Uptake of Sulfate from Ambient Water by Freshwater Animals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Animals
2.2. Test Water
2.3. Sampling Methods
2.3.1. Measurements of Animals and Water
2.3.2. Chemical Analysis of Test Water and Animals
2.3.3. Compartmental Analysis to Calculate the Influx and Efflux of Sulfate
2.3.4. Calculations
2.3.5. Statistical Analysis
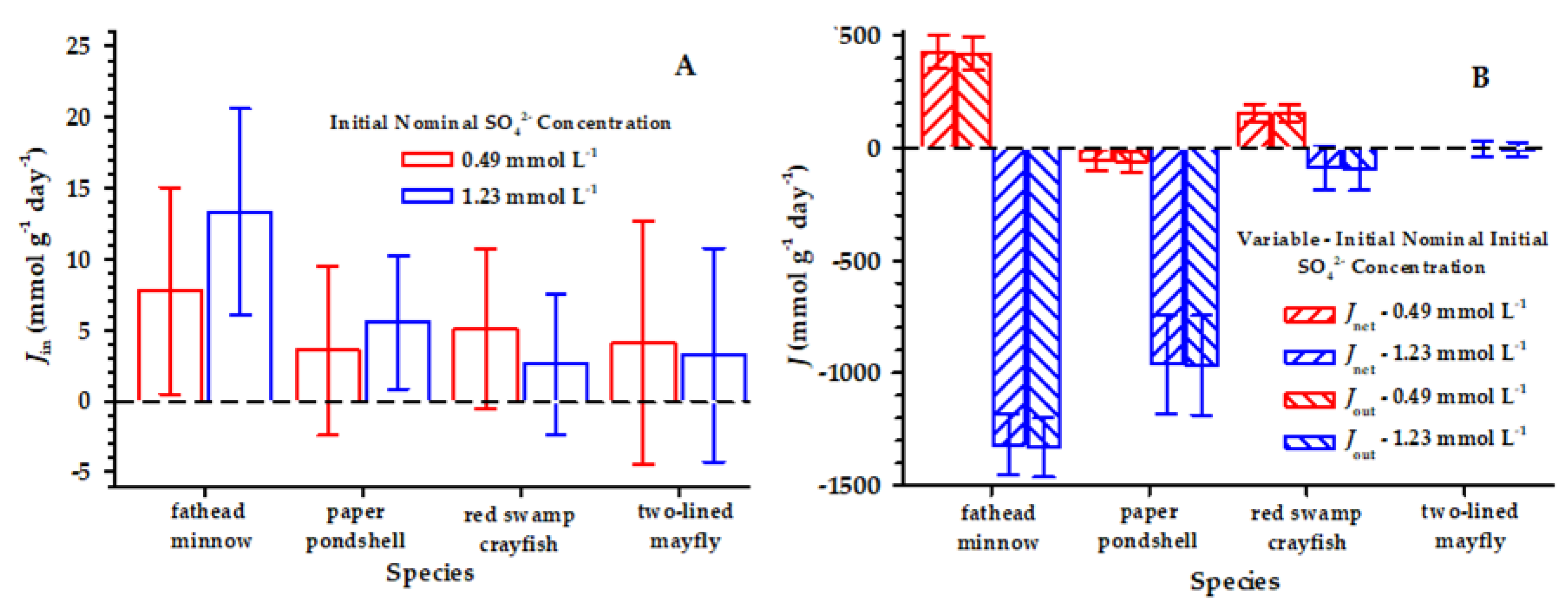
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data
References
- Griffith, M.; Zheng, L.; Cormier, S.M. Using extirpation to evaluate ionic tolerance of freshwater fish. Environ. Toxicol. Chem. 2017, 37, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.M.; Suter, G.; Zheng, L. Derivation of a benchmark for freshwater ionic strength. Environ. Toxicol. Chem. 2012, 32, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elphick, J.R.; Bergh, K.D.; Bailey, H.C. Chronic toxicity of chloride to freshwater species: Effects of hardness and implications for water quality guidelines. Environ. Toxicol. Chem. 2010, 30, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lasier, P.J.; Hardin, I.R. Observed and predicted reproduction ofCeriodaphnia dubiaexposed to chloride, sulfate, and bicarbonate. Environ. Toxicol. Chem. 2010, 29, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Harper, D.D. The chronic toxicity of sodium bicarbonate, a major component of coal bed natural gas produced waters. Environ. Toxicol. Chem. 2014, 33, 532–540. [Google Scholar] [CrossRef]
- Soucek, D.J.; Linton, T.K.; Tarr, C.D.; Dickinson, A.; Wickramanayake, N.; Delos, C.G.; Cruz, L.A. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 2011, 30, 930–938. [Google Scholar] [CrossRef]
- Griffith, M.; Norton, S.B.; Alexander, L.C.; Pollard, A.I.; LeDuc, S.D. The effects of mountaintop mines and valley fills on the physicochemical quality of stream ecosystems in the central Appalachians: A review. Sci. Total Environ. 2012, 417, 1–12. [Google Scholar] [CrossRef]
- Cowie, R.; Williams, M.W.; Wireman, M.; Runkel, R.L. Use of Natural and Applied Tracers to Guide Targeted Remediation Efforts in an Acid Mine Drainage System, Colorado Rockies, USA. Water 2014, 6, 745–777. [Google Scholar] [CrossRef] [Green Version]
- Nordstrom, D.K. Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Iii, C.A.C.; Brightbill, R.A.; Langland, M.J. Abandoned Mine Drainage in the Swatara Creek Basin, Southern Anthracite Coalfield, Pennsylvania, USA: 1. Stream Water Quality Trends Coinciding with the Return of Fish. Mine Water Environ. 2010, 29, 176–199. [Google Scholar] [CrossRef] [Green Version]
