The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review
Abstract
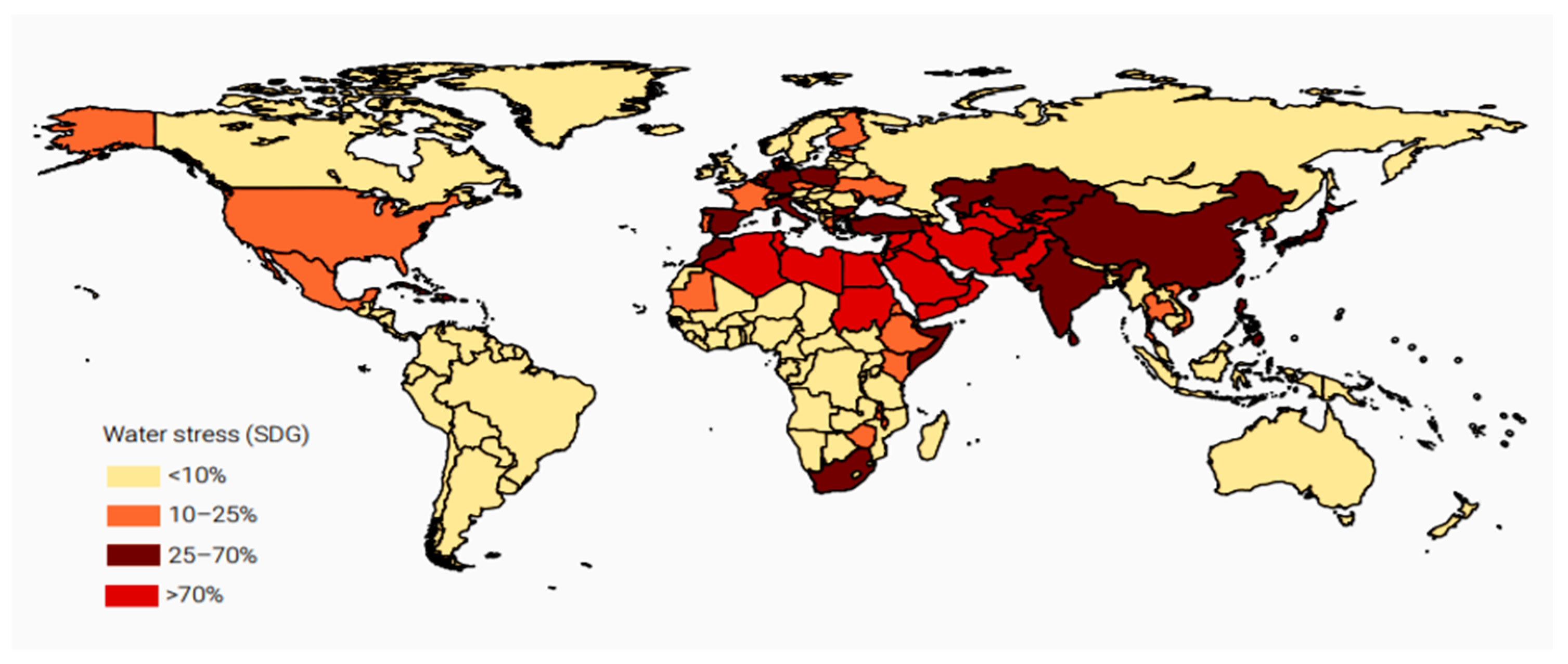
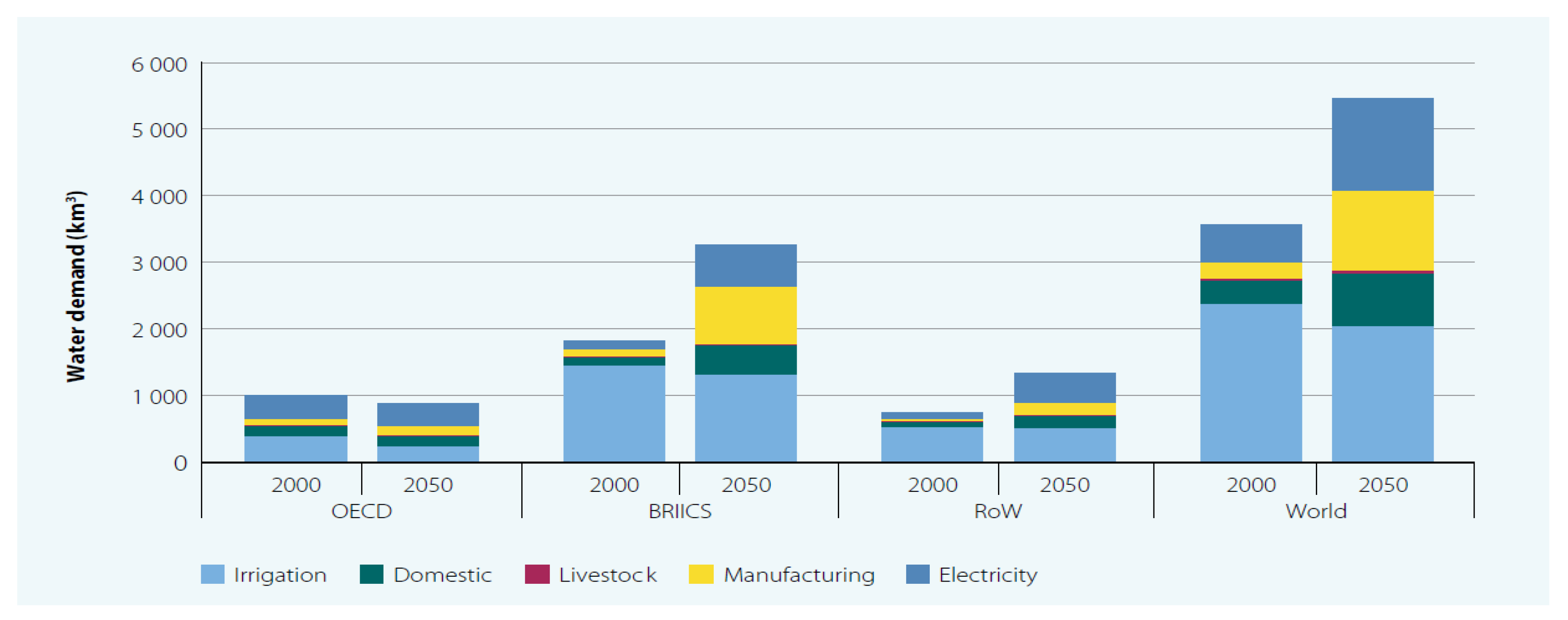
1. Introduction
- TFWW: Total freshwater withdrawn
- TRWR: Total renewable freshwater resources
- EFR: Environmental Flow requirements which are defined as a quantity, timing, and quality of water flows required to sustain freshwater supplies for maintaining life.
