Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode
Abstract
1. Introduction
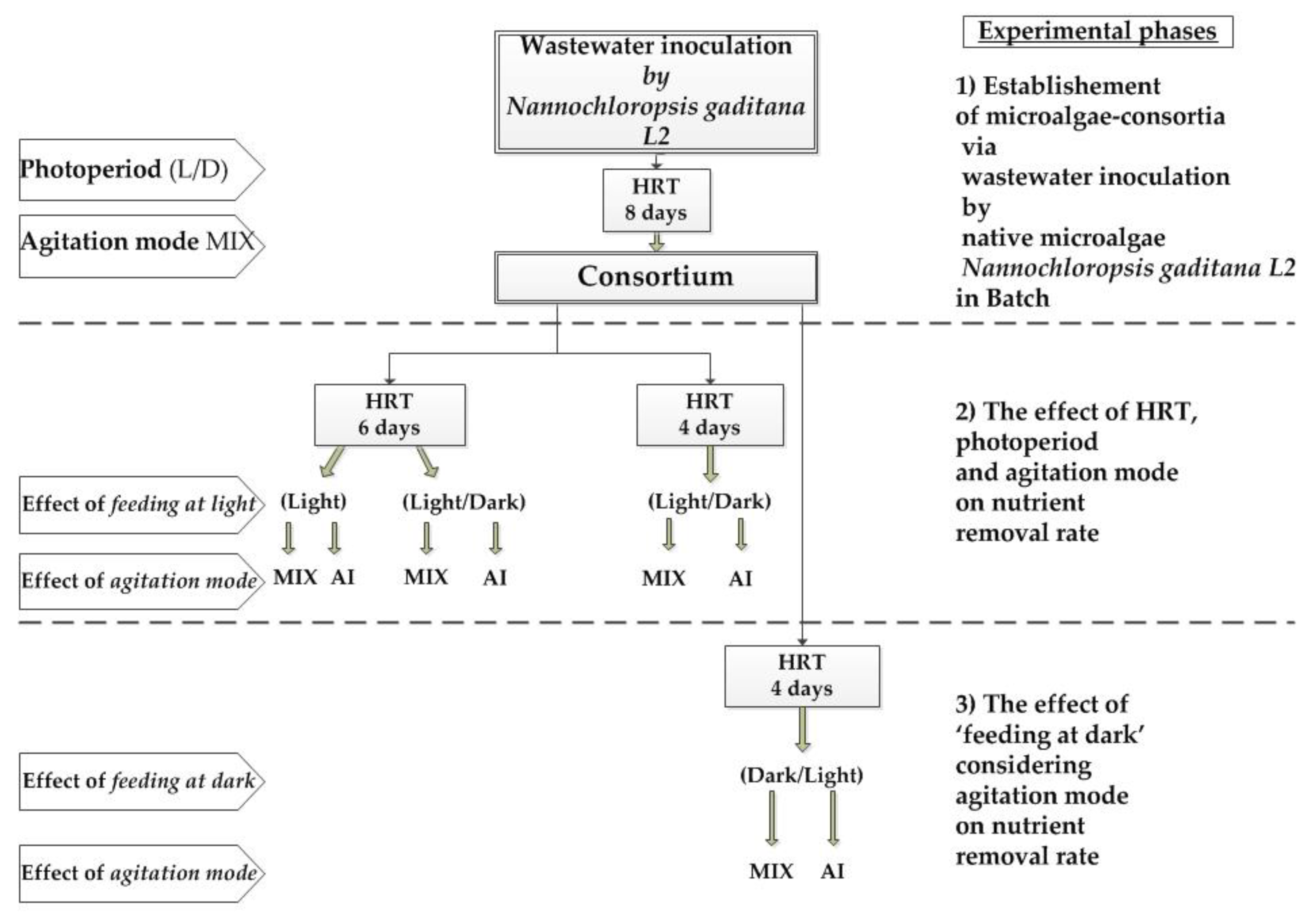
2. Materials and Methods
2.1. Composition of Raw Wastewater
2.2. Enrichment by the Algal Strain Nannochloropsis Gaditana L2
2.3. Bioreactor Set-up and Operations
2.4. Consortiums Establishment
2.5. Chemical Analysis and Data Processing
3. Results
3.1. Establishment of the Consortium by Nannochloropsis Gaditana L2
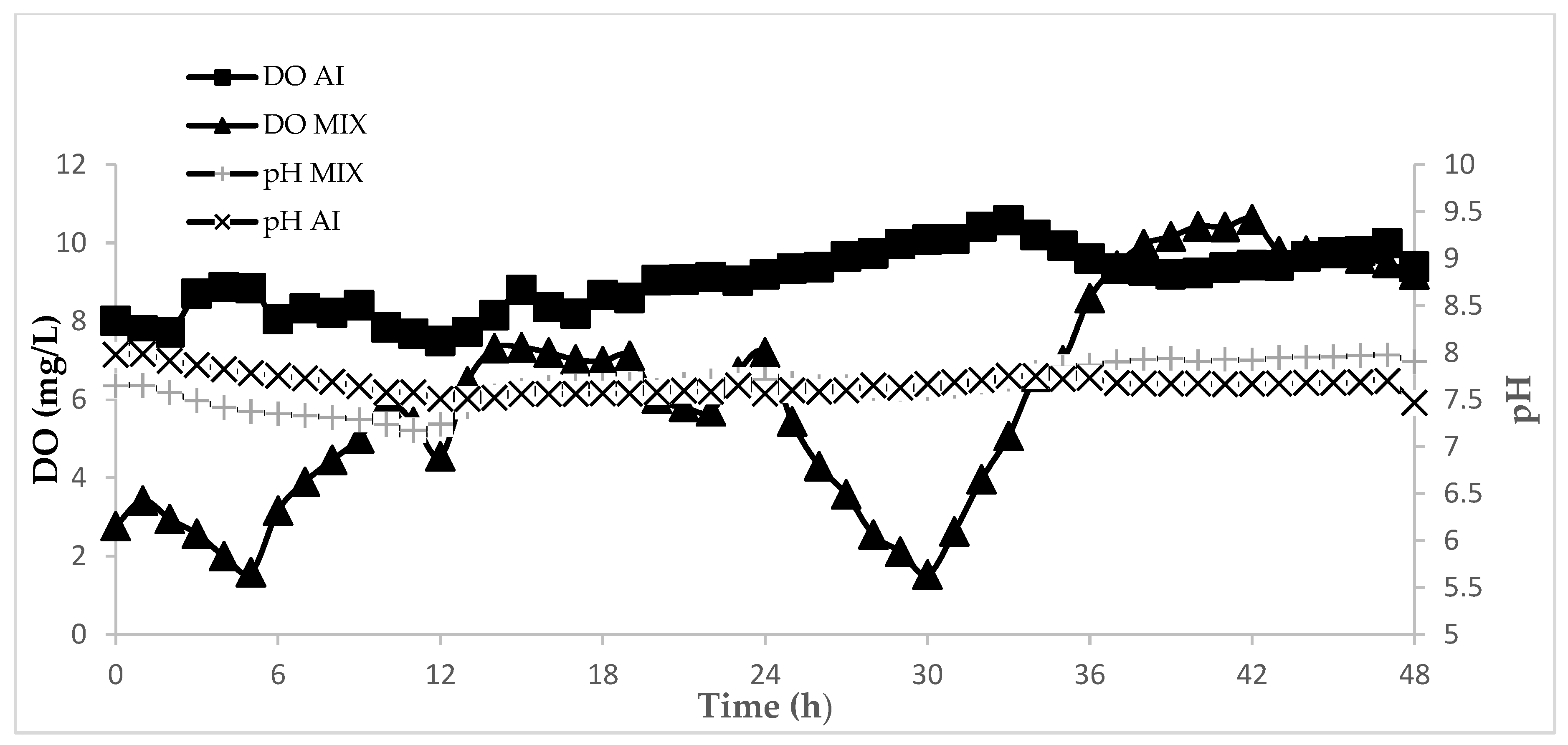
3.2. The Effect of Hydraulic Retention Time and Agitation Mode
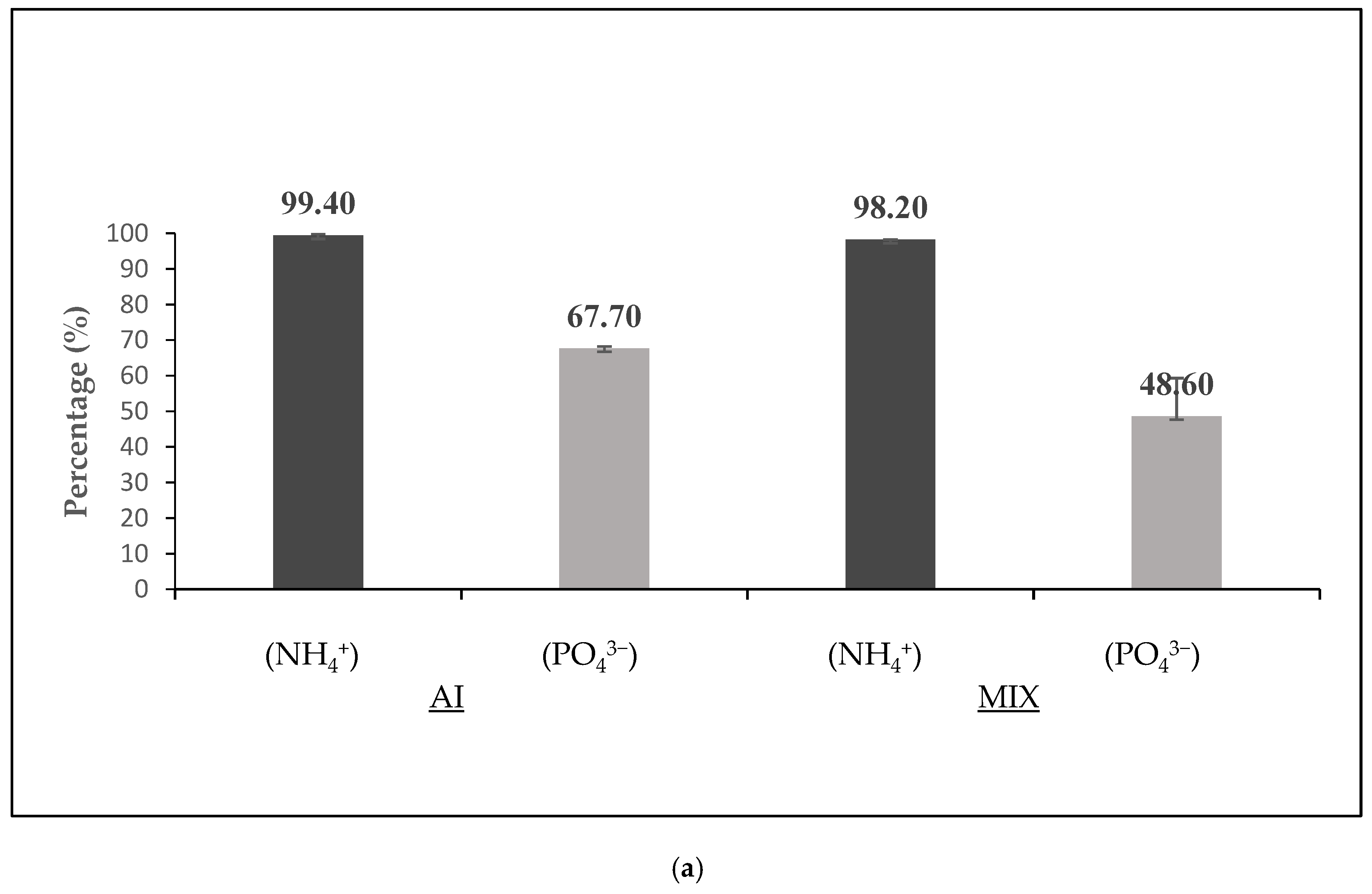
3.3. The Effect of Feeding at Dark on the SBR Process
3.4. The Interaction Associated with the Addition of Nannochloropsis Gaditana L2
3.4.1. TKN and COD Removal Rates
3.4.2. Microscopic Observation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Influence of Flue Gas Sparging on the Performance of High Rate Algae Ponds Treating Agro-Industrial Wastewaters. J. Hazard. Mater. 2010, 179, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; van der Graaf, J.H.J.M.; van Lier, J.B. Specific Energy Consumption of Membrane Bioreactor (MBR) for Sewage Treatment. Water Sci. Technol. 2012, 65, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Foladori, P.; Vaccari, M.; Vitali, F. Energy Audit in Small Wastewater Treatment Plants: Methodology, Energy Consumption Indicators, and Lessons Learned. Water Sci. Technol. 2015, 72, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, J.; Remiszewska-Skwarek, A.; Duda, S.; Łagód, G. Aeration Process in Bioreactors as the Main Energy Consumer in a Wastewater Treatment Plant. Review of Solutions and Methods of Process Optimization. Processes 2019, 7, 311. [Google Scholar] [CrossRef]