- Elphick, J.R.; Davies, M.; Gilron, G.; Canaria, E.C.; Lo, B.; Bailey, H.C. An aquatic toxicological evaluation of sulfate: The case for considering hardness as a modifying factor in setting water quality guidelines. Environ. Toxicol. Chem. 2010, 30, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.D.; Hall, K.J. Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environ. Toxicol. Chem. 2007, 26, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Soucek, D.J. Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ. Toxicol. Chem. 2007, 26, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Soucek, D.J.; Dickinson, A. Full-life chronic toxicity of sodium salts to the mayfly Neocloeon triangulifer in tests with laboratory cultured food. Environ. Toxicol. Chem. 2015, 34, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Horisberger, J.-D. Recent Insights into the Structure and Mechanism of the Sodium Pump. Physiology 2004, 19, 377–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanis, G.; Esbaugh, A.J.; Perry, S.F. Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J. Exp. Boil. 2008, 211, 2467–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullikuth, A.; Aimanova, K.; Kang’Ethe, W.; Sanders, H.R.; Gill, S.S. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J. Exp. Boil. 2006, 209, 3529–3544. [Google Scholar] [CrossRef] [Green Version]
- Soucek, D.J.; Mount, D.R.; Dickinson, A.; Hockett, J.R. Influence of dilution water ionic composition on acute major ion toxicity to the mayfly Neocloeon triangulifer. Environ. Toxicol. Chem. 2018, 37, 1330–1339. [Google Scholar] [CrossRef]
- Erickson, R.J.; Mount, D.R.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Peterson, K.N. The acute toxicity of major ion salts to Ceriodaphnia dubia. II. Empirical relationships in binary salt mixtures. Environ. Toxicol. Chem. 2016, 36, 1525–1537. [Google Scholar] [CrossRef]
- Mount, D.R.; Erickson, R.J.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Peterson, K.N.; Polaske, Z.M.; Wisniewski, S.; et al. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. influence of background water chemistry. Environ. Toxicol. Chem. 2016, 35, 3039–3057. [Google Scholar] [CrossRef]
- Griffith, M. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: Teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 2016, 36, 576–600. [Google Scholar] [CrossRef] [PubMed]
- Romeu, F.G.; Maetz, J. The Mechanism of Sodium and Chloride Uptake by the Gills of a Fresh-Water Fish, Carassius auratus. J. Gen. Physiol. 1964, 47, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- De Renzis, G.; Maetz, J. Studies on the mechanism of chloride absorption by the goldfish gill: Relation with acid-base regulation. J. Exp. Biol. 1973, 59, 339–358. [Google Scholar]
- Shaw, J. The absorption of sodium ions by the crayfish, Astacus pallipes Lereboullet: II. The effect of the external anion. J. Exp. Biol. 1960, 37, 534–547. [Google Scholar]
- McMahon, B.; Stuart, S. The physiological problems of crayfish in acid waters. In Acid Toxicity and Aquatic Animals; Cambridge University Press (CUP): Cambridge, UK, 1989; Volume 34, pp. 171–200. [Google Scholar]
