The Hydrogeochemical Characteristics of Groundwater Subjected to Seawater Intrusion in the Archipelago, Korea
Abstract
1. Introduction
2. Study Area and Methods
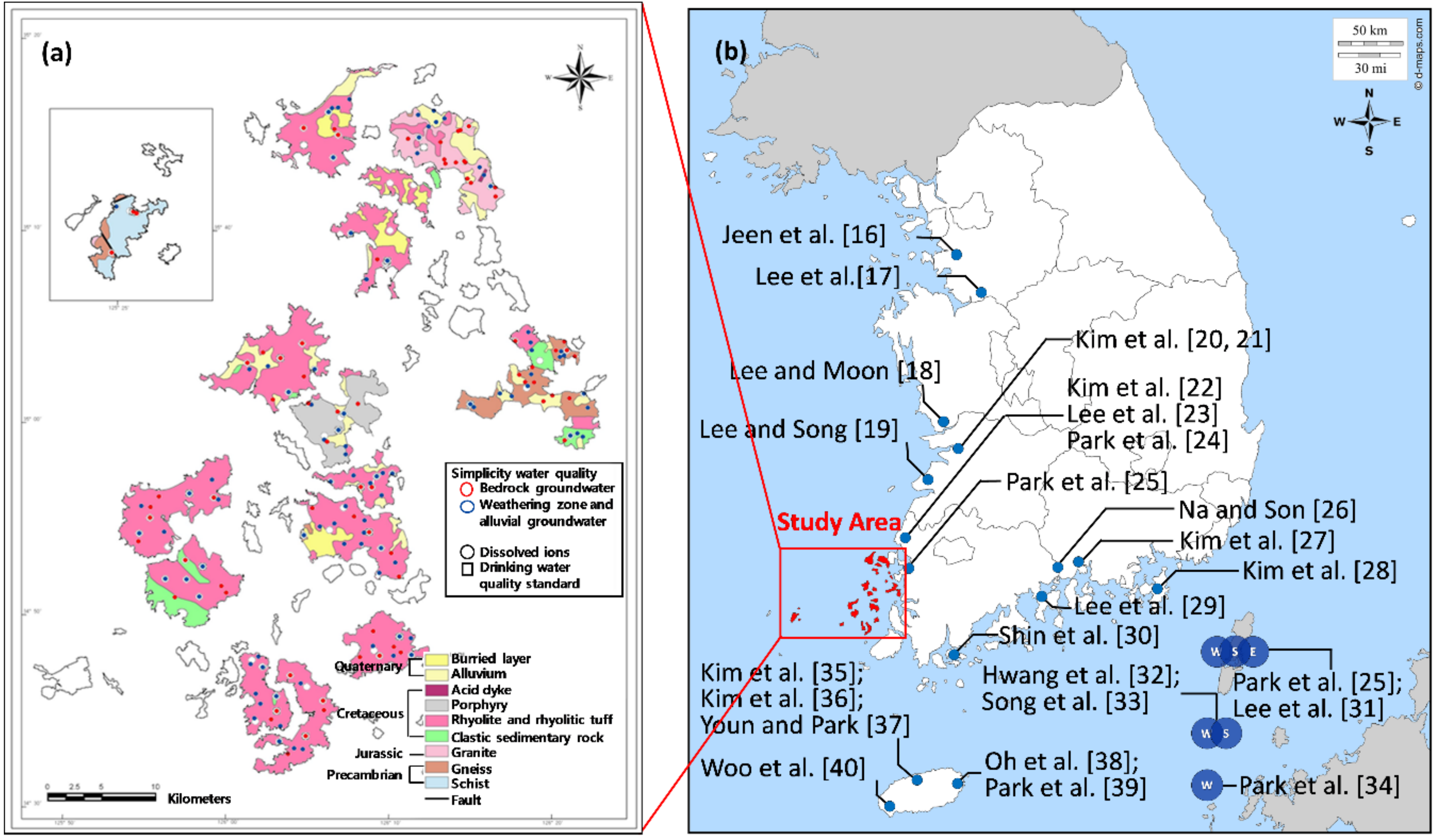
2.1. Study Area
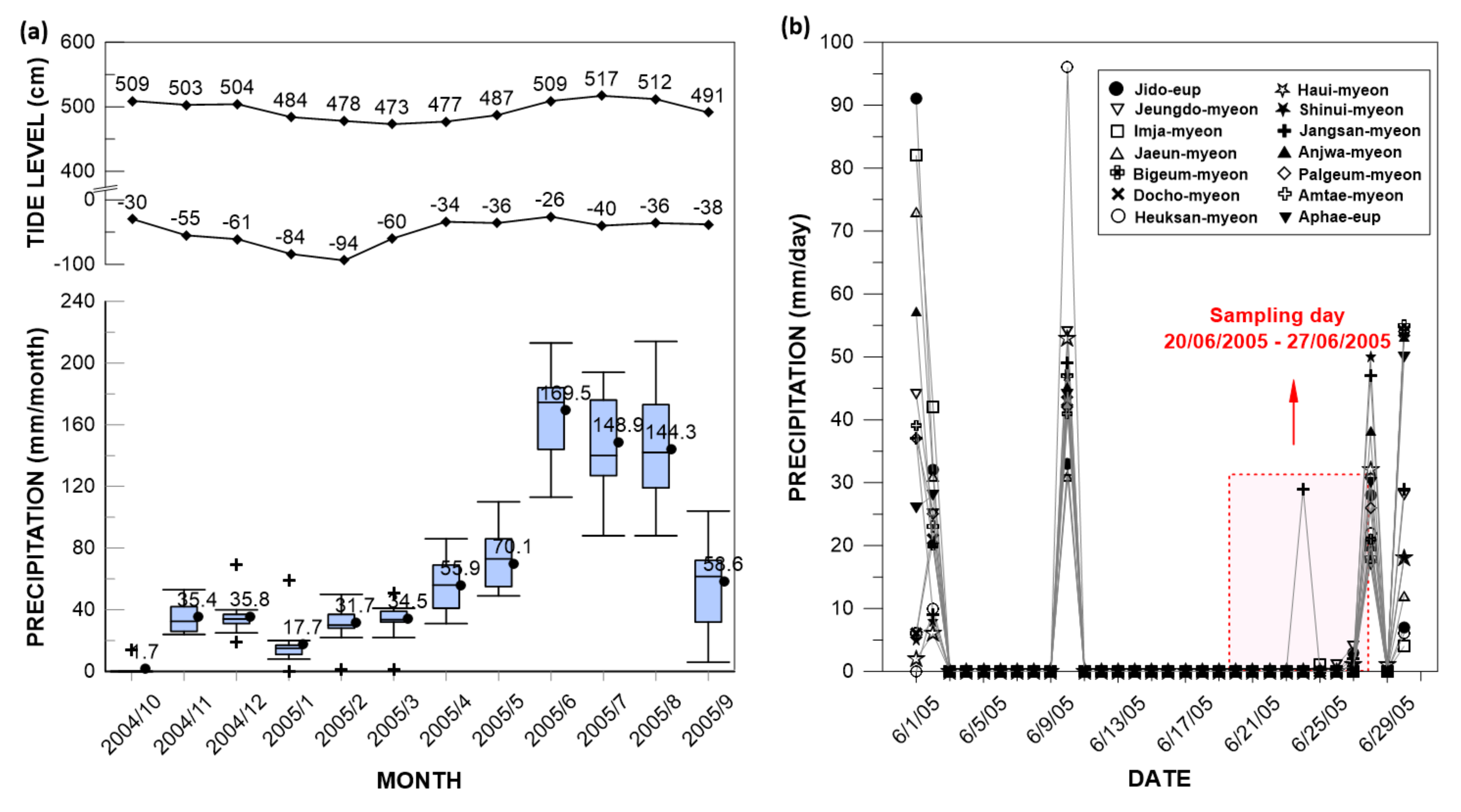
2.2. Sampling and Chemical Analysis
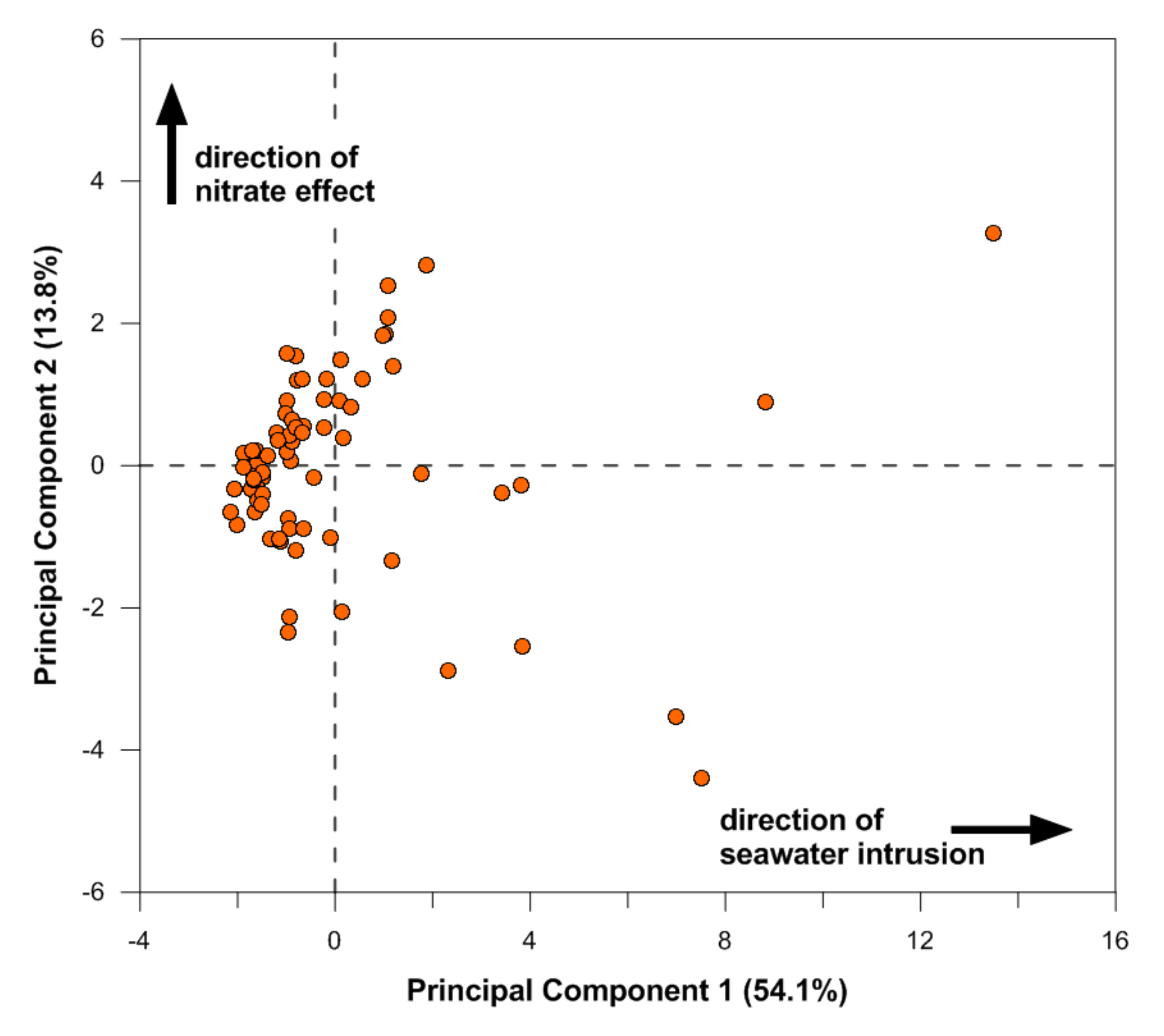
2.3. Principal Component Analysis (PCA)
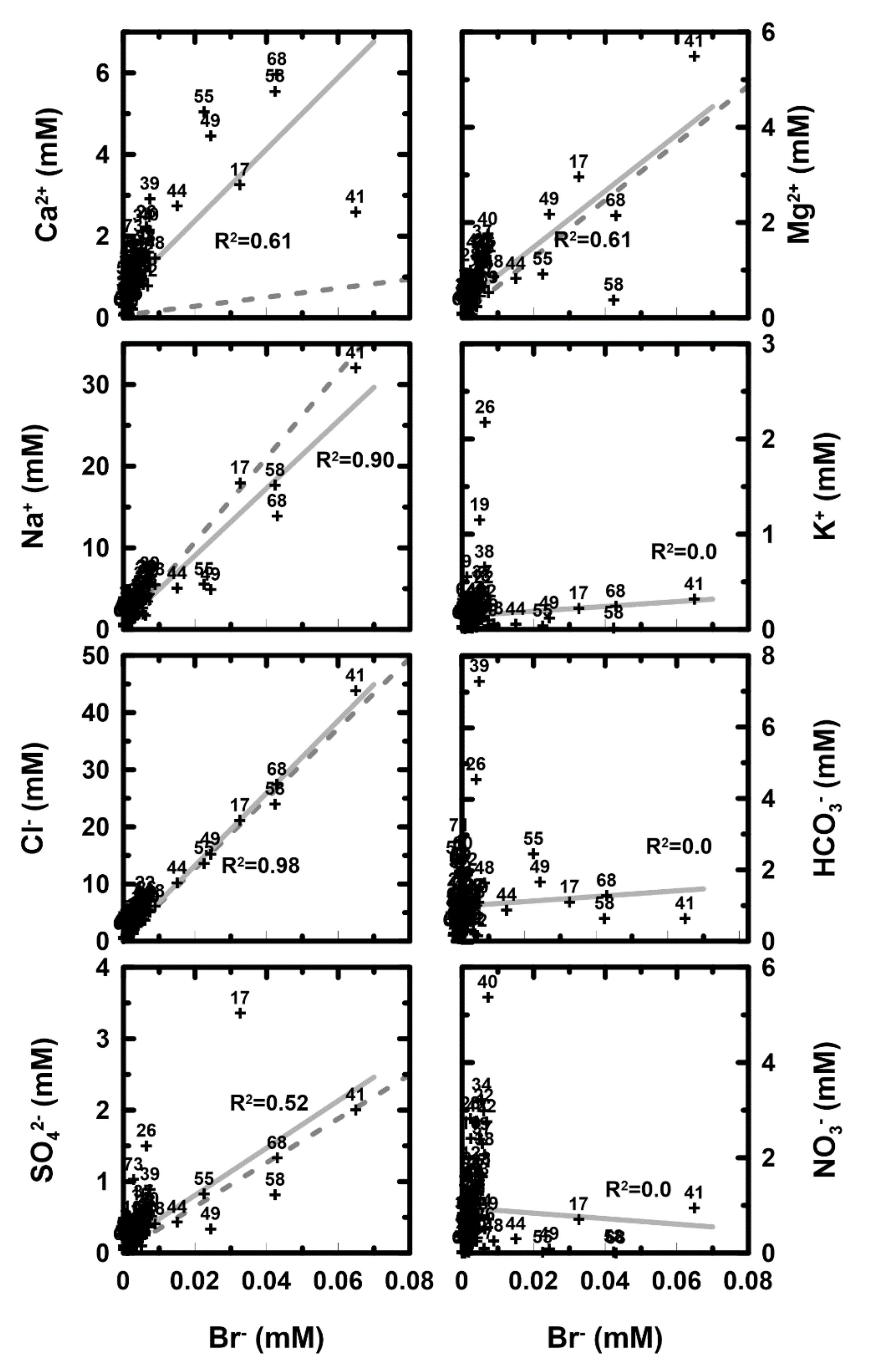
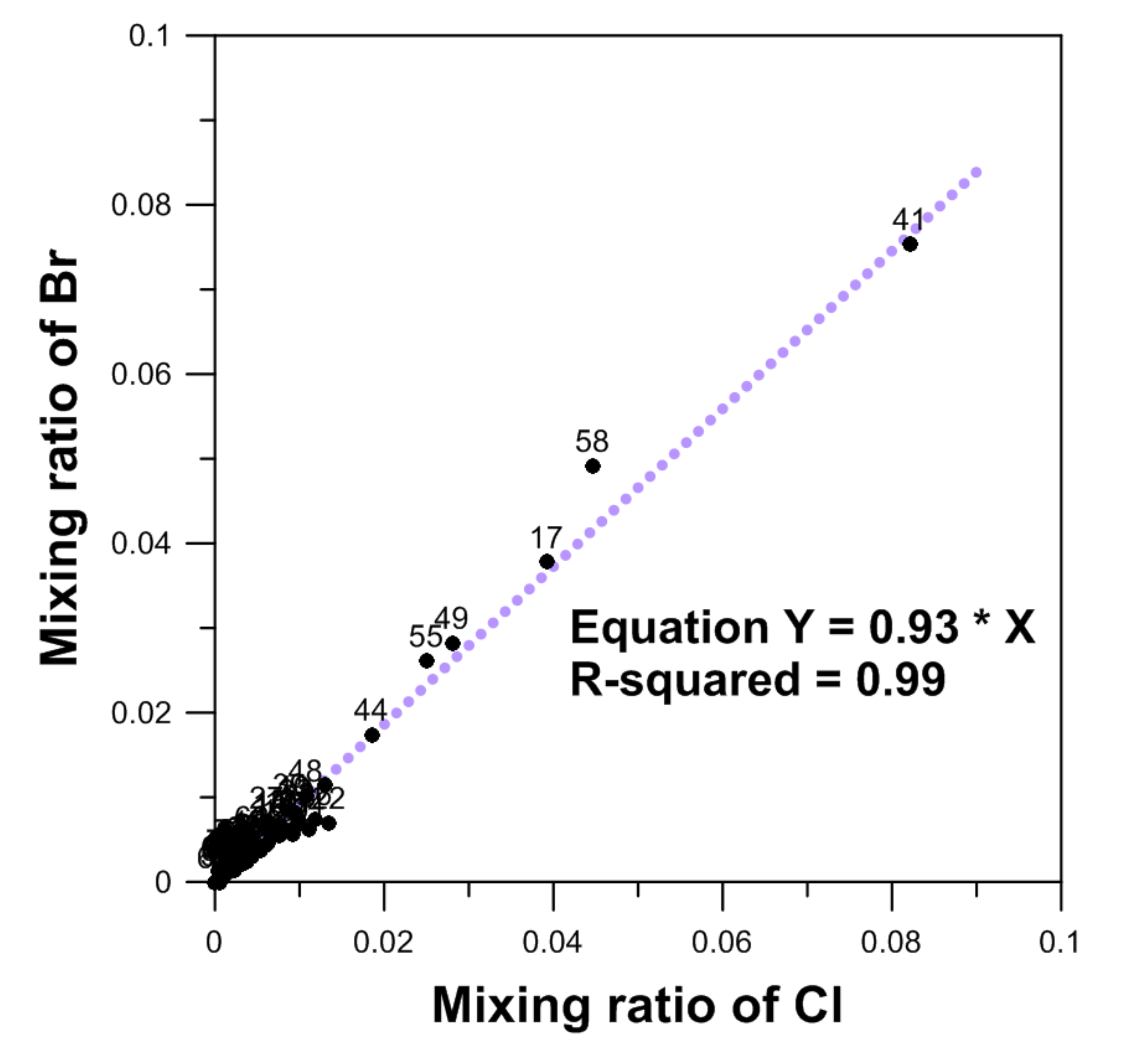
2.4. Ioninc Detas by Mixing Ratio
3. Results
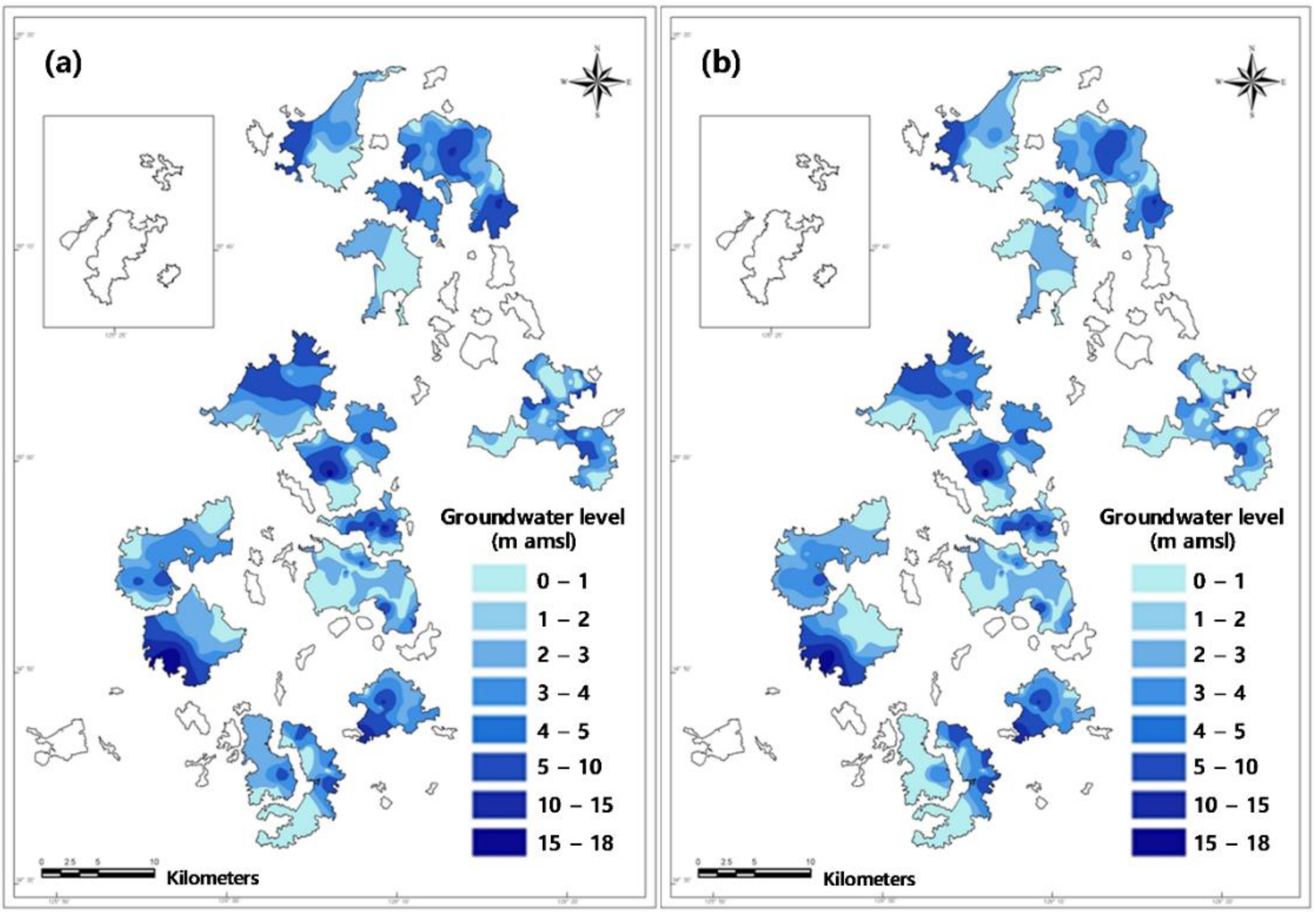
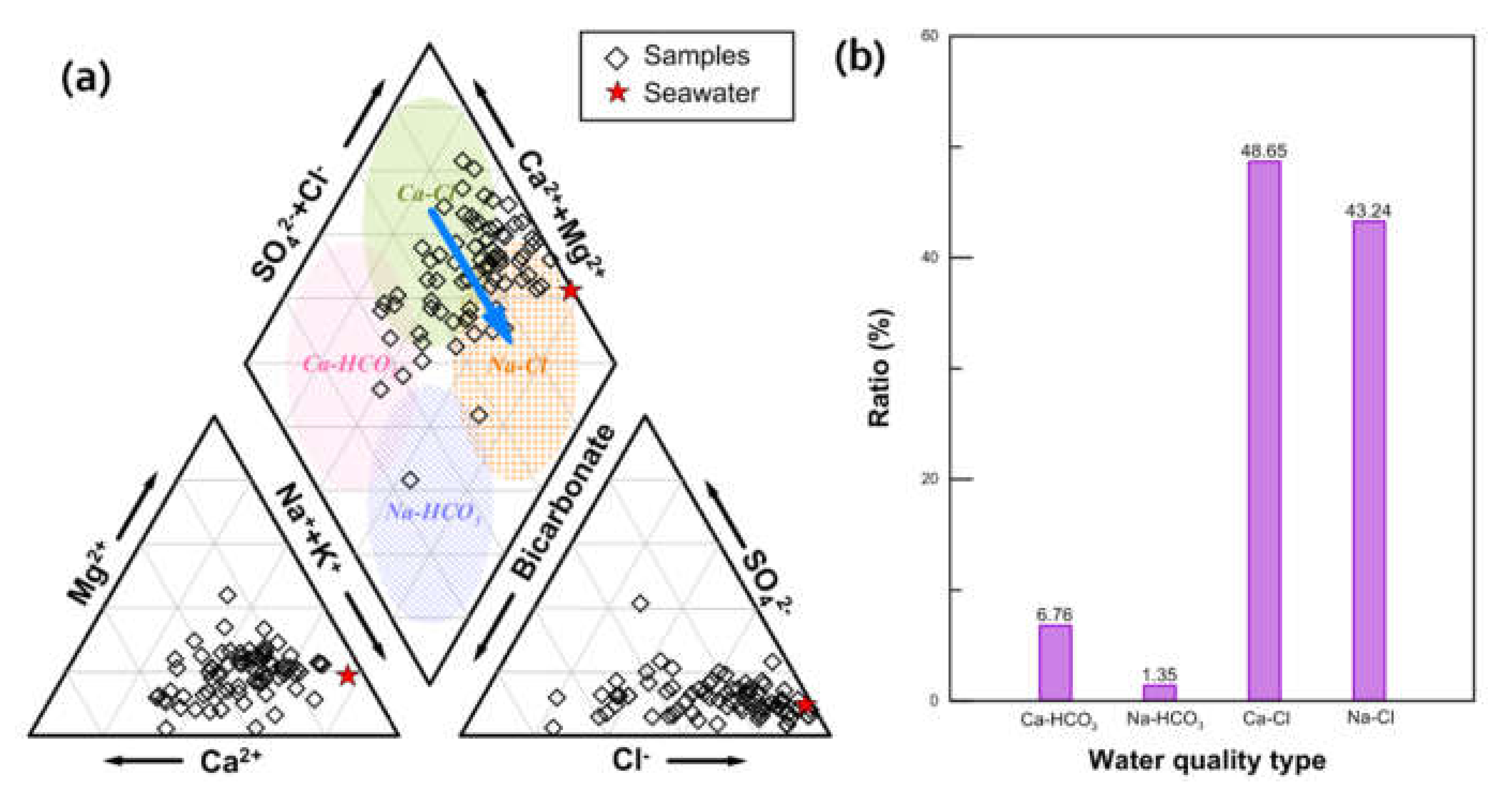
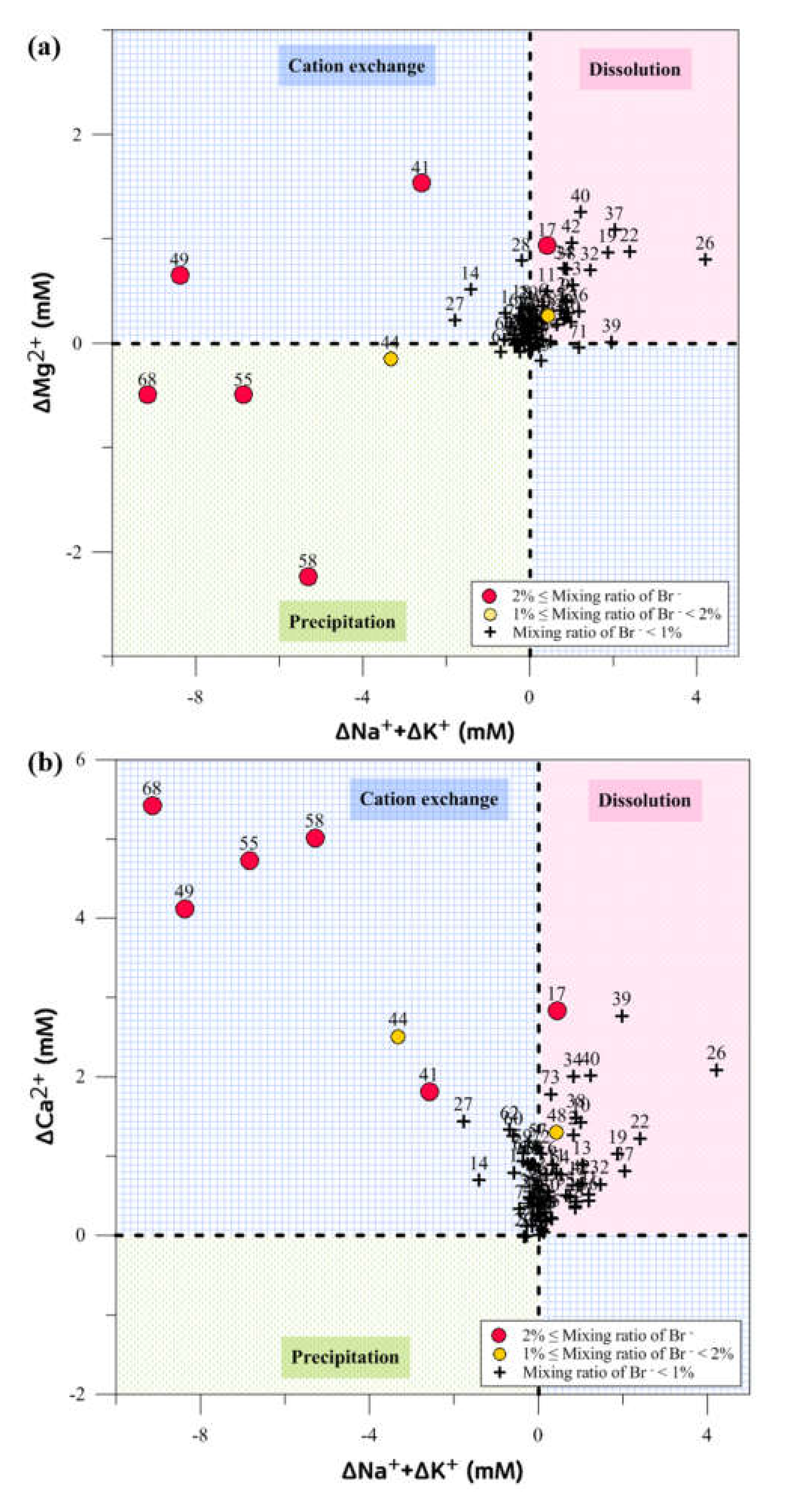
4. Discussion
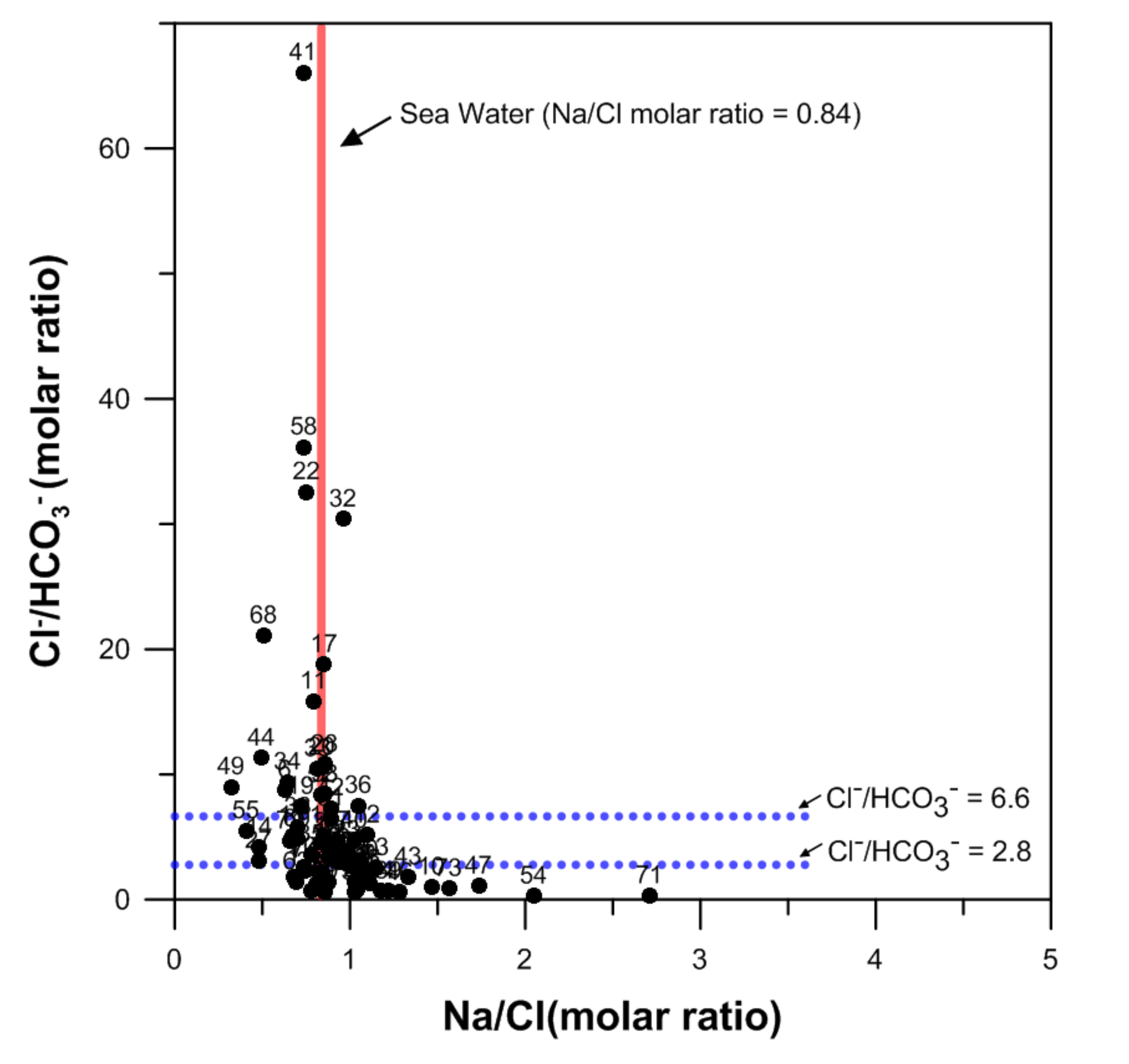
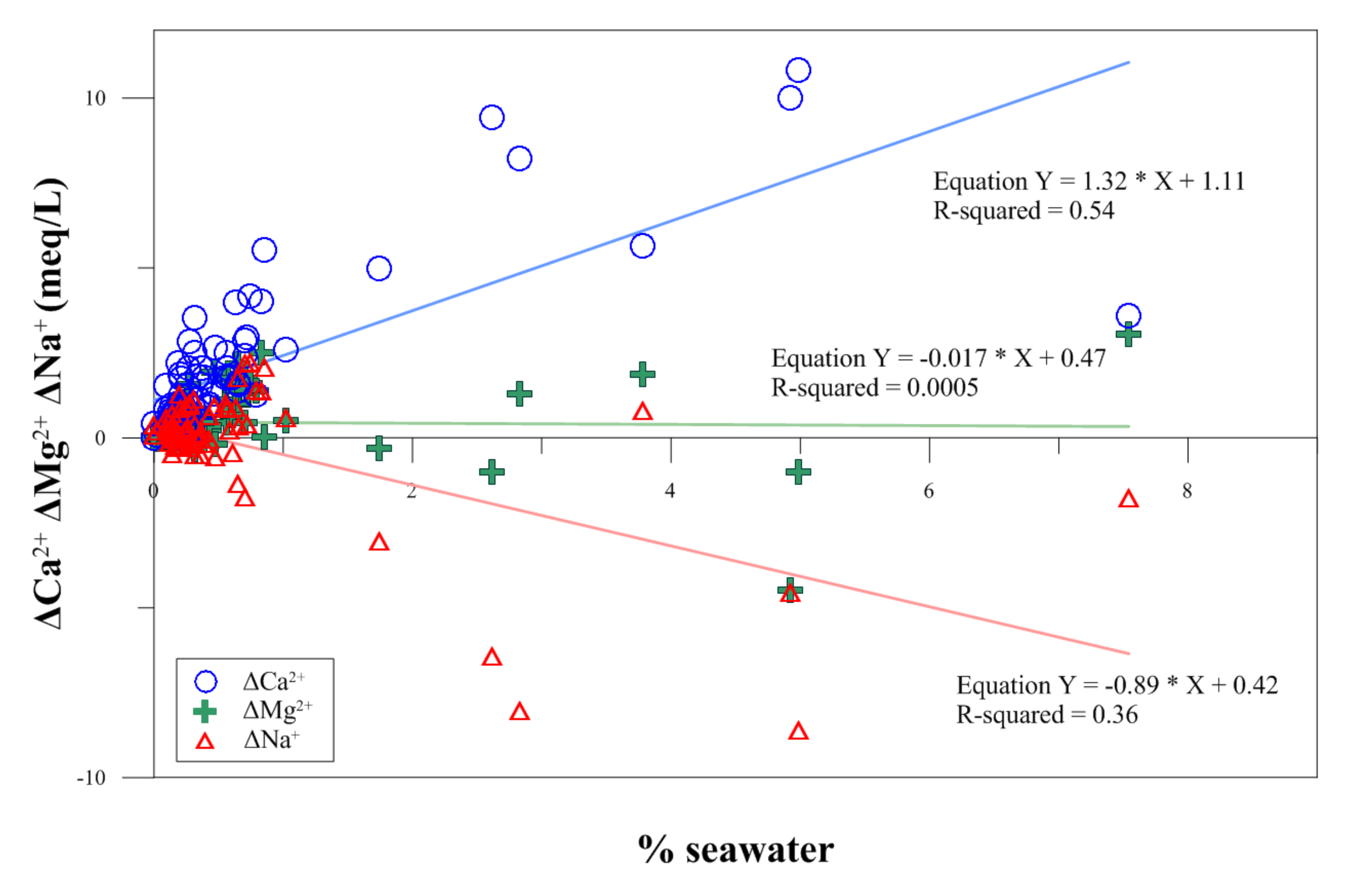
4.1. The Mixing Ratio between Groundwater and Seawater
4.2. Hydrogeochemical Processes by Seawater Intrusion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, M.S.; Nyvang, V.; Jakobsen, R.; Postma, D. Geochemical processes and solute transport at the seawater/freshwater interface of a sandy aquifer. Geochim. Cosmochim. Acta 2005, 69, 3979–3994. [Google Scholar] [CrossRef]
- Werner, A.D.; Simmons, C.T. Impact of sea-level rise on sea water intrusion in coastal aquifer. Groundwater 2009, 47, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiao, J.J.; Liang, W.; Kuang, X. Hydrogeochmical characteristics in coastal groundwater mixing zone. Appl. Geochem. 2017, 85, 49–60. [Google Scholar] [CrossRef]
- Kazakis, N.; Pavlou, A.; Vargemezis, G.; Voudouris, K.S.; Soulios, G.; Pliakas, F.; Tsokas, G. Seawater intrusion mapping using electrical resistivity tomography and hydrochemical data. An application in the coastal area of eastern Thermaikos Gulf, Greece. Sci. Total Environ. 2016, 543, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Alfarrah, N.; Walraevens, K. Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions. Water 2018, 10, 143. [Google Scholar] [CrossRef]