- Rosso, D.; Larson, L.E.; Stenstrom, M.K. Aeration of Large-Scale Municipal Wastewater Treatment Plants: State of the Art. Water Sci. Technol. 2008, 57, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant Protection and Growth Stimulation by Microorganisms: Biotechnological Applications of Bacilli in Agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Tang, C.-C.; Zuo, W.; Tian, Y.; Sun, N.; Wang, Z.-W.; Zhang, J. Effect of Aeration Rate on Performance and Stability of Algal-Bacterial Symbiosis System to Treat Domestic Wastewater in Sequencing Batch Reactors. Bioresour. Technol. 2016, 222, 156–164. [Google Scholar] [CrossRef]
- Manai, I.; Miladi, B.; El Mselmi, A.; Smaali, I.; Ben Hassen, A.; Hamdi, M.; Bouallagui, H. Industrial Textile Effluent Decolourization in Stirred and Static Batch Cultures of a New Fungal Strain Chaetomium Globosum IMA1 KJ472923. J. Environ. Manag. 2016, 170, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meneses, Y.E.; Stratton, J.; Wang, B. Acclimation of Consortium of Micro-Algae Help Removal of Organic Pollutants from Meat Processing Wastewater. J. Clean. Prod. 2019, 214, 95–102. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Nessenzia, M.; Andreottola, G. Enhanced Nitrogen Removal and Energy Saving in a Microalgal–Bacterial Consortium Treating Real Municipal Wastewater. Water Sci. Technol. 2018, 78, 174–182. [Google Scholar] [CrossRef]
- Katooli, M.H.; Aslani, A.; Razi Astaraee, F.; Mazzuca Sobczuk, T.; Bakhtiar, A. Multi-Criteria Analysis of Microalgae Production in Iran. Biofuels 2019, 1–7. [Google Scholar] [CrossRef]