- Dietz, T.H.; Udoetok, A.S.; Cherry, J.S.; Byrne, H.S.A. Kidney function and sulfate uptake and loss in the freshwater bivalve Toxolasma texasensis. Boil. Bull. 2000, 199, 14–20. [Google Scholar] [CrossRef]
- Larsen, E.H.; Simonsen, K. Sulfate transport in toad skin: Evidence for mitochondria-rich cell pathways in common with halide ions. Comp. Biochem. Physiol. Part A Physiol. 1988, 90, 709–714. [Google Scholar] [CrossRef]
- Scheibener, S.; Conley, J.M.; Buchwalter, D.B. Sulfate transport kinetics and toxicity are modulated by sodium in aquatic insects. Aquat. Toxicol. 2017, 190, 62–69. [Google Scholar] [CrossRef]
- Buchwalter, D.B.; Scheibener, S.; Chou, H.; Soucek, D.; Elphick, J. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Philos. Trans. R. Soc. B Boil. Sci. 2018, 374, 20180013. [Google Scholar] [CrossRef] [Green Version]
- Dietz, T.H.; Byrne, R.A. Measurement of sulfate uptake and loss in the freshwater bivalve Dreissena polymorpha using a semi-microassay. Can. J. Zool 1999, 77, 331–336. [Google Scholar] [CrossRef]
- Potts, W.T.W. The inorganic composition of the blood of Mytilus edulis and Anodonta cygnea. J. Exp. Biol. 1954, 31, 376–385. [Google Scholar]
- Malley, D.F.; Huebner, J.D.; Donkersloot, K. Effects on ionic composition of blood and tissues ofAnodonta grandis grandis (Bivalvia) of an addition of aluminum and acid to a lake. Arch. Environ. Contam. Toxicol. 1988, 17, 479–491. [Google Scholar] [CrossRef]
- Andrews, P. Über den Blutchemismus des Flukrebses Orconectes limosus und seine Vernderung im Laufe des Jahres. J. Comp. Physiol. A 1967, 57, 7–43. [Google Scholar] [CrossRef]
- Wood, C.M.; McMahon, B. Mechanisms of acid-base and ionoregulation in white suckers (Catostomus commersoni) in natural soft water. J. Comp. Physiol. B 1984, 154, 35–46. [Google Scholar] [CrossRef]
- Nakada, T.; Zandi-Nejad, K.; Kurita, Y.; Kudo, H.; Broumand, V.; Kwon, C.Y.; Mercado, A.; Mount, D.B.; Hirose, S. Roles of Slc13a1 and Slc26a1 sulfate transporters of eel kidney in sulfate homeostasis and osmoregulation in freshwater. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R575–R585. [Google Scholar] [CrossRef] [Green Version]
- Linker, P.H. Glycosaminoglycans in Anodonta californiensis, a Freshwater Mussel. Boil. Bull. 1993, 185, 263–276. [Google Scholar] [CrossRef]
- Luquet, G.; Fernández, M.S.; Badou, A.; Guichard, N.; Le Roy, N.; Corneillat, M.; Alcaraz, G.; Arias, J.L. Comparative Ultrastructure and Carbohydrate Composition of Gastroliths from Astacidae, Cambaridae and Parastacidae Freshwater Crayfish (Crustacea, Decapoda). Biomolecules 2012, 3, 18–38. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K.; Özbek, S. Evolution of glycosaminoglycans. Commun. Integr. Boil. 2011, 4, 150–158. [Google Scholar] [CrossRef]
