A Transport-Phenomena Approach to Model Hydrodynamic Cavitation of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Summary of Reported Experimental Investigations
2.2. Mathematical Modeling
3. Results and Discussion
3.1. Experimental Observations
3.2. Simulation Results
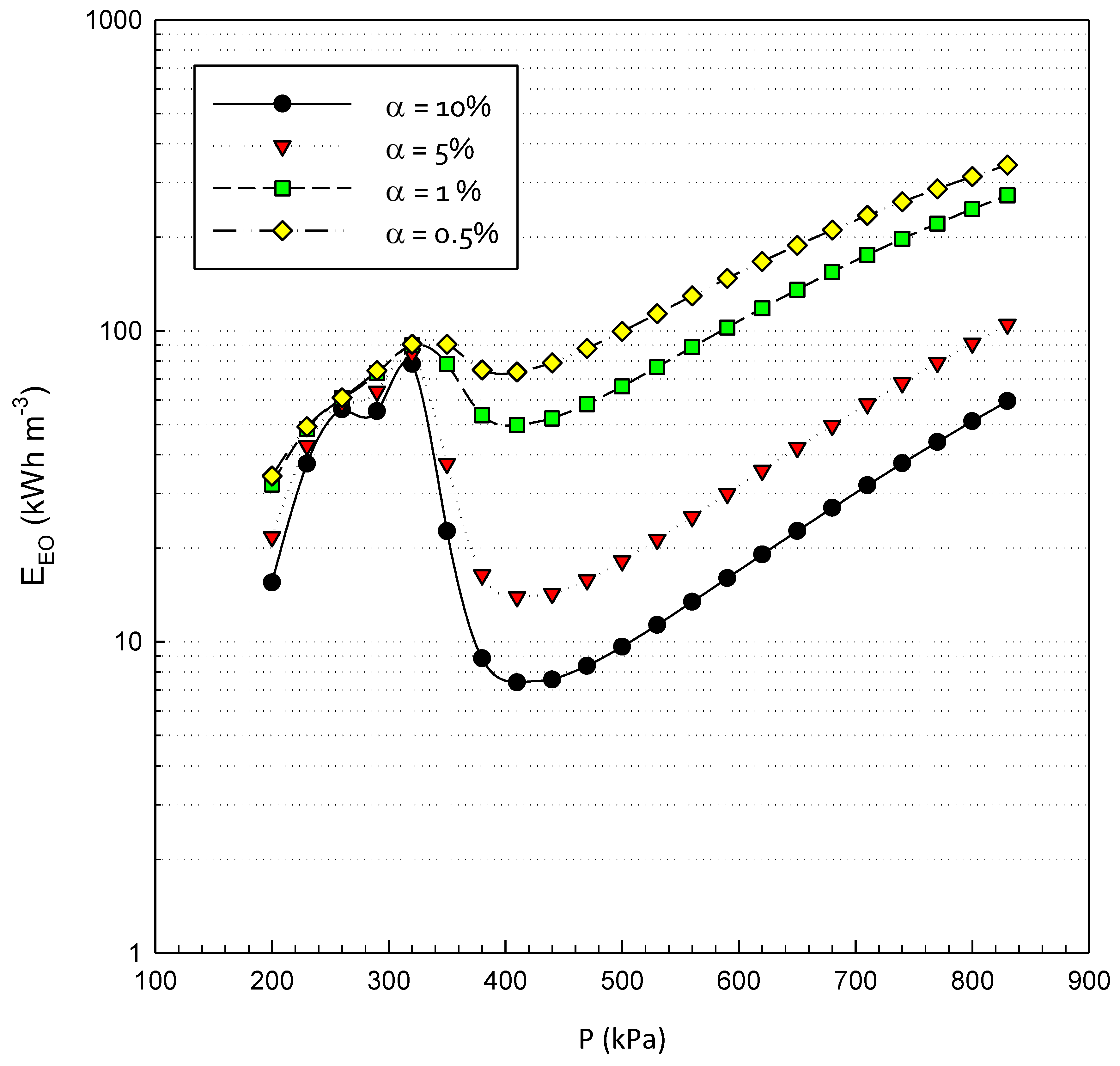
3.3. Energy Efficiency
4. Conclusions
- To implement this model, by fitting ad hoc experimental results, to estimate the model parameters that cannot be directly know from the literature.
- To improve the model for volatile compounds undergoing thermal degradation into the bubble environment.
- To evaluate the scavenging effect by means of ad hoc experiments and model fitting on the variable α.
- To test the model and to optimize the HC process for different geometries.
- To address the energy efficiency and the theoretical limit of HC from a thermodynamic point of view.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Cv | cavitation number (-) |
| C0 | pollutant initial concentration (mg/L) |
| C | Pollutant concentration (mg /L) |
| Da | Damköhler number (-) |
| DL | diffusivity (cm2 s−1) |
| dp | pipe inner diameter (m) |
| do | diameter of the constriction (m) |
| EEO | energy efficiency per order (kWh m−3) |
| kobs | degradation rate constant (min−1) |
| kOH | ∙OH reaction rate (M−1 s−1) |
| L | length of the cavitating device (m) |
| N | bubble size distribution (#bubbles m−1 m−3) |
| Np | pump power (W) |
| nb | number density of bubbles (#bubbles m−3) |
| p2 | recovered pressure (Pa) |
| Pe | Pectlet number |
| Pin | inlet pressure (Pa) |
| pmin | minimum pressure (Pa) |
| pv | water vapor pressure (Pa) |
| Q | flow rate (m3 s−1) |
| R0 | initial bubble radius (μm) |
| St | Stanton number (-) |
| V | reactor volume (m3) |
| u | liquid velocity (m s−1) |
| vo | liquid velocity at vena contracta (m s−1) |
| z | independent variable—space (m) |
| α | radicals’ scavenging factor (-) |
| β | orifice to pipe ratio (-) |
| ∆P | pressure drop across the reactor (Pa) |
| ηp | pump overall efficiency (-) |
| ΠOH | global hydroxyl radical production (mol m−3 s−1) |
| πOH | specific hydroxyl radical production (mol/bubble) |
| dimensionless length (-) | |
| dimensionless concentration (-) | |
| ρ | water density (kg m−3) |
References
- Maršálek, B.; Maršálková, E.; Odehnalová, K.; Pochylý, F.; Rudolf, P.; Stahel, P.; Rahel, J.; Čech, J.; Fialová, S.; Zezulka, Š. Removal of Microcystis aeruginosa through the Combined Effect of Plasma Discharge and Hydrodynamic Cavitation. Water 2020, 12, 8. [Google Scholar] [CrossRef]
