Biogeochemistry of Mediterranean Wetlands: A Review about the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions
Abstract
:1. Introduction
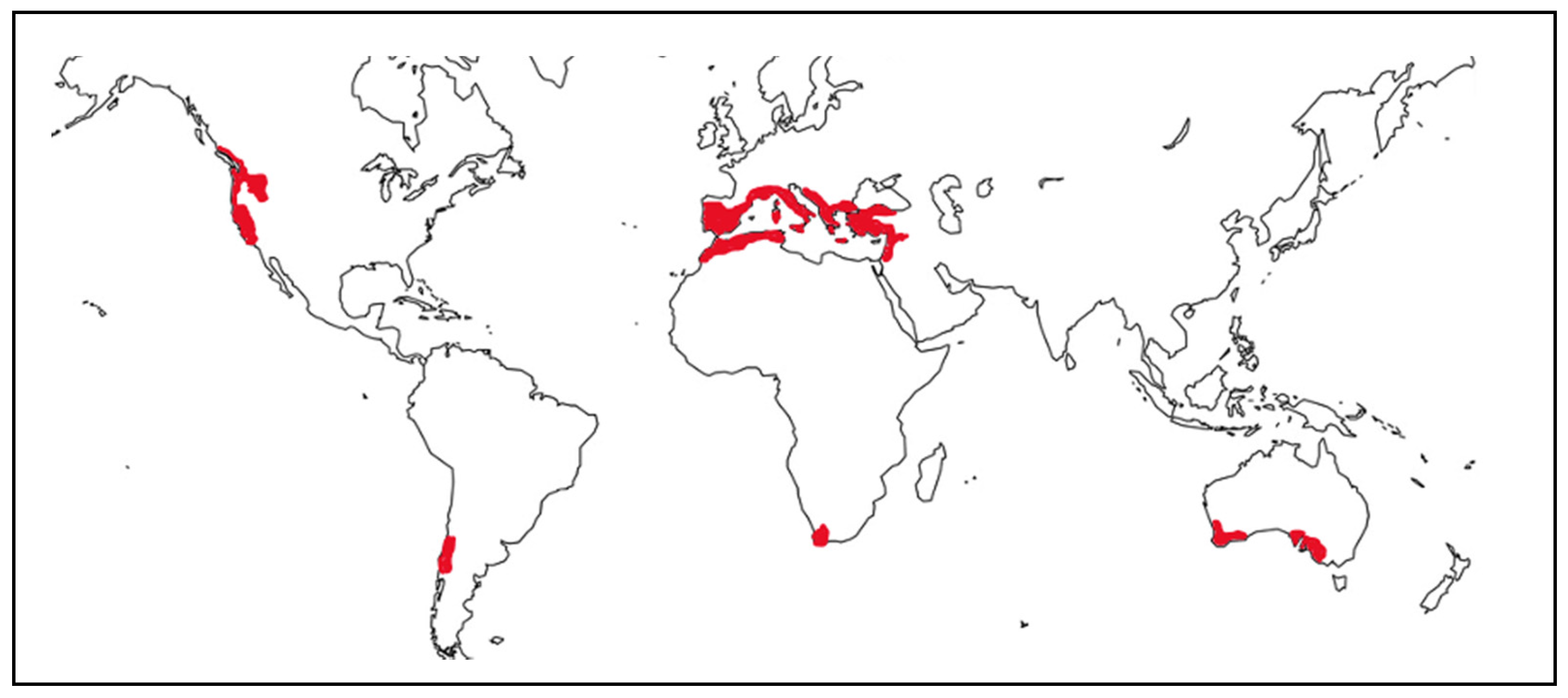
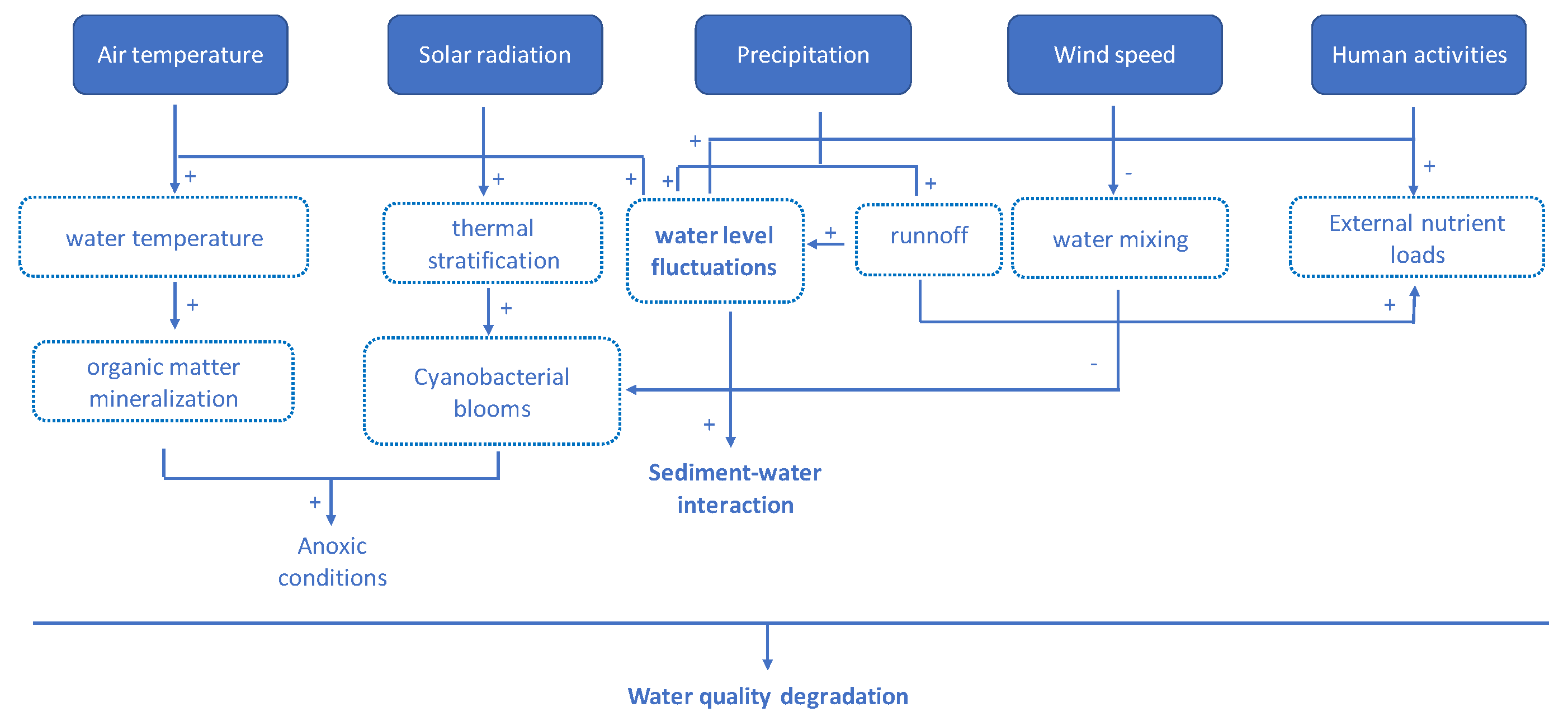
2. Sensitivity of Mediterranean Wetlands to Global Change Factors
3. The Sediment as an Essential Compartment of Mediterranean Wetlands
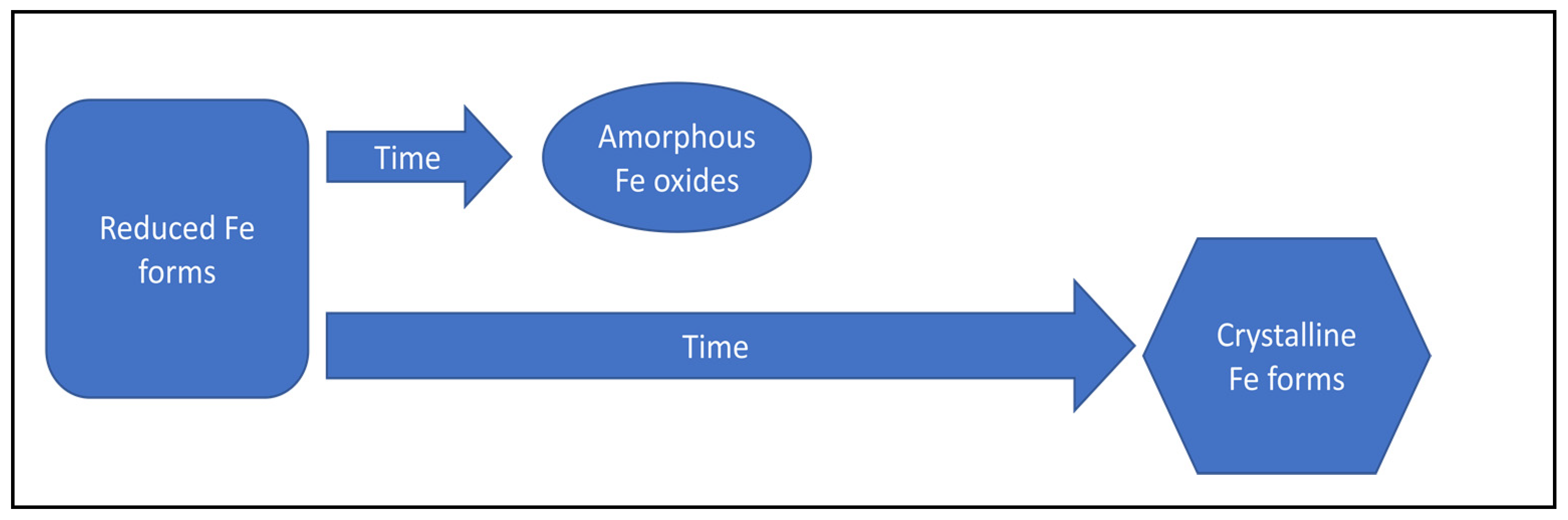
4. Impact of Sediment Desiccation and Re-Flooding on the Phosphorus Cycle
| Mechanism | Consequence | References | |
|---|---|---|---|
| Chemical | Increase in ferric oxyhydroxides concentration | Increase of phosphorus adsorption capacity | [111] |
| Transformation of amorphous iron oxides into more crystalline forms of iron oxides | Decrease of phosphorus adsorption capacity | [118,125] | |
| Increase in iron crystallinity | Decrease of phosphorus adsorption capacity | [114] | |
| Phosphate precipitation with CaCO3 as calcium concentration increases | Increase in sedimentary phosphorus concentration | [97] | |
| Physical | Increase of specific surface area | Increase of phosphorus adsorption capacity | [115] |
| Shifts towards larger particles | Decrease of phosphorus adsorption capacity | [110,126,127,135] | |
| Biological | A reduction in organic matter concentration inferring a more intense mineralization | Decrease of phosphorus adsorption capacity | [110,113] |
| Mechanism | References |
|---|---|
| Sediment resuspension | [138] |
| Breakdown of sedimentary organic phosphorus | [126] |
| Breakdown of sedimentary bacterial phosphorus | [127] |
| Shifts in bacterial communities, C-limitation as a result of the air exposure and ageing of minerals adsorbing phosphorus | [139] |
| Aging of Fe(OOH) decrease phosphorus affinity. Biological processes such as organic matter decomposition | [133] |
| Immobilized bacterial phosphorus upon desiccation is released | [140] |
| Loss of amorphous iron oxides upon desiccation | [110] |
| Enhanced mineralization rates, enhanced reduction of iron hydroxides and a general loss in sorption capacity due to increased crystallinity of iron hydroxides | [137] |
| Loss of organic matter upon desiccation | [113] |
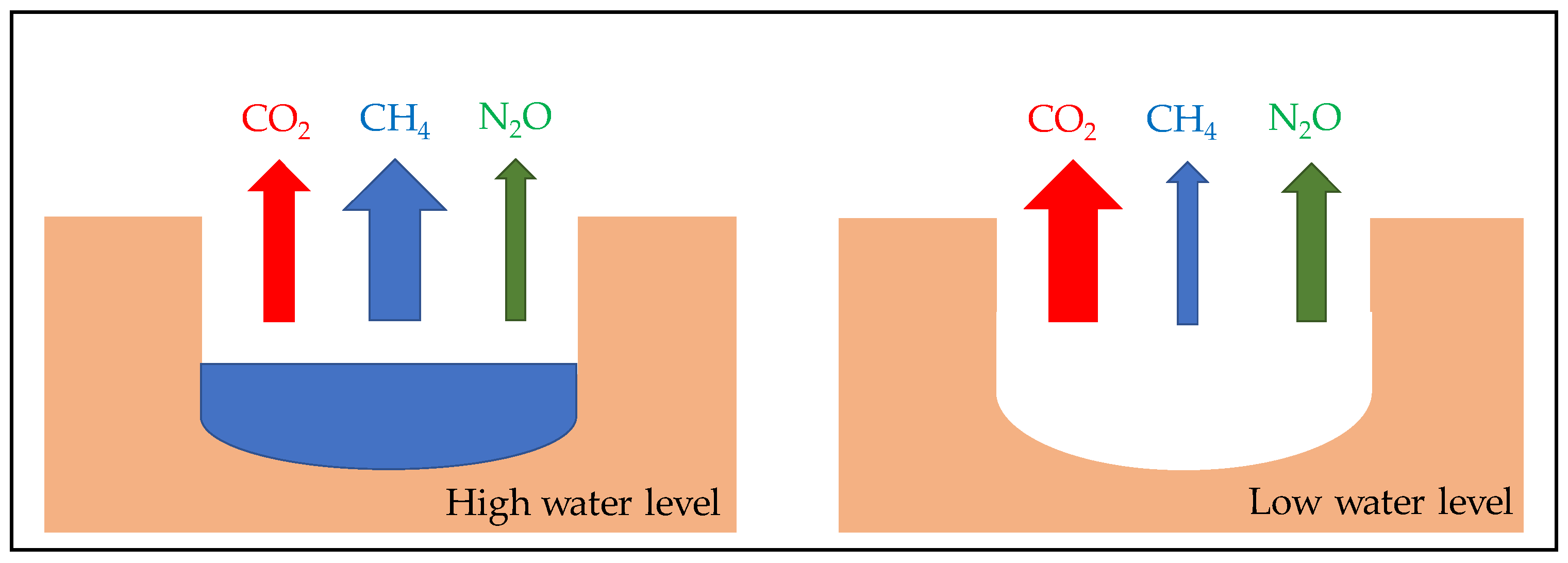
5. Consequence of Sediment Desiccation and Re-Flooding on Greenhouse Gases Emissions
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef] [Green Version]
- Greeson, P.E.; Clark, J.R.; Clark, J.E. Wetland Functions and Values: The State of Our Understanding; American Water Resources Association: Minneapolis, MN, USA, 1979. [Google Scholar]
- Clarkson, B.R.; Ausseil, A.E.; Gerbeaux, P. Wetland ecosystem services. In Ecosystem Services in New Zealand—Conditions and Trends; Dymond, J.R., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013. [Google Scholar]
- Ramsar. Factsheet 7: Wetland Products; Ramsar Convention Secretariat: Gland, Switzerland, 2009. [Google Scholar]
- Fisher, J.; Acreman, M.C. Wetland nutrient removal: A review of the evidence. Hydrol. Earth System Sci. 1999, 8, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Tanner, C.C.; Sukias, J.P.S. Multi-year nutrient removal performance of three constructed wetlands intercepting drainage flows from grazed pastures. J. Environ. Qual. 2011, 40, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.J.; Vaithiyanathan, P. Biochemical dynamics II: Cycling and storage of phosphorus in wetlands. In The Welands Handbook; Maltby, E., Barker, T., Eds.; Wiley-Blackwell: Oxford, UK, 2009; pp. 228–248. [Google Scholar]
- Reddy, K.R.; DeLaune, R.; Craft, C.B. Nutrients in wetlands: Implications to water quality under changing climatic conditions. Final. Rep. Front. Environ. Sci. 2010, 8, 55. [Google Scholar]
- Jackson, C.R.; Thompson, J.A.; Kolka, R.K. Wetland soils, hydrology and geomorphology. In Ecology of Freshwater and Estuarine Wetlands; Batzer, D., Sharitz, R., Eds.; University of California Press: Berkeley, CA, USA, 2014; pp. 23–60. [Google Scholar]
- Cherry, J.A. Ecology of wetland ecosystems: Water, substrate, and life. Nat. Educ. Knowl. 2011, 3, 16. [Google Scholar]
- U.S. EPA. Connectivity of Streams and Wetlands to Downstream Waters: A Review and Synthesis of the Scientific Evidence; Final Report; U.S. Environmental Protection Agency: Washington, DC, USA, 2015; EPA/600/R-14/475F.
- Meng, L.; Roulet, N.; Zhuang, Q.; Christensen, T.R.; Frolking, S. Focus on the impact of climate change on wetland ecosystems and carbon dynamics. Environ. Res. Lett. 2016, 11, 100201. [Google Scholar] [CrossRef]
- Walpole, M.; Davidson, N. Stop draining the swamp: It’s time to tackle wetland loss. Oryx 2018, 52, 595–596. [Google Scholar] [CrossRef] [Green Version]
- Bowden, W.D. The biogeochemistry of nitrogen in freshwater wetlands. Biogeochemistry 1987, 4, 313–348. [Google Scholar] [CrossRef]
- Reddy, K.R.; DÁngelo, E.M.; Harris, W.G. Biogeochemistry of wetlands. In Handbook of Soil Science; Sumer, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 89–119. [Google Scholar]
