Interacting Effects of Polystyrene Microplastics and the Antidepressant Amitriptyline on Early Life Stages of Brown Trout (Salmo trutta f. fario)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Test Substances
2.3. Exposure and Sampling of Brown Trout
2.3.1. Experiment 1a: Exposure of Embryos and Sac-Fry Stages
2.3.2. Experiment 1b: Exposure of Fry
2.3.3. Experiment 2
2.4. Chemical Analyses
2.5. Development Parameters
2.6. Scanning Electron Microscopy
2.7. Behavior
2.8. Level of Lipid Peroxides
2.9. Activity of Superoxide Dismutase
2.10. Neurotoxicity
2.11. Statistical Analysis
2.12. Animal Welfare
2.13. Credibility of Data
3. Results
3.1. Experiment 1a: Exposure of Embryos and Sac-Fry Stages
3.2. Experiment 1b: Exposure of Fry
3.3. Experiment 2
4. Discussion
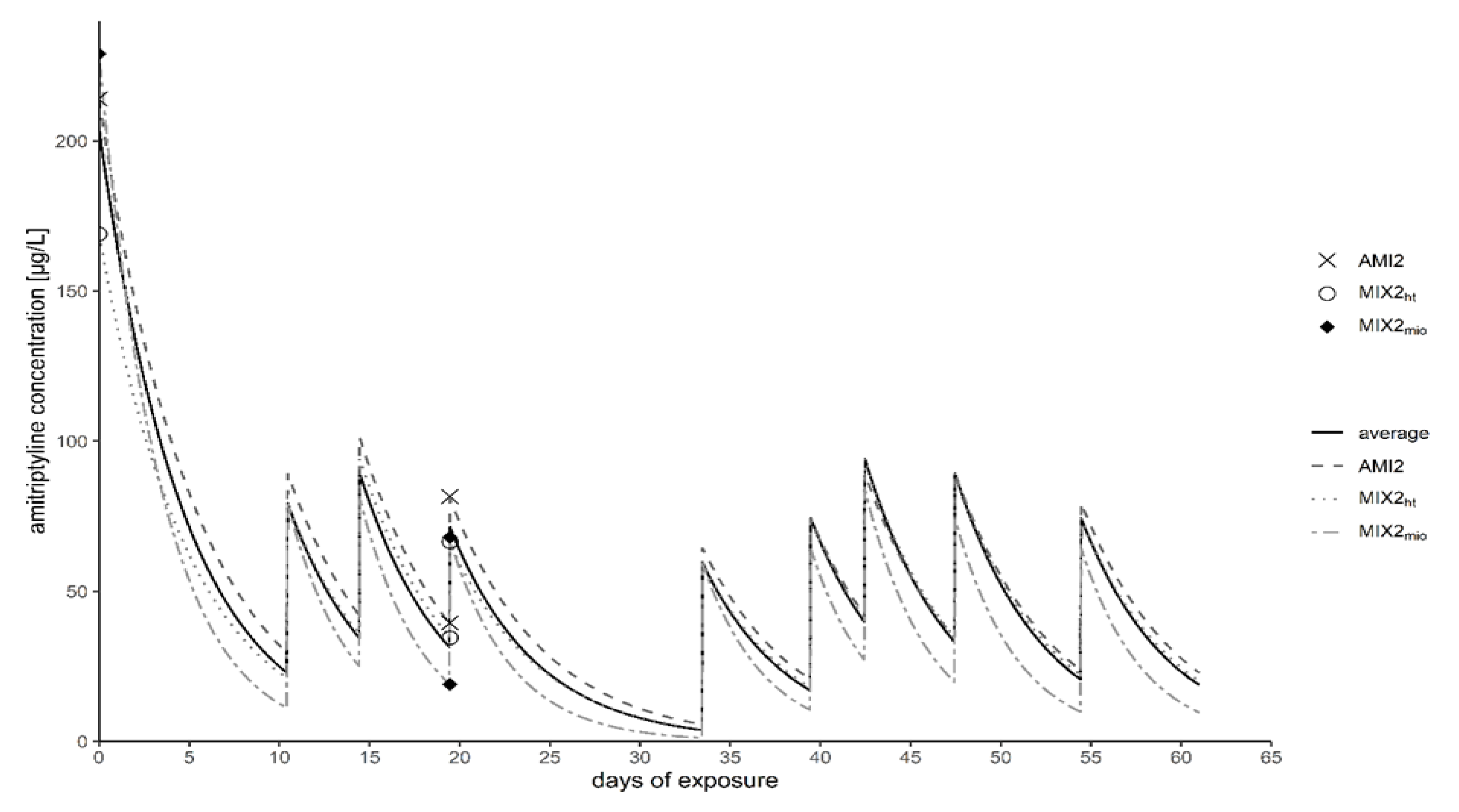
4.1. Amitriptyline Concentration
4.2. Effects of MP
4.3. Effects of Amitriptyline
4.4. Co-Exposure of MP and Amitriptyline
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Claessens, M.; Meester, S.D.; Landuyt, L.V.; Clerck, K.D.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; Van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, G.; Lu, L.; Zheng, Y.; Zhang, Q.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Low level of polystyrene microplastics decreases early developmental toxicity of phenanthrene on marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 385, 121586. [Google Scholar] [CrossRef] [PubMed]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed]
- Van Pomeren, M.; Brun, N.R.; Peijnenburg, W.J.G.M.; Vijver, M.G. Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages. Aquat. Toxicol. 2017, 190, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of nano- and microplastics for freshwater ecosystems: A critical review. Trac. Trends Anal. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhang, N.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.; Liu, H.-P.; Xu, Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018, 195, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A. Why are early life stages of aquatic organisms more sensitive to toxicants than adults? In New Insights into Toxicity and Drug Testing; Gowder, S.J.T., Ed.; IntechOpen: London, UK, 2013; pp. 49–62. [Google Scholar] [CrossRef]
- Malafaia, G.; De Souza, A.M.; Pereira, A.C.; Gonçalves, S.; Da Costa Araújo, A.P.; Ribeiro, R.X.; Rocha, T.L. Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total Environ. 2020, 700, 134867. [Google Scholar] [CrossRef] [PubMed]