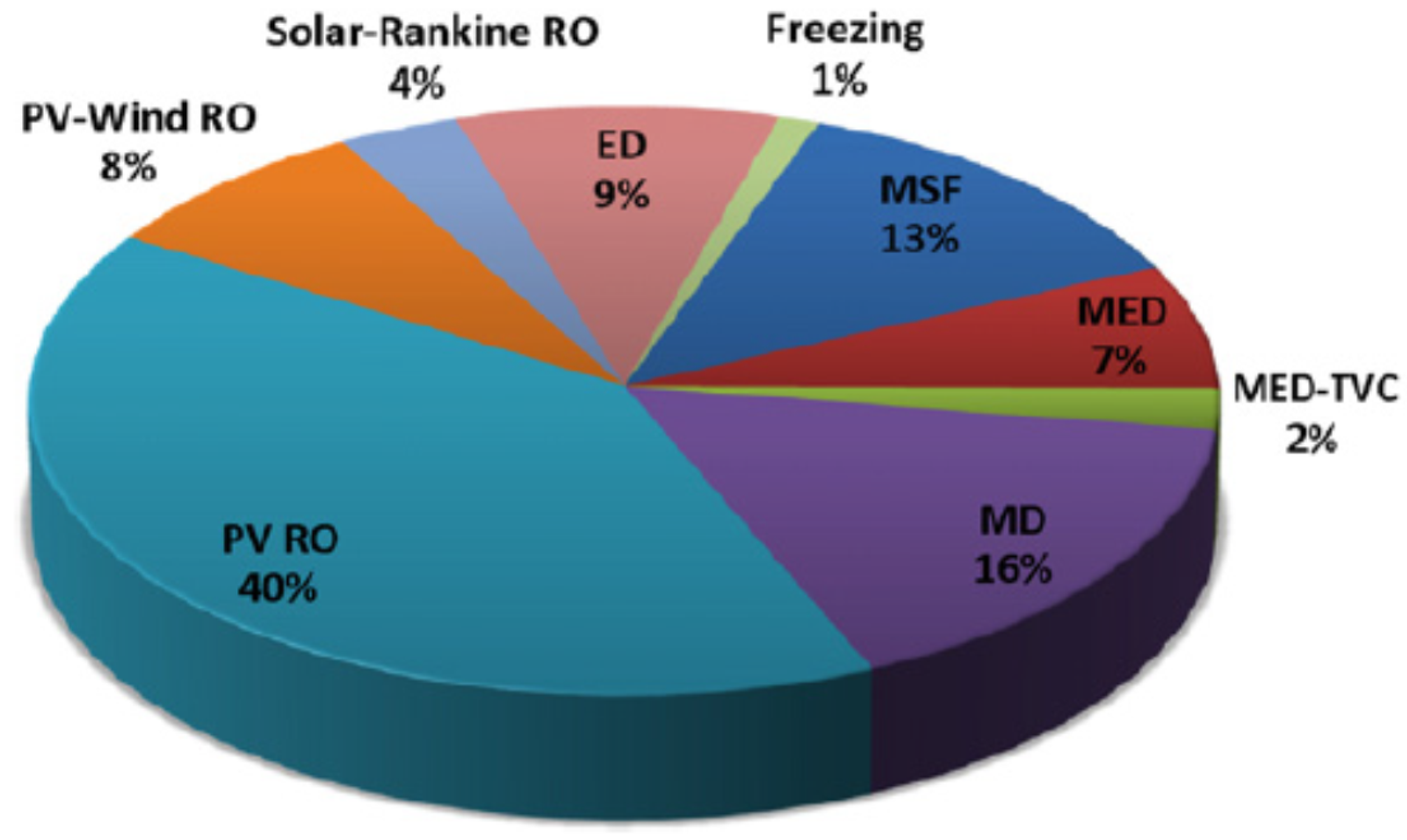
2. Assessment of Desalination Processes
2.1. Pre-Treatment of Intake Water
2.2. Desalination Technologies
2.3. Post-Treatment of Water Permeate
3. Role of Nanofluids in Energy-Efficient Desalination
3.1. Basics of Nanofluids
3.2. Application of Nanofluids for Thermal Desalination
4. Energy Consumption Economics and Environment Analysis of Renewable Energy-Based Desalination
4.1. Brine Discharge Mitigation
4.2. Microplastic Pollution
5. Opportunity to Develop Renewable Energy Powered Cost-Competitive Sustainable Desalination
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Adsorption Desalination |
| CAPEX | Capital Expenditure |
| DAF | Dissolved Air Flotation |
| ED | Electrodialysis |
| TO | Transformer Oil |
| IDA | International Desalination Association |
| TFWW | Total Freshwater Withdrawn |
| TRWR | Total renewable freshwater withdrawn |
| EFR | Environmental Flow Requirements |
| GCC | Gulf Cooperation Council |
| GOR | Gain Output Ratio |
| KSA | Kingdom of Saudi Arabia |
| UAE | United Arab Emirates |
| MED | Multi-Effect Distillation |
| MSF | Multi-Stage Flash |
| MENA | the Middle East and North Africa |
| MEDAD | Multiple-Effect Distillation Adsorption Desalination |
| PPM | Parts Per Million |
| LCOW | Levelized Cost of Water |
| LNPP | Large-Sized Nuclear Power Plant |
| SHNP | Small Sized Nuclear Heat Only Plant |
| MD | Membrane Desalination |
| MVC | Mechanical Vapour Compression |
| TVC | Thermal Vapour Compression |
| NF | Nanofiltration |
| MWCNTs | Multi-Wall Carbon Nano Tubes |
| GO | Graphene Oxide |
| MgO | Magnesium Oxide |
| T-55 | Therminol 55 Oil |
| OPEX | Operational Expenditures/Operational Expenses |
| PR | Performance Ratio |
| RO | Reverse Osmosis |
| BSA | Bovine Serum Albumin |
| DDA | Dodecyl Amine |
| SD | Solar Distillation/Solar Desalination |
| SBS | Sodium Meta Bi Sulphite |
| SDGs | Sustainable Development Goals |
| SWCC | Seawater Cooling Circuit |
| TDS | Total Dissolved Solids |
| TOC | Total Organic Compounds |
| TAC | Total Annual Cost |
| UNDESA | United Nations Department of Economic and Social Affairs |
| VOC | Volatile Organic Compounds |
| CSP | Concentrated Solar Power |
| EIA | US Energy Information Administration |
| IEA | International Energy Agency |
| IRENA | International Renewable Energy Agency |
| USD | United States Dollar |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| IWMI | International Water Management Institute |
| IPCC | Intergovernmental Panel on Climate Change |
| FAO | Food and Agriculture Organization of the United Nations |
| OECD | Organization for Economic Co-operation and Development |
| ROW | Rest of the World |
| BRIICS | Brazil, Russia, India, China and South Africa |
| PV | Photovoltaics |
| CO2 | Carbon Dioxide |
| KMnO4 | Potassium Permanganate |
| IX | Ion Exchange |
| EDR | Electrodialysis Reversal Desalination |
| ERD | Energy recovery device |
| TDS | Total Dissolved Solids |
| SAR | Sodium Adsorption Ratio |
| MGD | Million Gallons Per Day |
| MgO | Magnesium Oxide |
| NaCl | Sodium Chloride |
| NaOH | Sodium Hydroxide |
| HCl | Hydrochloric acid |
| CaCO3 | Calcium Carbonate |
| Na2CO3 | Sodium Carbonate |
| MgCO3 | Magnesium Carbonate |
| CaCl2 | Calcium Chloride |
| NaHCO3 | Sodium Bi-Carbonate |
| Al2O3 | Aluminium Oxide |
| GO | Graphene Oxide |
| TiO2 | Titanium Dioxide |
| CNT | Carbon Nano Tube |
| SEM | Scanning Electron Microscope |
| SiO2 | Silicon Dioxide |
| Au | Gold |
| Ag | Silver |
| MPa | Mega Pascal (unit of pressure; 1 MPa = 10 bar) |
| USA | the United States of America |
| ZnO | Zinc Oxide |
| Fe3O4 | Magnetite |
| Fe2O3 | Iron (III) oxide |
| SnO2 | Tin (IV) oxide |
| CuO | Copper (II) oxide |
| CuO2 | Copper Peroxide |
| MED-TVC | Multi-Effect Distillation-Thermal Vapour Compression |
| IBM | International Business Machines |
| QAPCO | Qatar Petrochemical Company |
| KWh | Kilo-Watt-Hour (Unit of energy) |
| km3 | Cubic Kilometre (1E + 9 cubic meter) |
References
- World Data Lab, Water Scarcity Clock. 2019. Available online: https://worldwater.io/?utm_source=google&utm_medium=search&utm_campaign=WaterscarcityData&campaignid=6444167483&adgroupid=77198318295&adid=376808482554&gclid=Cj0KCQiAl5zwBRCTARIsAIrukdNoi-wWAxdiHfZz8CuBTge4MXwf3fGOqByGFwziwrWDYr5ikX7BiqAaAliPEALw_wcB (accessed on 31 December 2019).
- The United Nations World Water Report, 2016. Water and Jobs. Available online: http://unesdoc.unesco.org/images/0024/002439/243938e.pdf (accessed on 29 October 2018).
- Dickinson, B. In 20 Years, Water Demand Will Exceed Supply by 40 Percent (Population and Climate Change Will Put a Strain on Our Water Supply). ZDNet-Innovation. 28 February 2011. Available online: https://www.zdnet.com/article/in-20-years-water-demand-will-exceed-supply-by-40-percent/ (accessed on 9 April 2018).
- Ghaffour, N.; Bundschuh, J.; Mahmoudi, H.; Goosen, M.F.A. Renewable energy-driven desalination technologies: A comprehensive review of challenges and potential applications of integrated systems. Desalination 2015, 356, 94–114. [Google Scholar] [CrossRef]
- International Desalination Association. Desalination by the Numbers. 2019. Available online: https://idadesal.org/ (accessed on 13 May 2019).
- Jones, E.; Qadir, M.; Van Vliet, M.T.H.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- UN-Water. Clean Water and Sanitation–Progress on Level of Water Stress (Global Baseline for SDG Indicator 6.4.2). Food and Agriculture Organization of the United Nations. 2018. Available online: http://www.unwater.org/publications/progress-on-level-of-water-stress-642/ (accessed on 13 May 2019).