- Gogate, P.R.; Kabadi, A.M. A Review of Applications of Cavitation in Biochemical Engineering/Biotechnology. Biochem. Eng. J. 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitation: An Auxiliary Technique in Wastewater Treatment Schemes. Adv. Environ. Res. 2002, 6, 335–358. [Google Scholar] [CrossRef]
- Franc, J.-P.; Michel, J.-M. Fundamentals of Cavitation; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernandez-Gonzalez, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitational Reactors for Process Intensification of Chemical Processing Applications: A Critical Review. Chem. Eng. Process. 2008, 47, 515–527. [Google Scholar] [CrossRef]
- Young, F.R. Cavitation; McGraw-Hill Book Co Ltd.: London, UK, 1989. [Google Scholar]
- Gogate, P.R.; Patil, P.N. Sonochemical Reactors, Topics in Current Chemistry; Springer International Publishing: Cham, Switzerland, 2016; Volume 374, pp. 1–27. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar] [CrossRef]
- Saharan, V.K.; Pinjari, D.V.; Gogate, P.R.; Pandit, A.B. Industrial Wastewater Treatment, Recycling and Reuse; Advanced Oxidation Technologies for Wastewater Treatment: An Overview; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar] [CrossRef]
- Carpenter, J.; Badve, M.; Rajoriya, S.; George, S.; Saharan, V.K.; Pandit, A.B. Hydrodynamic Cavitation: An Emerging Technology for the Intensification of Various Chemical and Physical Processes in a Chemical Process Industry. Rev. Chem. Eng. 2016, 33, 433–468. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Engineering Design Method for Cavitational Reactors: II. Hydrodynamic Cavitatiom. AIChE J. 2000, 46, 1641–1649. [Google Scholar] [CrossRef]
- Gogate, P.R.; Tatake, P.A.; Kanthale, P.M.; Pandit, A.B. Mapping of Sonochemical Reactors: Review, Analysis, and Experimental Verification. AIChE J. 2002, 48, 1542–1560. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater Treatment Using Hybrid Treatment Schemes Based on Cavitation and Fenton Chemistry: A Review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- González-García, J.; Sáez, V.; Tudela, I.; Díez-Garcia, M.I.; Esclapez, M.D.; Louisnard, O. Sonochemical Treatment of Water Polluted by Chlorinated Organocompounds. A Review. Water 2010, 2, 28–74. [Google Scholar] [CrossRef]
- Wang, C.; Ying, Z.; Ma, M.; Huo, M.; Yang, W. Degradation of Micropollutants by UV–Chlorine Treatment in Reclaimed Water: pH Effects, Formation of Disinfectant Byproducts, and Toxicity Assay. Water 2019, 11, 2639. [Google Scholar] [CrossRef]