- LeMoine, C.M.R.; Kelleher, B.M.; Lagarde, R.; Northam, C.; Elebute, O.O.; Cassone, B.J. Transcriptional effects of polyethylene microplastics ingestion in developing zebrafish (Danio rerio). Environ. Pollut 2018, 243, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Damberg, J.; Dave, G.; Larsson, Å. Leachates from plastic consumer products—Screening for toxicity with Daphnia magna. Chemosphere 2009, 74, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.; Oliviero, M.; Romano, V.; Dumontet, S.; Manzo, S.; Liu, G. Ecotoxicological assessment of virgin plastic pellet leachates in freshwater matrices. J. Environ. Account. Manag. 2018, 6, 345–353. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Rehse, S.; Kloas, W.; Zarfl, C. Microplastics reduce short-term effects of environmental contaminants. Part I: Effects of bisphenol A on freshwater zooplankton are lower in presence of polyamide particles. Int. J. Environ. Res. Public Health 2018, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Guilhermino, L.; Vieira, L.R.; Ribeiro, D.; Tavares, A.S.; Cardoso, V.; Alves, A.; Almeida, J.M. Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Sci. Total Environ. 2018, 622–623, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Nematdoost Haghi, B.; Banaee, M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int. J. Environ. Sci. Technol. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, B.; Ghosh, U. Differential bioavailability of polychlorinated biphenyls associated with environmental particles: Microplastic in comparison to wood, coal and biochar. Environ. Pollut. 2017, 220, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Breyer-Pfaff, U. The Metabolic Fate of Amitriptyline, Nortriptyline and Amitriptylinoxide in Man. Drug Metab. Rev. 2004, 36, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, R.; Gott, B.M.; Conway, C.R. Tricyclic antidepressants and monoamine oxidase Inhibitors: Are They Too Old for a New Look? In Antidepressants: From Biogenic Amines to Newe Mechanisms of Action; Macaluso, M., Preskorn, S.H., Eds.; Springer: Cham, Switzerland, 2019; Volume 250. [Google Scholar]
- Schwabe, U.; Paffrath, D.; Ludwig, W.-D.; Klauber, J. Arzneiverordnungs-Report 2019–Aktuelle Daten, Kosten, Trends und Kommentare; Springer: Berlin, Germany, 2019; p. 927. [Google Scholar]
- Snyder, S.H.; Yamamura, H.I. Antidepressants and the muscarinic acetylcholine receptor. Arch. Gen. Psychiatry 1977, 34, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Shapiro, D.A.; George, S.R.; Setola, V.; Lee, D.K.; Cheng, R.; Rauser, L.; Lee, S.P.; Lynch, K.R.; Roth, B.L.; et al. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001, 59, 427. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-W.; Liu, X.; Chan, C.-B.; Weinshenker, D.; Hall, R.A.; Xiao, G.; Ye, K. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem. Biol. 2009, 16, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Rudorfer, M.V.; Potter, W.Z. Metabolism of tricyclic antidepressants. Cell. Mol. Neurobiol. 1999, 19, 373–409. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-D.; Li, J.; Li, J.-J.; Liu, M.; Yan, D.-Z.; Shi, W.