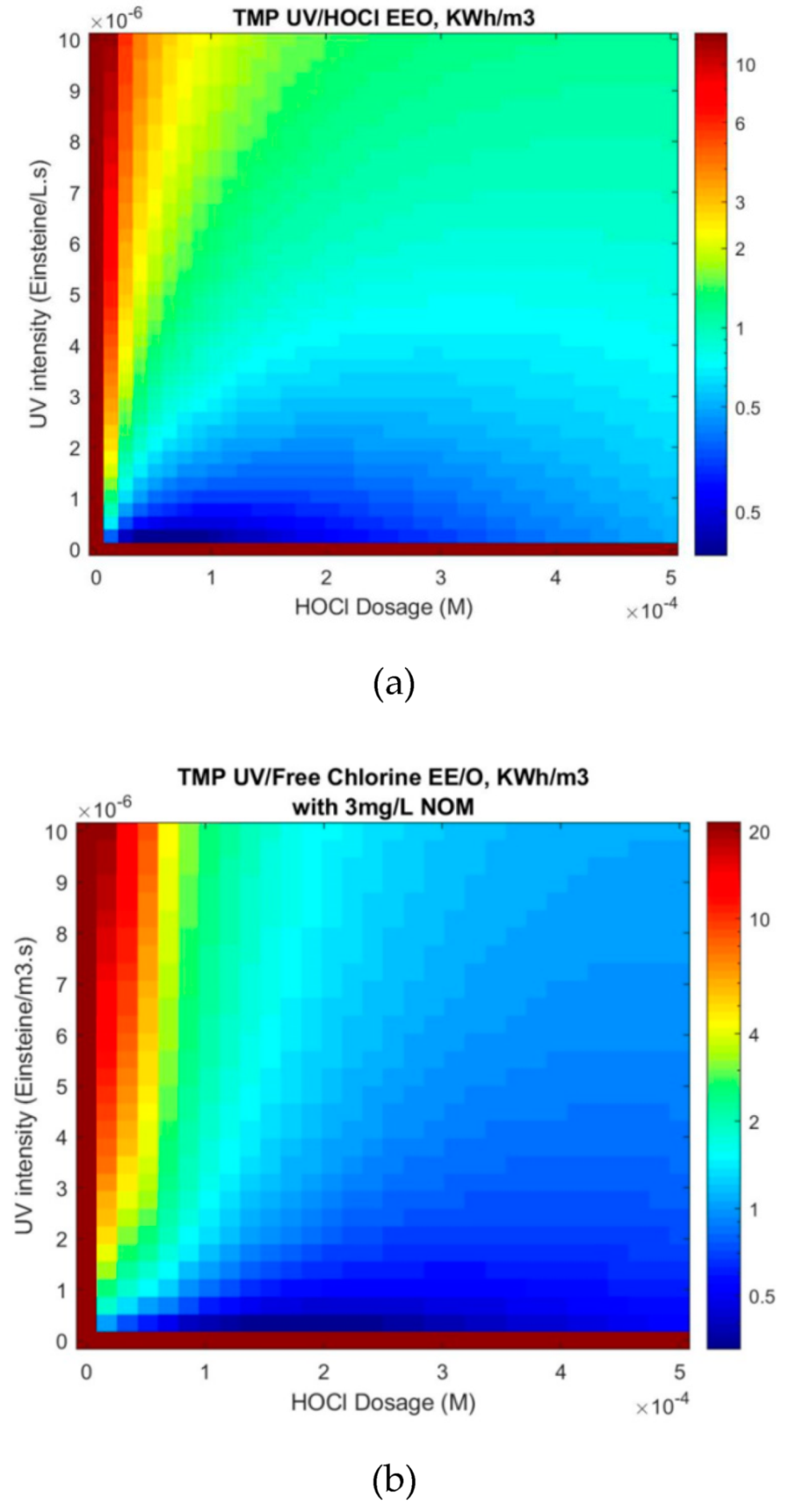
3.2. Effect of Oxidant Dosage on UV/Free Chlorine Process
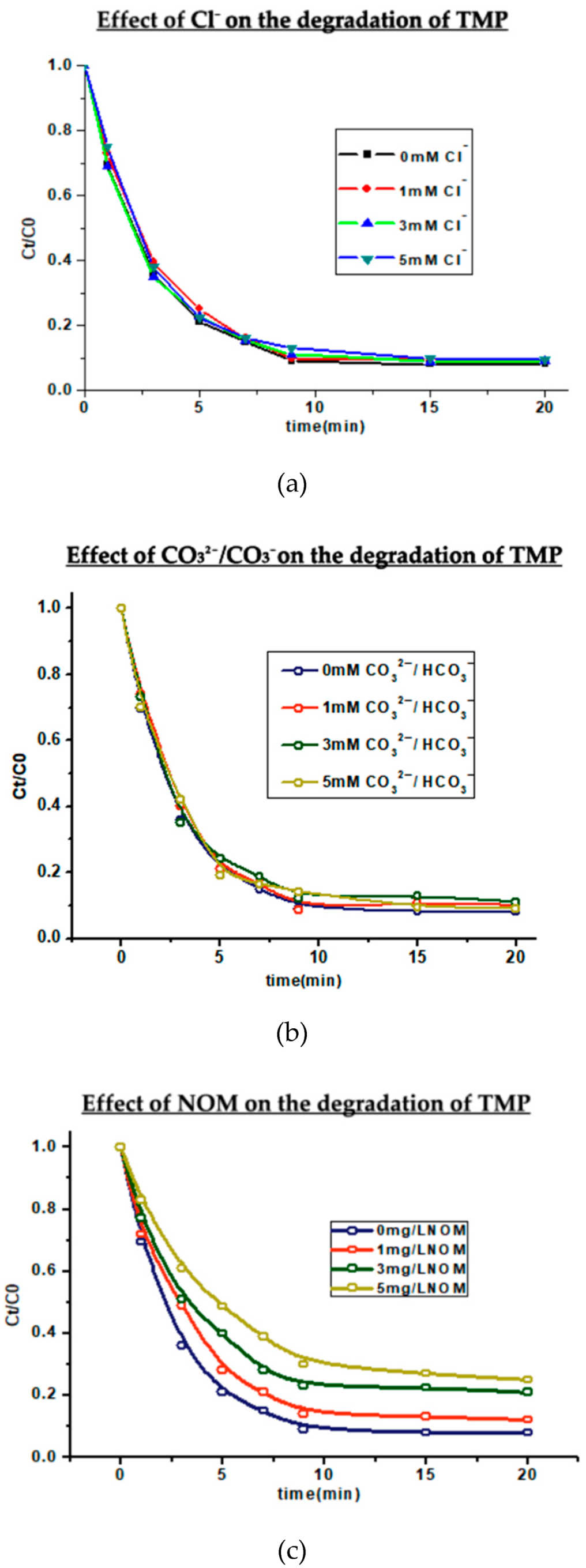
3.3. Effect of Chloride Ion on the UV/Free Chlorine Process
3.4. Effect of Bicarbonate/Carbonate on the UV/Free Chlorine Process
3.5. Effect of NOM on the UV/Free Chlorine Process
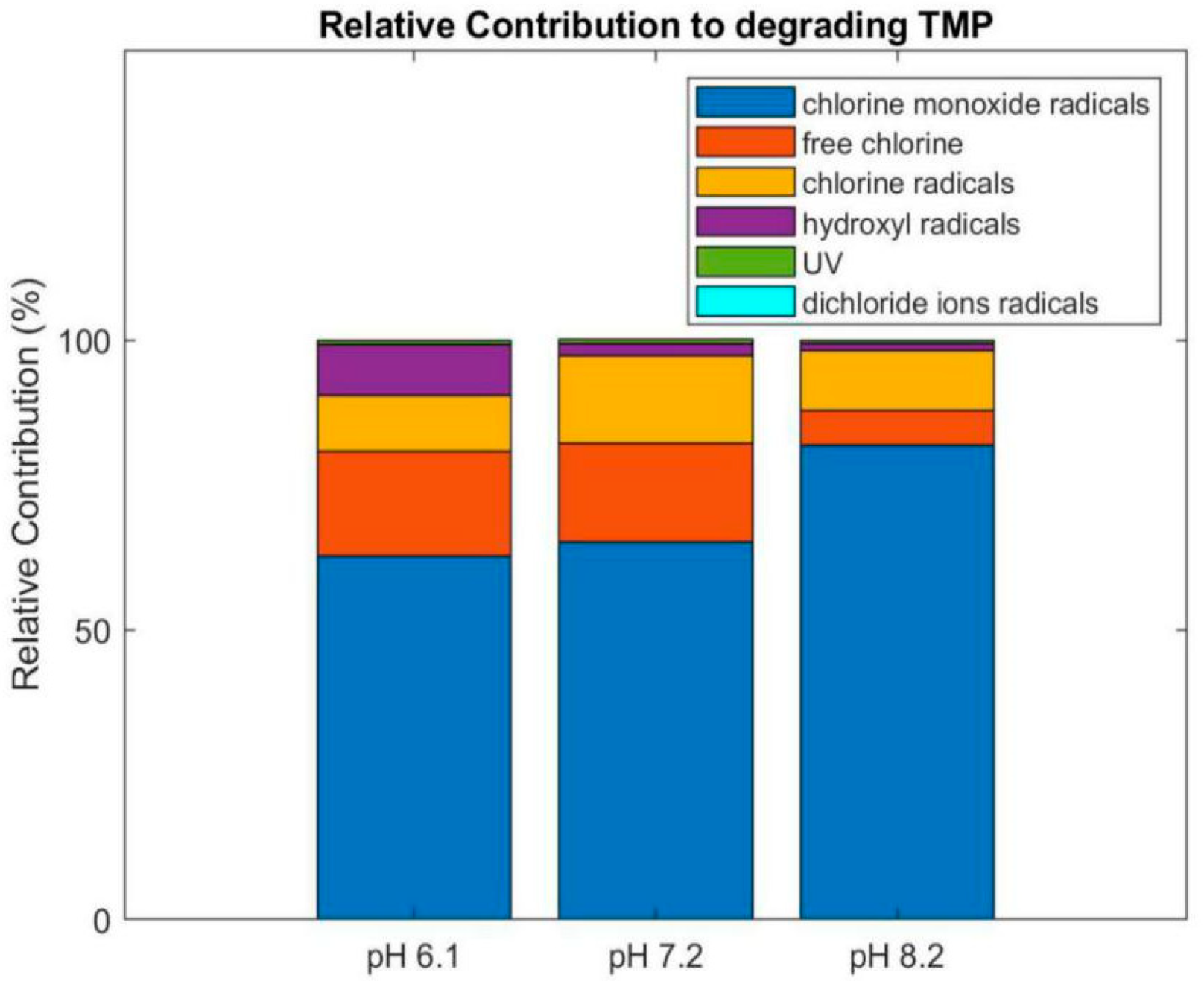
3.6. Relative Contribution of the Reactive Species, Photolysis and Chlorination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Gui, N.; Gao, N.; Zhang, Y.; Zhu, M. Research progress on advanced oxidative degradation of antibiotics in water. Sichuan Environ. 2014, 33, 146–153. [Google Scholar]
- Eliopoulos, G.M.; Moellering, R.C.J. Antibiotics in Laboratory Medicine; Lorian, V., Ed.; Waverly and Wilkins: Baltimore, MD, USA, 1996; pp. 331–396. [Google Scholar]
- Wu, Z.; Fang, J.; Xiang, Y.; Shang, C. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways. Water Res. 2016, 104, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, L.; Coscarelli, E.; Khaksari, M.; Mazzoleni, L.R.; Minakata, D. Transformations of dissolved organic matter induced by UV photolysis, hydroxyl radicals, chlorine radicals, and sulfate radicals in aqueous-phase UV-based