Real-Time Estimation of Disinfection By-Products through Differential UV Absorbance
Abstract
:1. Introduction
2. Materials and Methods
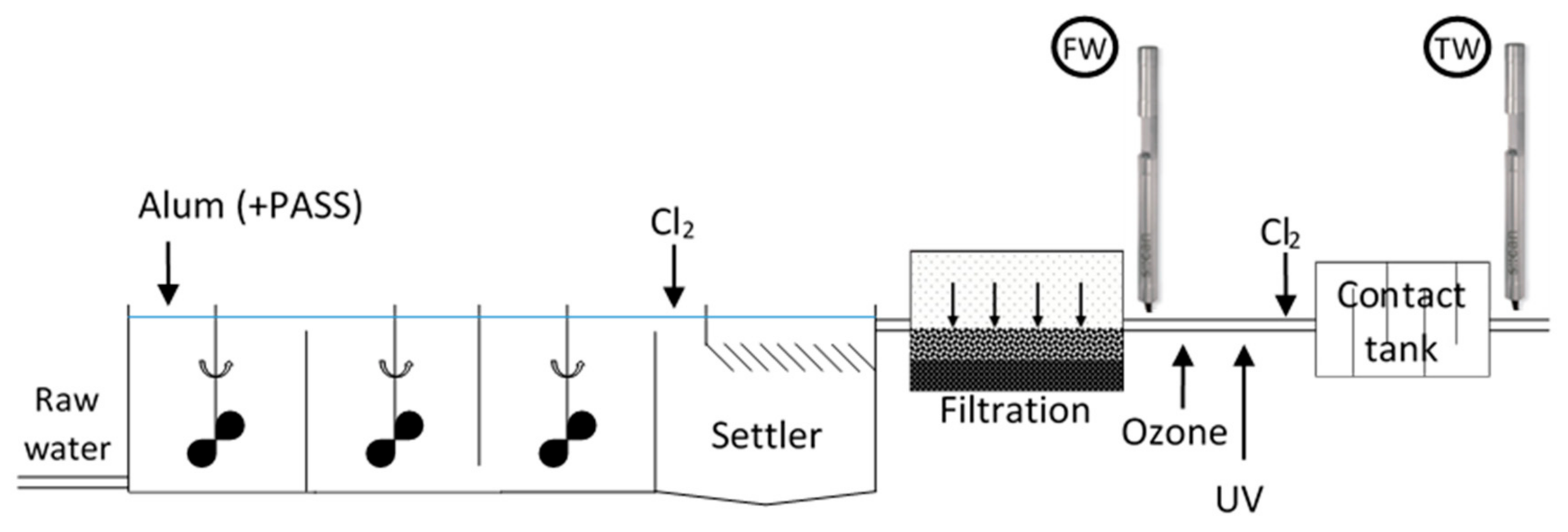
2.1. Experimental Approach
2.2. Field
2.2.1. Study Area
2.2.2. Field Instrumentation
2.2.3. Sampling Campaign
2.3. Laboratory
2.3.1. Laboratory Reference Spectrophotometer
2.3.2. Water Chlorination
2.3.3. Water Quality Analysis
3. Results
3.1. Water Quality Characteristics
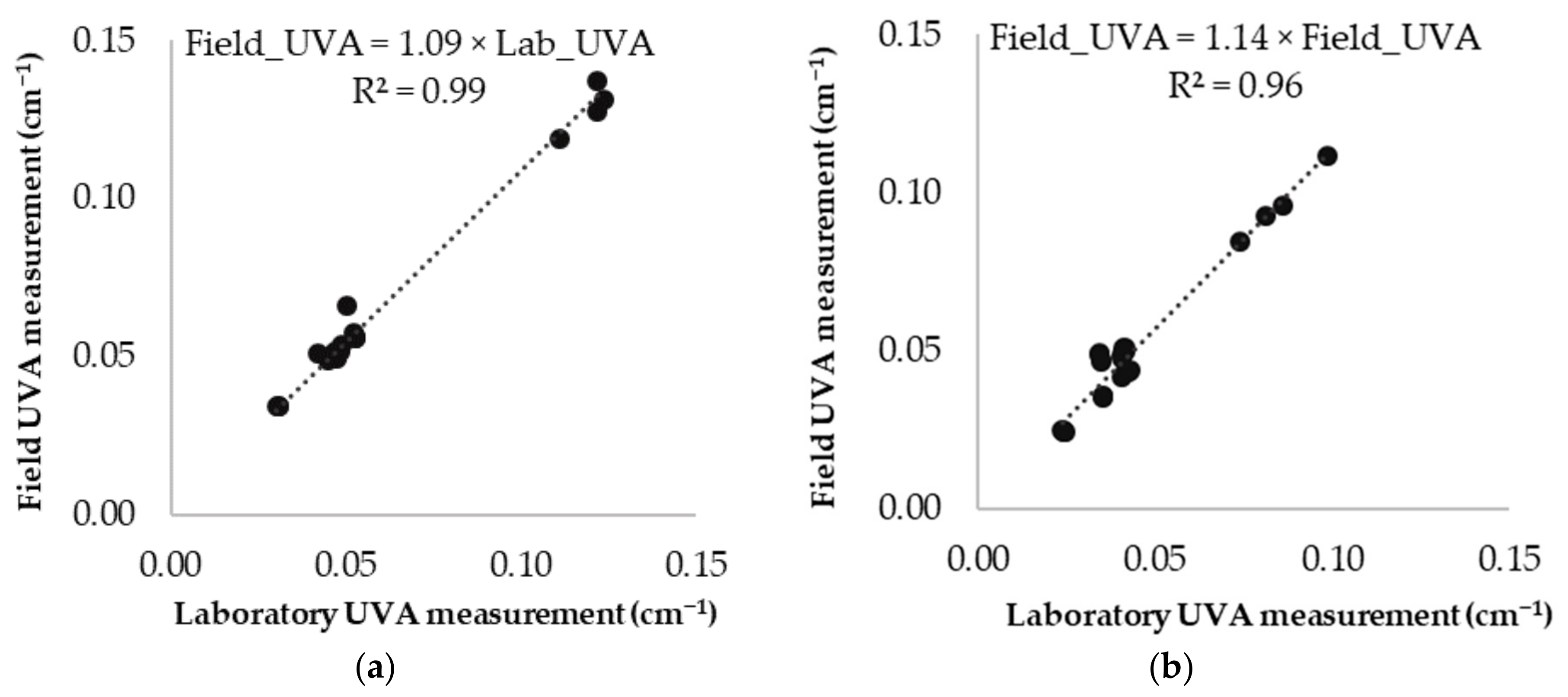
3.2. Validation of UVA Measurements between the Field and the Laboratory Spectrophotometers