- Mahlknecht, J.; Merchan, D.; Rosner, M.; Meixner, A.; Ledesma-Ruiz, R. Assessing seawater intrusion in an arid coastal aquifer under high anthropogenic influence using major constituents, Sr and B isotopes in groundwater. Sci. Total Environ. 2017, 587, 282–295. [Google Scholar] [CrossRef]
- Mondal, N.C.; Singh, V.P.; Singh, V.S.; Saxena, V.K. Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J. Hydrol. 2010, 388, 100–111. [Google Scholar] [CrossRef]
- Vallejos, A.; Daniele, L.; Sola, F.; Molina, L.; Pulido-Bosch, A. Anthropic-induced salinization in a dolomite coastal aquifer. Hydrogeochemical processes. J. Geochem. Explor. 2020, 209, 106438. [Google Scholar] [CrossRef]
- Sánchez-Martos, F.; Pulido-Bosch, A.; Molina-Sánchez, L.; Vallejos-Izquierdo, A. Identification of the origin of salinization in groundwater using minor ions (Lower Andarax, Southeast Spain). Sci. Total Environ. 2002, 297, 43–58. [Google Scholar] [CrossRef]
- Vengosh, A.; Rosenthal, E. Saline groundwater in Israel: Its bearing on the water crisis in the country. J. Hydrol. 1994, 156, 389–430. [Google Scholar] [CrossRef]
- Giménez-Forcada, E.; Bencini, A.; Pranzini, G. Hydrogeochemical considerations about the origin of groundwater salinization in some coastal plains of Elba Island (Tuscany, Italy). Environ. Geochem. Health 2010, 32, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Singaraja, C.; Chidambaram, S.; Prasanna, M.V.; Thivya, C.; Thilagavathi, R. Statistical analysis of the hydrogeochemical evolution of groundwater in hard rock coastal aquifers of Thoothukudi district in Tamil Nadu, India. Environ. Earth Sci. 2014, 71, 451–464. [Google Scholar] [CrossRef]
- Chang, S.W. A review of recent research into coastal groundwater problems and associated case studies. J. Eng. Geol. 2014, 24, 597–608. [Google Scholar] [CrossRef][Green Version]