advanced oxidation processes. Water Res. 2018, 135, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; Garcia, C.; Andre, K. Feasibility studies: UV/chlorine advanced oxidation treatment for the removal of emerging contaminants. Water Res. 2011, 45, 6371–6380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chu, W. Photo-assisted degradation of 2, 4, 5-trichlorophenoxyacetic acid by Fe (II)-catalyzed activation of Oxone process: The role of UV irradiation, reaction mechanism and mineralization. Appl. Catal. B Environ. 2012, 123, 151–161. [Google Scholar] [CrossRef]
- Wang, D.; Bolton, J.R.; Hofmann, R. Medium pressure UV combined with chlorine advanced oxidation for trichloroethylene destruction in a model water. Water Res. 2012, 46, 4677–4686. [Google Scholar] [CrossRef]
- Wang, T.X.; Margerum, D.W. Kinetics of reversible chlorine hydrolysis—temperature-dependence and general acid base-assisted mechanisms. Inorg. Chem. 1994, 33, 1050–1055. [Google Scholar] [CrossRef]
- Dodd, M.C.; Huang, C. Aqueous chlorination of the antibacterial agent trimethoprim: Reaction kinetics and pathways. Water Res. 2007, 41, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Klaning, U.K.; Wolff, T. Laser flash photolysis of HClO, C1O-, HBrO, and BrO-in Aqueous Solution Reactions of C1- and Br-Atoms. Berichte Bunsenges. Phys. Chem. Phys. 1985, 89, 243–245. [Google Scholar]