-Y.; Xu, G. Occurrence and source analysis of selected antidepressants and their metabolites in municipal wastewater and receiving surface water. Environ. Sci. Process. Impacts 2018, 20, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Ferrey, M.L.; Heiskary, S.; Grace, R.; Hamilton, M.C.; Lueck, A. Pharmaceuticals and other anthropogenic tracers in surface water: A randomized survey of 50 Minnesota lakes. Environ. Toxicol. Chem. 2015, 34, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of Basic Antidepressants and Their N-Desmethyl Metabolites in Raw Sewage and Wastewater Using Solid-Phase Extraction and Liquid Chromatography−Tandem Mass Spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.V.; Da Silva, F.M.A.; Langford, K.H.; De Souza, A.D.L.; Nizzeto, L.; Waichman, A.V. Screening for selected human pharmaceuticals and cocaine in the urban streams of Manaus, Amazonas, Brazil. Jawra J. Am. Water Resour. Assoc. 2014, 50, 302–308. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Multi-residue analysis of pharmaceutical compounds in aqueous samples. J. Chromatogr. A 2008, 1177, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Meshalkina, D.A.; Efimova, E.V.; Morzherin, Y.Y.; Kalueff, A.V. Acute effects of amitriptyline on adult zebrafish: Potential relevance to antidepressant drug screening and modeling human toxidromes. Neurotoxicol. Teratol. 2017, 62, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; Kysil, E.V.; Antonova, K.A.; Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Gainetdinov, R.R.; Alekseeva, P.A.; Kalueff, A.V. The effects of chronic amitriptyline on zebrafish behavior and monoamine neurochemistry. Neurochem. Res. 2018, 43, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Wu, M.; Liu, S.; Chen, B.; Pan, C.; Yang, M.; Wang, K.-J. Suppressive immunoregulatory effects of three antidepressants via inhibition of the nuclear factor-κB activation assessed using primary macrophages of carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2017, 322, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth inhibition and coordinated physiological regulation of zebrafish (Danio rerio) embryos upon sublethal exposure to antidepressant amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Marsalek, P.; Prokes, M.; Tichy, F.; Skladana, M.; Fiorino, E.; Mikula, P.; et al. Effects of selected tricyclic antidepressants on early-life stages of common carp (Cyprinus carpio). Chemosphere 2017, 185, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Zikova, A.; Blahova, J.; Svobodova, Z.; Chloupek, P.; Kloas, W. mRNA expression of antioxidant and biotransformation enzymes in zebrafish (Danio rerio) embryos after exposure to the tricyclic antidepressant amitriptyline. Chemosphere 2019, 217, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.J.; Sirotkin, H.I.; McElroy, A.E. Varying the exposure period and duration of neuroactive pharmaceuticals and their metabolites modulates effects on the visual motor response in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2019, 72, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Hodkovicova, N.; Urbanova, M.; Örn, S.; Blahova, J.; Svobodova, Z.; Faldyna, M.; Chloupek, P.; Briedikova, K.; Carlsson, G. Effects of antidepressants with different modes of action on early life stages of fish and amphibians. Environ. Pollut. 