- Rezanezhad, F.; McCarter, C.P.R.; Lennartz, B. Editorial: Wetland Biogeochemistry: Response to Environmental Change. Front. Environ. Sci. 2020, 8, 55. [Google Scholar] [CrossRef]
- Dunne, E.J.; Reddy, K.R. Phosphorus biogeochemistry of wetlands in agricultural watersheds. In Nutrient Management in Agricultural Watersheds: A Wetland Solution; Dunne, E.J., Reddy, R., Carton, O.T., Eds.; Wageningen Academic Publishers: Wageninhem, The Netherlands, 2005; pp. 105–119. [Google Scholar]
- Leberger, R.; Geijzendorffer, I.R.; Gaget, E.; Gwelmami, A.; Galewski, T.; Pereira, H.M.; Guerra, C.A. Mediterranean wetland conservation in the context of climate and land cover change. Reg. Environ. Chang. 2020, 20, 67. [Google Scholar] [CrossRef]
- Mooney, H.A.; Kalin Arroyo, M.T.; Bond, W.J.; Canadell, J.; Hobbs, R.J.; Lavorel, S.; Nelson, R.P. Mediterranean climate ecosystems. In Global Diversity in a Changing Environment; Scenarios for the 21st Century; Chapin, F.S., III, Sala, O.E., Huber-Sannwald, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 157–199. [Google Scholar]
- Álvarez-Cobelas, M.; Rojo, C.; Angeler, D. Mediterranean limnology: Current status, gaps and the future. J. Limnol. 2005, 64, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W. Eutrophication and recovery in expenmental lakes. Science 1974, 184, 897–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, G.D.; Welch, E.B.; Peterson, S.A.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bridgham, S.D.J.; Patrick Megonigal, J.K.; Keller, N.; Bliss, B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916. [Google Scholar]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, A. Carbon storage and fluxes within freshwaters wetlands: A critical review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Bennett, E.M. Reconsideration of the planetary boundary for phosphorus. Environ. Res. Lett. 2011, 6, 014009. [Google Scholar] [CrossRef]
- IPCC. The physical sciences basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M., Canziani, O., Palutkof, J., Van der Linden, P., Hanson, C., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 2005, 17, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Milly, P.C.D.; Dunne, K.A.; Vecchia, A.V. Global pattern of trends in stream flow and water availability in a changing climate. Nature 2006, 438, 347–350. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Phil. Trans. R. Soc. B 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.; Scholes, R.; Ash, N. Millenium Ecosystem Assessment. Ecosystems and Human Well-Being: Current State and Trends; Island Press: Washington, DC, USA, 2005; Volume 1. [Google Scholar]
- Huntington, T.G. Evidence for intensification of the global water cycle: Review and synthesis. J. Hydrol. 2006, 319, 83–95. [Google Scholar] [CrossRef]
- Park, Y.; Cho, K.H.; Kang, J.H.; Lee, S.W.; Kim, J.H. Developing a flow control strategy to reduce nutrient load in a reclaimed multireservoir system using a 2D hydrodynamic and water quality model. Sci. Total Environ. 2014, 466–467, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Sharabian, M.; Ahmad, S.; Karakouzian, M. Climate Change and Eutrophication: A Short Review. Eng. Technol. Appl. Sci. Res. 2018, 8, 3668–3672. [Google Scholar] [CrossRef]
- Meyer, J.L.; Sale, M.J.; Muiholland, P.J.; LeRoy Poff, N. Impacts of climate change on aquatic ecosystems functioning and health. J. Am. Water Resour. Assoc. 1999, 35, 1373–1386. [Google Scholar] [CrossRef]
- Covich, A.P.; Fritz, S.C.; Lamb, P.J.; Marzol, R.D.; Matthews, W.; Poiani, K.A.; Prepas, E.E.; Richman, M.B.; Winter, T.C. Potential effects of climate change on aquatic ecosystems of the Great Plains of North America. Hydrol. Process. 1997, 11, 993–1021. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The Value of the World’s Ecosystem Services and Natural Capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Balmford, A.; Bruner, A.; Cooper, P.; Costanza, R.; Farber, S.; Green, R.E.; Jenkins, M.; Jefferiss, P.; Jessamy, V.; Madden, J.; et al. Economic Reasons for Conserving Wild Nature. Science 2002, 297, 950–953. [Google Scholar] [CrossRef] [Green Version]
- Finlayson, M.; Cruz, R.D.; Davidson, N.; Alder, J.; Cork, S.; de Groot, R.S.; Lévêque, C.; Milton, G.R.; Peterson, G.; Pritchard, D.; et al. Millennium Ecosystem Assessment: Ecosystems and Human Well-Being: Wetlands and Water Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Ramírez, F.; Rodríguez, C.; Seoane, J.; Figuerola, J.; Bustamante, J. How will climate change affect endangered Mediterranean waterbirds? PLoS ONE 2018, 13, e0192702. [Google Scholar] [CrossRef] [Green Version]