- Cruz, J.V.; Coutinho, R.; Pacheco, D.; Cymbron, R.; Antunes, P.; Freire, P.; Mendes, S. Groundwater salinization in the Azores archipelago (Portugal). Environ. Earth Sci. 2011, 62, 1273–1285. [Google Scholar] [CrossRef]
- Ministry of Land, Infrastructure and Transportation (MOLIT); K-Water and Korea Institute of Geoscience and Mineral Resources (KIGAM). Report On the Basic Groundwater Investigation of Shinan; MOLIT: Sejong, Korea, 2005. (In Korean)
- Jeen, S.W.; Kim, J.M.; Ko, K.S.; Yum, B.W.; Chang, H.W. Hydrogeochemical characteristics of groundwater in a mid-western coastal aquifer system, Korea. Geosci. J. 2001, 5, 339–348. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Kim, H.-M.; Chang, H.W. Statistical approach to determine the salinized ground water flow path and Hydrogeochemical features around the underground LPG cavern, Korea. Hydrol. Process. 2007, 21, 3615–3626. [Google Scholar] [CrossRef]
- Lee, B.J.; Moon, S.H. Integrated approach for evaluating the characteristics of seawater intrusion using factor analysis and time series analysis: Seocheon-Gunsan area. J. Geol. Soc. Korea 2008, 44, 219–232. [Google Scholar]
- Lee, J.Y.; Song, S.H. Groundwater chemistry and ionic ratios in a western coastal aquifer of Buan, Korea: Implication for seawater intrusion. Geosci. J. 2007, 11, 259–270. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, R.H.; Lee, J.; Chang, H.W. Hydrogeochemical characterization of major factors affecting the quality of shallow groundwater in the coastal area at Kimje in South Korea. Environ. Geol. 2003, 44, 478–489. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Cheong, T.J.; Kim, R.H.; Koh, D.C.; Ryu, J.S.; Chang, H.W. Use of time series analysis for the identification of tidal effect on groundwater in the coastal area of Kimje, Korea. J. Hydrol. 2005, 300, 188–198. [Google Scholar] [CrossRef]