- Capocelli, M.; Joyce, E.; Lancia, A.; Mason, T.J.; Musmarra, D.; Prisciandaro, M. Sonochemical Degradation of Estradiols: Incidence of Ultrasonic Frequency. Chem. Eng. J. 2012, 210, 9–17. [Google Scholar] [CrossRef]
- Thanekar, P.; Gogate, P.R. Combined Hydrodynamic Cavitation Based Processes as an Efficient Treatment Option for Real Industrial Effluent. Ultrason. Sonochem. 2019, 53, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Chakinala, A.G.; Gogate, P.R.; Chand, R.; Bremner, D.H.; Molina, R.; Burgess, A.E. Intensification of Oxidation Capacity Using Chloroalkanes as Additives in Hydrodynamic and Acoustic Cavitation Reactors. Ultrason. Sonochem. 2008, 15, 64–170. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cai, J.; Huai, X.; Liu, B.; Guo, Z. Application of Hydrodynamic Cavitation to Wastewater Treatment. Chem. Eng. Technol. 2016, 39, 1363–1376. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Musmarra, D.; Lancia, A. Understanding the Physics of Advanced Oxidation in a Venturi Reactor. Chem. Eng. Trans. 2013, 32, 691–696. [Google Scholar] [CrossRef]
- Saharan, V.K.; Rizwani, M.A.; Malani, A.A.; Pandit, A.B. Effect of Geometry of Hydrodynamically Cavitating Device on Degradation of Orange-G. Ultrason. Sonochem. 2013, 20, 345–353. [Google Scholar] [CrossRef]
- Saharan, V.K.; Badve, M.P.; Pandit, A.B. Degradation of Reactive Red 120 Dye Using Hydrodynamic Cavitation. Chem. Eng. J. 2011, 178, 100–107. [Google Scholar] [CrossRef]
- Saharan, V.K.; Pandit, A.B.; Satish Kumar, P.S.; Anandan, S. Hydrodynamic Cavitation as an Advanced Oxidation Technique for the Degradation of Acid Red 88 Dye. Ind. Eng. Chem. Res. 2012, 51, 1981–1989. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Tortora, F.; Vegliò, F. Optimization of Hydrodynamic Cavitation Process of Azo Dye Reduction in the Presence of Metal Ions. J. Environ. Chem. Eng. 2018, 6, 6787–6796. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Gogate, P.R. Removal of P-Nitrophenol Using Hydrodynamic Cavitation and Fenton Chemistry at Pilot Scale Operation. Chem. Eng. J. 2010, 156, 77–82. [Google Scholar] [CrossRef]
- Thanekar, P.; Murugesan, P.; Gogate, P.R. Improvement in Biological Oxidation Process for the Removal of Dichlorvos from Aqueous Solutions Using Pretreatment Based on Hydrodynamic Cavitation. J. Water Process Eng. 2018, 23, 20–26. [Google Scholar] [CrossRef]
- Kumar, M.S.; Sonawane, S.H.; Pandit, A.B. Degradation of Methylene Blue Dye in Aqueous Solution Using Hydrodynamic Cavitation Based Hybrid Advanced Oxidation Processes. Chem. Eng. Process. 2017, 122, 288–295. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Degradation of Alachlor in Aqueous Solution by Using Hydrodynamic Cavitation. J. Hazard. Mater. 2009, 161, 202–207. [Google Scholar] [CrossRef]