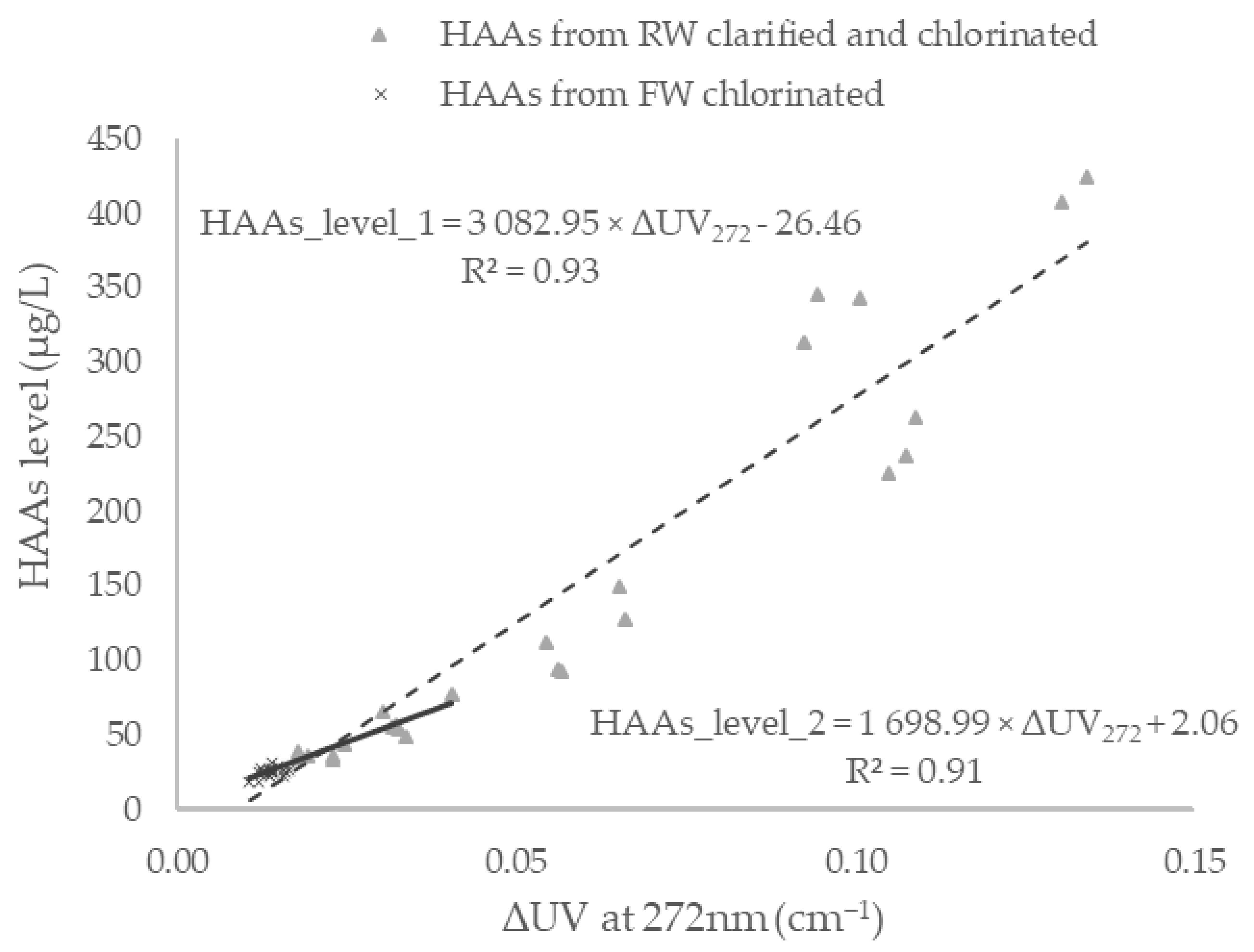
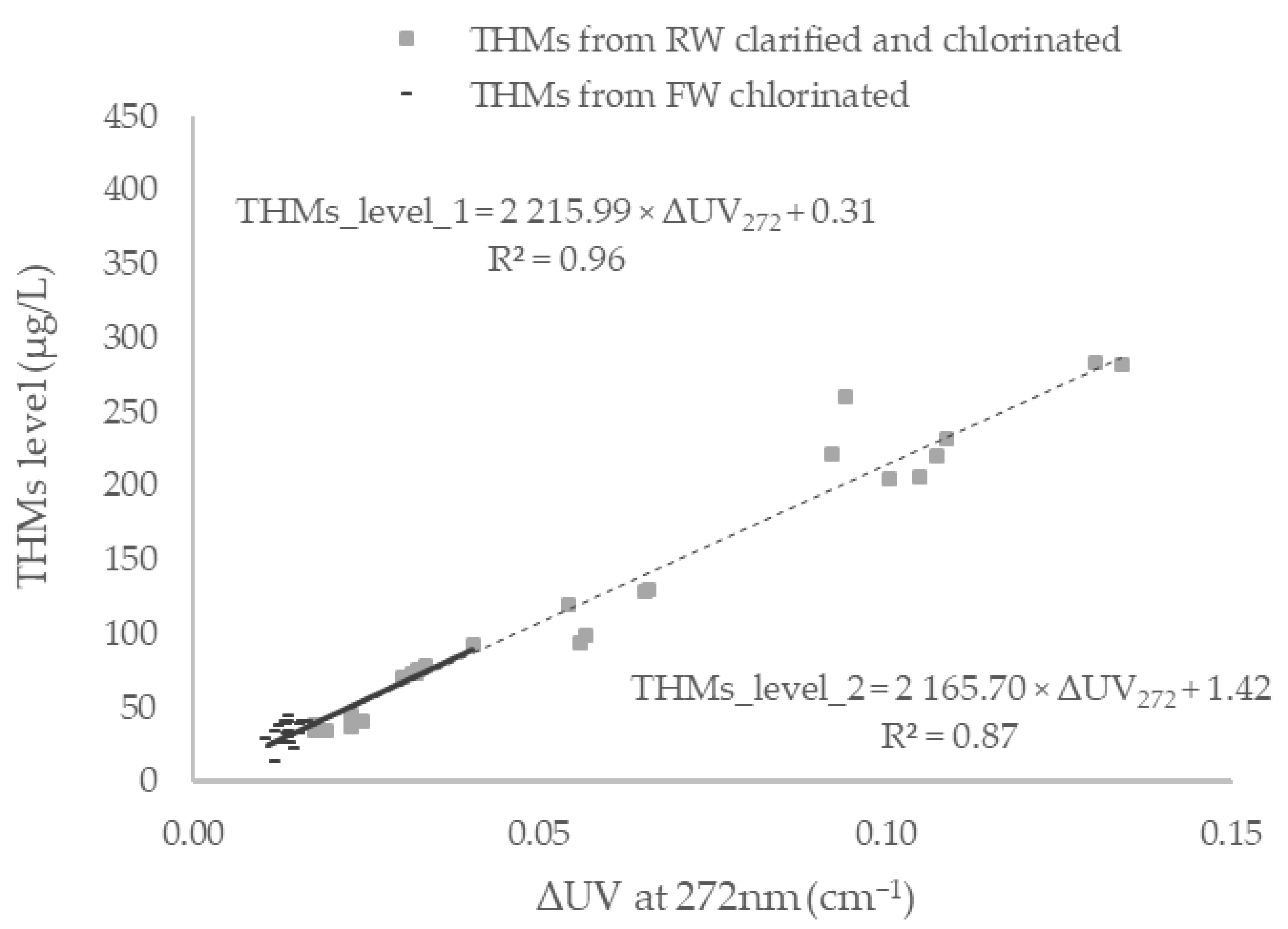
3.3. Lab-Based Estimations of DBPs
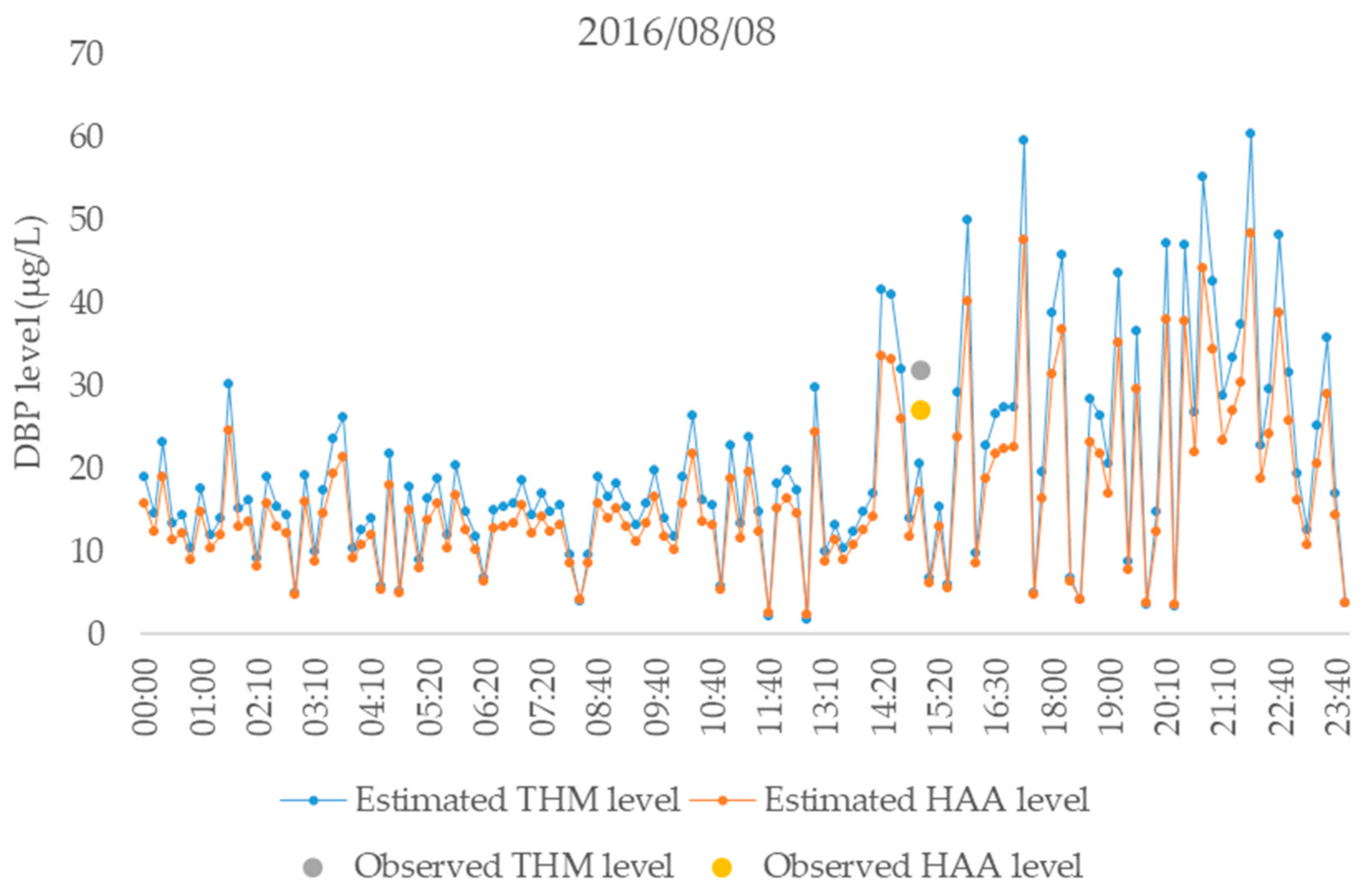
3.4. Determination of a Site-Specific Linear Calibration Curve for in Situ Real-Time Estimations of DBPs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rook, J.J. Formation of haloforms during the chlorination of natural water. Water Treat. Exam. 1974, 23, 234–243. [Google Scholar]
- Richardson, S.D. Disinfection By-Products: Formation and Occurrence in Drinking Water. Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier Science Inc.: Burlington, MA, USA, 2011. [Google Scholar]
- Yang, M.; Zhang, X. Current trends in the analysis and identification of emerging disinfection byproducts. Trends Environ. Anal. Chem. 2016, 10, 24–34. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Villanueva, C.; Cantor, K.P.; Grimalt, J.O.; Malats, N.; Silverman, D.; Tardon, A.; Garcia-Closas, R.; Serra, C.; Carrato, A.; Castaño-Vinyals, G.; et al. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering and swimming in pools. Am. J. Epidemiol. 2007, 165, 148–156. [Google Scholar] [CrossRef]
- Stalter, D.; O’Malley, E.; Von Gunten, U.; Escher, B.I. Fingerprinting the reactive toxicity pathways of 50 drinking water disinfection by-products. Water Res. 2016, 91, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Mouly, D.; Joulin, E.; Rosin, C.; Beaudeau, P.; Zeghnoun, A.; Olszewski-Ortar, A.; Munoz, J.-F.; Welté, B.; Joyeux, M.; Seux, R.; et al. Variations in trihalomethane levels in three French water distribution systems and the development of a predictive model. Water Res. 2010, 44, 5168–5179. [Google Scholar] [CrossRef]
- Singer, P.C. Occurrence of haloacetic acids in chlorinated drinking water. Water Sci. Technol. Water Supply 2002, 2, 487–492. [Google Scholar] [CrossRef]
- Guilherme, S.; Rodriguez, M.J. Occurrence of regulated and non-regulated disinfection by-products in small drinking water systems. Chemosphere 2014, 117, 425–432. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency-USEPA. Complying with the Stage 2 Disinfectant and Disinfection Byproducts Rule: Small Entity Compliance Guide; Office of Groundwater and Drinking Water: Washington, DC, USA, 2007.