- Geijzendorffer, I.R.; Beltrame, C.; Chazee, L.; Gaget, E.; Galewski, T.; Guelmami, A.; Perennou, C.; Popoff, N.; Guerra, C.A.; Leberger, R.; et al. A more effective Ramsar Convention for the conservation of Mediterranean wetlands. Front. Ecol. Evol. 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A. Climate change and interconnected risks to sustainable development in the mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Spanish National Ecosystem Assessment. Ecosystems and Biodiversity for Human Wellbeing. Synthesis of the Key Findings; Biodiversity Foundation of the Spanish Ministry of Agriculture, Food and Environment: Madrid, Spain, 2003.
- Eissa, A.E.; Zaki, M.M. The impact of global climatic changes on the aquatic environment. Procedia Environ. Sci. 2010, 4, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Baron, J.S.; Hall, E.K.; Nolan, B.T.; Finlay, J.C.; Bernhardt, E.S.; Harrison, J.A.; Chan, F.; Boyer, E.W. The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 2013, 114, 71–92. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeLaune, R.D. Biogeochemistry of Wetlands: Science and Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. The value of wetlands: Importance of scale and landscape setting. Ecol. Econ. 2000, 35, 25–33. [Google Scholar] [CrossRef]
- Coops, H.; Beklioglu, M.; Crisman, T.J. The role of water-level fluctuations in shallow lake ecosystems—Workshop conclusions. Hydrobiologia 2003, 506–509, 23–27. [Google Scholar] [CrossRef]
- Rhazi, L.; Grillas, P.; Toure, A.M.; Ham, L.T. Impact of land use in catchment and human activities on water, sediment and vegetation of Mediterranean temporary pools. Comptes Rendus de l’Acade´mie des Sciences Series III Sciences de la Vie 2001, 324, 165–177. [Google Scholar]
- Dodson, S.I.; Lillie, R.A.; Will-Wolf, S. Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecol. Appl. 2005, 15, 1191–1198. [Google Scholar] [CrossRef]
- Parra, G.; Jiménez-Melero, R.; Guerrero, F. Agricultural impacts on Mediterranean wetlands: The effects of pesticides on survival and hatching rates in copepods. Ann. Limnol. Int. J. Limnol. 2005, 41, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Angeler, D.G.; Viedma, O.; Sánchez-Carrillo, S.; Álvarez-Cobelas, M. Conservation issues of temporary wetland Branchiopoda (Anostraca, Notostraca: Crustacea) in a semiarid agricultural landscape: What spatial scales are relevant? Biol. Conserv. 2008, 141, 1224–1234. [Google Scholar] [CrossRef]
- García-Muñoz, E.; Gilbert, J.D.; Parra, G.; Guerrero, F. Wetlands classification for amphibian conservation in Mediterranean landscapes. Biodivers. Conserv. 2010, 19, 901–911. [Google Scholar] [CrossRef]
- Brinson, M.M.; Malvárez, A.I. Temperate freshwater wetlands: Types, status, and threats. Environ. Conserv. 2002, 29, 115–133. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.B.; García-Mora, M.R.; García-Novo, F. Small wetlands lost: A biological conservation hazard in Mediterranean landscapes. Environ. Conserv. 1999, 26, 190–199. [Google Scholar] [CrossRef]
- Zacharias, I.; Dimitriou, E.; Dekker, A.; Dorsman, E. Overview of temporary ponds in the Mediterranean region: Threats, management and conservation issues. J. Environ. Biol. 2007, 28, 1–9. [Google Scholar]
- Casas, J.J.; Toja, J.; Bonachela, S.; Fuentes, F.; Gallego, I.; Juan, D.; León, M.; Peñalver, P.; Pérez, C.; Sánchez, P. Artificial ponds in a Mediterranean region (Andalusia, southern Spain): Agricultural and environmental issues. Water Environ. J. 2011, 25, 308–317. [Google Scholar] [CrossRef]
- Gilbert, J.D.; de Vicente, I.; Ortega, F.; García-Muñoz, E.; Jiménez-Melero, R.; Parra, G.; Guerrero, F. Linking watershed land uses and crustacean assemblages in Mediterranean wetlands. Hydrobiologia 2017, 799, 181–191. [Google Scholar] [CrossRef]
- Gergel, S.E. Spatial and non-spatial factors: When do they affect landscape indicators of watershed loading? Landsc. Ecol. 2005, 20, 177–189. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Comín, F.A. Integrating objectives and scales for planning and implementing wetland restoration and creation in agricultural landscapes. J. Environ. Manag. 2010, 91, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, D.; Mander, U.; Comín, F.A.; Pedrocchi, C.; Uuemaa, E. Relationships between landscape pattern, wetland characteristics, and water quality in agricultural catchments. J. Environ. Qual. 2008, 37, 2170–2180. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Fasullo, J.T. Global warming due to increasing absorbed solar radiation. Geophys. Res. Lett. 2009, 36, L07706. [Google Scholar] [CrossRef] [Green Version]
- Randall, D.A.; Wood, R.A.; Bony, S.; Colman, R.; Fichefet, T.; Fyfe, J.; Kattsov, V.; Pitman, A.; Shukla, J.; Srinivasan, J.; et al. Climate models and their evaluation. In Climate Change 2007: The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller , H.L., Eds.; Cambridge University Press: New York, NY, USA, 2007; pp. 590–662. [Google Scholar]
- Frey, K.; Perovich, D.K.; Light, B. The spatial distribution of solar radiation under a melting Arctic sea ice cover. Geophys. Res. Lett. 2011, 38, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.G.; Bombardelli, F.A.; Schladow, S.G. Modeling linkages between sediment resuspension and water quality in a shallow, eutrophic, wind-exposed lake. Ecol. Model. 2009, 220, 1251–1265. [Google Scholar] [CrossRef]