- Gore, M.M.; Saharan, V.K.; Pinjari, D.V.; Chavan, P.V.; Pandit, A.B. Degradation of Reactive Orange 4 Dye Using Hydrodynamic Cavitation Based Hybrid Techniques. Ultrason. Sonochem. 2014, 21, 1075–1082. [Google Scholar] [CrossRef]
- Thanekar, P.; Panda, M.; Gogate, P.R. Degradation of Carbamazepine Using Hydrodynamic Cavitation Combined with Advanced Oxidation Processes. Ultrason. Sonochem. 2018, 40, 567–576. [Google Scholar] [CrossRef]
- Mishra, K.P.; Gogate, P.R. Intensification of Degradation of Rhodamine B Using Hydrodynamic Cavitation in the Presence of Additives. Sep. Purif. Technol. 2010, 75, 385–391. [Google Scholar] [CrossRef]
- Thanekar, P.; Gogate, P.R. Application of Hydrodynamic Cavitation Reactors for Treatment of Wastewater Containing Organic Pollutants: Intensification Using Hybrid Approaches. Fluids 2018, 3, 98. [Google Scholar] [CrossRef]
- Gogate, P.R.; Bhosale, G.S. Comparison of Effectiveness of Acoustic and Hydrodynamic Cavitation in Combined Treatment Schemes for Degradation of Dye Wastewaters. Chem. Eng. Process. 2013, 71, 59–69. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Hydrodynamic Cavitation of P-Nitrophenol: A Theoretical and Experimental Insight. Chem. Eng. J. 2014, 254, 1–8. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of 2,4-Dinitrophenol Using a Combination of Hydrodynamic Cavitation, Chemical and Advanced Oxidation Processes. Ultrason. Sonochem. 2013, 20, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.N.; Gogate, P.R. Degradation of Methyl Parathion Using Hydrodynamic Cavitation: Effect of Operating Parameters and Intensification Using Additives. Sep. Purif. Technol. 2012, 95, 172–179. [Google Scholar] [CrossRef]
- Randhavane, S.B. Comparing Geometric Parameters of a Hydrodynamic Cavitation Process Treating Pesticide Effluent. Environ. Eng. Res. 2018, 24, 318–323. [Google Scholar] [CrossRef]
- Musmarra, D.; Prisciandaro, M.; Capocelli, M.; Karatza, D.; Iovino, P.; Canzano, S.; Lancia, A. Degradation of Ibuprofen by Hydrodynamic Cavitation: Reaction Pathways and Effect of Operational Parameters. Ultrason. Sonochem. 2016, 29, 76–83. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Saharan, V.K.; Pinjari, D.; Sonawane, S.; Saini, D.; Pandit, A. Synergetic Effect of Combination of AOP’s (Hydrodynamic Cavitation and H2O2) on the Degradation of Neonicotinoid Class of Insecticide. J. Hazard. Mater. 2013, 261, 139–147. [Google Scholar] [CrossRef]