- Kim, R.H.; Kim, J.H.; Ryu, J.S.; Chang, H.W. Salinization properties of a shallow groundwater in a coastal reclaimed area, Yeonggwang, Korea. Environ. Geol. 2006, 49, 1180–1194. [Google Scholar] [CrossRef]
- Lee, B.J.; Hwang, S.H. Evaluation of characteristics of seawater intrusion based on the groundwater fluctuations: Baksu area, Yeonggwang-gun. J. Geol. Soc. Korea 2008, 44, 232–240. [Google Scholar]
- Park, K.G.; Shin, J.H.; Hwang, S.H.; Park, I.H. Fresh water injection test to mitigate seawater intrusion and geophysical monitoring in coastal area. Korean Soc. Earth Explor. Geophys. 2007, 10, 353–360. [Google Scholar]
- Park, Y.Y.; Lee, J.Y.; Kim, J.H.; Song, S.H. National scale evaluation of groundwater chemistry in Korea coastal aquifers: Evidences of seawater intrusion. Environ. Earth Sci. 2012, 66, 707–718. [Google Scholar] [CrossRef]
- Na, C.K.; Son, C.I. Groundwater quality and pollution characteristics at Seomjin river basin: Pollution source and risk assessment. Econ. Environ. Geol. 2005, 35, 261–272. [Google Scholar]
- Kim, H.J.; Hamm, S.Y.; Cheong, J.Y.; Lee, J.H. Characteristics of groundwater contamination caused by seawater intrusion and agricultural activity in sacheon and hadong areas, Republic of Korea. Econ. Environ. Geol. 2009, 42, 575–589. [Google Scholar]
- Kim, C.S.; Kim, K.S.; Bae, D.S.; Song, S.H. Hydrogeological characteristics of seawater intrusion in the coastal area. J. Korean Soc. Groundw. Environ. 1997, 4, 61–72. [Google Scholar]
- Lee, J.H.; Kim, R.H.; Chang, H.W. Interaction between groundwater quality and hydraulic head in an area around an underground LPG storage cavern, Korea. Environ. Geol. 2002, 13, 901–912. [Google Scholar] [CrossRef]
- Shin, I.H.; Park, C.Y.; Ahan, K.S.; Jeong, Y.J. Hydrogeochemistry of Groundwaters at the Gogum island area in Jeonnam, Korea. J. Korean Earth Sci. Soc. 2002, 23, 474–485. [Google Scholar]