- Eichelberger, S.C.J.; Nijssen, B.; Wood, A. Climate change effects on wind speed. N. Am. Windpower 2008, 7, 68–72. [Google Scholar]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Moreno-Ostos, E.; Rodrigues da Silva, S.L.; de Vicente, I.; Cruz-Pizarro, L. Inter annual and between-site variability in the occurrence of clear water phases in two shallow Mediterranean lakes. Aquat. Ecol. 2007, 41, 285–297. [Google Scholar] [CrossRef]
- Huber, V.; Wagner, C.D.; Gerten, R.A. To bloom or not to bloom: Contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef] [PubMed]
- de-los-Ríos-Mérida, J.; Reul, A.; Muñoz, M.; Arijo, S.; Tapia-Paniagua, S.; Rendón-Martos, M.; Guerrero, F. How efficient are semi-natural ponds in assimilating wastewater effluents? Application to Fuente de Piedra ramsar, Mediterranean Salt lake (South of Spain). Water 2017, 9, 600. [Google Scholar] [CrossRef] [Green Version]
- de-los-Ríos-Mérida, J.; Guerrero, F.; Arijo, S.; Muñoz, M.; Álvarez-Manzaneda, I.; García-Márquez, J.; Bautista, B.; Rendón-Martos, M.; Reul, A. Wastewater discharge through a stream into a Mediterranean Ramsar wetland: Evaluation and proposal of a nature-based treatment system. Sustainability 2021, 13, 3540. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I.S. Ecological Impacts of Excessive Water Level Fluctuations in Stratified Freshwater Lakes. Inland Waters 2011, 1, 47–59. [Google Scholar] [CrossRef]
- Wetzel, R.G. Land-water interfaces: Metabolic and limnological regulators. Verh. Int. Verein. Limnol. 1990, 24, 6–24. [Google Scholar] [CrossRef]
- Håkanson, L. On the relationship between lake trophic level and lake sediments. Water Res. 1984, 18, 303–314. [Google Scholar] [CrossRef]
- Kalff, J. Limnology, Inland Water Ecosystems; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Luque, J.A.; Juliá, R. Lake sediment response to land-use and climate change during the last 1000 years in the oligotrophic Lake Sanabria (northwest of Iberian Peninsula). Sed. Geol. 2002, 148, 343–355. [Google Scholar] [CrossRef]
- Schmidt, R.; Koinig, K.A.; Thompson, R.; Kamenik, C. A multi proxy core study of the last 7000 years of climate and alpine land-use impacts on an Austrian mountain lake (Unterer Landschitzsee, Niedere Tauern). Paleogeogr. Paleoclimatol. Paleoecol. 2002, 187, 101–120. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppensen, E.; Kristensen, P.; Sortkjǽr, O. Interactions between sediment and water in a shallow and hypertrophic lake: A study on phytoplankton collapses in Lake Søbygaard, Denmark. Hydrobiologia 1990, 191, 139–148. [Google Scholar] [CrossRef]
- Goedkoop, W.; Johnson, R.K. Pelagic-benthic coupling: Profundal benthic community response to spring diatom deposition in mesotrophic Lake Erken. Limnol. Oceanogr. 1996, 41, 636–647. [Google Scholar] [CrossRef]
- Relexans, J.C. Measurement of the respiratory electron system (ETS) activity in marine sediments: State of the art and interpretation. I. Methodology and review of literature data. Mar. Ecol. Prog. Ser. 1996, 136, 277–287. [Google Scholar] [CrossRef] [Green Version]
- de Vicente, I.; Amores, V.; Guerrero, F.; Cruz-Pizarro, L. Contrasting factors controlling microbial respiratory activity in the sediment of two adjacent Mediterranean wetlands. Naturwissenschaften 2010, 97, 627–635. [Google Scholar] [CrossRef]
- de Vicente, I.; Guerrero, F.; Cruz-Pizarro, L. Chemical composition of wetland sediments as an integrator of trophic state. Aquat. Ecosyst. Health Manag. 2010, 13, 99–103. [Google Scholar] [CrossRef]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Kratzer, C.R.; Brezonik, P.L. A Carlson-type trophic state index for nitrogen in Florida lakes. Water Res. Bull. 1981, 17, 713–715. [Google Scholar] [CrossRef]
- Gilbert, J.D.; Guerrero, F.; Jiménez-Melero, R.; de Vicente, I. Is the bioproduction number a good index of the trophic state in Mediterranean wetlands? Knowl. Manag. Aquat. Ecosyst. 2015, 416, 05. [Google Scholar] [CrossRef] [Green Version]
- Boström, B.; Jansson, M.; Forsberg, C. Phosphorus release from lake sediments. Arch. Hydrobiol. Beih. Ergeb. Limnol. 1982, 18, 5–59. [Google Scholar]
- Ryding, S.O. Chemical and microbiological processes as regulators of the exchange of substances between sediments and water in shallow eutrophic lakes. Int. Revue ges. Hydrobiol. 1985, 70, 657–702. [Google Scholar] [CrossRef]
- Boström, B.; Andersen, J.M.; Fleischer, S.; Jansson, M. Exchange of phosphorus across the sediment-water interface. Hydrobiologia 1988, 170, 229–244. [Google Scholar] [CrossRef]
- Marsden, M.W. Lake restoration by reducing external phosphorus loading: The influence of sediment phosphorus release. Fresh. Biol. 1989, 21, 139–162. [Google Scholar] [CrossRef]