- Kumar, M.S.; Sonawane, S.H.; Bhanvase, B.A.; Bethi, B. Treatment of Ternary Dye Wastewater by Hydrodynamic Cavitation Combined with Other Advanced Oxidation Processes (AOP’s). J. Water Process Eng. 2018, 23, 250–256. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Vegliò, F. Effect of the Hydrodynamic Cavitation for the Treatment of Industrial Wastewater. Chem. Eng. Trans. 2018, 67, 529–534. [Google Scholar] [CrossRef]
- Barik, A.J.; Gogate, P.R. Degradation of 4-Chloro 2-Aminophenol Using a Novel Combined Process Based on Hydrodynamic Cavitation, UV Photolysis and Ozone. Ultrason. Sonochem. 2016, 30, 70–78. [Google Scholar] [CrossRef]
- Moholkar, V.S.; Pandit, A.B. Bubble Behavior in Hydrodynamic Cavitation: Effect of Turbulence. AIChE J. 1997, 43, 1641–1648. [Google Scholar] [CrossRef]
- Moholkar, V.S.; Senthil Kumar, P.; Pandit, A.B. Hydrodynamic Cavitation for Sonochemical Effects. Ultrason. Sonochem. 1999, 6, 53–65. [Google Scholar] [CrossRef]
- Storey, B.D.; Szeri, J. Water vapour, sonoluminescence and sonochemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2000, 456, 1685–1709. [Google Scholar] [CrossRef]
- Toegel, R.; Gompf, B.; Pecha, R.; Lohse, D. Does Water Vapor Prevent Upscaling Sonoluminescence? Phys. Rev. Lett. 2000, 85, 3165–3168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Khanna, S.; Moholkar, V. Flow regime maps and optimization thereby of hydrodynamic cavitation reactors. AIChE J. 2012, 58, 3858–3866. [Google Scholar] [CrossRef]
- Sharma, A.; Gogate, P.; Mahulkar, A.; Pandit, A. Modeling of hydrodynamic cavitation reactors based on orifice plates considering hydrodynamics and chemical reactions occurring in bubble. Chem. Eng. J. 2008, 143, 201–209. [Google Scholar] [CrossRef]
- Krishnan, J.S.; Dwivedi, P.; Moholkar, V.S. Numerical Investigation into the Chemistry Induced by Hydrodynamic Cavitation. Ind. Eng. Chem. Res. 2006, 45, 1493–1504. [Google Scholar] [CrossRef]
- Capocelli, M.; Musmarra, D.; Prisciandaro, M.; Lancia, A. Chemical Effect of Hydrodynamic Cavitation: Simulation and Experimental Comparison. AIChE J. 2014, 60, 2566–2572. [Google Scholar] [CrossRef]
- Mishra, S.K.; Deymier, P.; Muralidharan, K.; Frantziskonis, G.; Pannala, S.; Simunovic, S. Modeling the coupling of reaction kinetics and hydrodynamics in a collapsing cavity. Ultrason. Sonochem. 2010, 17, 258–265. [Google Scholar] [CrossRef]
- Ozonek, J. Application of Hydrodynamic Cavitation in Environmental Engineering; CRC Press: London, UK, 2012. [Google Scholar]
- Dular, M.; Coutier-Delgosha, O. Thermodynamic effects during growth and collapse of a single cavitation bubble. J. Fluid Mech. 2013, 736, 4466. [Google Scholar] [CrossRef]