2019, 254, 112999. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Posthaus, H.; Bernet, D.; Wahli, T.; Burkhardt-Holm, P. Morphological organ alterations and infectious diseases in brown trout Salmo trutta and rainbow trout Oncorhynchus mykiss exposed to polluted river water. Dis. Aquat. Org. 2001, 44, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Klemetsen, A.; Amundsen, P.-A.; Dempson, J.B.; Jonsson, N.; Jonsson, B.; O’connell, M.; Mortensen, E. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecol. Freshw. Fish. 2003, 12, 1–59. [Google Scholar] [CrossRef]
- Cheng, J.; Flahaut, E.; Cheng, S.H. Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem. 2007, 26, 708–716. [Google Scholar] [CrossRef] [PubMed]
- EU. Council Directive 2006/88/EC. Off. J. Eur. Union 2006, L 328, 14–56. [Google Scholar]
- Eitzen, L.; Paul, S.; Braun, U.; Altmann, K.; Jekel, M.; Ruhl, A.S. The challenge in preparing particle suspensions for aquatic microplastic research. Environ. Res. 2019, 168, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR–Hydrophobic, Electronic, and Steric Constants; American Chemical Society: Washington, DC, USA, 1995; p. 167. [Google Scholar]
- OECD. Test. No. 212: Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages; OECD Publishing: Paris, France, 1998. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Willmore, W.G.; Storey, K.B. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radic. Biol. Med. 1995, 19, 271–280. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Geracitano, L.A.; Pinho, G.L.L.; Vinagre, T.M.; Faleiros, M.; Alciati, J.C.; Bianchini, A. Determination of Lipid Peroxides in Invertebrates Tissues Using the Fe(III) Xylenol Orange Complex Formation. Arch. Environ. Contam. Toxicol. 2003, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rault, M.; Collange, B.; Mazzia, C.; Capowiez, Y. Dynamics of acetylcholinesterase activity recovery in two earthworm species following exposure to ethyl-parathion. Soil Biol. Biochem. 2008, 40, 3086–3091. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Mazzia, C.; Capowiez, Y.; Rault, M. Carboxylesterase activity in earthworm gut contents: Potential (eco)toxicological implications. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Moermond, C.T.A.; Kase, R.; Korkaric, M.; Ågerstrand, M. CRED: Criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem. 2016, 35, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Degradation kinetics of pharmaceuticals and personal care products in surface waters: Photolysis vs biodegradation. Sci. Total Environ. 2017, 590–591, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Jiang, W.-T.; Li, Z.; Kuo, C.-Y.; Jean, J.-S.; Chen, W.-R.; Lv, G. Mechanism of amitriptyline adsorption on Ca-montmorillonite (SAz-2). J. Hazard. Mater. 