- Lee, Y.H.; Hong, W.H.; Lee, J.S. The vulnerability assessment of water supply through the analysis of emergency water shortage in cut off water supply-prone island area. J. Archit. Inst. Korea Plan. Des. 2014, 30, 253–260. [Google Scholar] [CrossRef]
- Hwang, S.H.; Park, K.G.; Shin, J.H.; Lee, S.K. Relationship between the groundwater resistivity and NaCl equivalent salinity in western and southern coastal areas, Korea. Geophys. Geophys. Explor. 2007, 10, 361–368. [Google Scholar]
- Song, S.H.; Lee, J.Y.; Yi, M.J. Evaluation of long-term data obtained from seawater intrusion monitoring network using variation type analysis. J. Korean Earth Sci. Soc. 2007, 28, 478–490. [Google Scholar] [CrossRef]
- Park, S.C.; Yun, S.T.; Chae, G.T.; Yoo, I.S.; Shin, K.S.; Heo, C.H.; Lee, S.K. Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J. Hydrol. 2005, 313, 182–194. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, K.S.; Koh, D.C.; Lee, D.H.; Lee, S.G.; Park, W.B.; Koh, G.W.; Woo, N.C. Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: A case study in Jeju volcanic island, Korea. J. Hydrol. 2003, 270, 282–294. [Google Scholar] [CrossRef]
- Kim, K.H.; Shin, J.Y.; Koh, E.H.; Koh, G.W.; Lee, K.K. Sea level rise around Jeju Island due to global warming and movement of groundwater/seawater interface in the eastern part of Jeju Island. J. Soil Groundw. Environ. 2009, 14, 68–79. [Google Scholar]
- Youn, J.S.; Park, S.W. Hydrochemical characteristics of spring water in Cheju Island. J. Korean Soc. Groundw. Environ. 1998, 5, 66–79. [Google Scholar]
- Oh, T.G.; Kim, Y.J.; Kim, K.S.; Kim, S.R. Nitrate and chloride characteristics in the groundwater in Jeju area. J. Environ. Res. 2006, 3, 1–14. [Google Scholar]