- Health Canada. Guidelines for Canadian Drinking Water Quality. In Guideline Technical Document—Trihalomethanes; Health Canada: Ottawa, ON, Canada, 2006. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality. In Guideline Technical Document–Haloacetic Acids; Health Canada: Ottawa, ON, Canada, 2008. [Google Scholar]
- Ministère du Développement Durable, de l’Environnement et de la Lutte contre les Changements Climatiques—MDDELCC. Règlement sur la Qualité de l’eau Potable; Éditeur Officiel du Québec: Québec, QC, Canada, 2012. Available online: http://legisquebec.gouv.qc.ca/fr/ShowDoc/cr/Q-2,%20r.%2040 (accessed on 10 September 2020).
- Ontario Regulation 169/03, 2017. Ontario Drinking Water Quality Standards, Safe Drinking Water Act, 2002, S.O. 2002, c. 32. 2017. Available online: https://www.ontario.ca/laws/statute/s02032 (accessed on 10 September 2020).
- The Standard Methods Organization. Method 5910 UV-Absorbing Organic Constituents. In Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Pifer, A.D.; Fairey, J.L. Suitability of Organic Matter Surrogates to Predict Trihalomethane Formation in Drinking. Water Environ. Eng. Sci. 2014, 31, 117–126. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Molognoni, D. Online monitoring of priority and dangerous pollutants in natural and urban waters: A state-of-the-art review. Manag. Environ. Qual. 2016, 27, 507–536. [Google Scholar] [CrossRef]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. Use of UV spectroscopy to study chlorination of natural organic matter. (ACS Symposium Series). Water Disinfect. Nat. Org. Matter. 1996, 649, 182–195. [Google Scholar]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. The decrease of UV absorbance as an indicator of TOX formation. Water Res. 1997, 31, 946–994. [Google Scholar] [CrossRef]
- Korshin, G.V.; Wu, W.W.; Benjamin, M.M.; Hemingway, O. Correlations between differential absorbance and the formation of individual DBPs. Water Res. 2002, 36, 3273–3282. [Google Scholar] [CrossRef]
- Li, C.; Benjamin, M.M.; Korshin, G.V. The relationship between TOX formation and spectral changes accompanying chlorination of pre-concentrated or fractionated NOM. Water Res. 2002, 36, 3265–3272. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G.A. Differential vs. absolute UV absorbance approaches in studying NOM reactivity in DBPs formation: comparison and applicability. Water Res. 2009, 43, 744–750. [Google Scholar] [CrossRef]
- Beauchamp, N.; Laflamme, O.; Simard, S.; Dorea, C.; Pelletier, G.; Bouchard, C.; Rodriguez, M.J. Relationships between DBP concentrations and differential UV absorbance in full-scale conditions. Water Res. 2018, 131, 110–121. [Google Scholar] [CrossRef]
- Özdemir, K.; Toröz, İ.; Uyak, V. Assessment of Trihalomethane Formation in Chlorinated Raw Waters with Differential UV Spectroscopy Approach. Sci. World J. 2013, 890854. [Google Scholar] [CrossRef]