- Cássaro, C.M.; Dietrich, C.P. Distribution of sulfated mucopolysaccharides in invertebrates. J. Boil. Chem. 1977, 252, 2254–2261. [Google Scholar]
- Hudson, B.H.; York, J.D. Roles for nucleotide phosphatases in sulfate assimilation and skeletal disease. Adv. Boil. Regul. 2012, 52, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, C.D.; Boles, J.W. The importance of 3′-phosphoadenosine 5‘-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997, 11, 404–418. [Google Scholar] [CrossRef]
- Sun, M.; Leyh, T.S. Channeling in Sulfate Activating Complexes†. Biochemistry 2006, 45, 11304–11311. [Google Scholar] [CrossRef] [PubMed]
- Boom, J.V.D.; Heider, D.; Martin, S.R.; Pastore, A.; Mueller, J.W. 3’-Phosphoadenosine 5’-phosphosulfate (PAPS) synthases, naturally fragile enzymes specifically stabilized by nucleotide binding. J. Boil. Chem. 2012, 287, 17645–17655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, T.J. Hyper-regulators: Life in fresh water. In Animal Osmoregulation; Oxford University Press (OUP): Oxford, UK, 2008; pp. 86–110. [Google Scholar]
- USEPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; U.S. Environmental Protection Agency, Ed.; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 2002.
- Gordon, D.A.; Smith, M.E.; Wratschko, M.; Agard, D.; Holden, L.; Wilcox, S.; Lazorchak, J. A new approach for the laboratory culture of the fathead minnow, Pimephales promelas. Environ. Toxicol. Chem. 2013, 33, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Lazorchak, J.; Herrin, L.E.; Brewer-Swartz, S.; Thoeny, W.T. A reformulated, reconstituted water for testing the freshwater amphipod, Hyalella azteca. Environ. Toxicol. Chem. 1997, 16, 1229–1233. [Google Scholar] [CrossRef]
- Wang, N.; Consbrock, R.A.; Ingersoll, C.G.; Hardesty, D.K.; Brumbaugh, W.G.; Hammer, E.; Bauer, C.R.; Mount, D.R. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ. Toxicol. Chem. 2015, 35, 115–127. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Effects of potassium on nitrate mediated alterations of osmoregulation in marine crabs. Aquat. Toxicol. 2007, 85, 202–208. [Google Scholar] [CrossRef]
- Dietz, T.H.; Wilcox, S.J.; Silverman, H.; Byrne, R.A. Effects of hyperosmotic challenge on the freshwater bivalve Dreissena polymorpha: Importance of K+. Can. J. Zool 1997, 75, 697–705. [Google Scholar] [CrossRef]
- Henry, M.; Chester, D.; Mauck, W. Role of artificial burrows inHexageniatoxicity tests: Recommendations for protocol development. Environ. Toxicol. Chem. 1986, 5, 553–559. [Google Scholar] [CrossRef]
- Revesz, K.; Coplen, T.B. Determination of the δ34S of Total Sulfur in Solids: RSIL Lab Code 1800. In Techniques and Methods; US Geological Survey: Reston, VA, USA, 2006; pp. 1–31. [Google Scholar]
- USEPA. Methods for the Determination of Inorganic Substances in Environmental Samples; U.S. Environmental Protection Agency, Office of Research and Development: Cincinnati, OH, USA, 1993.