2014, 277, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Stockwell, C.; Niles, J.; Minegar, S.; Li, Z.; Jiang, W.-T. Uptake and retention of amitriptyline by kaolinite. J. Colloid Interface Sci. 2013, 411, 198–203. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Lange, A.; Tyler, C.R.; Hill, E.M. Concentrating mixtures of neuroactive pharmaceuticals and altered neurotransmitter levels in the brain of fish exposed to a wastewater effluent. Sci. Total Environ. 2018, 621, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Gagné, F.; Louis, S.; Čejka, P.; Sauvé, S. Distribution of antidepressants and their metabolites in brook trout exposed to municipal wastewaters before and after ozone treatment—Evidence of biological effects. Chemosphere 2011, 83, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Muir, D.; Simmons, D.; Wang, X.; Peart, T.; Villella, M.; Miller, J.; Sherry, J. Bioaccumulation of pharmaceuticals and personal care product chemicals in fish exposed to wastewater effluent in an urban wetland. Sci. Rep. 2017, 7, 16999. [Google Scholar] [CrossRef] [PubMed]
- Ziarrusta, H.; Mijangos, L.; Izagirre, U.; Plassmann, M.M.; Benskin, J.P.; Anakabe, E.; Olivares, M.; Zuloaga, O. Bioconcentration and biotransformation of amitriptyline in gilt-head bream. Environ. Sci. Technol. 2017, 51, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Grierson, J.P.; Neville, A.C. Helicoidal architecture of fish eggshell. Tissue Cell 1981, 13, 819–830. [Google Scholar] [CrossRef]
- Killeen, J.; McLay, H.A.; Johnston, I.A. Development in Salmo trutta at different temperatures, with a quantitative scoring method for intraspecific comparisons. J. Fish Biol. 1999, 55, 382–404. [Google Scholar] [CrossRef]
- Schmieg, H.; Huppertsberg, S.; Knepper, T.P.; Krais, S.; Reitter, K.; Rezbach, F.; Ruhl, A.S.; Köhler, H.-R.; Triebskorn, R. Polystyrene microplastics do not affect juvenile brown trout (Salmo trutta f. fario) or modulate effects of the pesticide methiocarb. Environ. Sci. Eur. 2020, 32, 49. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.; Vieira, L.R.; Guilhermino, L. Neurotoxicity, behavior, and lethal effects of cadmium, microplastics, and their mixtures on Pomatoschistus microps juveniles from two wild populations exposed under laboratory conditions―Implications to environmental and human risk assessment. Int. J. Environ. Res. Public Health 2019, 16, 2857. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Fonte, E.; Soares, M.E.; Carvalho, F.; Guilhermino, L. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: Gold nanoparticles, microplastics and temperature. Aquat. Toxicol. 2016, 170, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tosetto, L.; Williamson, J.E.; Brown, C. Trophic transfer of microplastics does not affect fish personality. Anim. Behav. 2017, 123, 159–167. [Google Scholar] [CrossRef]