- Ben Yahmed, N.; Jmel, M.A.; Ben Alaya, M.; Bouallagui, H.; Marzouki, M.N.; Smaali, I. A Biorefinery Concept Using the Green Macroalgae Chaetomorpha Linum for the Coproduction of Bioethanol and Biogas. Energy Convers. Manag. 2016, 119, 257–265. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Simultaneous Microalgae Cultivation and Wastewater Treatment in Submerged Membrane Photobioreactors: A Review. Algal Res. 2017, 24, 425–437. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, H.; Song, C.; Jahng, D. Co-Culture of Microalgae and Enriched Nitrifying Bacteria for Energy-Efficient Nitrification. Biochem. Eng. J. 2019, 152, 107385. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Sialve, B.; Molinuevo-Salces, B. Anaerobic Digestion of Microalgal Biomass: Challenges, Opportunities and Research Needs. Bioresour. Technol. 2015, 198, 896–906. [Google Scholar] [CrossRef]
- González-Fernández, C.; Molinuevo-Salces, B.; García-González, M.C. Nitrogen Transformations under Different Conditions in Open Ponds by Means of Microalgae–Bacteria Consortium Treating Pig Slurry. Bioresour. Technol. 2011, 102, 960–966. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced Growth and Lipid Production of Microalgae under Mixotrophic Culture Condition: Effect of Light Intensity, Glucose Concentration and Fed-Batch Cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef]
- Yoshioka, T.; Saijo, Y. Photoinhibition and Recovery of NH4+-Oxidizing Bacteria and NO2--Oxidizing Bacteria. J. Gen. Appl. Microbiol. 1984, 30, 151–166. [Google Scholar] [CrossRef]