- Tester, J.W.; Drake, E.M.; Driscoll, M.J.; Golay, M.W. Sustainable Energy: Choosing Among Options; MIT Press: London, UK, 2012. [Google Scholar]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2006, 3, 1167990. [Google Scholar] [CrossRef]
- Burn, S.; Hoang, M.; Zarzo, D.; Olewniak, F.; Campos, E.; Bolto, B.; Barron, O. Desalination techniques—A review of the opportunities for desalination in agriculture. Desalination 2015, 364, 2–16. [Google Scholar] [CrossRef]
- Lenntech, B.V. Processes for Pre-Treatment of Seawater. Available online: https://www.lenntech.com/processes/desalination/pretreatment/general/desalination-pretreatment.htm (accessed on 9 April 2018).
- Dawoud, M.A.; Al Mulla, M.M. Environmental Impacts of Seawater Desalination: Arabian Gulf Case Study. Int. J. Environ. Sustain. 2012, 1, 22–37. [Google Scholar] [CrossRef]
- Condorchem envitech. Evaporation Systems for Water Desalination. Available online: https://blog-en.condorchem.com/evaporation-systems-water-desalination/#.XNk3uY4zZPb (accessed on 13 May 2019).
- DME Desalination Institute. Classification of Desalination Technologies. Available online: https://www.dme-gmbh.de/desalination-technology/technology-basics/classification-of-desalination-technologies/ (accessed on 13 May 2019).
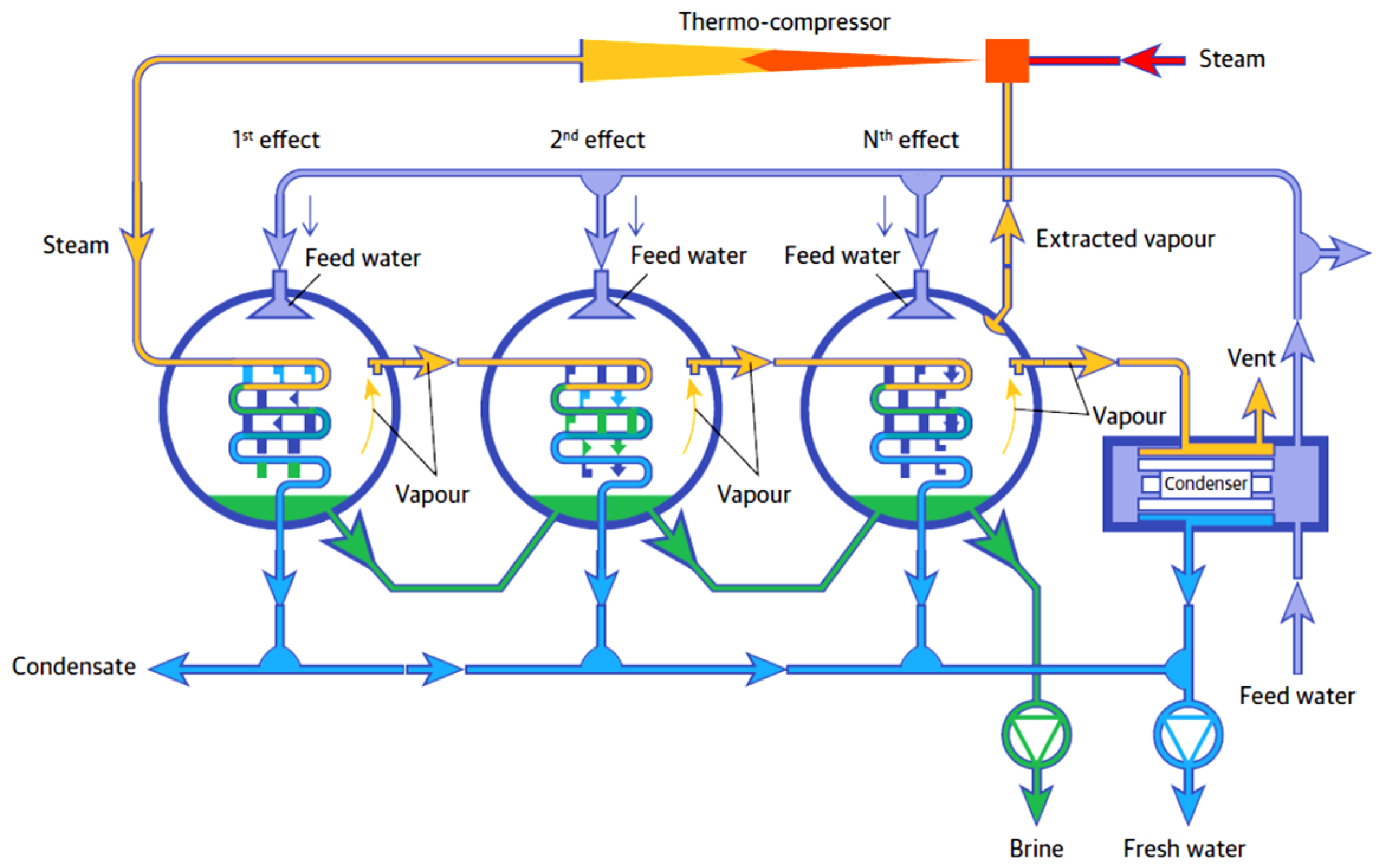
- SIDEM Veolia. Multiple Effect Distillation Process. Available online: http://www.sidem-desalination.com/Process/Thermal-desalination-MED/MED/Process/ (accessed on 13 May 2019).
- Voutchkov, N. Desalination Engineering-Planning and Design; Water Globe Consulting, LLC: WEF Press (For the Water Quality Professional): Water Reuse Association (Sustainable Solutions for a Thirsty Planet)-Mc Graw Hill: Florida, FL, USA, 2013; ISBN 978-0-07-177716-2. [Google Scholar]
- Finger, C.R. From the Sea to Your House: Post-Treatment of Desalinated Water, Abengoa Solar. 2014. Available online: http://www.theenergyofchange.com/sea-house-post-treatment-of-desalinated-water (accessed on 14 May 2019).
- Lenntech. SEAWATER DESALINATION: Post-Treatment Processes. 2019. Available online: https://www.lenntech.com/processes/desalination/post-treatment/general/desalination-postreatment.htm (accessed on 14 May 2019).
- Lenntech. Desalination Post-treatment: Neutralization/Remineralization for Drinking Water. 2019. Available online: https://www.lenntech.com/processes/desalination/post-treatment/post-treatments/remineralization.htm (accessed on 14 May 2019).