- Li, C.W.; Korshin, G.V.; Benjamin, M.M. Monitoring DBP formation with differential UV spectroscopy: a new application uses differential UV spectroscopy to monitor DBP formation easily, rapidly, and inexpensively. J. Am. Water Works Assn. 1998, 90, 88–100. [Google Scholar] [CrossRef]
- Chow, A.T.; Dahlgren, R.A.; Zhang, Q.; Wong, P.K. Relationships between specific UVA and THMs precursors of different carbon sources. J. Water Supply Res. Technol. AQUA 2008, 57, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, N.; Dorea, C.C.; Bouchard, C.; Rodriguez, M. Use of differential UV absorbance to estimate concentrations of chlorinated disinfection by-product in drinking water: Critical review and research needs. Crit. Rev. Env. Sci. Technol. 2018, 48, 210–241. [Google Scholar] [CrossRef]
- Mercier, N.; Bouchard, C.; Dorea, C.C. Online estimation of disinfection by products through differential UV spectrophotometry: Potential & challenges. In Proceedings of the 30th Eastern Canadian Symposium on Water Quality Research, Ottawa, ON, Canada, 27 May 2016. [Google Scholar]
- Légaré-Julien, F.; Bouchard, C.; Dorea, C.C. Application of Differential UV Spectrophotometry for the Estimation of Disinfection By-products. In Proceedings of the AWWA Water Quality & Technology Conference, Indianapolis, IN, USA, 13–17 November 2016. [Google Scholar]
- Korshin, G.V.; Chang, H.-S. Spectroscopic Studies of the Roles of Distinct Chromophores in NOM Chlorination and DBP Formation. In Disinfection By-Products in Drinking Water. J. Am. Chem. Soc. 2008, 995, 158–171. [Google Scholar]
- Uyak, V.; Demirbas, K.D. Formation of Disinfection Byproducts (DBPs) in Surface Water Sources: Differential Ultraviolet (UV) Absorbance Approach. Environ. Forensics. 2014, 15, 52–65. [Google Scholar] [CrossRef]
- Available online: http://www.s-can.at/ (accessed on 21 May 2020).
- Edzwald, J.K.; Becker, W.C.; Wattier, K.L. Surrogate parameters for monitoring organic matter and THM precursors. J. Am. Water Works Assn. 1985, 77, 122–132. [Google Scholar] [CrossRef]
- Roccaro, P.; Chang, H.-S.; Vagliasindi, F.G.A.; Korshin, G.V. Differential absorbance study of effects of temperature on chlorine consumption and formation of disinfection by-products in chlorinated water. Water Res. 2008, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, P.; Vagliasindi, F.G.A.; Korshin, G.V. Comparison of the performance of spectroscopic indices developed to quantify the halogenation of natural organic matter at varying chlorine concentrations, reaction times and temperatures. In: Disinfection By-Products in Drinking Water. J. Am. Chem. Soc. 2008, 995, 198–212. [Google Scholar]
- Yan, M.; Korshin, G.V.; Chang, H.-S. Examination of disinfection by-product (DBP) formation in source waters: A study using log-transformed differential spectra. Water Res. 2014, 50, 179–188. [Google Scholar] [CrossRef]
- Guilherme, S.; Rodriguez, M.J. Short-term spatial and temporal variability of disinfection by-product occurrence in small drinking water systems. Sci. Total Environ. 2015, 518–519, 280–289. [Google Scholar] [CrossRef]