- Gupta, A. Simultaneous Carbon and Nitrogen Removal from High Strength Domestic Wastewater in an Aerobic RBC Biofilm. Water Res. 2001, 35, 1714–1722. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and Harvesting of Microalgae for Wastewater Treatment, Biofuels, and Bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- Kunjapur, A.M.; Eldridge, R.B. Photobioreactor Design for Commercial Biofuel Production from Microalgae. Ind. Eng. Chem. Res. 2010, 49, 3516–3526. [Google Scholar] [CrossRef]
- Alagha, O.; Allazem, A.; Bukhari, A.A.; Anil, I.; Mu’azu, N.D. Suitability of SBR for Wastewater Treatment and Reuse: Pilot-Scale Reactor Operated in Different Anoxic Conditions. IJERPH 2020, 17, 1617. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, R.; Paul, A.; Lewandowski, M. Improving SBR Performance Alongside with Cost Reduction through Optimizing Biological Processes and Dissolved Oxygen Concentration Trajectory. Appl. Sci. 2019, 9, 2268. [Google Scholar] [CrossRef]
- Puig Broch, S. Operation and Control of SBR Processes for Enhanced Biological Nutrient Removal from Wastewater; Universitat de Girona: Girona, Spain, 2007. [Google Scholar]
- Szaja, A.; Łagód, G.; Jaromin-Gleń, K.; Montusiewicz, A. The Effect of Bioaugmentation with Archaea on the Oxygen Uptake Rate in a Sequencing Batch Reactor. Water 2018, 10, 575. [Google Scholar] [CrossRef]
- Real, A.; Garcia-Martinez, A.M.; Pidre, J.R.; Coello, M.D.; Aragon, C.A. Environmental Assessment of Two Small Scale Wastewater Treatment Systems: SBR vs CAS. Water Pract. Technol. 2017, 12, 549–556. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, R.K. Sequencing Batch Reactor Technology for Biological Wastewater Treatment: A Review. Asia Pac. J. Chem. Eng. 2011, 6, 3–13. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Andreottola, G. Evolution of Real Municipal Wastewater Treatment in Photobioreactors and Microalgae-Bacteria Consortia Using Real-Time Parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Jazzar, S.; Berrejeb, N.; Messaoud, C.; Marzouki, M.N.; Smaali, I. Growth Parameters, Photosynthetic Performance, and Biochemical Characterization of Newly Isolated Green Microalgae in Response to Culture Condition Variations. Appl. Biochem. Biotechnol. 2016, 179, 1290–1308. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Marine Microalgae Selection and Culture Conditions Optimization for Biodiesel Production. Bioresour. Technol. 2013, 134, 353–361. [Google Scholar] [CrossRef]
- Oswald, W.J. Micro-Algae and Wastewater Treatment; Cambridge University Press: Cambridge, UK, 1988; pp. 305–328. [Google Scholar]
- Zhou, L.; Wu, F.; Zhao, Z.; Wang, B. Effects of Environmental Factors on Nitrogen and Phosphorus Removal by Chlorella Vularis in Wastewater. Curr. Biotechnol. 2015, 5, 60–65. [Google Scholar]
- Osundeko, O.; Dean, A.P.; Davies, H.; Pittman, J.K. Acclimation of Microalgae to Wastewater Environments Involves Increased Oxidative Stress Tolerance Activity. Plant Cell Physiol. 2014, 55, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic Cooperation between Wastewater-Born Algae and Activated Sludge for Wastewater Treatment: Influence of Algae and Sludge Inoculation Ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, F.; Bellucci, M.; Fantasia, T.; Ficara, E.; Mezzanotte, V. Interactions between Microalgae and Bacteria in the Treatment of Wastewater from Milk Whey Processing. Water 2020, 12, 297. [Google Scholar] [CrossRef]