- Sas, H. Lake Restoration by Reduction of Nutrient Loading: Expectations, Experiences and Extrapolations; Academia Verlag Richarz: St. Augustin, Germany, 1989. [Google Scholar]
- Ryding, S.O.; Rast, W. El Control de la Eutrofización en Lagos y Pantanos; Pirámide: Madrid, Spain, 1992. [Google Scholar]
- Harper, D. Eutrophication of Freshwaters. Principles, Problems and Restoration; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Istvánovics, V.; Somlyódy, L. Changes in the cycling of phosphorus in the Upper Kis Balaton Reservoir following external load reduction. Fresh. Biol. 1999, 41, 147–165. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 1999, 408–409, 145–152. [Google Scholar] [CrossRef]
- Schauser, I.; Lewandowski, J.; Hupfer, M. Decision support for the selection of an appropriate in-lake measure to influence the phosphorus retention in sediments. Water Res. 2003, 37, 801–812. [Google Scholar] [CrossRef]
- Golterman, H.L. The Chemistry of Phosphate and Nitrogen Compounds in Sediments; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2004. [Google Scholar]
- Lürling, M.; van Oosterhout, F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef]
- Hupfer, M.; Hilt, S. Lake restoration. In Ecological Engineering, Encyclopedia of Ecology; Jørgensen, S.E., Fath, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Meis, S.; Spears, B.M.; Maberly, S.C.; O’Malley, M.B.; Perkins, R.G. Sediment amendment with Phoslock® in Clatto Reservoir (Dundee, UK): Investigating changes in sediment elemental composition and phosphorus fractionation. J. Environ. Manag. 2012, 93, 185–193. [Google Scholar] [CrossRef]
- Yamada, T.M.; Sueitt, A.P.; Beraldo, D.A.; Botta, C.M.; Fadini, P.S.; Nascimento, M.R.; Faria, B.M.; Mozeto, A.A. Calcium nitrate addition to control the internal load of phosphorus from sediments of a tropical eutrophic reservoir: Microcosm experiments. Water Res. 2012, 46, 6463–6475. [Google Scholar] [CrossRef] [PubMed]
- Spears, B.M.; Meis, S.; Anderson, A.; Kellou, M. Comparison of phosphorus (P) removal properties of materials proposed for the control of sediment p release in UK lakes. Sci. Total Environ. 2013, 442, 103–110. [Google Scholar] [CrossRef]
- Funes, A.; de Vicente, J.; Cruz-Pizarro, L.; Alvarez-Manzaneda, I.; de Vicente, I. Magnetic microparticles as a new tool for lake restoration: A microcosm experiment for evaluating the impact on phosphorus fluxes and sedimentary phosphorus pools. Water Res. 2016, 89, 366–374. [Google Scholar] [CrossRef]
- Jensen, H.S.; Andersen, F.Ø. Importance of temperature, nitrate and pH for phosphorus release from aerobic sediments of four shallow eutrophic lakes. Limnol. Oceanogr. 1992, 37, 577–589. [Google Scholar] [CrossRef]
- Søndergaard, M.; Kristensen, P.; Jeppesesen, E. Phosphorus release from resuspended sediment in the shallow and windexposed Lake Arresø, Denmark. Hydrobiologia 1992, 228, 91–99. [Google Scholar] [CrossRef]
- Rydin, E.; Welch, E.B. Dosing Alum to Wisconsin lake sediments based on in vitro formation of aluminum bound phosphate. Lake Reserv. Manag. 1999, 15, 324–331. [Google Scholar] [CrossRef]
- Egemose, S.; Wauer, G.; Kleeberg, A. Resuspension behavior of aluminum treated lake sediments-effects of ageing and pH. Hydrobiologia 2009, 636, 203–217. [Google Scholar] [CrossRef]
- Marsh, P.; Lesack, L.F.W. The hydrologic regime of perched lakes in the Mackenzie Delta: Potential responses to climate change. Limnol. Oceanogr. 1996, 41, 849–856. [Google Scholar] [CrossRef]
- Batzer, D.; Sharitz, R. Ecology of Freshwater and Estuarine Wetlands; University of California Press: Berkeley, CA, USA, 2006. [Google Scholar]
- de Vicente, I.; Andersen, F.Ø.; Hansen, H.C.B.; Cruz-Pizarro, L.; Jensen, H.S. Water level fluctuations may decrease phosphate adsorption capacity of the sediment in oligotrophic high mountain lakes. Hydrobiologia 2010, 651, 253–264. [Google Scholar] [CrossRef]
- De Groot, C.J.; VanWijck, C. The impact of desiccation of a freshwatermarsh (Garcines Nord, Camarge, France) on sediment–water–vegetation interactions. Part 1: The sediment chemistry. Hydrobiologia 1993, 252, 83–94. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Mitchell, A.M.; Rees, G.N. The effects of in situ drying on sediment-phosphate interactions in sediments from an old wetland. Hydrobiologia 2000, 431, 3–12. [Google Scholar] [CrossRef]
- Gilbert, J.D.; Guerrero, F.; de Vicente, I. Sediment desiccation as a driver of phosphate availability in the water column of Mediterranean wetlands. Sci. Total Environ. 2014, 466–467, 965–975. [Google Scholar] [CrossRef]
- Attygalla, N.W.; Baldwin, D.S.; Silvester, E.; Kappen, P.; Whitworth, K.L. The severity of sediment desiccation affects the adsorption characteristics and speciation of phosphorus. Environ. Sci. Process. Impacts 2016, 18, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.; Shaw, T.C. Effect of drying soil on the measurement of phosphate adsorption. Commun. Soil Sci. Plant Anal. 1980, 11, 347–353. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S. Effects of air-drying on the adsorption and desorption of phosphate and levels of extractable phosphate in a group of New Zealand acid soils. Geoderma 1985, 35, 145–157. [Google Scholar] [CrossRef]