- Šarc, A.; Stepišnik-Perdih, T.; Petkovšek, M.; Dular, M. The issue of cavitation number value in studies of water treatment by hydrodynamic cavitation. Ultrason. Sonochem. 2016, 34, 51–59. [Google Scholar] [CrossRef]
- Ladino, J.A.; Herrera, J.; Malagon, D.; Prisciandaro, M.; Piemonte, V.; Capocelli, M. Biodiesel production via hydrodynamic cavitation: Numerical study of new geometrical arrangements. Chem. Eng. Trans. 2016, 50, 319–324. [Google Scholar] [CrossRef]
- Delale, C.F.; Okita, K.; Matsumoto, Y. Steady-State Cavitating Nozzle Flows With Nucleation. J. Fluids Eng. 2005, 127, 770–777. [Google Scholar] [CrossRef]
- Yan, Y.; Thorpe, R.B. Flow regime transitions due to cavitation in the flow through an orifice. Int. J. Multiph. Flow 1990, 16, 1023–1045. [Google Scholar] [CrossRef]
- Bashir, T.A.; Soni, A.G.; Mahulkar, A.V.; Pandit, A.B. The CFD driven optimization of a modified venturi for cavitational activity. Can. J. Chem. Eng. 2011, 89, 1366–1375. [Google Scholar] [CrossRef]
- Kanthale, M.; Gogate, P.R.; Pandit, A.B.; Wilhelm, A.M. Dynamics of cavitational bubbles and design of a hydrodynamic cavitational reactor: Cluster approach. Ultrason. Sonochem. 2005, 12, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Brenner, C.E. Models of cavitation event rate. Proceedings of International Symposium of Cavitation, Deauville, France, 2–5 May 1995; pp. 321–328. [Google Scholar]
- Liu, Z.; Sato, K.; Brennen, C. Cavitation nuclei population dynamics in a water tunnel. ASME 1993, 153, 119–125. [Google Scholar]
- Ferrari, A. Fluid Dynamics of acoustic and hydrodynamic cavitation in hydraulic power systems. Proc. R. Soc. A 2017, 473, 20160345. [Google Scholar] [CrossRef]
- Besagni, G.; Inzoli, F.; Ziegenhein, T. Two-Phase Bubble Columns: A Comprehensive Review. Chem. Eng. 2018, 2, 13. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Factors influencing the ultrasonic degradation of emerging compounds: ANN analysis. Chem. Eng. Trans. 2014, 39, 1777–1782. [Google Scholar]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Application of ANN to hydrodynamic cavitation: Preliminary results on process efficiency evaluation. Chem. Eng. Trans. 2014, 36, 199–204. [Google Scholar]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Piemonte, V.; Barba, D. A technical-economical approach to promote the water treatment & reuse processes. J. Clean. Prod. 2019, 207, 85–96. [Google Scholar]