- Zhang, W.; Zhou, S.; Sun, J.; Meng, X.; Luo, J.; Zhou, D.; Crittenden, J.C. Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ. Sci. Technol. 2018, 52, 7380–7389. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Jiang, J.; Ma, J.; Yang, Y.; Liu, W.; Liu, Y. Degradation of atrazine by UV/chlorine: Efficiency, influencing factors, and products. Water Res. 2016, 90, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yin, K.; Liu, C.; Luo, J.; Crittenden, J.; Zhang, W.; Luo, S.; He, Q.; Deng, Y.; Liu, H. The role of reactive oxygen species and carbonate radical in oxcarbazepine degradation via UV, UV/H2O2: Kinetics, mechanisms and toxicity evaluation. Water Res. 2018, 147, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wu, Z.; Shang, C.; Yao, B.; Hou, S.; Yang, X.; Song, W.; Fang, J. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
| pH | HOCl (Mole %) | OCl− (Mole %) | TMP+2 (Mole %) | TMP+ (Mole %) | TMP (Mole %) |
|---|---|---|---|---|---|
| 5 | 99.7060 | 0.2940 | 1.5480 | 97.6760 | 0.7760 |
| 6 | 97.1330 | 2.8670 | 0.1470 | 92.5050 | 7.3480 |
| 7 | 77.2130 | 22.7870 | 0.0090 | 55.7260 | 44.2650 |
| 8 | 25.3090 | 74.6910 | 0.0002 | 11.1816 | 88.8183 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, Q.; Fu, Y.; Ran, Z.; Crittenden, J.C.; Zhang, W.; Wang, H. Degradation of Trimethoprim Using the UV/Free Chlorine Process: Influencing Factors and Optimal Operating Conditions. Water 2021, 13, 1656. https://doi.org/10.3390/w13121656
Wang B, Zhang Q, Fu Y, Ran Z, Crittenden JC, Zhang W, Wang H. Degradation of Trimethoprim Using the UV/Free Chlorine Process: Influencing Factors and Optimal Operating Conditions. Water. 2021; 13(12):1656. https://doi.org/10.3390/w13121656
Chicago/Turabian StyleWang, Bing, Qi Zhang, Yongdi Fu, Zhilin Ran, John C. Crittenden, Weiqiu Zhang, and Haipei Wang. 2021. "Degradation of Trimethoprim Using the UV/Free Chlorine Process: Influencing Factors and Optimal Operating Conditions" Water 13, no. 12: 1656. https://doi.org/10.3390/w13121656
APA StyleWang, B., Zhang, Q., Fu, Y., Ran, Z., Crittenden, J. C., Zhang, W., & Wang, H. (2021). Degradation of Trimethoprim Using the UV/Free Chlorine Process: Influencing Factors and Optimal Operating Conditions. Water, 13(12), 1656. https://doi.org/10.3390/w13121656