- De Groot, C.J.; Fabre, A. The impact of desiccation of a freshwater marsh (Garcines Nord, Camargue, France) on the sediment–water–vegetation interactions. Part 3: The fractional composition and the phosphate adsorption characteristics of the sediment. Hydrobiologia 1993, 252, 105–116. [Google Scholar] [CrossRef]
- Baldwin, D.S. Effects of exposure to air and subsequent drying on the phosphate sorption characteristics of sediments from a eutrophic reservoir. Limnol. Oceanogr. 1996, 41, 1725–1732. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Mitchell, A.M. The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river-floodplain systems: A synthesis. Regul. Rivers Res. Manag. 2000, 16, 457–467. [Google Scholar] [CrossRef]
- Einsele, W. Über die Beziehungen des Eisenkreislaufs zum Phosphatkreislauf imm eutrophen See. Arch. Hydrobiol. 1936, 29, 664–686. [Google Scholar]
- Mortimer, C.H. The exchange of dissolved substances between mud and water in lakes. I. J. Ecol. 1941, 30, 280–329. [Google Scholar] [CrossRef]
- Gächter, R.; Müller, B. Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface? Limnol. Oceanogr. 2003, 48, 929–933. [Google Scholar] [CrossRef]
- Rothe, M.; Frederichs, T.; Eder, M.; Kleeberg, A.; Hupfer, M. Evidence for vivianite formation and its contribution to long-term phosphorus retention in a recent lake sediment: A novel analytical approach. Biogeosciences 2014, 11, 5169–5180. [Google Scholar] [CrossRef] [Green Version]
- Rothe, M.; Kleeberg, A.; Grüneberg, B.; Friese, K.; Pérez-Mayo, M.; Hupfer, M. Sedimentary sulphur:iron ratio indicates vivianite occurrence: A study from two contrasting freshwater systems. PLoS ONE 2015, 10, e0143737. [Google Scholar] [CrossRef]
- Lijklema, L. Interaction of orthophosphate with iron (III) and aluminium hydroxides. Environ. Sci. Technol. 1980, 14, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Twinch, A.J. Phosphate exchange characteristics of wet and dried sediment samples from a hypertrophic reservoir: Implications for the measurements of sediment phosphorus status. Water Res. 1987, 21, 1225–1230. [Google Scholar] [CrossRef]
- Qiu, S.; McComb, A.J. Interrelations between iron extractability and phosphate sorption in reflooded airdried sediments. Hydrobiologia 2002, 472, 39–44. [Google Scholar] [CrossRef]
- Selig, U. Particle size-related phosphate binding and P-release at the sediment–water interface in a shallow German lake. Hydrobiologia 2003, 492, 107–118. [Google Scholar] [CrossRef]
- Darke, A.K.; Walbridge, M.R. Al and Fe biogeochemistry in a floodplain forest: Implications for P retention. Biogeochemistry 2000, 51, 1–32. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson-Penitence Hall: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bruland, G.L.; Richardson, C.J. An assessment of the phosphorus retention capacity of wetlands in the Painter Creek Watershed, Minnesota, USA. Water Air Soil Pollut. 2006, 171, 169–184. [Google Scholar] [CrossRef]
- Novak, J.M.; Watts, D.W. Phosphorus sorption by sediments in southeastern Coastal Plain in-stream wetland. J. Environ. Qual. 2006, 35, 1975–1982. [Google Scholar] [CrossRef] [Green Version]
- Watts, C.J. The effect of organic matter on sedimentary phosphorus release in an Australian reservoir. Hydrobiologia 2000, 43, 13–25. [Google Scholar] [CrossRef]
- Sparling, G.P.; Whale, K.N.; Ramsay, A.J. Quantifying the contribution from the soil microbail biomass to the extractable P levels of fresh and air-dried soils. Aust. J. Soil. Res. 1985, 23, 613–621. [Google Scholar] [CrossRef]
- Qiu, S.; McComb, A.J. Effects of oxygen concentration on phosphorus release from reflooded air-dried wetland sediments. Aust. J. Mar. Freshw. Res. 1994, 45, 1319–1328. [Google Scholar] [CrossRef]
- Qiu, S.; McComb, A.J. Planktonic and microbial contributions to phosphorus release from fresh and air-dried sediments. Mar. Freshw. Res. 1995, 46, 1039–1045. [Google Scholar] [CrossRef]
- Schönbrunner, I.M.; Preiner, S.; Hein, T. Impact of drying and re-flooding of sediment on phosphorus dynamics of river–floodplain systems. Sci. Total Environ. 2012, 432, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Fabre, A. Experimental studies on some factors influencing phosphorus solubilization in connection with the drawdown of a reservoir. Hydrobiologia 1988, 159, 153–158. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Baldwin, D.S. Effects of desiccation/oxidation on the potential for bacterially mediated P release from sediments. Limnol. Oceanogr. 1998, 43, 481–487. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphorus solubilization in rewetted soils. Nature 2001, 411, 258. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- WCD. Dams and Development: A New Framework for Decision-Making: The Report of the World Commission on Dams; Earthscan Publications Ltd.: London, UK, 2000. [Google Scholar]