- Kim, I.S.; Hwang, M.; Choi, C. Membrane-Based Desalination Technology for Energy Efficiency and Cost Reduction. In Desalination Sustainability (A Technical, Socioeconomic, and Environmental Approach); Elsevier: Amsterdam, The Netherlands, 2017; pp. 31–74. [Google Scholar] [CrossRef]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on the specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Gavarrell, P.G. Thermal Energy Storage for High Temperature Applications. Ph.D. Thesis, Departamento de Ingeniería Energética, Escuela Superior de Ingeniería, Universidad de Sevilla, Sevilla, Spain, 2017. [Google Scholar]
- Zhang, Z.; Cai, J.; Chen, F.; Li, H.; Zhang, W.; Qi, W. Progress in enhancement of CO2 absorption by nanofluids: A mini-review of mechanisms and current status. Renew. Energy 2018, 118, 527–535. [Google Scholar] [CrossRef]
- Kasaeian, A.; Daneshazarian, R.; Mahian, O.; Kolsi, L.; Chamkha, A.J.; Wongwises, S.; Pop, I. Nanofluid flow and heat transfer in porous media: A review of the latest developments. Int. J. Heat Mass Transf. 2017, 107, 778–791. [Google Scholar] [CrossRef]
- Sharshir, S.W.; Guilong, P.; Lirong, W.; Nuo, Y.; Essa, F.A.; Elsheikh, A.H.; Mohamed, S.I.T.; Kabeel, A.E. Enhancing the solar still performance using nanofluids and glass cover cooling: Experimental study. Appl. Therm. Eng. 2017, 113, 684–693. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X.; Liang, H. Application of recoverable carbon nanotube nanofluids in solar desalination system: An experimental investigation. Desalination 2017. [Google Scholar] [CrossRef]
- Wang, M.; Fang, G.; Liu, P.; Zhou, D.; Ma, C.; Zhang, D.; Zhan, J. Fe3O4 @β-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl. Catal. B Environ. 2016, 188, 113–122. [Google Scholar] [CrossRef]
- Shao, L.; Wang, X.F.; Ren, Y.M.; Wang, S.F.; Zhong, J.R.; Chu, M.F.; Tang, H.; Luo, L.Z.; Xie, D.H. Facile fabrication of magnetic cucurbit [6]uril/graphene oxide composite and application for uranium removal. Chem. Eng. J. 2016, 286, 311–319. [Google Scholar] [CrossRef]
- Kabeel, A.E.; El-Said, E.M.S. Applicability of flashing desalination technique for small-scale needs using a novel integrated system coupled with the nanofluid-based solar collector. Desalination 2014, 333, 10–22. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X.; Li, L. Experimental investigation of SiC nanofluids for solar distillation system: Stability, optical properties and thermal conductivity with saline water-based fluid. Int. J. Heat Mass Transf. 2017, 107, 264–270. [Google Scholar] [CrossRef]
- Arunkumar, T.; Raj, K.; Denkenberger, D.; Velraj, R. Heat carrier nanofluids in solar still–A review. Desalin. Water Treat. 2018, 130, 1–16. [Google Scholar] [CrossRef]
- Sahota, L.; Tiwari, G.N. Effect of Al2O3 nanoparticles on the performance of passive double slope solar still. Sol. Energy 2016, 130, 260–272. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Omara, Z.M.; Essa, F.A. Numerical investigation of modified solar still using nanofluids and external condenser. J. Taiwan Inst. Chem. Eng. 2017, 75, 77–86. [Google Scholar] [CrossRef]
- Elango, T.; Kannan, A.; Murugavel, K.K. Performance studies on single basin single slope solar still with different water nanofluids. Desalination 2015, 360, 45–51. [Google Scholar] [CrossRef]
- Gupta, B.; Shankar, P.; Sharma, R.; Baredar, P.T. Performance enhancement using nanoparticles in the modified, passive solar still. Procedia Technol. 2016, 25, 1209–1216. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Omara, Z.M.; Essa, F.A.; Abdullah, A.S.; Arunkumar, T.; Sathyamurthy, R. Augmentation of a solar still distillate yield via black absorber plate coated with black nanoparticles. Alex. Eng. J. 2017, 56, 433–438. [Google Scholar] [CrossRef]
- Sain, M.K.; Kumawat, G. Performance enhancement of single slope solar still using nano-particles mixed with black paint. Adv. Nanosci. Technol. 2015, 1, 55–65. [Google Scholar]
- Negewo, D.; Bekele Christopher WS-World Bank Group. The Role of Desalination in an Increasingly Water-Scarce World; Report ID: 135312, 1; Technical Paper-Water Global Practice: Washington, DC, USA, March 2019; Available online: http://documents.worldbank.org/curated/en/476041552622967264/pdf/135312-WP-PUBLIC-14-3-2019-12-3-35-W.pdf (accessed on 23 May 2019).
- Nisan, S.; Benzarti, N. A Comprehensive Economic Evaluation of Integrated Desalination Systems, Using Fossil-Fuelled and Nuclear Energies and Including Their Environmental Costs; INIS 41 and Issue 6, Reference number: 41021704 and Reference record: 41021686; International Atomic Energy Agency (IAEA): Vienna, Austria, 2009; Available online: https://www-pub.iaea.org/MTCD/publications/PDF/P_1354_CD/PDF/P_1354.pdf (accessed on 23 May 2019).
- Mansour, S.; Arafat, H.A.; Hasan, S.W. Brine Management in Desalination Plants–Desalination Sustainability-Technical, Socioeconomic, and Environmental Approach; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809791-5. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Marzooqi, F.A.; Kaabi, M.A.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Rosado, D.; Bakouri, H.E.; Riaza, A.; Bernaola, F.J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- The Great Bubble Barrier. 2019. Available online: https://thegreatbubblebarrier.com/en/bubble-barrier-en/ (accessed on 31 December 2019).
- TU Delft. The Ocean Clean Up. 2017. Available online: https://www.delta.tudelft.nl/article/ocean-cleanup-will-it-deliver (accessed on 31 December 2019).
- Membracon, U.K. What’s the Difference between Microfiltration, Ultrafiltration and Nanofiltration. 2019. Available online: https://www.membracon.co.uk/blog/whats-the-difference-between-microfiltration-ultrafiltration-and-nanofiltration/ (accessed on 31 December 2019).
- Bitar, R.W.; Ahmad, A. Issam Fares Institute for Public Policy and International Affairs; solar vs. Nuclear Which is Cheaper for Water Desalination. Policy Brief February 2017. Available online: http://website.aub.edu.lb/ifi/publications/Documents/policy_memos/2016-2017/20170215_solar_nuclear.pdf (accessed on 9 April 2018).
- Jung, Y.H.; Jeong, Y.H.; Choi, J.; Wibisono, A.F.; Lee, J.I.; Cheon, N.H. Feasibility study of a small-sized nuclear heat-only plant dedicated to desalination in the UAE. Desalination 2014, 337, 83–97. [Google Scholar] [CrossRef]
- Frantz, C.; Seifert, B. Thermal analysis of a multi-effect distillation plant powered by a solar tower plant. International Conference on Concentrating Solar Power and Chemical Energy Systems, SolarPACES 2014. Energy Procedia 2015, 69, 1928–1937. [Google Scholar] [CrossRef]
- Ali, M.T.; Fath, H.E.S.; Armstrong, P.R. A comprehensive techno-economical review of indirect solar desalination. Renew. Sustain. Energy Rev. 2011, 15, 4187–4199. [Google Scholar] [CrossRef]
- Shatat, M.; Riffat, S.; Ghabayen, S. State of the art water desalination technologies using conventional and sustainable energy sources. In Proceedings of the 4th International Engineering Conference–Towards Engineering of the 21st Century, Gaza, Palestine, 15–16 October 2012; Available online: http://research.iugaza.edu.ps/files/2153.PDF (accessed on 9 September 2018).
- Casimiro, S.; Cardoso, J.; Alarcón-Padilla, D.; Turchi, C.; Ioakimidis, C.; Farinha Mendes, J. Modellingmulti-effect distillation powered by CSP in TRNSYS. Energy Procedia 2014, 49, 2241–2250. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Assad, M.E.H.; Sayed, E.T.; Soudan, B. Recent progress in the use of renewable energy sources to power water desalination plants. Desalination 2018, 435, 97–113. [Google Scholar] [CrossRef]
- Hassabou, A.H.; Spinnler, M.; Polifke, W. Techno-economic Analysis of Medium and Large-scale Desalination Plants Driven by Concentrated Solar Systems in the Mena Region. Energy Procedia 2013, 42, 735–744. [Google Scholar] [CrossRef]
- Beitelmal, A.H.; Fabris, D. Off-the-grid solar-powered portable desalination system. Appl. Therm. Eng. 2015, 85, 172–178. [Google Scholar] [CrossRef]
- Farshchi, P. (Deputy for the Marine Environment at the Department of Environment Iran). Persian Gulf Salinity Rising. Financial Tribune. 31 May 2016. Available online: https://financialtribune.com/articles/people-environment/42562/persian-gulf-salinity-rising (accessed on 9 April 2018).
- Todorova, V. Desalination Threat to the Growing Gulf. The National UAE. 2009. Available online: https://www.thenational.ae/uae/environment/desalination-threat-to-the-growing-gulf-1.553346vtodorova@thenational.ae (accessed on 9 April 2018).
- Li, Z.; Siddiqi, A.; Anadon, L.D.; Venky Narayanamurti, V. Towards sustainability in water-energy nexus: Ocean energy for seawater desalination. Renew. Sustain. Energy Rev. 2018, 82, 3833–3847. [Google Scholar] [CrossRef]
- IEA-ETSAP and IRENA. Technology Brief I12. March 2012. Available online: https://www.ctc-n.org/sites/www.ctc-n.org/files/resources/irena-etsap_tech_brief_i12_water-desalination.pdf (accessed on 31 October 2018).
- Alkaisi, A.; Mossad, R.; Barforoush, A.S. A review of the water desalination systems integrated with renewable energy. 1st International Conference on Energy and Power, ICEP2016, 14–16 December 2016, RMIT University, Melbourne, Australia. Energy Procedia 2017, 110, 268–274. [Google Scholar] [CrossRef]
- Qiblawey, H.M.; Banat, F. Solar thermal desalination technologies. Desalination 2008, 220, 633–644. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Neumann, O.; Nordlander, P.; Halas, N.J. Solar thermal desalination as a nonlinear optical process. Proc. Natl. Acad. Sci. USA 2019, 116, 13182–13187. first published 17 June 2019. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.E.; Dall, E.P. Integrating desalination with concentrating solar thermal power: A Namibian case study. Renew. Energy 2018, 115, 423–432. [Google Scholar] [CrossRef]
- Ng, K.C.; Thu, K.; Oh, S.J.; Ang, L.; Shahzad, M.W.; Ismail, A.B. Recent developments in thermally-driven seawater desalination: Energy efficiency improvement by hybridization of the MED and AD cycles. Desalination 2015, 356, 255–270. [Google Scholar] [CrossRef]
- MEDAD Technologies Pte. Ltd.; Singapore, Reg No. 201111303 W, Year of Registration 2012, 6C Tanjong Rhu Road, #13-02, Singapore 438668. Available online: https://www.medad-tech.com/ (accessed on 22 January 2020).