- Critchell, K.; Hoogenboom, M.O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Hoogen). PLoS ONE 2018, 13, e0193308. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.; Gilson, A.; Lanctôt, C.; Besson, M.; Metian, M.; Lecchini, D. No Effect of Polystyrene Microplastics on Foraging Activity and Survival in a Post-larvae Coral-Reef Fish, Acanthurus triostegus. Bull. Environ. Contam. Toxicol. 2019, 102, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.R.; Guilhermino, L. Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): Changes in behavioural responses and reduction of swimming velocity and resistance time. Environ. Pollut. 2018, 236, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Guven, O.; Bach, L.; Munk, P.; Dinh, K.V.; Mariani, P.; Nielsen, T.G. Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquat. Toxicol. 2018, 198, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, S.; Hu, L.; Qu, H.; Pan, C.; Lei, P.; Shen, Y.; Yang, M. Global transcriptomic analysis of zebrafish in response to embryonic exposure to three antidepressants, amitriptyline, fluoxetine and mianserin. Aquat. Toxicol. 2017, 192, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Mannerström, M.; Toimela, T.; Ylikomi, T.; Tähti, H. The combined use of human neural and liver cell lines and mouse hepatocytes improves the predictability of the neurotoxicity of selected drugs. Toxicol. Lett. 2006, 165, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.C.; Rocha, J.B.T.; Morsch, V.M.; Neis, R.T.; Schetinger, M.R.C. Antidepressants inhibit human acetylcholinesterase and butyrylcholinesterase activity. Biochim. Biophys. Acta Mol. Basis Dis. 2002, 1587, 92–98. [Google Scholar] [CrossRef]
- Ziegler, M.; Knoll, S.; Köhler, H.R.; Tisler, S.; Huhn, C.; Zwiener, C.; Triebskorn, R. Impact of the antidepressant citalopram on the behaviour of two different life stages of brown trout. PeerJ 2020, 8, e8765. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Porseryd, T.; Porsch-Hällström, I.; Borg, B.; Roufidou, C.; Olsén, K.H. Developmental exposure to the SSRI citalopram causes long-lasting behavioural effects in the three-spined stickleback (Gasterosteus aculeatus). Ecotoxicology 2018, 27, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Meijide, F.J.; Da Cuña, R.H.; Prieto, J.P.; Dorelle, L.S.; Babay, P.A.; Lo Nostro, F.L. Effects of waterborne exposure to the antidepressant fluoxetine on swimming, shoaling and anxiety behaviours of the mosquitofish Gambusia holbrooki. Ecotoxicol. Environ. Saf. 2018, 163, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Buerkley, M.A.; Julius, M.L.; Vajda, A.M.; Norris, D.O.; Barber, L.B.; Furlong, E.T.; Schultz, M.M.; Schoenfuss, H.L. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2009, 28, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Santos, L.H.M.L.M.; Paula, J.R.; Camacho, C.; Pissarra, V.; Fogaça, F.; Barbosa, V.; Alves, R.; Ferreira, P.P.; Barceló, D.; et al. Differential behavioural responses to venlafaxine exposure route, warming and acidification in juvenile fish (Argyrosomus regius). Sci. Total Environ. 