- Park, N.S.; Koh, B.R.; Lim, Y.D. Impacts of fresh and saline groundwater development in Sungsan watershed, Jeju Island. J. Korea Water Resour. Assoc. 2013, 46, 783–794. [Google Scholar] [CrossRef]
- Woo, N.C.; Kim, H.D.; Lee, K.S.; Park, W.B.; Koh, G.W.; Moon, Y.S. Interpretation of groundwater system and contamination by water-quality monitoring in the Daejung watershed, Jeju Island. Econ. Environ. Geol. 2001, 34, 485–498. [Google Scholar]
- Chae, G.T.; Yun, S.T.; Yun, S.M.; Kim, K.H.; So, C.S. Seawater-freshwater mixing and resulting calcite dissolution: An example from a coastal alluvial aquifer in eastern South Korea. Hydrol. Sci. J. 2012, 57, 1672–1683. [Google Scholar] [CrossRef]
- Lee, J.; Ko, K.S.; Kim, J.M.; Chang, H.W. Multivariate statistical analysis of underground gas storage caverns on groundwater chemistry in Korea. Hydrol. Process. 2008, 22, 3410–3417. [Google Scholar] [CrossRef]
- Ko, K.S.; Lee, J.; Lee, K.K.; Chang, H.W. Multivariate statistical analysis for groundwater mixing ratios around underground storage caverns in Korea. Carbonates Evaporites 2010, 25, 35–42. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, J. Time series analysis for evaluating hydrological responses of pore-water pressure to rainfall in a slope. Hydrol. Sci. J. 2017, 62, 1412–1421. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Skårman, C.; Skårman, E. Multivariate mixing and mass balance (M3) calculations, a new tool for decoding hydrogeochemical information. Appl. Geochem. 1999, 14, 861–871. [Google Scholar] [CrossRef]
- Stetzenbach, K.J.; Farnham, I.M.; Hodge, V.F.; Johannesson, K.H. Using multivariate statistical analysis of groundwater major cation and trace element concentrations to evaluate groundwater flow in a regional aquifer. Hydrol. Process. 1999, 13, 2655–2673. [Google Scholar] [CrossRef]