- Kyaw, T.; Chakraborty, A.; Kim, Y.D.; Myat, A.; Saha, B.B.; Ng, K.C. Numerical simulation and performance investigation of an advanced adsorption desalination cycle. Desalination 2013, 308, 209–218. [Google Scholar] [CrossRef]
- Thu, K.; Kim, Y.D.; Shahzad, M.W.; Saththasivam, J.; Ng, K.C. Performance investigation of an advanced multi-effect adsorption desalination (MEAD) cycle. Appl. Energy 2015, 159, 469–477. [Google Scholar] [CrossRef]
- Mabrouk, A.; Abotaleb, A.; Tahir, F.; Koc, M.; Al-Rashid, A. High-Performance MED Desalination Plants Part I: Novel Design MED Evaporator; The International Desalination Association World Congress on Water Reuse and Desalination: São Paulo, Brazil, October 2017. [Google Scholar]
- Mabrouk, A.N.; Fath, H.E.S. Techno-economic study of a novel integrated thermal MSF–MED desalination technology. Desalination 2015, 371, 115–125. [Google Scholar] [CrossRef]
- Jani, H.K.; Modi, K.V. A review on numerous means of enhancing heat transfer rate in solar thermal-based desalination devices. Renew. Sustain. Energy Rev. 2018, 93, 302–317. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology to improve the performance of solar collectors–Recent advances and overview. Renew. Sustain. Energy Rev. 2016, 62, 767–792. [Google Scholar] [CrossRef]
- Singh, T.; Hussien, M.A.A.; Al-Ansari, T.; Saoud, K.; McKay, G. Critical review of solar thermal resources in GCC and application of nanofluids for the development of efficient and cost-effective CSP technologies. Renew. Sustain. Energy Rev. 2018, 91, 708–719. [Google Scholar] [CrossRef]
- Sharon, H.; Reddy, K.S. A review of solar energy-driven desalination technologies. Renew. Sustain. Energy Rev. 2015, 41, 1080–1118. [Google Scholar] [CrossRef]
- Reif, H.; Alhalabi, W. Solar-thermal powered desalination: Its significant challenges and potential. Renew. Sustain. Energy Rev. 2015, 48, 152–165. [Google Scholar] [CrossRef]
- Hassaboou, A.M.A. Combination Photovoltaic and Thermal Energy System. U.S. Patent Application. Pub. No. US 2017/0230000 A1, 10 August 2017. [Google Scholar]
- Al-Karaghouli, A.; Renne, D.; Kazmerski, L.L. Solar and wind opportunities for water desalination in the Arab regions. Renew. Sustain. Energy Rev. 2009, 13, 2397–2407. [Google Scholar] [CrossRef]
- El-Ghonemy, A.M.K. Future sustainable water desalination technologies for Saudi Arabia: A review. Renew. Sustain. Energy Rev. 2012, 16, 6566–6597. [Google Scholar] [CrossRef]
- Barau, A.S.; Al-Hosani, N. Prospects for environmental governance in addressing sustainability challenges of seawater desalination industry in the Arabian Gulf. Environ. Sci. Policy 2015, 50, 145–154. [Google Scholar] [CrossRef]
- Gude, V.G.; Nirmalakhandan, N.; Deng, S. Renewable and sustainable approaches for desalination. Renew. Sustain. Energy Rev. 2010, 14, 2641–2654. [Google Scholar] [CrossRef]
- Ghaffour, N.; Reddy, V.K.; Abu-Arabia, M. Technology development and application of solar energy in desalination: MEDRC contribution. Renew. Sustain. Energy Rev. 2011, 15, 4410–4415. [Google Scholar] [CrossRef]
- Goosen, M.F.A.; Mahmoudi, H.; Ghaffour, N. Today’s and Future Challenges in Applications of Renewable Energy Technologies for Desalination. J. Crit. Rev. Environ. Sci. Technol. 2014, 44. [Google Scholar] [CrossRef]
- Shahzad, M.W.; Burhan, M.; Ybyraiymkul, D.; Ng, K.C. Desalination processes efficiency and future roadmap. Entropy 2019, 21, 84. [Google Scholar] [CrossRef]
- Jama, M.; Singh, T.; Gamaleldin, S.M.; Koc, M.; Samara, A.; Isaifan, R.J.; Atieh, M.A. Critical Review on Nanofluids: Preparation, Characterization, and Applications; August 2016. J. Nanomater. 2016, 6717624. [Google Scholar] [CrossRef]
- Yang, X.-F.; Liu, Z.-H. Pool boiling heat transfer of functionalized nanofluid under sub-atmospheric pressures. Int. J. Therm. Sci. 2011, 50, 2402–2412. [Google Scholar] [CrossRef]
- Jamshidi, N.; Farhadi, M.; Ganji, D.D.; Sedighi, K. Experimental investigation on the viscosity of nanofluids. Int. J. Eng. Trans. B Appl. 2012, 25, 201–209. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Mamat, R.; Usri, N.A.; Najafi, G. Effect of titanium oxide nanofluid concentration on pressure drop. Arpn J. Eng. Appl. Sci. 2015, 10, 7815–7820. [Google Scholar]
- Kavitha, T.; Rajendran, A.; Durairajan, A. Synthesis, characterization of TiO2 nanopowder and water-based nanofluids using two-step method. Eur. J. Appl. Eng. Sci. Res. 2012, 1, 235–240. [Google Scholar]
- Chandrasekar, M.; Suresh, S.; Bose, A.C. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. Exp. Therm. Fluid Sci. 2010, 34, 210–216. [Google Scholar] [CrossRef]