- Arango, L.; Cuervo, F.M.; González-Sánchez, A.; Buitrón, G. Effect of Microalgae Inoculation on the Start-up of Microalgae–Bacteria Systems Treating Municipal, Piggery and Digestate Wastewaters. Water Sci. Technol. 2016, 73, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Larsdotter, K.; la Cour Jansen, J.; Dalhammar, G. Biologically Mediated Phosphorus Precipitation in Wastewater Treatment with Microalgae. Environ. Technol. 2007, 28, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Guieysse, B. Algal–Bacterial Processes for the Treatment of Hazardous Contaminants: A Review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Oh, H.-S.; Oh, H.-M.; Kim, H.-S.; Ahn, C.-Y. Two-Phase Photoperiodic Cultivation of Algal–Bacterial Consortia for High Biomass Production and Efficient Nutrient Removal from Municipal Wastewater. Bioresour. Technol. 2016, 200, 867–875. [Google Scholar] [CrossRef]
- Arcila, J.S.; Buitrón, G. Microalgae-Bacteria Aggregates: Effect of the Hydraulic Retention Time on the Municipal Wastewater Treatment, Biomass Settleability and Methane Potential: Microalgae-Bacteria Aggregates for Wastewater Treatment. J. Chem. Technol. Biotechnol. 2016, 91, 2862–2870. [Google Scholar] [CrossRef]
- Rocha, J.M.S.; Garcia, J.E.C.; Henriques, M.H.F. Growth Aspects of the Marine Microalga Nannochloropsis Gaditana. Biomol. Eng. 2003, 20, 237–242. [Google Scholar] [CrossRef]

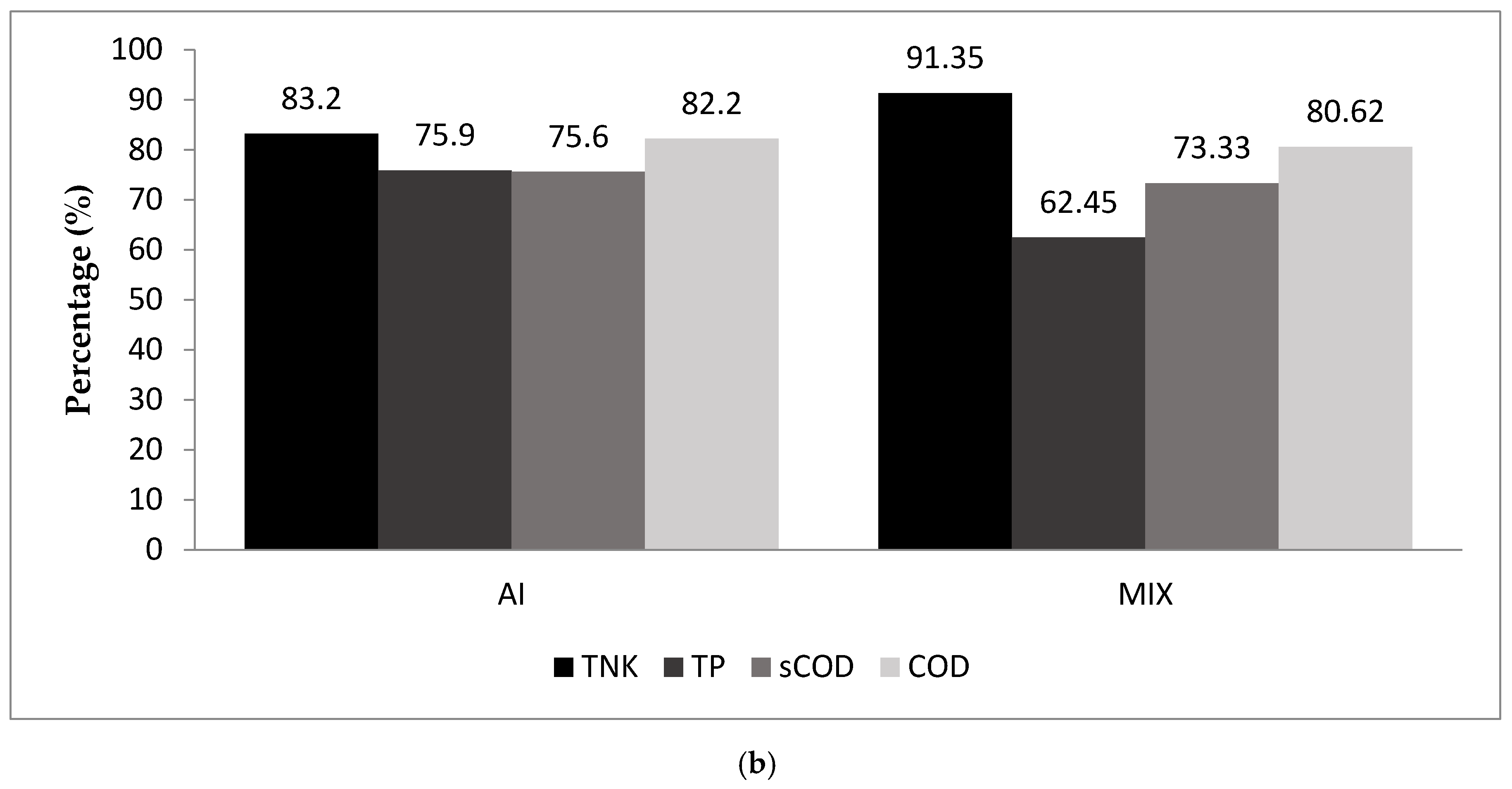
| Parameters | (mg/L) |
|---|---|
| TKN | 83.3 ± 27 |
| TP | 9.5 ± 3 |
| sCOD * | 137 ± 33 |
| COD | 498 ± 182 |