- Rencher, A.C. Methods of Multivariate Analysis; Wiley Series in Probability and Mathematical Statistics: Probability and Mathematical Statistics Section; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1995; ISBN 0471-571-520. [Google Scholar]
- Zghibi, A.; Zouhri, L.; Tarhouni, J.; Kouzana, L. Groundwater mineralization processes in Mediterranean semi-arid systems (Cap-Bon, North east of Tunisia): Hydrogeological and geochemical approaches. Hydrol. Process. 2013, 27, 3227–3239. [Google Scholar]
- Argamasilla, M.; Barberá, J.A.; Andreo, B. Factors controlling groundwater salinization and hydrogeochemical processes in coastal aquifers from southern Spain. Sci. Total Environ. 2017, 580, 50–68. [Google Scholar] [CrossRef]
- Revelle, R. Criteria for recognition of seawater in groundwater. Trans. Am. Geophys. Union 1941, 22, 593–597. [Google Scholar] [CrossRef]
- Faye, S.; Maloszewski, P.; Stichler, W.; Trimborn, P.; Faye, S.C.; Gaye, C.B. Groundwater salinization in the Saloum (Senegal) delta aquifer: Minor elements and isotopic indicators. Sci. Total Environ. 2005, 343, 243–259. [Google Scholar] [CrossRef]
- Giménez, E.; Morell, I. Hydrogeochemical analysis of salinization processes in the coastal aquifer of Oropesa (Castellon, Spain). Environ. Geol. 1997, 29, 118–131. [Google Scholar] [CrossRef]
- Santucci, L.; Carol, E.; Kruse, E. Identification of palaeo-seawater intrusion in groundwater using minor ions in a semi-confined aquifer of the Río de la Plata littoral (Argentina). Sci. Total Environ. 1640, 566, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koh, D.C.; Choo, M.K. Influences of fractionation of stable isotopic composition of rain and snowmelt on isotopic hydrograph separation. J. Korean Earth Sci. Soc. 2014, 35, 97–103. [Google Scholar] [CrossRef][Green Version]
| pH | Temp. | Electrical Conductivity (EC) | Total Dissolved Solids (TDS) | Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | NO3− | Br− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (uS/cm) | (mg/L) | |||||||||||