- Soltani, S.; Etemad, S.G.; Thibault, J. Pool boiling heat transfer of non-Newtonian nanofluids. Int. Commun. Heat Mass Transf. 2010, 37, 29–33. [Google Scholar] [CrossRef]
- Gharagozloo, P.E.; Goodson, K.E. Temperature-dependent aggregation and diffusion in nanofluids. Int. J. Heat Mass Transf. 2011, 54, 797–806. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Thermophysical and pool boiling characteristics of ZnO-ethylene glycol nanofluids. Int. J. Therm. Sci. 2012, 62, 61–70. [Google Scholar] [CrossRef]
- Priya, K.R.; Suganthi, K.S.; Rajan, K.S. Transport properties of ultra-low concentration CuO-water nanofluids containing non-spherical nanoparticles. Int. J. Heat Mass Transf. 2012, 55, 4734–4743. [Google Scholar] [CrossRef]
- Michael, J.J.; Iniyan, S. Performance analysis of a copper sheet laminated photovoltaic thermal collector using copper oxide—Water nanofluid. Sol. Energy 2015, 119, 439–451. [Google Scholar] [CrossRef]
- Kannadasan, N.; Ramanathan, K.; Suresh, S. Comparison of heat transfer and pressure drop in the horizontal and vertical helically coiled heat exchanger with CuO/water-based nanofluids. Exp. Therm. Fluid Sci. 2012, 42, 64–70. [Google Scholar] [CrossRef]
- Bahai, M.S.; Esfahany, M.N.; Etesami, N. Experimental investigation of pool boiling of Fe3O4/ethylene glycol-water nanofluid in an electric field. Int. J. Therm. Sci. 2012, 62, 149–153. [Google Scholar]
- Sundar, L.S.; Naik, M.T.; Sharma, K.V.; Singh, M.K.; Reddy, T.C.S. Experimental investigation of forced convection heat transfer and friction factor in a tube with Fe3O4 magnetic nanofluid. Exp. Therm. Fluid Sci. 2012, 37, 65–71. [Google Scholar] [CrossRef]
- Karimi, A.; Goharkhah, M.; Ashjaee, M.; Shafii, M.B. Thermal conductivity of Fe2O3 and Fe3O4 magnetic nanofluids under the influence of the magnetic field. Int. J. Thermophys. 2015, 36, 2720–2739. [Google Scholar] [CrossRef]
- Vermahmoudi, Y.; Peyghambarzadeh, S.M.; Hashemabadi, S.H.; Naraki, M. Experimental investigation on heat transfer performance of Fe2O3/water nanofluid in an air-finned heat exchanger. Eur. J. Mech. B/Fluids 2014, 44, 32–41. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Hu, H. Effect of surfactant additives on nucleate pool boiling heat transfer of refrigerant-based nanofluid. Exp. Therm. Fluid Sci. 2011, 35, 960–970. [Google Scholar] [CrossRef]
- Tamjid, E.; Guenther, B.H. Rheology and colloidal structure of silver nanoparticles dispersed in diethylene glycol. Powder Technol. 2010, 197, 49–53. [Google Scholar] [CrossRef]
- Parametthanuwat, T.; Rittidech, S.; Pattiya, A. A correlation to predict heat-transfer rates of a two-phase closed thermosyphon (TPCT) using silver nanofluid at normal operating conditions. Int. J. Heat Mass Transf. 2010, 53, 4960–4965. [Google Scholar] [CrossRef]
- John, J.; Thomas, L.; Kumar, B.R.; Kurian, A.; George, S.D. Shape-dependent heat transport through green synthesized gold nanofluids. J. Phys. D Appl. Phys. 2015, 48, 335301. [Google Scholar] [CrossRef]
- Soltaninejad, S.; Husin, M.S.; Sadrolhosseini, A.R.; Zamiri, R.; Zakaria, A.; Moksin, M.M.; Gharibshahi, E. Thermal diffusivity measurement of Au nanofluids of very low concentration by using photoflash technique. Measurement 2013, 46, 4321–4327. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Li, Y.; Yu, W. Nanofluids containing carbon nanotubes treated by the mechanochemical reaction. Thermochim. Acta 2008, 477, 21–24. [Google Scholar] [CrossRef]
- Garg, P.; Alvarado, J.L.; Marsh, C.; Carlson, T.A.; Kessler, D.A.; Annamalai, K. An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multiwall carbon nanotube-based aqueous nanofluids. Int. J. Heat Mass Transf. 2009, 52, 5090–5101. [Google Scholar] [CrossRef]
- Zhaoguo, M.; Daxiong, W.; Wang, L.; Zhu, H.; Li, Q. Carbon nanotube glycol nanofluids: Photo-thermal properties, thermal conductivities and rheological behavior. Particuology 2012, 10, 614–618. [Google Scholar]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Micale, G.; Helsen, J.; Papapetrou, M. REA Power: Use of desalination brine for power production through reverse electrodialysis. Desalin. Water Treat. 2015, 53, 3161–3169. [Google Scholar] [CrossRef]
- El-Naas, M.; QU-CENG Team Develops a New Method for ‘Reject Brine’ Management. The Peninsula–Qatar’s Daily Newspaper, 20 November 2017. Available online: https://www.thepeninsulaqatar.com/article/20/11/2017/QU-CENG-team-develops-new-method-for-%E2%80%98reject-brine%E2%80%99-management (accessed on 22 July 2019).