- Revesz, K.; Qi, H.; Coplen, T.B. Determination of the δ34S of sulfate in water; RSIL lab code 1951. In Techniques and Methods; US Geological Survey: Reston, VA, USA, 2006; pp. 1–31. [Google Scholar]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef]
- Cobelli, C.; Toffolo, G.; Bier, D.M.; Nosadini, R. Models to interpret kinetic data in stable isotope tracer studies. Am. J. Physiol. Metab. 1987, 253, E551–E564. [Google Scholar] [CrossRef]
- Cobelli, C.; Toffolo, G.; Foster, D.M. Tracer-to-tracee ratio for analysis of stable isotope tracer data: Link with radioactive kinetic formalism. Am. J. Physiol. Metab. 1992, 262, E968–E975. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M. Acid-base and ionic exchanges at gills and kidney after exhaustive exercise in the rainbow trout. J. Exp. Biol. 1988, 136, 461–481. [Google Scholar]
- Wood, C.M.; Wheatly, M.G.; Hōbe, H. The mechanisms of acid-base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia. III. Branchial exchanges. Respir. Physiol. 1984, 55, 175–192. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Dunson, W.A. Adaptations of sodium balance to low pH in a sunfish (Enneacanthus obesus) from naturally acidic waters. J. Comp. Physiol. B 1987, 157, 555–566. [Google Scholar] [CrossRef]
- Robinson, R.B. Reference and intercomparison materials for stable isotopes of light elements. In Proceedings of the a Consultants Meeting, Vienna, Austria, 1–3 December 1993; International Atomic Energy Agency: Vienna, Austria, 1995; pp. 39–45. [Google Scholar]
- Stobbart, R.H. The effect of some anions and cations upon the fluxes and net uptake of chloride in the larva of Aëdes aegypti (L.), and the nature of the uptake mechanisms for sodium and chloride. J. Exp. Boil. 1967, 47, 35–57. [Google Scholar]
- Chen, Y.-Y.; Lu, F.-I.; Hwang, P.-P. Comparisons of calcium regulation in fish larvae. J. Exp. Zool 2003, 295, 127–135. [Google Scholar] [CrossRef]
- Wilcox, S.; Dietz, T. Potassium transport in the freshwater bivalve Dreissena polymorpha. J. Exp. Boil. 1995, 198, 861–868. [Google Scholar]
- Frain, W.J. The effect of external sodium and calcium concentrations on sodium fluxes by salt-depleted and non-depleted minnows, Phoxinus phoxinus (L.). J. Exp. Biol. 1987, 131, 417–425. [Google Scholar]
- Chasiotis, H.; Kolosov, D.; Kelly, S. Permeability properties of the teleost gill epithelium under ion-poor conditions. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R727–R739. [Google Scholar] [CrossRef] [Green Version]
- Dietz, T.H.; Byrne, R.A.; Lynn, J.W.; Silverman, H. Paracellular solute uptake by the freshwater zebra mussel Dreissena polymorpha. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R300–R307. [Google Scholar] [CrossRef]
- McDonald, D.G.; Rogano, M.S. Ion Regulation by the Rainbow Trout, Salmo gairdneri, in Ion-Poor Water. Physiol. Zool 1986, 59, 318–331. [Google Scholar] [CrossRef]
- Wood, C.M.; Gilmour, K.M.; Pärt, P. Passive and active transport properties of a gill model, the cultured branchial epithelium of the freshwater rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 1998, 119, 87–96. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur Assimilation in Photosynthetic Organisms: Molecular Functions and Regulations of Transporters and Assimilatory Enzymes. Annu. Rev. Plant. Boil. 2011, 62, 157–184. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.V.; Moorhead, K.K. Characterization of riparian species and stream detritus using multiple stable isotopes. Oecologia 1996, 107, 232–238. [Google Scholar] [CrossRef]