2018, 634, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.; Hanslik, L.; Kämmer, N.; Braunbeck, T. The tox is in the detail: Technical fundamentals for designing, performing, and interpreting experiments on toxicity of microplastics and associated substances. Environ. Sci. Pollut. Res. 2020, 27, 22292–22318. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef] [PubMed]
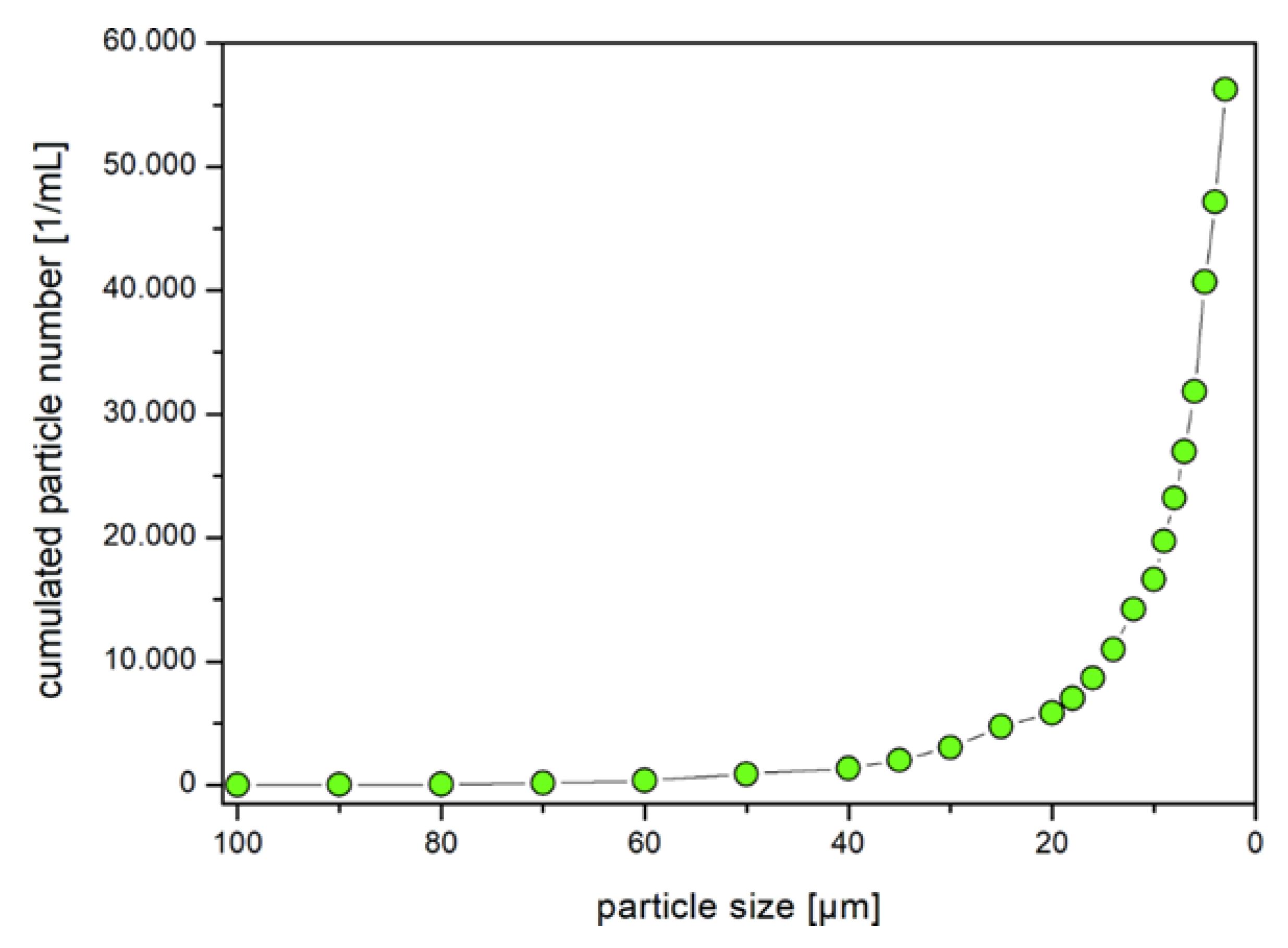
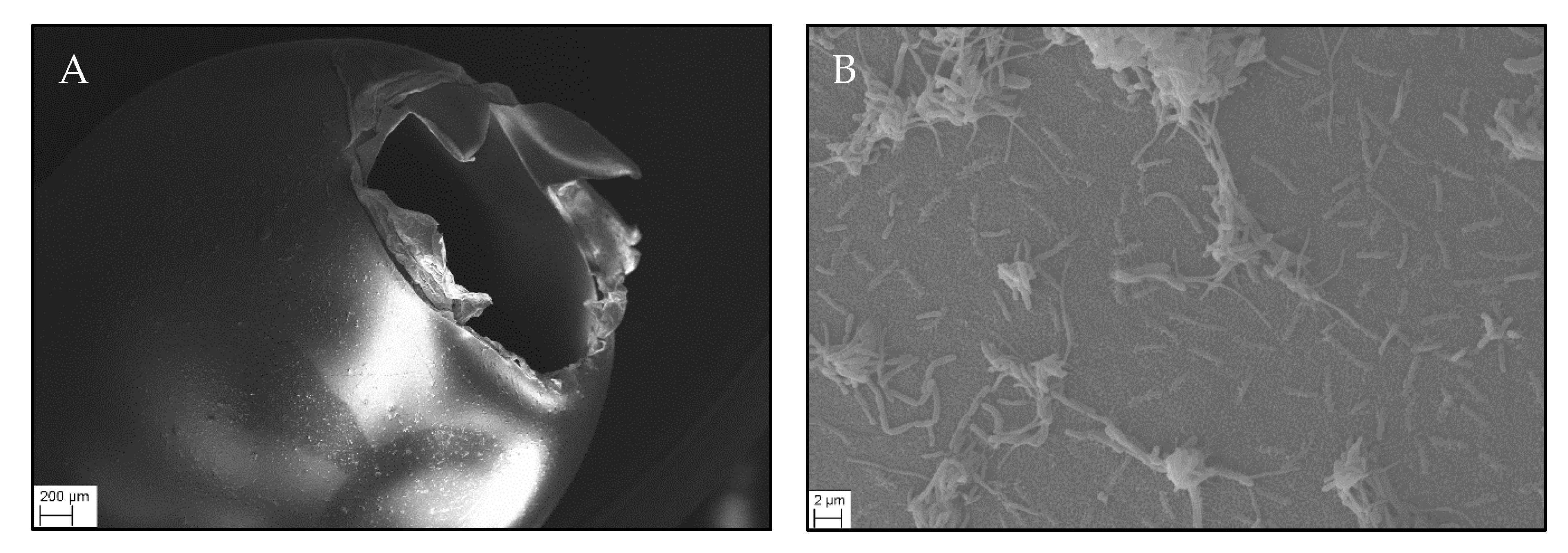

| Control (C1) | 100 Particles/L (MP1h) | 104 Particles/L (MP1tt) | 105 Particles/L (MP1ht) | |
|---|---|---|---|---|
| Mortality (%) | 1 ±2 | 1 ±2 | 1 ±2 | 1 ±2 |
| Time until eyed ova stage (dpf) | 39 ±1 | 39 ±1 | 40 ±3 | 40 ±2 |
| Time to hatch (dpf) | 75 ±3 | 73 ±3 | 73 ±2 | 73 ±3 |
| Heart rate (beats/min) | 51 ±2 | 56 ±3 | 52 ±2 | 53 ±3 |
| Length (cm) | 2.7 ±0.2 | 2.7 ±0.2 | 2.7 ±0.2 | 2.8 ±0.2 |
| Body mass (g) | 0.15 ±0.03 | 0.14 ±0.02 | 0.14 ±0.03 | 0.16 ±0.02 |
| Lipid peroxidation (CHP-equiv.) | 57.89 ±10.20 | 60.12 ±13.70 | 62.42 ±12.17 | 61.23 ±13.01 |
| Control (C1) | 100 Particles/L (MP1h) | 104 Particles/L (MP1tt) | 105 Particles/L (MP1ht) | |
|---|---|---|---|---|
| Mortality (%) | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 |
| Length (cm) | 3.0 ±0.1 | 2.9 ±0.2 | 3.0 ±0.2 | 2.9 ±0.2 |
| Body mass (g) | 0.20 ±0.02 | 0.17 ±0.04 | 0.20 ±0.04 | 0.19 ±0.04 |
| Lipid peroxidation (CHP-equiv.) | 58.28 ±15.46 | 65.46 ±22.14 | 52.30 ±18.77 | 58.57 ±15.51 |
| SOD (U/mL) | 95.43 ±28.47 | 105.60 ±42.84 | 96.15 ±31.87 | 98.60 ±30.56 |
| Nominal Concentration | Measured Concentration | |||
|---|---|---|---|---|
| Start of Experiment | Prior Water Exchange | After Water Exchange | ||
| C2 | 0 µg/L | <0.01 µg/L | <0.01 µg/L | <0.01 µg/L |
| MP2ht | 0 µg/L | <0.01 µg/L | <0.01 µg/L | <0.01 µg/L |
| MP2mio | 0 µg/L | <0.01 µg/L | <0.01 µg/L | <0.01 µg/L |
| AMI2 | 300 µg/L | 214 µg/L | 40 µg/L | 82 µg/L |
| MIX2ht | 300 µg/L | 169 µg/L | 35 µg/L | 67 µg/L |
| MIX2mio | 300 µg/L | 229 µg/L | 19 µg/L | 68 µg/L |
| Control (C2) | 105 Particles/L (MP2ht) | 106 Particles/L (MP2mio) | Amitriptyline (AMI2) | Amitriptyline + 105 Particles/L (MIX2ht) | Amitriptyline +106 Particles/L (MIX2mio) | |
|---|---|---|---|---|---|---|
| Mortality (%) | 0 ± 0 | 1 ± 1 | 0 ± 0 | 3 ± 4 | 2 ± 1 | 4 ± 3 |
| Time to hatch (days of exposure) | 12 ± 2 | 12 ± 1 p = 0.786 | 11 ± 2 p = 0.580 | 10 ± 2 p< 0.001 | 10 ± 2 p< 0.001 | 10 ±2 p< 0.001 |
| Heart rate (beat /min) | 56 ± 4 | 55 ± 2 | 56 ± 2 | 56 ± 1 | 54 ± 4 | 55 ± 2 |
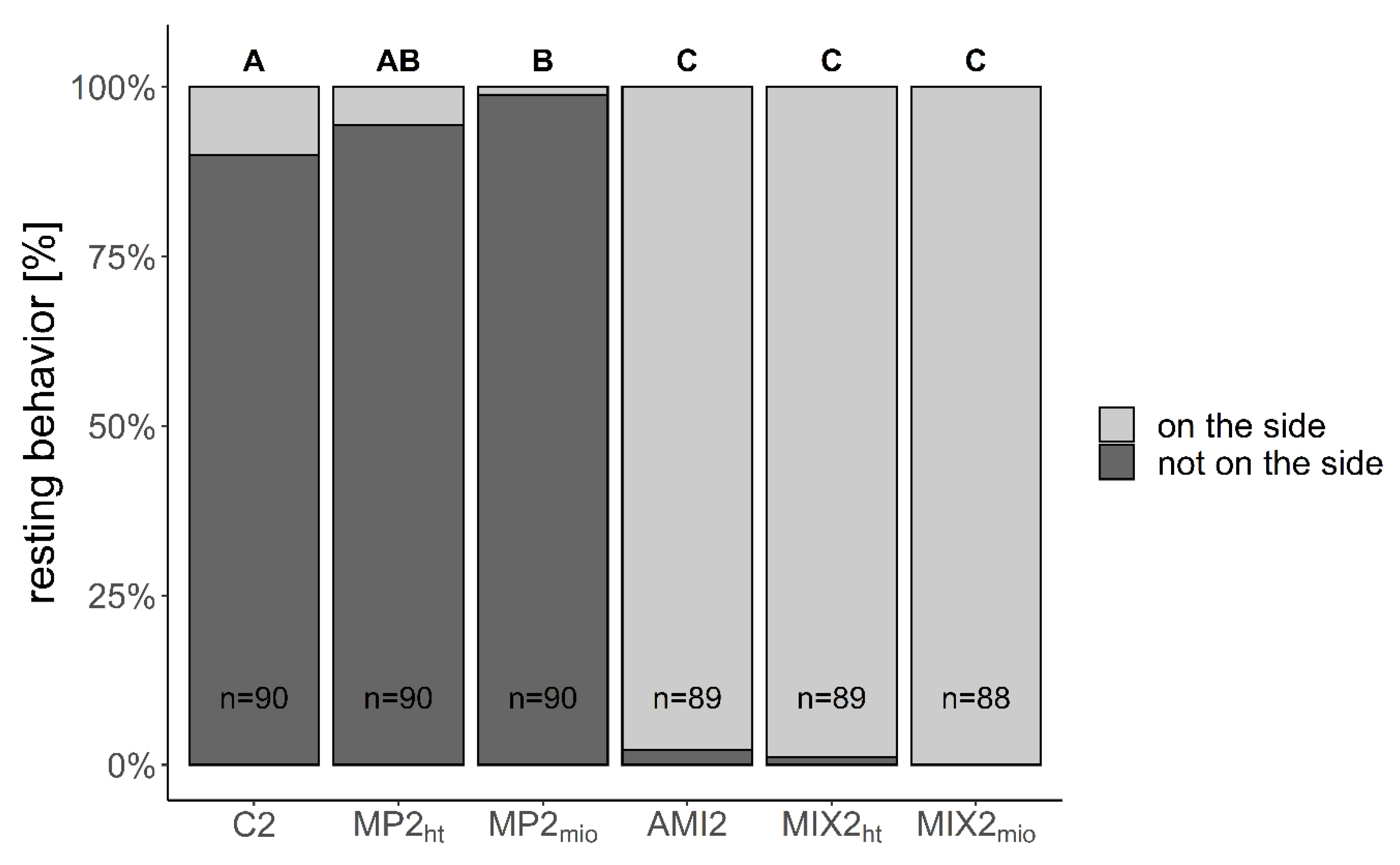
| Larvae resting on their side (%) | 10.0 ± 11.9 | 5.6 ± 4.2 p = 0.405 | 1.1 ± 1.6 p= 0.018 | 97.8 ± 3.1 p< 0.001 | 98.9 ± 1.6 p< 0.001 | 100.0 ± 0.0 p< 0.001 |
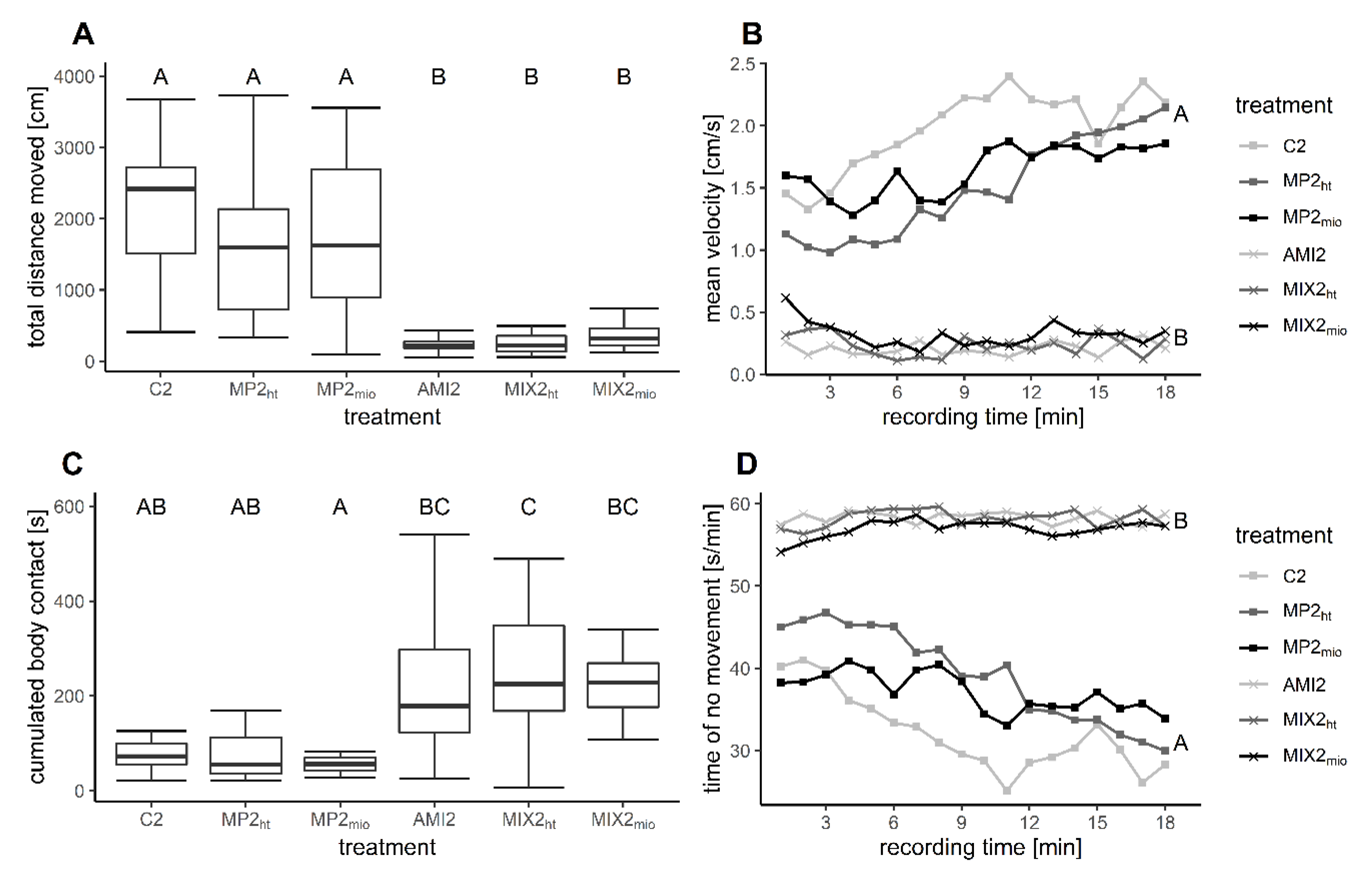
| Total distance moved (cm) | 2135 ± 862 | 1617 ± 1008 p = 0.385 | 1772 ± 1103 p = 0.756 | 224 ± 95 p< 0.001 | 253 ± 136 p< 0.001 | 345 ± 165 p< 0.001 |