- El-Naas, M. Process for the Capture of Carbon Dioxide and Desalination. U.S. Patent Application Patent No. US 10,118,843 B2, 6 November 2018. [Google Scholar]
- Hughes, W.B. Method of Providing Fertilizer from Brines. U.S. Patent Application Patent Number 4,576,627, 18 March 1986. [Google Scholar]
- Wijayarathne, U.; Perera, S.A.S.; Wasalathilake, K.; Vidanage, P. Development of a Multi-Nutrient Fertilizer from Liquid Waste of Solar Salt Manufacturing Process. In Proceedings of the IEEE Xplore–2015 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 7–8 April 2015. [Google Scholar] [CrossRef]
- Zachary, J.; Layman, C.M. Adding Desalination to Solar Hybrid and Fossil Plants-Bechtel Power Corp. 2010. Available online: https://www.powermag.com/adding-desalination-to-solar-hybrid-and-fossil-plants/?pagenum=5 (accessed on 2 January 2020).


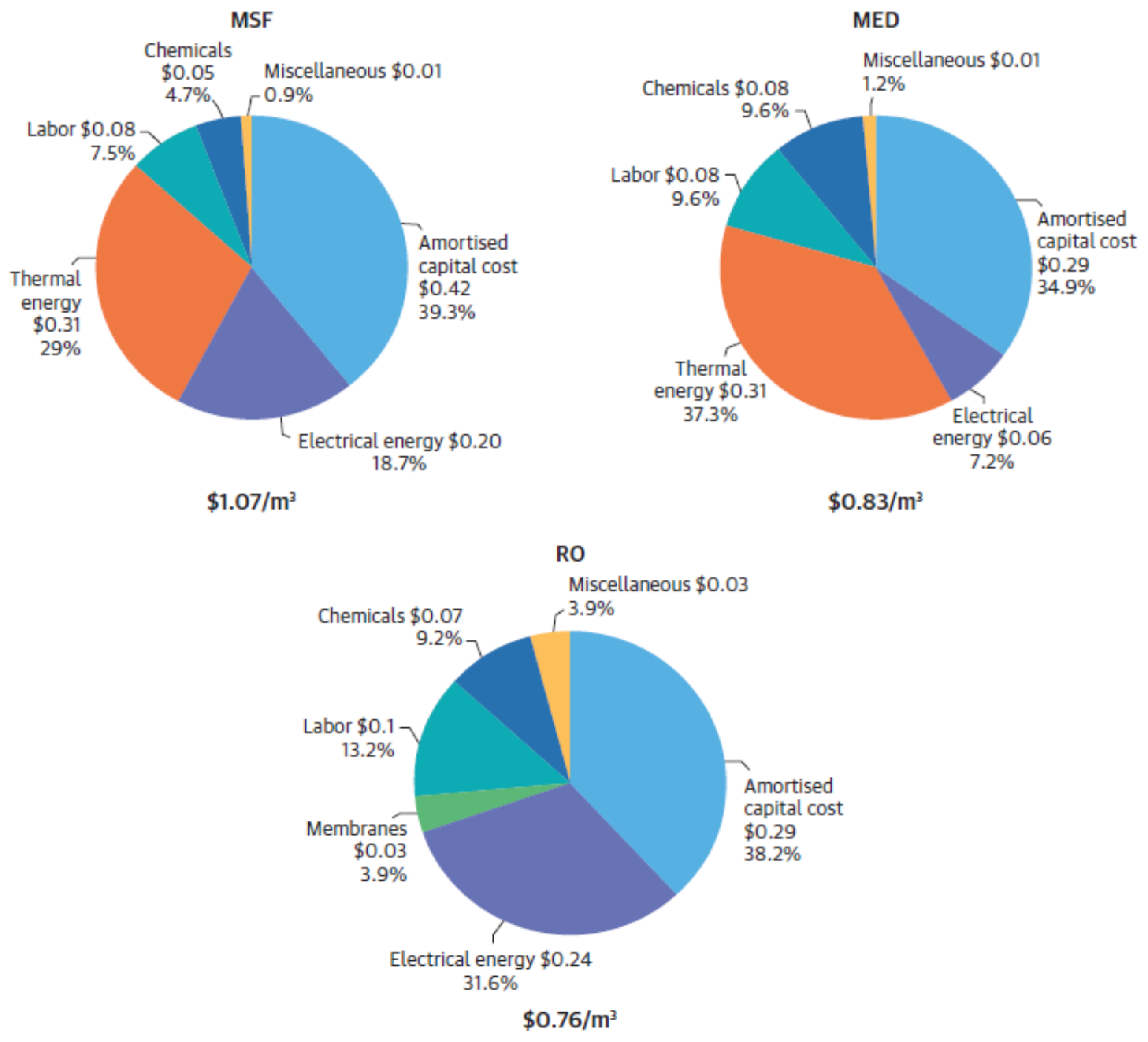
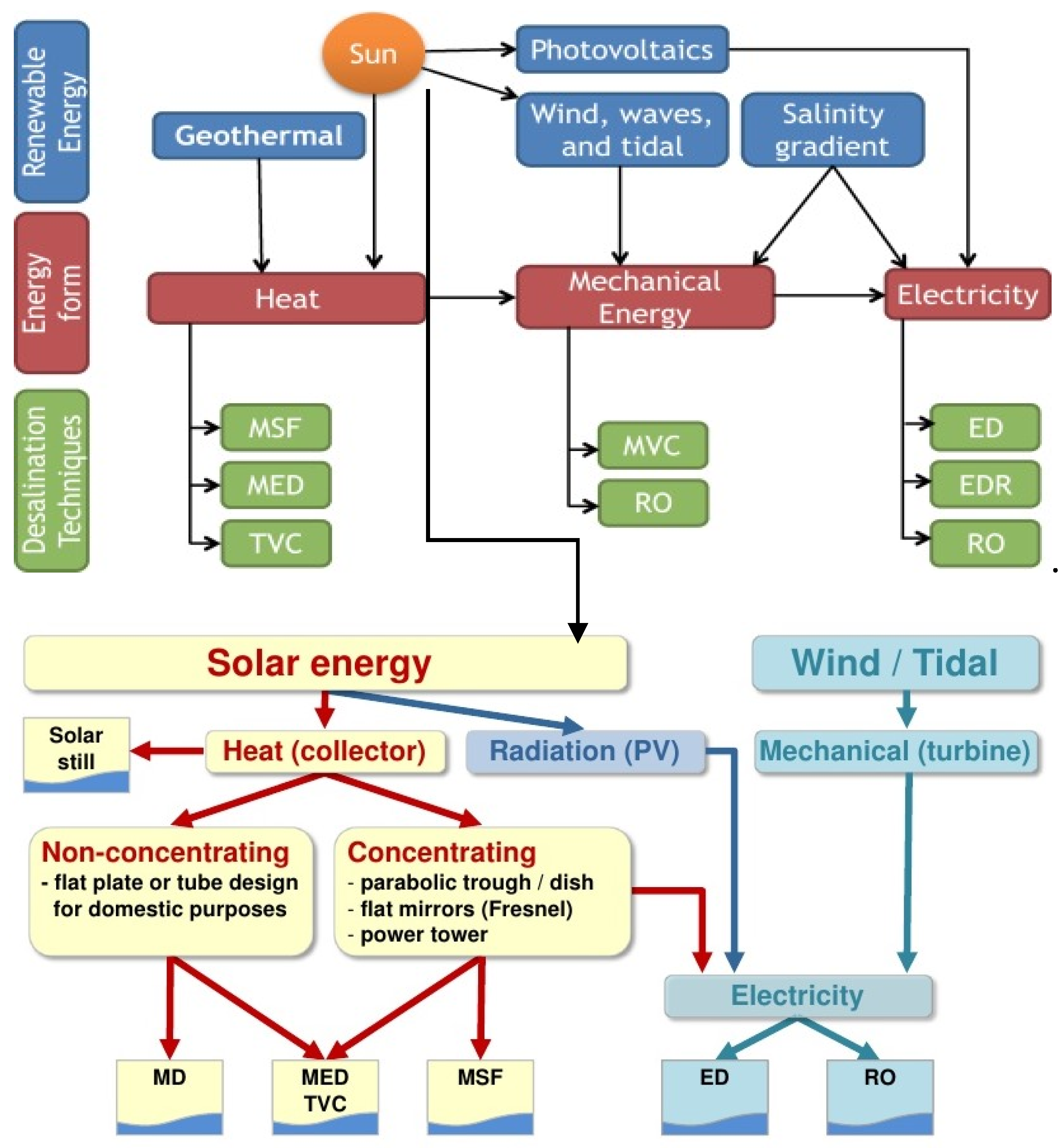
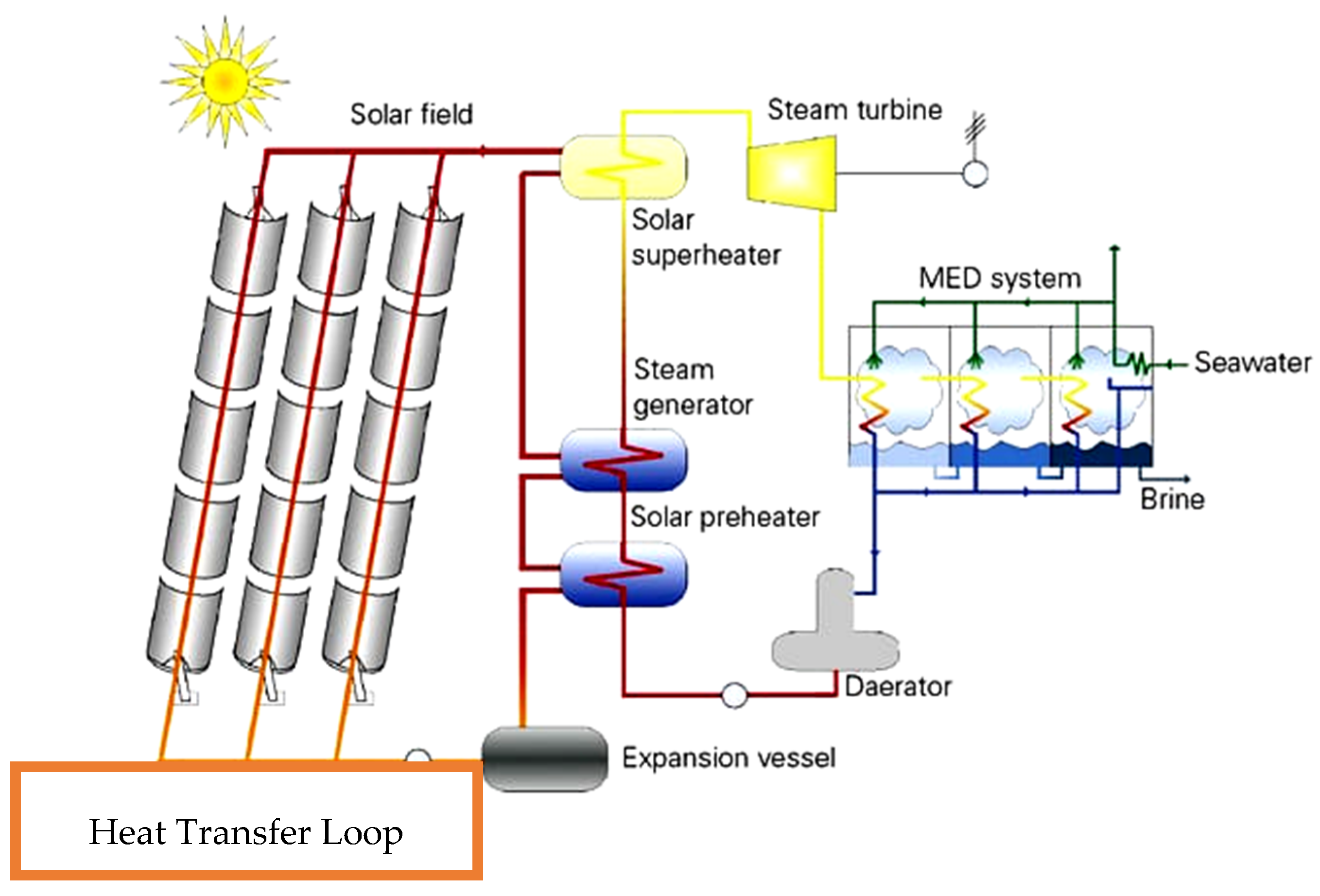
| Fouling | Cause | Appropriate Pre-Treatment |
|---|---|---|
| Biological | Bacteria, Microorganisms, Viruses, Protozoan | Chlorination |
| Colloidal | Organic and inorganic complexes, colloidal particles, micro-algae | Coagulation + filtration Optional: flocculation/sedimentation |
| Mineral | Barium, Calcium, Magnesium, or Strontium sulphates or carbonates | Antiscalant dosing; Acidification |
| Organic | Natural Organic Matter (NOM): Fulvic and Humic acids, biopolymers | Coagulation + filtration + Activated carbon adsorption; Coagulation + Ultra-filtration |
| Particle | Sand, Clay (Turbidity & Suspended Solids) | Filtration |
| Oxidant | Chlorine, Ozone, KMnO4 | Oxidant Scavenger dosing: Sodium (meta) bisulphite and Granulated Activated Carbon |
| Separation Mechanism | Energy | Process | Technology |
|---|---|---|---|
| Phase Change | Thermal + Electrical | Evaporation | Multi-Stage Flash (MSF), Multi-Effect Distillation (MED), Thermal Vapour Compression (TVC), Solar distillation, Ocean Thermal Energy Conversion (OTEC) (Liquid/Gas-phase change) |
| Crystallization | Freezing and Formation of Hydrates (Liquid/Solid-phase change) | ||
| Evaporation and filtration | Membrane Distillation (MD) (Liquid/Gas phase change) | ||
| Mechanical | Evaporation | Mechanical Vapour Compression (MVC) (Liquid/Gas phase change) | |
| No phase change (Single Phase) | Mechanical | Filtration | Reverse Osmosis (RO) and Nanofiltration (Solute diffusion) |
| Electrical | Selective filtration (Ionic migration) | Electro Dialysis (ED) (Electrochemical separation) | |
| Chemical | Exchange | Ion Exchange (IX) and Extraction (Electrochemical separation) |
| Process | Drinking-Water | Irrigation Water | Process Water |
|---|---|---|---|
| Removal of NaCl | Second pass RO | - | Second pass RO |
| Re-mineralization | Calcium and Magnesium addition | Calcium and Magnesium addition | - |
| Neutralization (pH = 7) | NaOH/HCl injection | NaOH/HCl injection | NaOH/HCl injection |
| Boron Removal | NaOH + second pass RO | Boron specific ion exchange resin | - |
| Disinfection | Required | Not Required | Not Required |
| Remineralization Process | Description | Minerals | Water Quality |
|---|---|---|---|
| 1 | Blending with 1% clarified seawater + pH neutralization | 15 mgL−1 Magnesium + 5 mgL−1 Calcium + 125 mgL−1 Sodium+ 220 mgL−1 Chlorine + 25 mgL−1 sulphate salts pH 7–7.5 | Medium |
| 2 | CO2 addition + Calcite Limestone (CaCO3, MgO) percolation + Na2CO3 | 80 mgL−1 CaCO3 pH 7–7.5 | Good |
| 3 | CO2 addition + Dolomite Limestone (CaCO3, MgCO3) percolation + Na2CO3 | 80 mgL−1 CaCO3 + MgCO3 pH 7–7.5 | Very Good |
| 4 | CaCl2 + NaHCO3 addition | 100 mgL−1 CaCO3 + 100 mgL−1 Sodium + 50 mgL−1 Chlorine pH 7–7.5 | Medium |
| Base Fluid | Nanofluid | Nanoparticles Thermal Conductivity (W × (mK)−1) | Volume Concentration (%) | Observation | Ref. |
|---|---|---|---|---|---|
| Water | Al2O3 | 46 | 0.04, 0.08, 0.12 | For 0.12%, the productivity enhanced by 16.83% | [32] |
| Water | CuO | 17.6 | 0.02 | Efficiency of solar still increased by 84.16% for CuO and 73.85% for Al2O3 | [33] |
| Al2O3 | 46 | 0.02 | |||
| Water |
| 46 | 0.05–0.1 | 29.95% | [34] |
| 29 | 0.05–0.1 | 12.67% | ||
| 6 | 0.05–0.1 | ---------- | ||
| 1.34–1.38 | 0.05–0.1 | 18.63% | ||
| Productivity enhancement | |||||
| Water | CuO | 17.6 | 0.12 | 22.5% in the total productivity | [35] |
| Mix with black paint | CuO2 | 76.5 | 10–40 | 25% increment in productivity | [36] |
| Mixed with black paint | Al2O3 | 46 | ------- | 38.09% increment in productivity | [37] |
| Water | Graphite Flakes | 129 | 0.125–2 | Productivity increased by 53.95% for graphite flakes while 44.91% increment was observed for CuO nanofluids | [38] |