- Colussi, A.J.; Weavers, L.K.; Hoffmann, M.R. Chemical Bubble Dynamics and Quantitative Sonochemistry. J. Phys. Chem. A 1998, 102, 6927–6934. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, M.; Sun, Y.; Chen, Z.; Duan, H.; Liu, D. Application and Evaluation of Energy Conservation Technologies in Wastewater Treatment Plants. Appl. Sci. 2019, 9, 4501. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment-A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Von Gunten, U. Oxidation processes in water treatment: Are we on track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]
- Chonga, M.N.; Sharma, A.K.; Burn, S.; Saint, C.P. Feasibility study on the application of advanced oxidation technologies for decentralised wastewater treatment. J. Clean. Prod. 2012, 35, 230–238. [Google Scholar] [CrossRef]
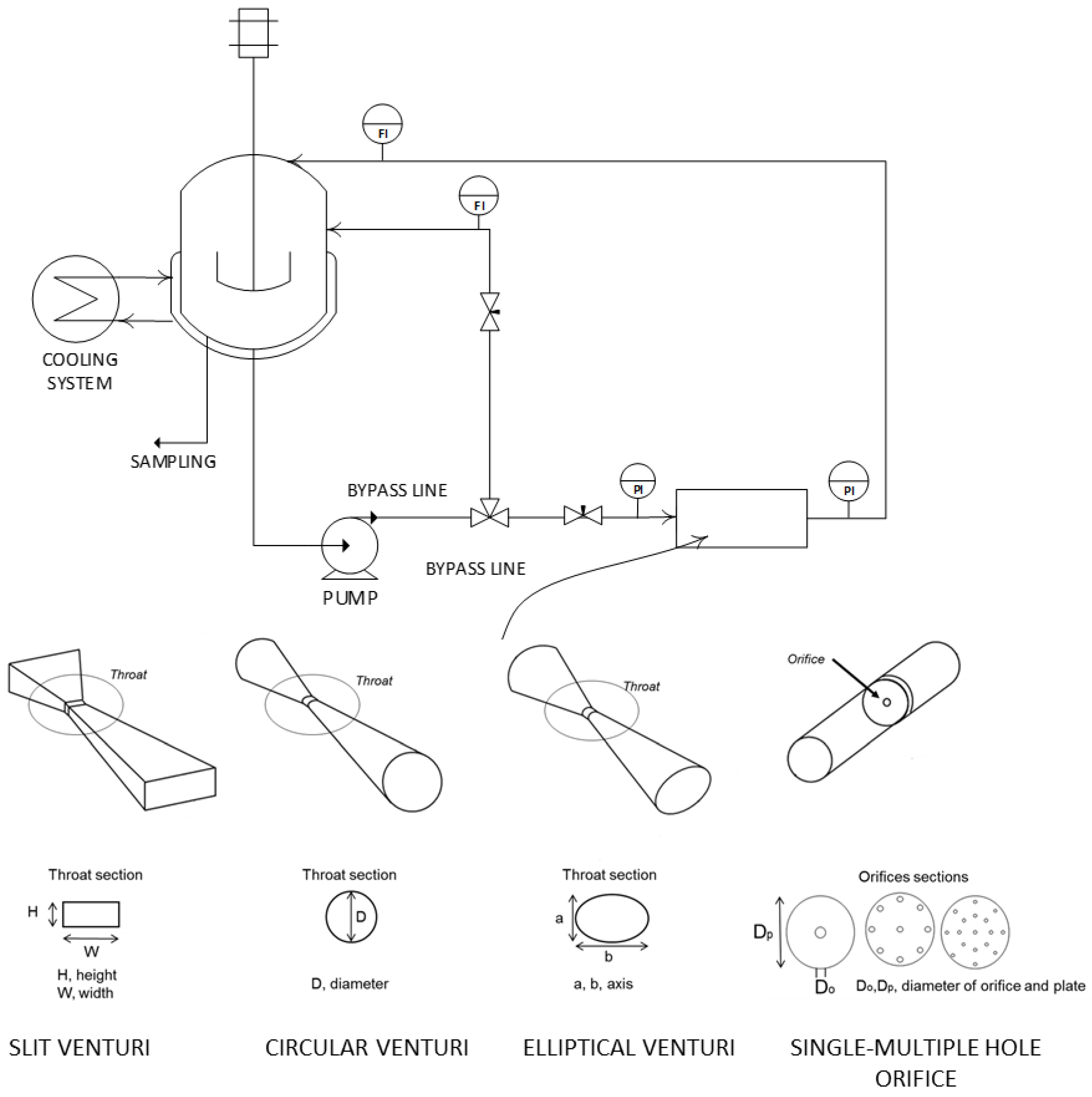
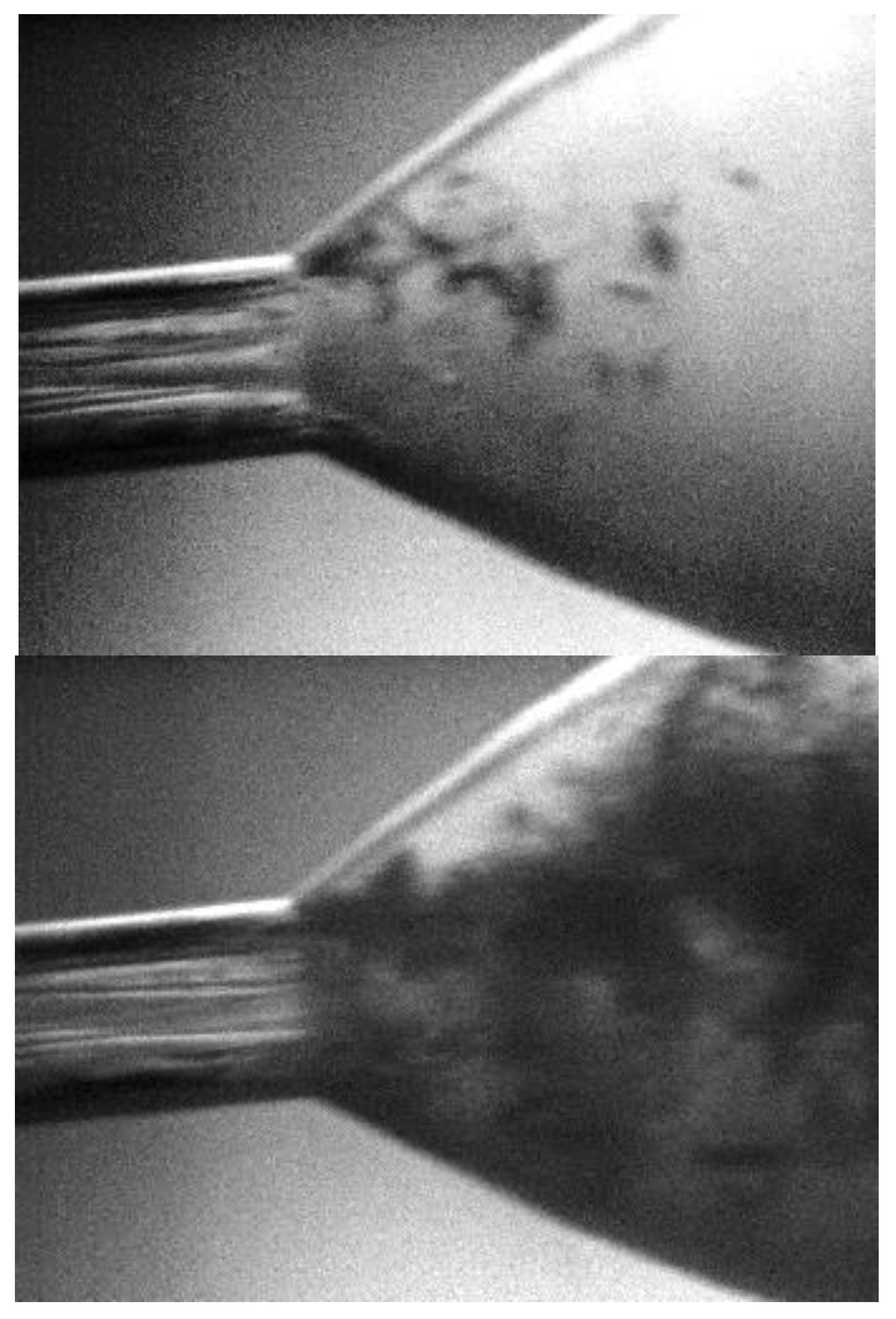
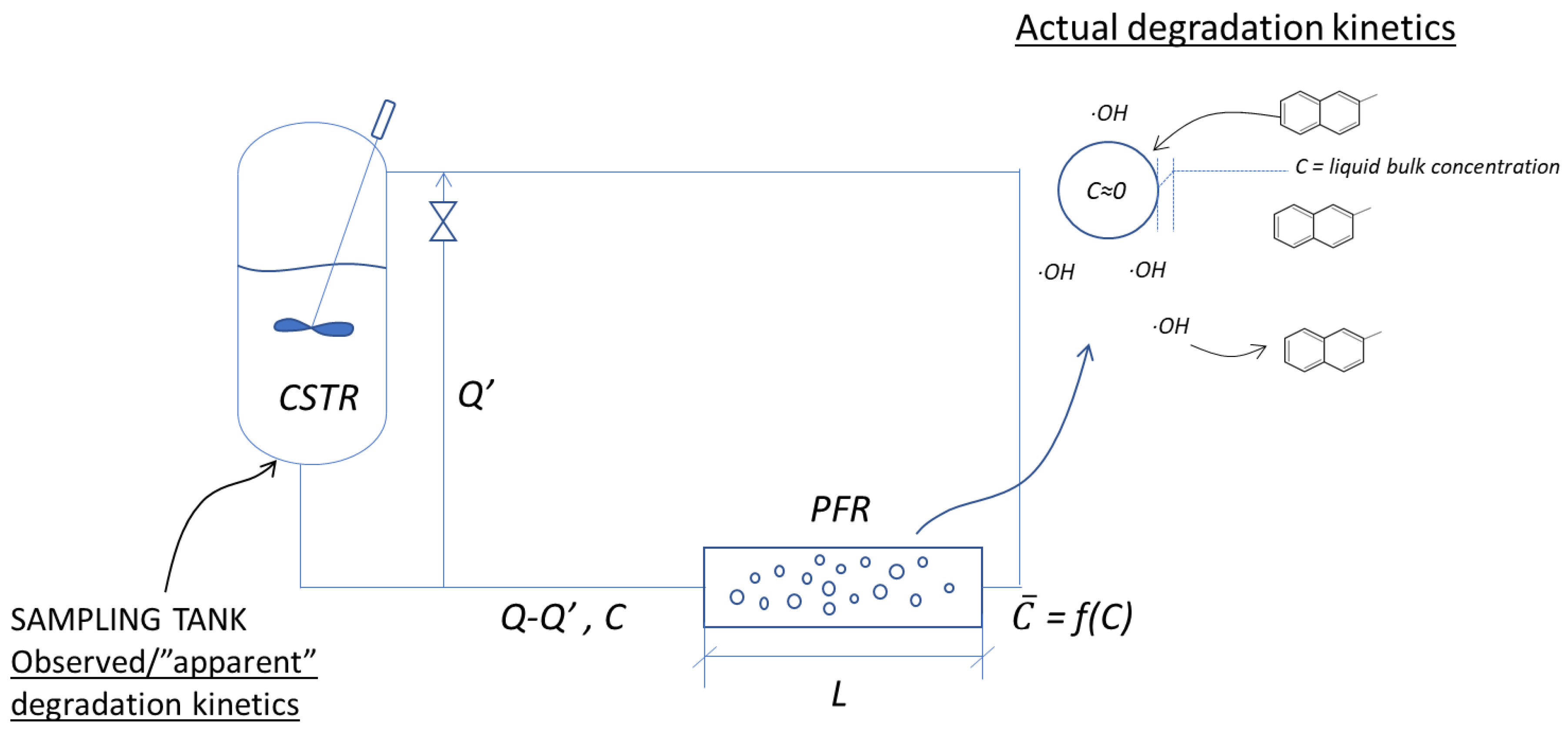
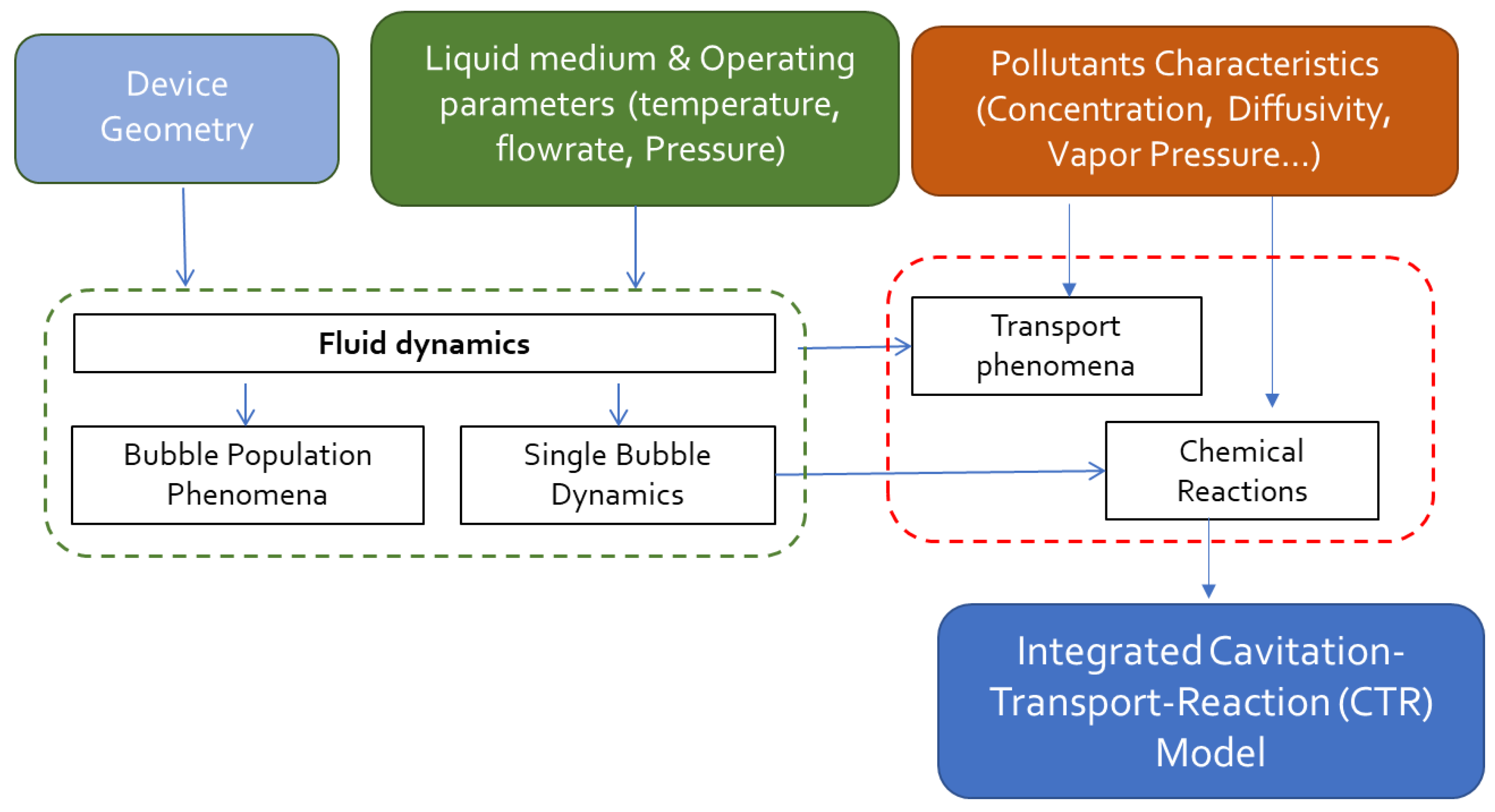


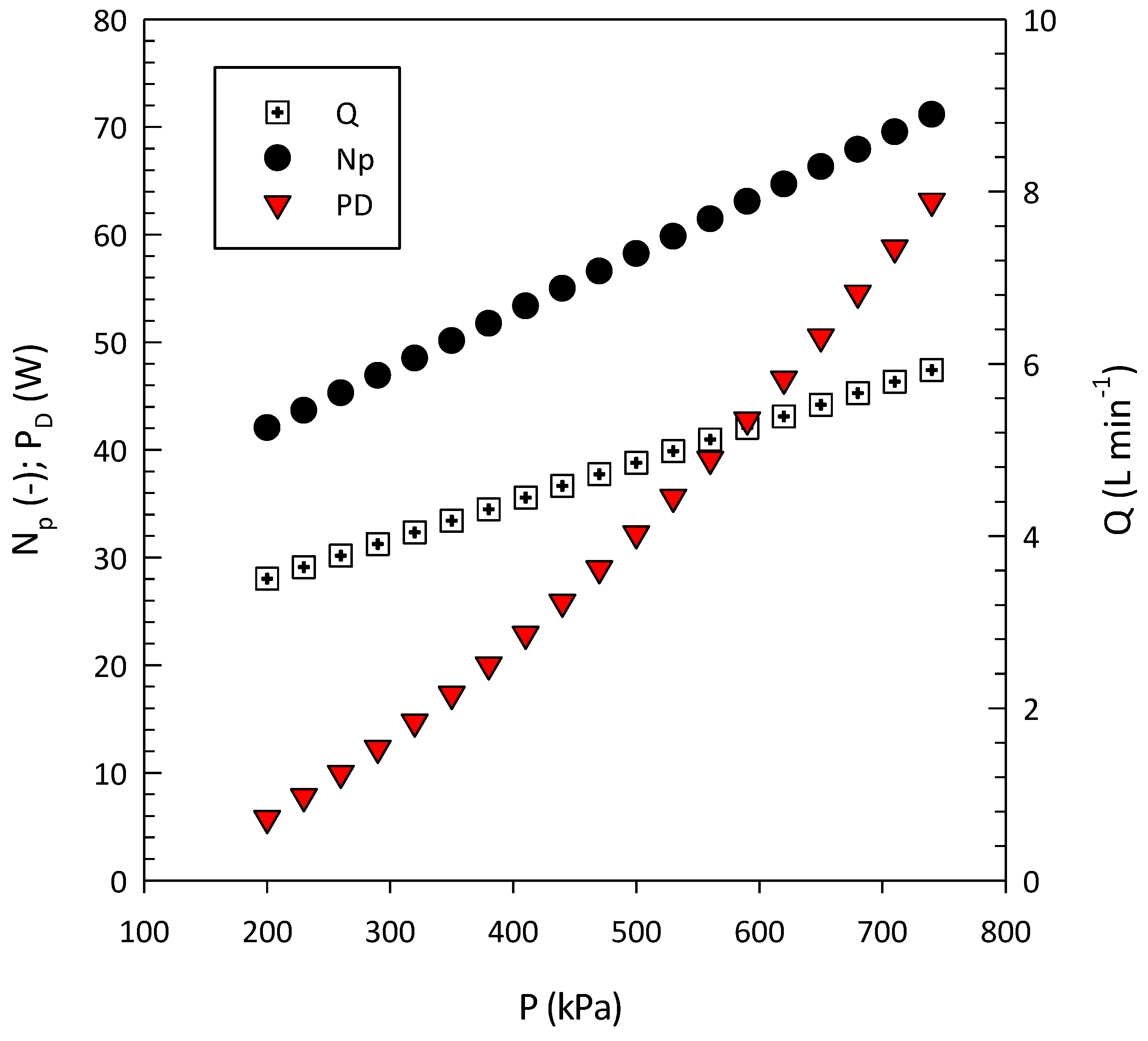
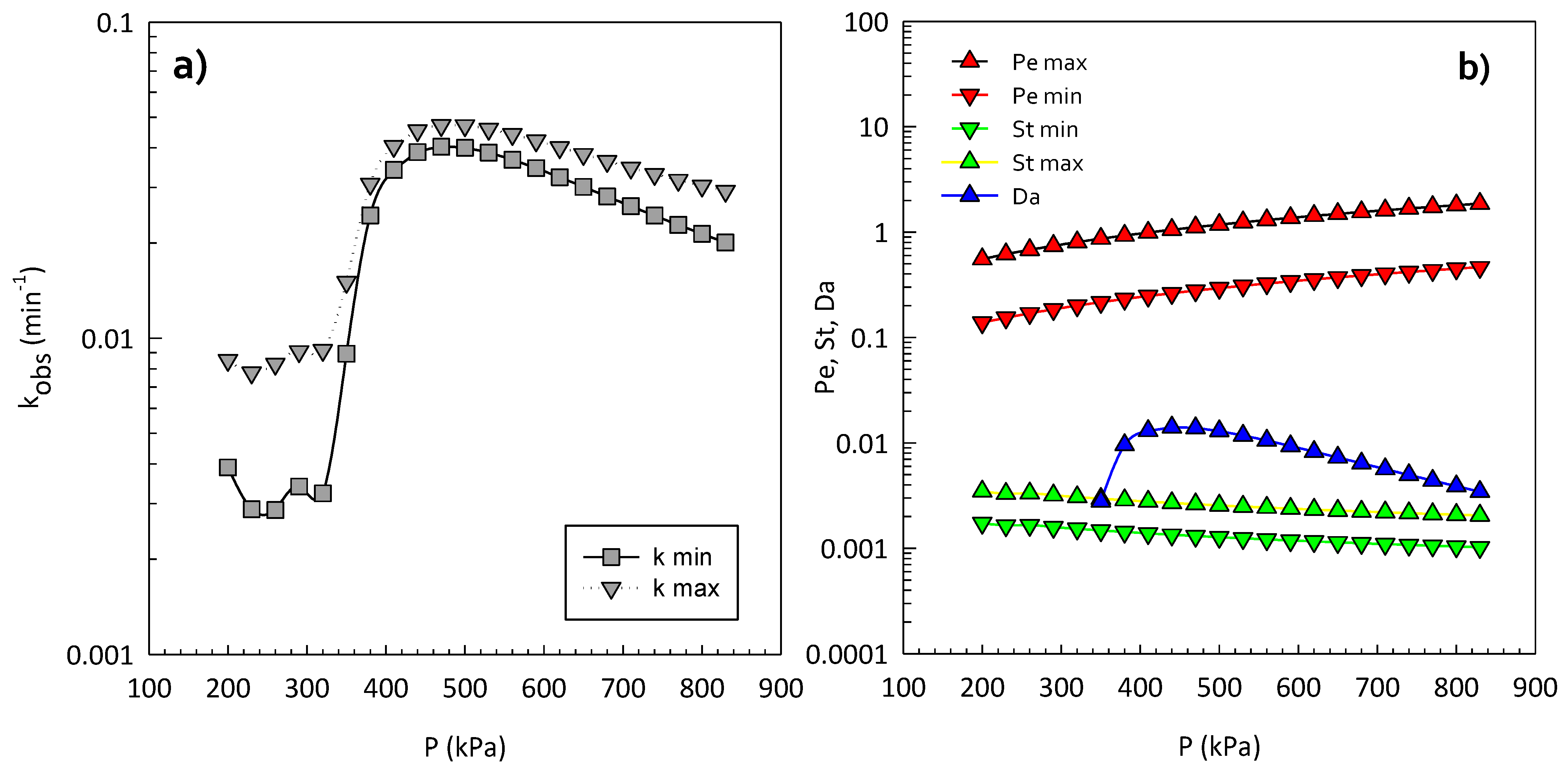
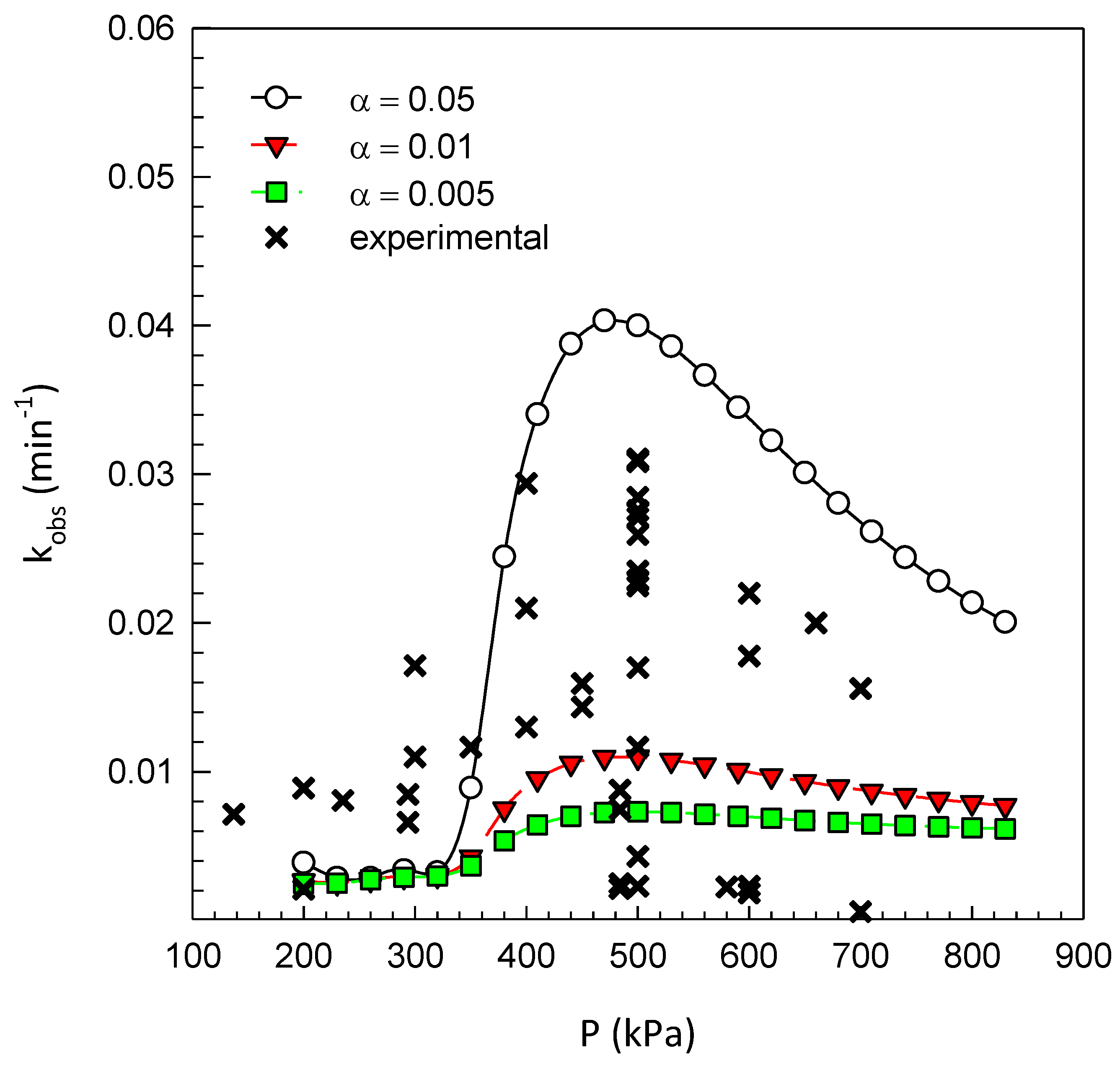
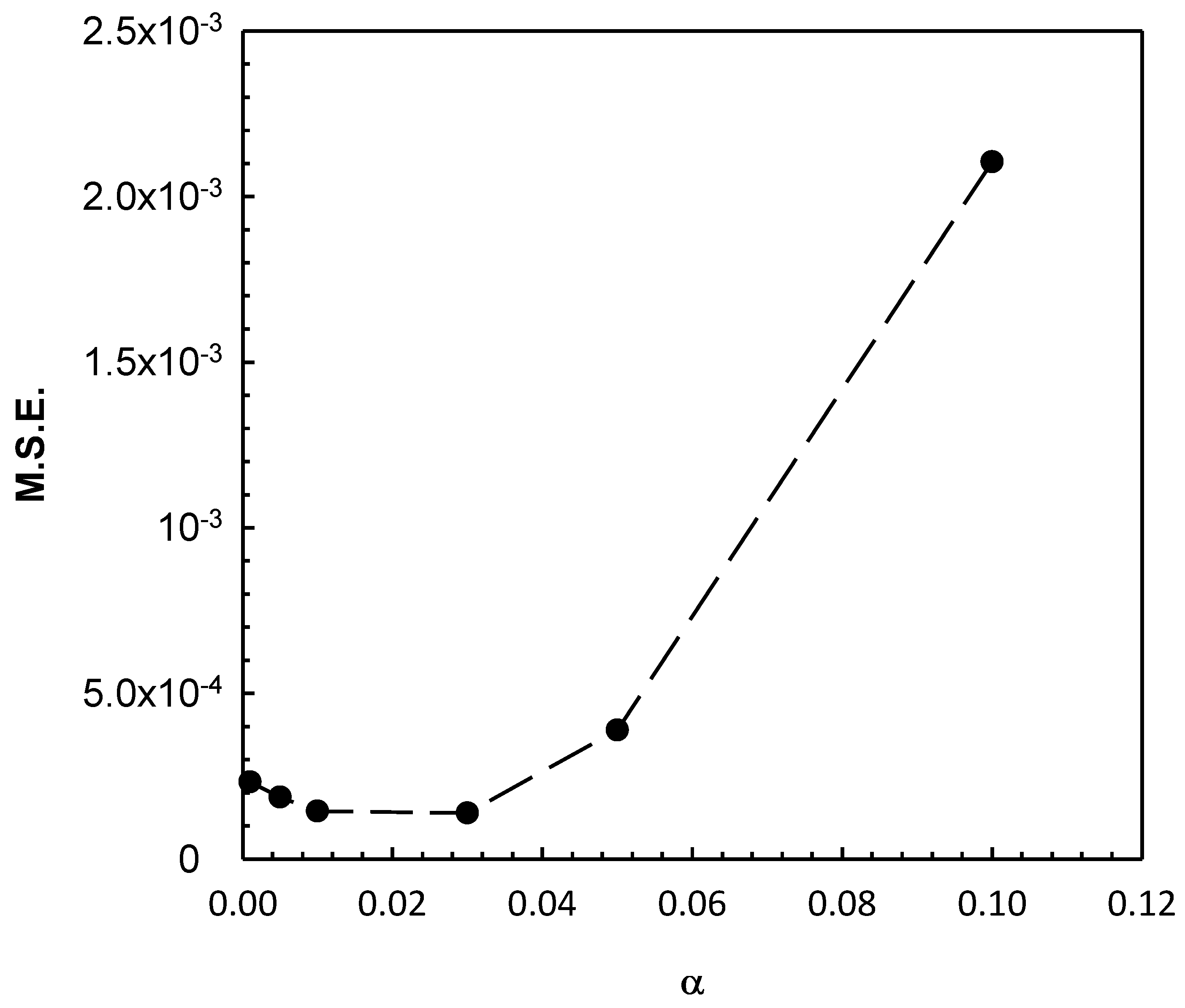
| Cavitating Device | Pollutant | T (°C) | C0 (mg/L) | Pin (bar) | pH | Cv | K (min−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| Venturi | Orange-G | 32 | 13.6–67.9 | 3–7 | 2–9 | 0.11–0.21 | 5.25 × 10−4–3.10 × 10−2 | Saharan et al., 2013 [22] |
| Venturi | Reactive Red 120 | 35 | 50 | 1–7 | 2–9 | 0.10–0.44 | 1.55 × 10−4–7.56 × 10−3 | Saharan et al., 2011 [23] |
| Venturi | Acid red 88 | 30 | 20–60 | 3–7 | 2–10 | 0.11–0.21 | 5.80 × 10−4–2.35 × 10−2 | Saharan et al., 2011 [24] |
| Venturi | Methyl orange | 20 | 5 | 2–6.6 | 2–4 | 0.27–0.5 | 2.87 × 10−3–2.20 × 10−2 | Innocenzi et al., 2018 [25] |
| Orifice | p-Nitrophenol | 30 | 5000 | 0.4–2.9 | 6 | 0.26–0.53 | 2.58 × 10−3–7.96 × 10−3 | Pradhan and Gogate, 2010 [26] |
| Circular Venturi | 5000–10,000 | 0.46–0.56 | 3.29 × 10−3–8.48 × 10−3 | |||||
| Circular Venturi | Methylene blue dye | 30 | 50 | 1–8 | 1–7.8 | 0.09–0.45 | 3.63 × 10−4–3.42 × 10−3 | Kumar et al., 2017 [28] |
| Circular Venturi | Reactive Orange 4 dye | 30 | 40 | 3–8 | 2 | 0.095–0.21 | 2.58 × 10−3–4.91 × 10−3 | Gore et al., 2014 [30] |
| Slit Venturi | Dichlorvos | 30 | 10–50 | 4–7 | 3–9 | 3–5 | 1.1 × 10−3–5.0 × 10−3 | Thanekar et al., 2018 [31] |
| Venturi | Rhodamine B | 30–40 | 10 | 2.9–5.8 | 2.5–11 | 0.07–0.20 | 1.26 × 10−3–8.77 × 10−3 | Mishra and Gogate, 2010 [32] |
| Orifice | 30 | 4.78 | 0.07–0.16 | 1.87 × 10−3–2.09 × 10−3 | ||||
| Slit Venturi | Carbamazepine | 10 | 35 | 3–5 | 3–11.6 | 6.00 × 10−4–3.8 × 10−3 | Thanekar et al., 2018 [33] | |
| Orifice | Orange Acid II | 20 | 20 | 3–7 | 2–8 | 2.24 × 10−3–5.67 × 10−3 | Gogate and Boshale, 2013 [34] | |