- Mercier Shanks, C.; Sérodes, J.-B.; Rodriguez, M.J. Spatio-temporal variability of non-regulated disinfection by-products within a drinking water distribution network. Water Res. 2013, 47, 3231–3243. [Google Scholar] [CrossRef]
- Beauchamp, N.; Dorea, C.C.; Bouchard, C.; Rodriguez, M. Multi-wavelength models expand the validity of DBP-differential absorbance relationships in drinking water. Water Res. 2019, 158, 61–71. [Google Scholar] [CrossRef]

| Parameter | Alum Dose (mg/L) | Cl × UVA = Cl2 (mg/L) × UVA254 (cm−1) × 10 | pH | Contact Time (h) |
|---|---|---|---|---|
| Values | 20/40 | 0.25/0.5/1.0/1.5/2.0/3.0/4.0/6.0 | 6.5/7.0/7.5 | 2 |
| Parameter | Cl × UVA = Cl2 (mg/L) × UVA254 (cm−1) × 10 | pH | Contact Time (h) |
|---|---|---|---|
| Values | 1.0/1.5/2.0/3.0/4.0 | 6.5/7.0/7.5 | 2 |
| Raw Water (RW) | Filtered Water (FW) | Treated Water (TW) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD * | Range | Mean ± SD * | Range | Mean ± SD * | Range | |
| pH | 7.6 ± 0.4 | 6.8–9.0 | 6.6 ± 0.4 | 4.8–7.2 | 6.6 ± 0.4 | 4.9–7.1 |
| Turbidity (NTU) | 9.2 ± 13.5 | 1.4–64.7 | 0.24 ± 0.19 | 0.07–0.73 | 0.19 ± 0.20 | 0.07–1.10 |
| DOC (mg/L) | 9.2 ± 3.6 | 5.3–19.6 | 3.0 ± 1.0 | 1.7–6.8 | 3.0 ± 1.0 | 1.7–6.4 |
| UVA at 254 nm (cm−1) | 0.34 ± 0.14 | 0.18–0.77 | 0.05 ± 0.02 | 0.03–0.14 | 0.04 ± 0.02 | 0.03–0.12 |
| SUVA | 3.8 ± 0.7 | 2.7–5.2 | 1.7 ± 0.1 | 1.5–2.1 | 1.5 ± 0.1 | 1.3–1.8 |
| Free chlorine (mg/L) | - | - | 0.02 ± 0.02 | 0.00–0.05 | 1.14 ± 0.16 | 0.85–1.44 |
| Total chlorine (mg/L) | - | - | 0.31 ± 0.20 | 0.01–0.80 | 1.39 ± 0.16 | 1.13–1.68 |
| Type of Model | DBPs | R2 |
|---|---|---|
| Large-scale model | HAAs | 0.93 |
| THMs | 0.96 | |
| Limited-scale model | HAAs | 0.91 |
| THMs | 0.87 |
| Type of Model | DBPs | Models | DUVA Level Range (cm−1) | DBPs Level Range (µg/L) | Uncertainty |
|---|---|---|---|---|---|
| Large-scale model | HAAs | HAA6 = 3 082.95 × (1/1.09 × UVAFW272 − 1/1.14 × UVATW272) − 26.46 | 0.01–0.13 | 18–424 | 77% |
| THMs | THM4 = 2 215.99 × (1/1.09 × UVAFW272 − 1/1.14 × UVATW272) + 0.31 | 0.01–0.13 | 12–283 | 32% | |
| Limited scale model | HAAs | HAA6 = 1 698.99 × (1/1.09 × UVAFW272 − 1/1.14 × UVATW272) + 2.06 | 0.02–0.04 | 33–77 | 23% |
| THMs | THM4 = 2 165.70 × (1/1.09 × UVAFW272 − 1/1.14 × UVATW272) + 1.42 | 0.02–0.04 | 34–92 | 32% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stéphanie, G.; Caetano, D. Real-Time Estimation of Disinfection By-Products through Differential UV Absorbance. Water 2020, 12, 2536. https://doi.org/10.3390/w12092536
Stéphanie G, Caetano D. Real-Time Estimation of Disinfection By-Products through Differential UV Absorbance. Water. 2020; 12(9):2536. https://doi.org/10.3390/w12092536
Chicago/Turabian StyleStéphanie, Guilherme, and Dorea Caetano. 2020. "Real-Time Estimation of Disinfection By-Products through Differential UV Absorbance" Water 12, no. 9: 2536. https://doi.org/10.3390/w12092536
APA StyleStéphanie, G., & Caetano, D. (2020). Real-Time Estimation of Disinfection By-Products through Differential UV Absorbance. Water, 12(9), 2536. https://doi.org/10.3390/w12092536