| CuO | 17.6 | ||||
| S.No. | Type of Power Plants | MSF (Million t × yr−1) | RO (Million t × yr−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | SOx | NOx | Particles | CO2 | SOx | NOx | Particles | ||
| 1 | Coal Fired | 264.5 | 0.33 | 0.54 | 0.04 | 32.2 | 0.04 | 0.07 | 0.005 |
| 2 | Oil Fired | 216.2 | 1.31 | 0.30 | 0.03 | 25.7 | 0.16 | 0.04 | 0.003 |
| 3 | Gas Turbine | 141.6 | 0.01 | 0.23 | 0.01 | 12.9 | 0.001 | 0.02 | 0.001 |
| Type | Base Fluid | Synthesis Process | Particle Loading (Vol %) | Particle Size (nm) | Dispersion Method | Stability | Ref |
|---|---|---|---|---|---|---|---|
| SiO2 | Water | Two-step | 10 | NR | 3-Glycid oxyl proyl tri-methy oxy silane (mass ratio of silane to silica = 0.115) + solution kept at 50 C for 12 h | 12 months | [83] |
| SiO2 | DI water | Two-step | NR | NR | Oscillated in an ultrasonic bath for 12 h | Several days | [83] |
| SiO2 | EG, water, EG/water solutions, TO/water solution | Two-step | NR | 10 (for 600 m2/g specific surface area) | Ultrasonic disrupter technique | NR (Not Reported) | [84] |
| TiO2 | EG/Water | Two-step | 0.5–1.5 | 50 | Ultrasonic bath for 2 h | NR | [85] |
| TiO2 | Water | Two-step | 1, 1.5, and 2.0 | 6 | Mixture sonicated in magnetic stirrer followed by ultrasonic vibration for 2 h | NR | [86] |
| Al2O3 | Water | Two-step | NR | NR | Ultrasonic pulses of 100 W and 36 ± 3 kHz for six hours and pH = 4.8 | Several weeks | [87,88] |
| Al2O3 | Water | Two-step | 20 | 40.2 | Mixture diluted by 1% nitric acid + sonication continuously four hours at 60 Hz and 130 W | One week | [89] |
| ZnO | Water | Two-step | NR | 150–80 | Acetyl acetone + sonication for 10 min | 9–12 months | [88] |
| ZnO | EG | Two-step | 0.005 and 0.0375 | <50 | Intense Ultrasonication (200 W) for 100 h | NR | [90] |
| CuO | Water | Two-step | NR | NR | Ultrasonication for 6 h + surfactant Tiron (CuO: Tiron = 2.5:1). Zeta potential = 30 mV ensured | NR | [91] |
| CuO | Distilled Water | Two-step | 0.05 vol % | NR | Sodium dodecylbenzene sulphonate (SDBS) of 10 wt % of nanoparticles (reason: to prevent immediate settlement as CuO NPs have a high density (6310 kg/m3) compared to water (995 kg/m3) + sonication for 60 min | Best with SDBS | [92] |
| CuO | Water | Two-step | NR | NR | Ultrasonic (100 W) for 4 h | 25 days | [93] |
| Fe3O4 | H2O+EG (50%: 50%) | Two-step | NR | NR | Sonicated for two hours with vigorous agitation for 30 min | More than 8 h | [94] |
| Fe3O4 | Water | Two-step | NR | NR | Sonication for 2 h + pH = 3 (use sulfuric acid (H2SO4)) | NR | [95] |
| α-Fe2O3 | Water | Two-step | 0.25, 0.5, 1, 2, 3, & 4 | 20–40 | Sonication + tetra methyl ammonium hydroxide | NR | [96] |
| Fe2O3 | Water | Two-step | 0.02 vol | 40 | PH = 11.1 + PEG as surfactant + magnetic stirring for 1 h | 7 days | [97] |
| Cu | DI water | NR | 9 | NR | Laureate salts + ultrasonic vibration | 30 h | [98] |
| Cu | NR | Two-step | NR | NR | Sodium dodecyl sulfate, cetyl trimethyl ammonium bromide, and sorbitan monooleate | 24 h | [99] |
| Ag | DEG | Two-step | NR | NR | Continuous stirring & agitation for 5 min by an ultrasonic agitator | NR | [100] |
| Ag | NR | Two-step | NR | NR | Ultrasonic bath for 3 h | 48 h | [101] |
| Au | Distilled Water | Two-step | NR | NR | Solution boiled for 10 min at 80 °C | NR | [102] |
| Au | Water (laser ablation) | One-step | NR | NR | Stirred magnetically | NR | [103] |
| MWCNT (treated with hydrophilic functional groups) | NR | Two-step | NR | NR | NR | Several months | [104] |
| CNTs | Water | Two-step | NR | NR | 0.2 wt % chitosan | 2 months | [105] |
| CNTs | Glycol | Two-step | NR | NR | Ultrasonic vibration (Gum Arabic, Tween 80, and CTAB) | >2 months | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, T.; Atieh, M.A.; Al-Ansari, T.; Mohammad, A.W.; McKay, G. The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review. Water 2020, 12, 2002. https://doi.org/10.3390/w12072002
Singh T, Atieh MA, Al-Ansari T, Mohammad AW, McKay G. The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review. Water. 2020; 12(7):2002. https://doi.org/10.3390/w12072002
Chicago/Turabian StyleSingh, Tejvir, Muataz Ali Atieh, Tareq Al-Ansari, Abdul Wahab Mohammad, and Gordon McKay. 2020. "The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review" Water 12, no. 7: 2002. https://doi.org/10.3390/w12072002
APA StyleSingh, T., Atieh, M. A., Al-Ansari, T., Mohammad, A. W., & McKay, G. (2020). The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review. Water, 12(7), 2002. https://doi.org/10.3390/w12072002