| Venturi | p-Nitrophenol | 30 | 0.001 | 2–7 | 3.5–8 | 0.16–0.57 | 6.05 × 10−4–1.59 × 10−2 | Capocelli et al., 2014 [35] |
| Orifice plate | 2,4-dinitrophenol | 30–40 | 20 | 3–6 | 3–11 | - | 7.7 × 10−4–1.11 × 10−3 | Bagal et al., 2013 [36] |
| Orifice | Methyl parathion | 30 | 20–100 | 1–8 | 2.2–8.2 | 1.40 × 10−3–5.42 × 10−3 | Patil and Gogate, 2012 [37] | |
| Orifice plate | Chlorpyrifos | 31–39 | 0.11 | 3–8 | 3–10 | 0.35–3.47 | 8.02 × 10−3–4.14 × 10−2 | Randhavane, 2019 [38] |
| Circular Venturi | Imidacloprid | 32 | 25 | 3–20 | 2.7–7.5 | 0.051–0.215 | 2.42 × 10−4–2.89 × 10−3 | Raut-Jadhav et al., 2014 [40] |
| Orifice | Ternary dyes | 30 | 30 | 2–8 | 2–9 | 0.10–0.47 | 2.72 × 10−3–4.52 × 10−2 | Kumar et al., 2018 [41] |
| Venturi | Tetramethyl ammonium hydroxide | 20 | 2000 | 3.25–6 | 3–7 | 0.26–0.4 | 2.94 × 10−3–4.14 × 10−2 | Innocenzi et al., 2018 [42] |
| Orifice | 2-Amino-4-chlorophenol | 35 | 20 | 2–4 | 3–8 | 0.27–0.76 | 1.7 × 10−3–1.9 × 10−3 | Barik et al., 2016 [43] |
| Variable | Description | Values |
|---|---|---|
| D (m) | Pipe diameter | 0.012 |
| D0 (m) | Orifice (constriction) diameter | 0.002 |
| Ld | Length of the divergent section | 0.024 |
| V (L) | Liquid volume | 1.5 |
| R0 (μm) | Initial bubble radius | 50–150 |
| πOH | Hydroxyl radical production at the collapse stage | Equation (5) |
| kLa (s−1) | Mass transfer coefficient | 0.5–10 × 10−2 |
| Dax (m2 s−1) | Axial Dispersion (eddy diffusivity) | 0.02 |
| kOH (M−1 s−1) | Hydroxyl radical—attack reaction rate | 109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capocelli, M.; De Crescenzo, C.; Karatza, D.; Lancia, A.; Musmarra, D.; Piemonte, V.; Prisciandaro, M. A Transport-Phenomena Approach to Model Hydrodynamic Cavitation of Organic Pollutants. Water 2020, 12, 1564. https://doi.org/10.3390/w12061564
Capocelli M, De Crescenzo C, Karatza D, Lancia A, Musmarra D, Piemonte V, Prisciandaro M. A Transport-Phenomena Approach to Model Hydrodynamic Cavitation of Organic Pollutants. Water. 2020; 12(6):1564. https://doi.org/10.3390/w12061564
Chicago/Turabian StyleCapocelli, Mauro, Carmen De Crescenzo, Despina Karatza, Amedeo Lancia, Dino Musmarra, Vincenzo Piemonte, and Marina Prisciandaro. 2020. "A Transport-Phenomena Approach to Model Hydrodynamic Cavitation of Organic Pollutants" Water 12, no. 6: 1564. https://doi.org/10.3390/w12061564
APA StyleCapocelli, M., De Crescenzo, C., Karatza, D., Lancia, A., Musmarra, D., Piemonte, V., & Prisciandaro, M. (2020). A Transport-Phenomena Approach to Model Hydrodynamic Cavitation of Organic Pollutants. Water, 12(6), 1564. https://doi.org/10.3390/w12061564