| Min. | 5.3 | 14.7 | 120 | 72.7 | 9.3 | 0.3 | 2.5 | 1.6 | 13.6 | 5.9 | 3.3 | 0.2 | 0.003 |
| Median | 6.4 | 16.9 | 513.5 | 335.6 | 42.1 | 2.4 | 32 | 10.4 | 73.7 | 43.7 | 19.7 | 44.2 | 0.2 |
| Max. | 8.4 | 20.7 | 4800 | 2870 | 737.2 | 85 | 238.5 | 133.6 | 1554.7 | 445.9 | 322.7 | 333 | 5.19 |
| Mean | 6.6 | 17.2 | 778.2 | 479.1 | 75.6 | 6.1 | 45.9 | 15.9 | 154.4 | 63.4 | 33.3 | 58.5 | 0.44 |
| Standard Deviation | 0.6 | 1.33 | 805.2 | 460.3 | 107.5 | 11.6 | 48.4 | 19.2 | 243.6 | 64 | 47.5 | 60.9 | 0.79 |
| Variables | PC 1 | PC 2 |
|---|---|---|
| TDS | 0.37 | 0.03 |
| EC | 0.37 | 0.03 |
| Cl− | 0.36 | 0.00 |
| Ca2+ | 0.30 | −0.27 |
| Mg2+ | 0.33 | 0.30 |
| Na+ | 0.36 | 0.05 |
| K+ | 0.10 | 0.02 |
| SO42− | 0.32 | 0.01 |
| Br− | 0.33 | 0.04 |
| HCO3− | 0.08 | −0.35 |
| SiO2 | 0.03 | 0.33 |
| NO3− | 0.03 | 0.54 |
| F− | 0.16 | −0.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.; Koh, D.-C.; Jung, H.; Lee, J. The Hydrogeochemical Characteristics of Groundwater Subjected to Seawater Intrusion in the Archipelago, Korea. Water 2020, 12, 1542. https://doi.org/10.3390/w12061542
Shin K, Koh D-C, Jung H, Lee J. The Hydrogeochemical Characteristics of Groundwater Subjected to Seawater Intrusion in the Archipelago, Korea. Water. 2020; 12(6):1542. https://doi.org/10.3390/w12061542
Chicago/Turabian StyleShin, Kyungsun, Dong-Chan Koh, Hyejung Jung, and Jeonghoon Lee. 2020. "The Hydrogeochemical Characteristics of Groundwater Subjected to Seawater Intrusion in the Archipelago, Korea" Water 12, no. 6: 1542. https://doi.org/10.3390/w12061542
APA StyleShin, K., Koh, D.-C., Jung, H., & Lee, J. (2020). The Hydrogeochemical Characteristics of Groundwater Subjected to Seawater Intrusion in the Archipelago, Korea. Water, 12(6), 1542. https://doi.org/10.3390/w12061542