- Markovich, D.; Romano, A.; Storelli, C.; Verri, T. Functional and structural characterization of the zebrafish Na+-sulfate cotransporter 1 (NaS1) cDNA and gene (slc13a1). Physiol. Genom. 2008, 34, 256–264. [Google Scholar] [CrossRef]
- Katoh, F.; Tresguerres, M.; Lee, K.M.; Kaneko, T.; Aida, K.; Goss, G.G. Cloning of rainbow trout SLC26A1: Involvement in renal sulfate secretion. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R1468–R1478. [Google Scholar] [CrossRef] [Green Version]
- Maddrell, S.H.P.; Phillips, J.E. Active transport of sulphate ions by the Malpighian tubules of larvae of the mosquito Aedes campestris. J. Exp. Biol. 1975, 62, 367–378. [Google Scholar]
- Maddrell, S.H.P.; Phillips, J.E. Induction of sulphate transport and hormonal control of fluid secretion by Malpighian Tubules of larvae of the mosquito Aedes taeniorhynchus. J. Exp. Biol. 1978, 72, 181–202. [Google Scholar]
- Gerencser, G.A.; Cattey, M.A.; Ahearn, G.A. Sulfate/oxalate exchange by lobster hepatopancreatic basolateral membrane vesicles. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R572–R577. [Google Scholar] [CrossRef]
- Gerencser, G.A.; Ahearn, G.A.; Cattey, M.A. Antiport-driven sulfate secretion in an invertebrate epithelium. J. Exp. Zool 1996, 275, 269–276. [Google Scholar] [CrossRef]
- Cattey, M.A.; Gerencser, G.A.; Ahearn, G.A. Electrogenic H(+)-regulated sulfate-chloride exchange in lobster hepatopancreatic brush-border membrane vesicles. Am. J. Physiol. Integr. Comp. Physiol. 1992, 262, R255–R262. [Google Scholar] [CrossRef] [PubMed]
- Cattey, M.A.; Gerencser, G.A.; Ahearn, G.A. Electrogenic Coupling of Sulfate Secretion to Chloride Transport in Lobster Hepatopancreas. In Blood and Tissue Oxygen Carriers; Springer Science and Business Media LLC: Berlin, Germany, 1994; Volume 19, pp. 109–120. [Google Scholar]
- Horng, J.-L.; Hwang, P.-P.; Shih, T.-H.; Wen, Z.-H.; Lin, C.-S.; Lin, L.-Y. Chloride transport in mitochondrion-rich cells of euryhaline tilapia (Oreochromis mossambicus) larvae. Am. J. Physiol. Physiol. 2009, 297, C845–C854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, K.P.; O’Brien, S.; Evans, D.H.; Toop, T.; Edwards, S. Immunolocalization of Na+/K+-ATPase, carbonic anhydrase II, and vacuolar H+-ATPase in the gills of freshwater adult lampreys, Geotria australis. J. Exp. Zool 2004, 301, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Filippov, V.; Aimanova, K.; Gill, S.S. Expression of an Aedes aegypti cation-chloride cotransporter and its Drosophila homologues. Insect Mol. Boil. 2003, 12, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wheatly, M.G. Characterization and expression of plasma membrane Ca2+ ATPase (PMCA3) in the crayfish Procambarus clarkii antennal gland during molting. J. Exp. Boil. 2004, 207, 2991–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankley, G.T.; Bennett, R.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
| Water | Salt | mg L−1 | Ions | mmol L−1 |
|---|---|---|---|---|
| Acclimation | CaCO3 | 71.0 | Na+ | 0.54 |
| NaHCO3 | 4.0 | K+ | 0.05 | |
| MgCl2·6H20 | 59.4 | Ca2+ | 0.71 | |
| KCl | 3.5 | Mg2+ | 0.29 | |