- León-Palmero, E. Greenhouse Gases in Reservoirs: From Watershed to Functional Genes. Ph.D. Thesis, University of Granada, Granada, Spain, 2021. [Google Scholar]
- Butler, J.H.; Montzka, S.A. The NOAA Annual Greenhouse Gas Index (AGGI); NOAA Earth System Research Laboratories (ESRL): Boulder, CO, USA, 2019.
- Lashof, D.A.; Ahuja, D.R. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.; Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 2011, 331, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deemer, B.R.; Harrison, J.A.; Li, S.; Beaulieu, J.J.; DelSontro, T.; Barros, N.; Bezerra-Neto, J.F.; Powers, S.M.; dos Santos, M.A.; Vonk, J.A. Greenhouse gas emissions from reservoir water surfaces: A new global synthesis. BioScience 2016, 66, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.H.; Casson, N.J.; Christel, S.T.; Crawford, J.T.; Loken, L.C.; Oliver, S.K. The ecology of methane in streams and rivers: Patterns, controls, and global significance. Ecol. Monogr. 2016, 86, 146–171. [Google Scholar] [CrossRef]
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.G.; Dlugokencky, E.J.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 2016, 8, 697–751. [Google Scholar] [CrossRef] [Green Version]
- Segers, R. Methane production and methane consumption: A review of processes underlying wetland methane fluxes. Biogeochemistry 1998, 41, 23–51. [Google Scholar] [CrossRef]
- Oswald, K.; Milucka, J.; Brand, A.; Littmann, S.; Wehrli, B.; Kuypers, M.M.M.; Schubert, C.J. Light-dependent aerobic methane oxidation reduces methane emissions from seasonally stratified lakes. PLoS ONE 2015, 10, e0132574. [Google Scholar] [CrossRef]
- Oswald, K.; Jegge, C.; Tischer, J.; Berg, J.; Brand, A.; Miracle, M.R.; Soria, X.; Vicente, E.; Lehmann, M.F.; Zopfi, J.; et al. Methanotrophy under versatile conditions in the water column of the ferruginous meromictic lake La Cruz (Spain). Front. Microbiol. 2016, 7, 1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.W.; McGinnis, D.F.; Ionescu, D.; Grossart, H.P. Methane production in oxic lake waters potentially increases aquatic methane flux to air. Environ. Sci. Technol. Lett. 2016, 3, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Schubert, C.J.; Wehrli, B. Contribution of methane formation and methane oxidation to methane emission from freshwater systems. In Biogenesis of Hydrocarbons; Stams, A.J.M., Sousa, D., Eds.; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Thalasso, F.; Sepulveda-Jauregui, A.; Gandois, L.; Martinez-Cruz, K.; Gerardo-Nieto, O.; Astorga-Espana, M.S.; Teisserenc, R.; Lavergne, C.; Tananaev, N.; Barret, M.; et al. Sub-oxycline methane oxidation can fully uptake CH4 produced in sediments: Case study of a lake in Siberia. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Pina-Ochoa, E.; Alvarez-Cobelas, M. Denitrification in aquatic environments: A cross-system analysis. Biogeochemistry 2006, 81, 111–130. [Google Scholar] [CrossRef]
- Moseman-Valtierra, S.; Gonzalez, R.; Kroeger, K.D.; Tang, J.; Chao, W.C.; Crusius, J.; Bratton, J.; Green, A.; Shelton, J. Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O. Atmos. Environ. 2011, 45, 4390–4397. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Kroeze, C. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Glob. Biogeochem. Cycles 1998, 12, 93–113. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.; Laanbroek, H.; Oenema, O. Nitrous oxide production in grassland soils: Assessing the contribution of nitrifier denitrification. Soil Biol. Biochem. 2004, 36, 229–236. [Google Scholar] [CrossRef]
- Davidson, E.A. Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Sci. Soc. Am. J. 1992, 56, 95–102. [Google Scholar] [CrossRef]
- Xu, C.; Wong, V.N.L.; Reef, R.E. Effect of inundation on greenhouse gas emissions from temperate coastal wetland soils with different vegetation types in southern Australia. Sci.Total Environ. 2021, 763, 142949. [Google Scholar] [CrossRef]
- Heil, J.; Wolf, B.; Bruggemann, N.; Emmenegger, L.; Tuzson, B.; Vereecken, H.; Mohn, J. Site-specific 15N isotopic signatures of abiotically produced N2O. Geochim. Cosmochim. Acta 2014, 139, 72–82. [Google Scholar] [CrossRef]
- Zhu-Barker, X.; Cavazos, A.R.; Ostrom, N.E.; Horwath, W.R.; Glass, J.B. The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 2015, 126, 251–267. [Google Scholar] [CrossRef]
- Soler-Jofra, A.; Stevens, B.; Hoekstra, M.; Picioreanu, C.; Sorokin, D.; van Loosdrecht, M.C.; Perez, J. Importance of abiotic hydroxylamine conversion on nitrous oxide emissions during nitritation of reject water. Chem. Eng. J. 2016, 287, 720–726. [Google Scholar] [CrossRef]
- Liu, S.; Han, P.; Hink, L.; Prosser, J.I.; Wagner, M.; Bruggemann, N. Abiotic conversion of extracellular NH2OH contributes to N2O emission during ammonia oxidation. Environ. Sci. Technol. 2017, 51, 13122–13132. [Google Scholar] [CrossRef] [Green Version]