| TSS | 361 ± 91 |
| Batch 1 | Batch 2 | Batch 3 | Removal Rate % * | ||||
|---|---|---|---|---|---|---|---|
| Parameters (mg/L) | T0 | TF | T0 | TF | T0 | TF | |
| TKN | 60.5 | 19.2 | 69 | 11.7 | 72.1 | 12.8 | 78.84 |
| TP | 7.2 | 3.8 | 4.9 | 3.9 | 8.4 | 3.8 | 47.22 |
| COD | 395 | 206 | 263 | 183 | 481 | 156 | 53.67 |
| sCOD | 181 | 92 | 113 | 78 | 157 | 83 | 54.14 |
| HRT 6 | HRT 4 | |||||
|---|---|---|---|---|---|---|
| (L) | (L/D) | (L/D) | ||||
| Parameters | MIX | AI | MIX | AI | MIX | AI |
| TKN% | 55.2 | 92 | 80.6 | 74.5 | 68.3 | 91 |
| TP% | 59.67 | 24.63 | 45.8 | 38 | 47.2 | 29.5 |
| sCOD% | 57.69 | 22 | 56.9 | 10.31 | 49.2 | 22.5 |
| COD% | 78.44 | 74.78 | 60.5 | 61.4 | 47.8 | 75.9 |
| Removal Rate (%) | |||
|---|---|---|---|
| Time (Day) | TKN | COD | |
| WW | 7 | 16.3 | 25 |
| L2 + sterilized WW | 7 | 30 | 39 |
| L2 + WW + consortium | 3 | 86 | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhedhbi, E.; Khelifi, N.; Foladori, P.; Smaali, I. Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode. Water 2020, 12, 1893. https://doi.org/10.3390/w12071893
Mhedhbi E, Khelifi N, Foladori P, Smaali I. Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode. Water. 2020; 12(7):1893. https://doi.org/10.3390/w12071893
Chicago/Turabian StyleMhedhbi, Emna, Nadia Khelifi, Paola Foladori, and Issam Smaali. 2020. "Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode" Water 12, no. 7: 1893. https://doi.org/10.3390/w12071893
APA StyleMhedhbi, E., Khelifi, N., Foladori, P., & Smaali, I. (2020). Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode. Water, 12(7), 1893. https://doi.org/10.3390/w12071893