| Na2SO4 | 35.0 | HCO3− | 1.47 | |
| Cl− | 0.34 | |||
| SO42− | 0.25 | |||
| Hardness (mg/L, CaCO4) | 100.2 | |||
| Molar [Na+]/[K+] | 11.5 | |||
| 0.49 mmol L−1 SO42− | Na2SO4 | 70.0 | Na+ | 1.03 |
| SO42− | 0.49 | |||
| Hardness (mg/L, CaCO4) | 100.2 | |||
| Molar [Na+]/[K+] | 22.0 | |||
| 1.23 mmol L−1 SO42− | Na2SO4 | 175.0 | Na+ | 2.51 |
| SO42− | 1.23 | |||
| Hardness (mg L−1, CaCO4) | 100.2 | |||
| [Na+]/[K+] | 53.5 |
| Variable | Species | 0.49 mmol L−1 | 1.23 mmol L−1 | ||
|---|---|---|---|---|---|
| n | Value | n | Value | ||
| [SO42−] (mmol L−1) | Fathead minnow | 5 | 0.52 ± 0.03 | 5 | 1.67 ± 0.03 |
| Paper pondshell | 5 | 0.35 ± 0.01 | 5 | 0.98 ± 0.06 | |
| Red swamp crayfish | 10 | 0.45 ± 0.01 | 10 | 1.15 ± 0.01 | |
| Two-lined mayfly | 1 | 0.48 | 3 | 1.19 ± 0.04 | |
| ẟ(34S/32S) (‰) | Fathead minnow | 5 | +1377.6 ± 1.3 | 5 | +1433.9 ± 0.8 |
| Paper pondshell | 5 | +1432.7 ± 34.1 | 5 | +1452.6 ± 0.5 | |
| Red swamp crayfish | 5 | +1475.6 ± 2.8 | 5 | +1461.3 ± 1.0 | |
| Two-lined mayfly | 5 | +1411.6 ± 3.0 | 5 | +1365.7 ± 4.4 | |
| Species | Target SO42− Concentration | J value | Variable | Proportion of Variance |
|---|---|---|---|---|
| Fathead minnow | 0.49 mmol L−1 | Jin | 0.603 | |
| Jout | 0.089 | |||
| 0.015 | ||||
| Jnet | 0.090 | |||
| 1.23 mmol L−1 | Jin | 0.584 | ||
| Jout | 0.583 | |||
| 0.006 | ||||
| Jnet | 0.058 | |||
| Paper pondshell | 0.49 mmol L−1 | Jin | 0.172 | |
| Jout | 0.046 | |||
| 0.021 | ||||
| Jnet | 0.047 | |||
| 1.23 mmol L−1 | Jin | 0.519 | ||
| Jout | 0.059 | |||
| 0.001 | ||||
| Jnet | 0.059 | |||
| Red swamp crayfish | 0.49 mmol L−1 | Jin | 0.582 | |
| 0.028 | ||||
| Jout | 0.012 | |||
| Jnet | 0.072 | |||
| 1.23 mmol L−1 | Jin | 0.747 | ||
| 0.002 | ||||
| Jout | 0.002 | |||
| Jnet | 0.005 | |||
| Two-lined mayfly | 0.49 mmol L−1 | Jin | 0.890 | |
| 1.23 mmol L−1 | Jin | 0.941 | ||
| Jout | 0.346 | |||
| 0.084 | ||||
| Jnet | 0.380 |
| Species | Target SO42− Concentration | H0: mJin = 0 t-Value (df, p) | H0: mJout = 0 t-Value (df, p) | H0: mJnet = 0 t-Value (df, p) |
|---|---|---|---|---|
| Fathead minnow | 0.49 mmol/L | 5.32 (24, <0.0001) | 27.88 (24, <0.0001) | 28.60 (24, <0.0001) |
| 1.23 mmol/L | 9.15 (24, <0.0001) | −49.38 (23, <0.0001) | −48.51 (23, <0.0001) | |
| Paper pondshell | 0.49 mmol/L | 2.98 (24, 0.0066) | −6.38 (24, <0.0001) | −6.04 (24, <0.0001) |
| 1.23 mmol/L | 5.91 (24, <0.0001) | −21.78 (24, <0.0001) | −21.74 (24, <0.0001) | |
| Red swamp crayfish | 0.49 mmol/L | 4.50 (24, 0.0001) | 19.26 (24, <0.0001) | 20.34 (24, <0.0001) |
| 1.23 mmol/L | 2.60 (24, 0.0155) | −4.80 (24, <0.0001) | −4.68 (24, <0.0001) | |
| Two-lined mayfly | 0.49 mmol/L | 1.95 (17, 0.0680) | ||
| 1.23 mmol/L | 1.97 (21, 0.0623) | −0.33 (2, 0.77) | −0.16 (2, 0.89) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffith, M.B.; Lazorchak, J.M.; Haring, H. Uptake of Sulfate from Ambient Water by Freshwater Animals. Water 2020, 12, 1496. https://doi.org/10.3390/w12051496
Griffith MB, Lazorchak JM, Haring H. Uptake of Sulfate from Ambient Water by Freshwater Animals. Water. 2020; 12(5):1496. https://doi.org/10.3390/w12051496
Chicago/Turabian StyleGriffith, Michael B., James M. Lazorchak, and Herman Haring. 2020. "Uptake of Sulfate from Ambient Water by Freshwater Animals" Water 12, no. 5: 1496. https://doi.org/10.3390/w12051496