- Wankel, S.D.; Ziebis, W.; Buchwald, C.; Charoenpong, C.; Beer, D.; de Dentinger, J.; Xu, Z.; Zengler, K. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediment. Nat. Commun. 2017, 8, 15595. [Google Scholar] [CrossRef]
- Bastviken, D.; Cole, J.J.; Pace, M.L.; Tranvik, L.J. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambrigde, UK, 2013; pp. 659–708. [Google Scholar]
- Koffi, N.; Bergamashi, P.; Alkama, R.; Cescatti, A. An observation-constrained assessment of the climate sensitivity and future trajectories of wetlands methane emissions. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melton, J.R. Present state of global wetland extent and wetland methane modelling: Conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 2013, 10, 753–788. [Google Scholar] [CrossRef] [Green Version]
- Whiting, G.J.; Chanton, J.P. Primary production control of methane emission from wetlands. Nature 1993, 364, 794–795. [Google Scholar] [CrossRef]
- Fennessy, M.S.; Wardrop, D.H.; Moon, J.B.; Wilson, S.; Craft, C. Soil carbon sequestration in freshwater wetlands varies across a gradient of ecological condition and by ecoregion. Ecol. Eng. 2017, 114, 129–136. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef]
- Scholz, O.; Gawne, B.E.N.; Ebner, B.; Ellis, I. The effects of drying and re-flooding on nutrient availability in ephemeral deflation basin lakes in western New South Wales, Australia. River Res. Appl. 2002, 18, 185–196. [Google Scholar] [CrossRef]
- Fromin, N.; Pinay, G.; Montuelle, B.; Landais, D.; Ourcival, J.M.; Joffre, R.; Lensi, R. Impact of seasonal sediment desiccation and rewetting on microbial processes involved in greenhouse gas emissions. Ecohydrology 2010, 3, 339–348. [Google Scholar] [CrossRef]
- Miller Miller, A.E.; Schimel, J.P.; Meixne, T.; Sickman, J.O.; Melack, J.M. Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol. Biochem. 2005, 37, 2195–2204. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Baldwin, D.S. The effects of sediment desiccation on the potential for nitrification, denitrification and methanogenesis in an Australian reservoir. Hydrobiologia 1999, 392, 3–11. [Google Scholar] [CrossRef]
- Shao, X.; Sheng, X.; Wu, M.; Wu, H.; Ning, X. Methane production potential and emission at different water levels in the restored reed wetland of Hangzhou Bay. PLoS ONE 2017. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Han, G.; Li, J.; Song, W.; Qu, W.; Eller, F.; Wang, J.; Jiang, C. Responses of soil CO2 and CH4 emissions to changing water table level in a coastal wetland. J. Clean. Prod. 2020, 269, 122316. [Google Scholar] [CrossRef]
- León-Palmero, E.; Contreras-Ruiz, A.; Sierra, A.; Morales-Baquero, R.; Reche, I. Dissolved CH4 coupled to photosynthetic picoeukaryotes in oxic waters and to cumulative chlorophyll a in anoxic waters of reservoirs. Biogeosciences 2020, 17, 3223–3245. [Google Scholar] [CrossRef]
- León-Palmero, E.; Morales-Baquero, R.; Reche, I. Greenhouse gas fluxes from reservoirs determined by watershed lithology, morphometry, and anthropogenic pressure. Environ. Res. Lett. 2020, 15, 044012. [Google Scholar] [CrossRef]
- Maeck, A.; Hofmann, H.; Lorke, A. Pumping methane out of aquatic sediments: Ebullition forcing mechanisms in an impounded river. Biogeosciences 2014, 11, 2925–2938. [Google Scholar] [CrossRef] [Green Version]
- Kiene, R.P. Production and consumption of methane in aquatic systems. In Microbial Production and of Greenhouse Gases: Methane, Nitrogen Oxides, and Halomethanes; Rogers, J.E., Whitman, W.B., Eds.; American Society for Microbiology: Washington, DC, USA, 1991; pp. 111–146. [Google Scholar]
- Thornton, K.W.; Kimm, B.L.; Payne, F.E. Reservoir Limnology: Ecological Perspectives; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Franzluebbers, K.; Weaver, R.W.; Juo, A.S.R.; Franzluebbers, A.J. Carbon and nitrogen mineralization from cowpea plants part decomposing in moist and in repeatedly dried and wetted soil. Soil Biol. Biochem. 1994, 26, 1379–1387. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vicente, I. Biogeochemistry of Mediterranean Wetlands: A Review about the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions. Water 2021, 13, 1510. https://doi.org/10.3390/w13111510
de Vicente I. Biogeochemistry of Mediterranean Wetlands: A Review about the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions. Water. 2021; 13(11):1510. https://doi.org/10.3390/w13111510
Chicago/Turabian Stylede Vicente, Inmaculada. 2021. "Biogeochemistry of Mediterranean Wetlands: A Review about the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions" Water 13, no. 11: 1510. https://doi.org/10.3390/w13111510
APA Stylede Vicente, I. (2021). Biogeochemistry of Mediterranean Wetlands: A Review about the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions. Water, 13(11), 1510. https://doi.org/10.3390/w13111510