| Body contact (s) | 75 ± 31 | 77 ± 49 p = 1 | 56 ± 17 p = 0.999 | 217 ± 137 p = 0.082 | 241 ± 137 p= 0.023 | 222 ± 63 p = 0.066 |
| Mean velocity (cm/s) | 2.0 ± 0.8 | 1.5 ± 0.9 p = 0.129 | 1.6 ± 1.0 p = 0.505 | 0.2 ± 0.1 p< 0.001 | 0.2 ± 0.1 p< 0.001 | 0.3 ± 0.2 p< 0.001 |
| No movement (s) | 579 ± 207 | 706 ± 243 p = 0.628 | 668 ± 282 p = 0.902 | 1050 ± 20 p< 0.001 | 1049 ± 28 p< 0.001 | 1025 ± 36 p< 0.001 |
| Length (cm) | 2.7 ± 0.1 | 2.6 ± 0.1 p = 0.603 | 2.7 ± 0.1 p = 1 | 2.4 ± 0.1 p< 0.001 | 2.4 ± 0.1 p< 0.001 | 2.4 ± 0.1 p< 0.001 |
| Body mass (g) | 0.14 ± 0.03 | 0.14 ± 0.02 p = 1 | 0.15 ± 0.02 p = 0.963 | 0.11 ± 0.02 p< 0.001 | 0.11 ± 0.02 p< 0.001 | 0.12 ± 0.02 p< 0.001 |
| Lipid peroxidation (CHP-equiv.) | 18.59 ± 2.62 | 19.61 ± 4.01 | 18.04 ± 2.46 | 19.05 ± 2.40 | 19.24 ± 3.71 | 19.29 ± 2.53 |
| SOD (U/mL) | 117.54 ± 28.89 | 122.62 ± 28.11 | 121.55 ± 24.58 | 137.88 ± 33.58 | 133.66 ± 29.16 | 129.09 ± 31.48 |
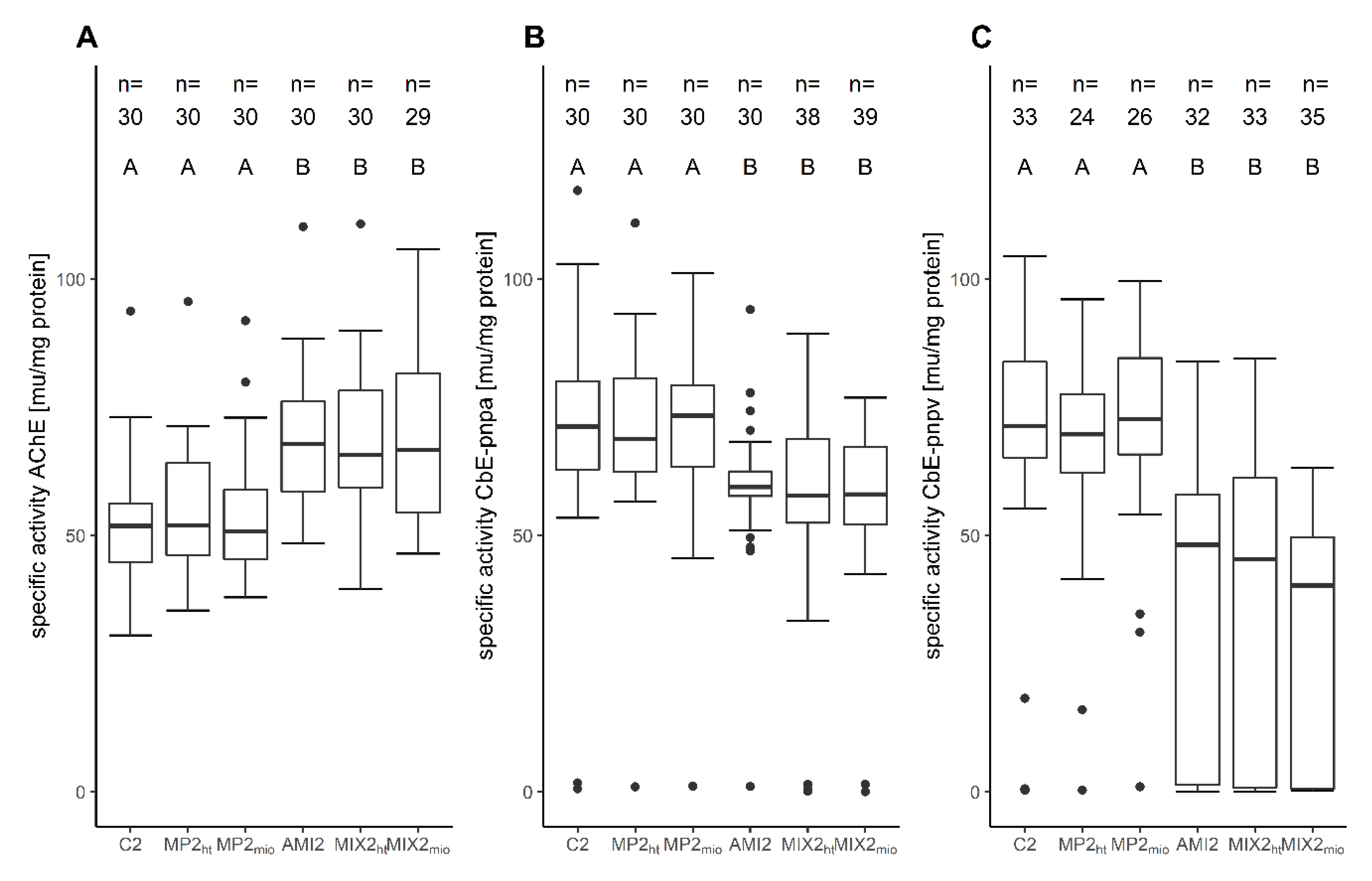
| AChE activity (mu/mg protein) | 52.46 ± 12.66 | 54.48 ± 13.04 p = 0.997 | 53.69 ± 12.39 p = 0.999 | 68.29 ± 13.59 p= 0.008 | 68.53 ± 14.70 p= 0.008 | 68.87 ± 14.78 p= 0.008 |
| CbE-pnpa activity (mu/mg protein) | 69.21 ± 22.85 | 70.25 ± 17.96 p = 0.915 | 70.15 ± 17.54 p = 0.915 | 59.23 ± 14.10 p= 0.001 | 54.11 ± 23.21 p= 0.001 | 57.22 ± 15.89 p= 0.008 |
| CbE-pnpv activity (mu/mg protein) | 70.28 ± 24.04 | 66.15 ± 21.79 p = 0.859 | 70.43 ± 21.51 p = 1 | 39.12 ± 26.11 p< 0.001 | 38.27 ± 27.86 p< 0.001 | 31.33 ± 23.59 p< 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmieg, H.; Burmester, J.K.Y.; Krais, S.; Ruhl, A.S.; Tisler, S.; Zwiener, C.; Köhler, H.-R.; Triebskorn, R. Interacting Effects of Polystyrene Microplastics and the Antidepressant Amitriptyline on Early Life Stages of Brown Trout (Salmo trutta f. fario). Water 2020, 12, 2361. https://doi.org/10.3390/w12092361
Schmieg H, Burmester JKY, Krais S, Ruhl AS, Tisler S, Zwiener C, Köhler H-R, Triebskorn R. Interacting Effects of Polystyrene Microplastics and the Antidepressant Amitriptyline on Early Life Stages of Brown Trout (Salmo trutta f. fario). Water. 2020; 12(9):2361. https://doi.org/10.3390/w12092361
Chicago/Turabian StyleSchmieg, Hannah, Janne K.Y. Burmester, Stefanie Krais, Aki S. Ruhl, Selina Tisler, Christian Zwiener, Heinz-R. Köhler, and Rita Triebskorn. 2020. "Interacting Effects of Polystyrene Microplastics and the Antidepressant Amitriptyline on Early Life Stages of Brown Trout (Salmo trutta f. fario)" Water 12, no. 9: 2361. https://doi.org/10.3390/w12092361
APA StyleSchmieg, H., Burmester, J. K. Y., Krais, S., Ruhl, A. S., Tisler, S., Zwiener, C., Köhler, H.-R., & Triebskorn, R. (2020). Interacting Effects of Polystyrene Microplastics and the Antidepressant Amitriptyline on Early Life Stages of Brown Trout (Salmo trutta f. fario). Water, 12(9), 2361. https://doi.org/10.3390/w12092361





