Upcycling of Electroplating Sludge to Prepare Erdite-Bearing Nanorods for the Adsorption of Heavy Metals from Electroplating Wastewater Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electroplating Sludge Pre-Treatment
2.2. Hydrothermal Conversion of Sludge
2.3. Heavy Metal Release
2.4. Electroplating Wastewater Treatment
2.5. Kinetic Experiment
2.6. Adsorbent Regeneration
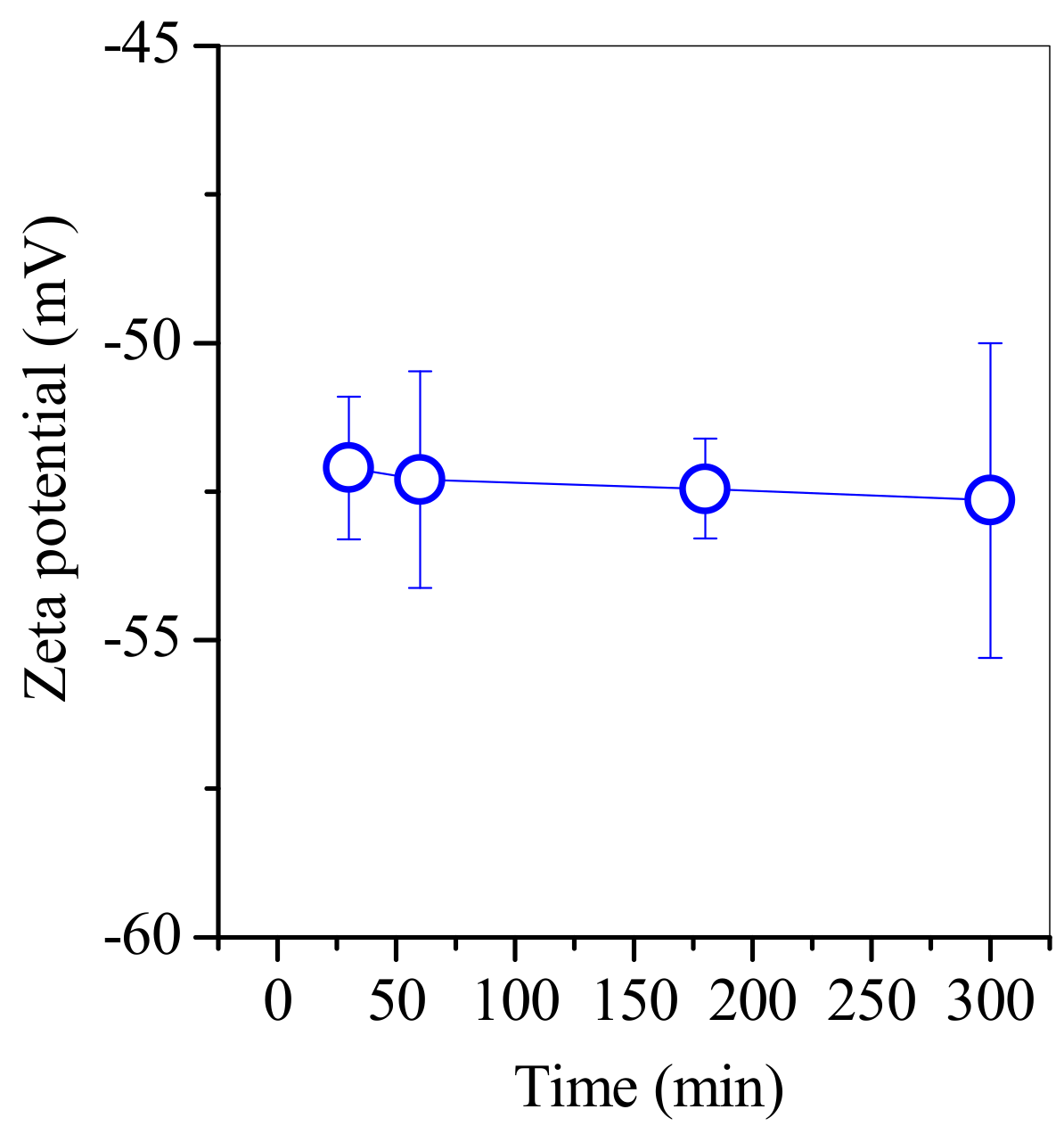
2.7. Zeta Potential Measurement
2.8. Characterisation
3. Results and Discussion
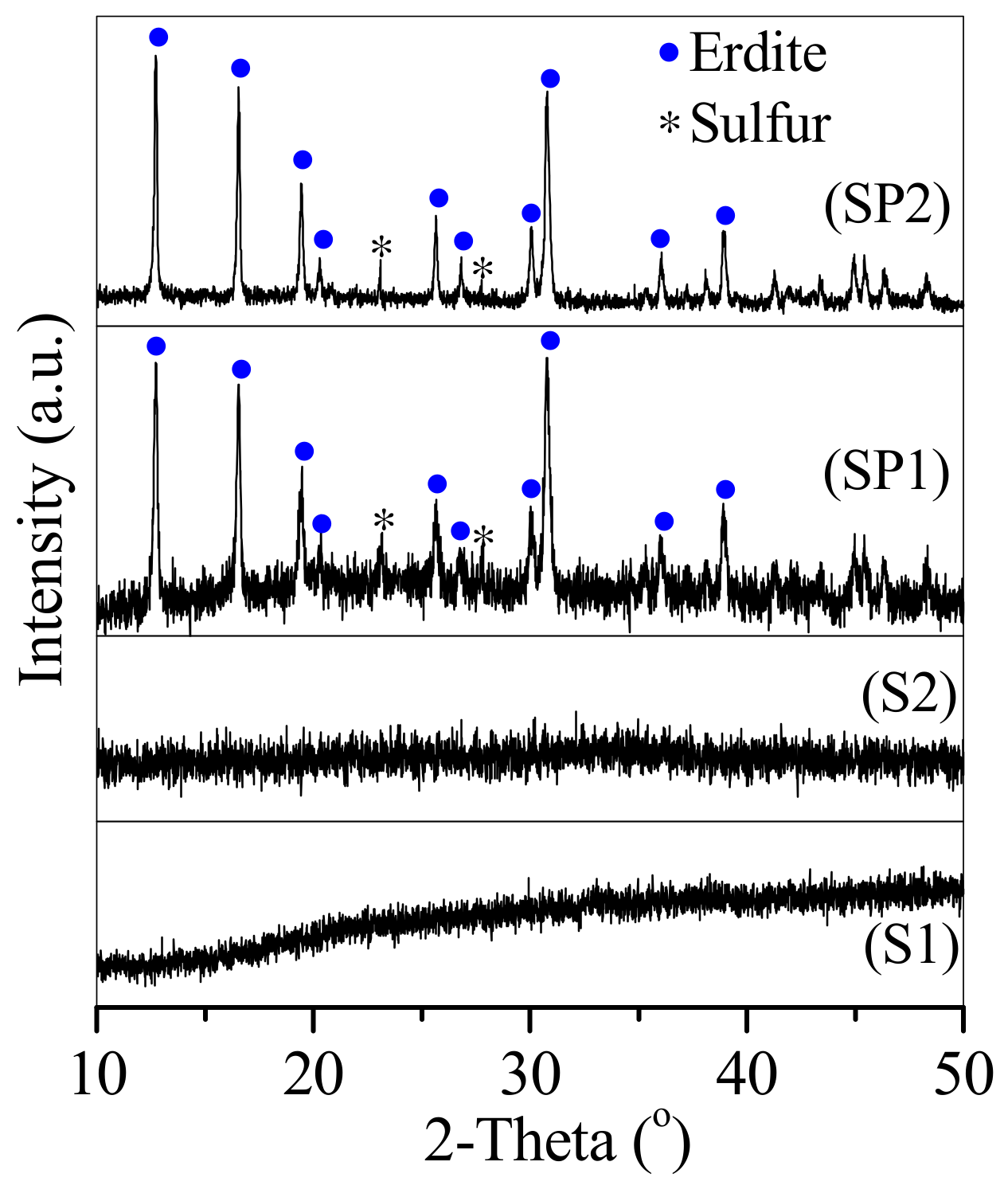
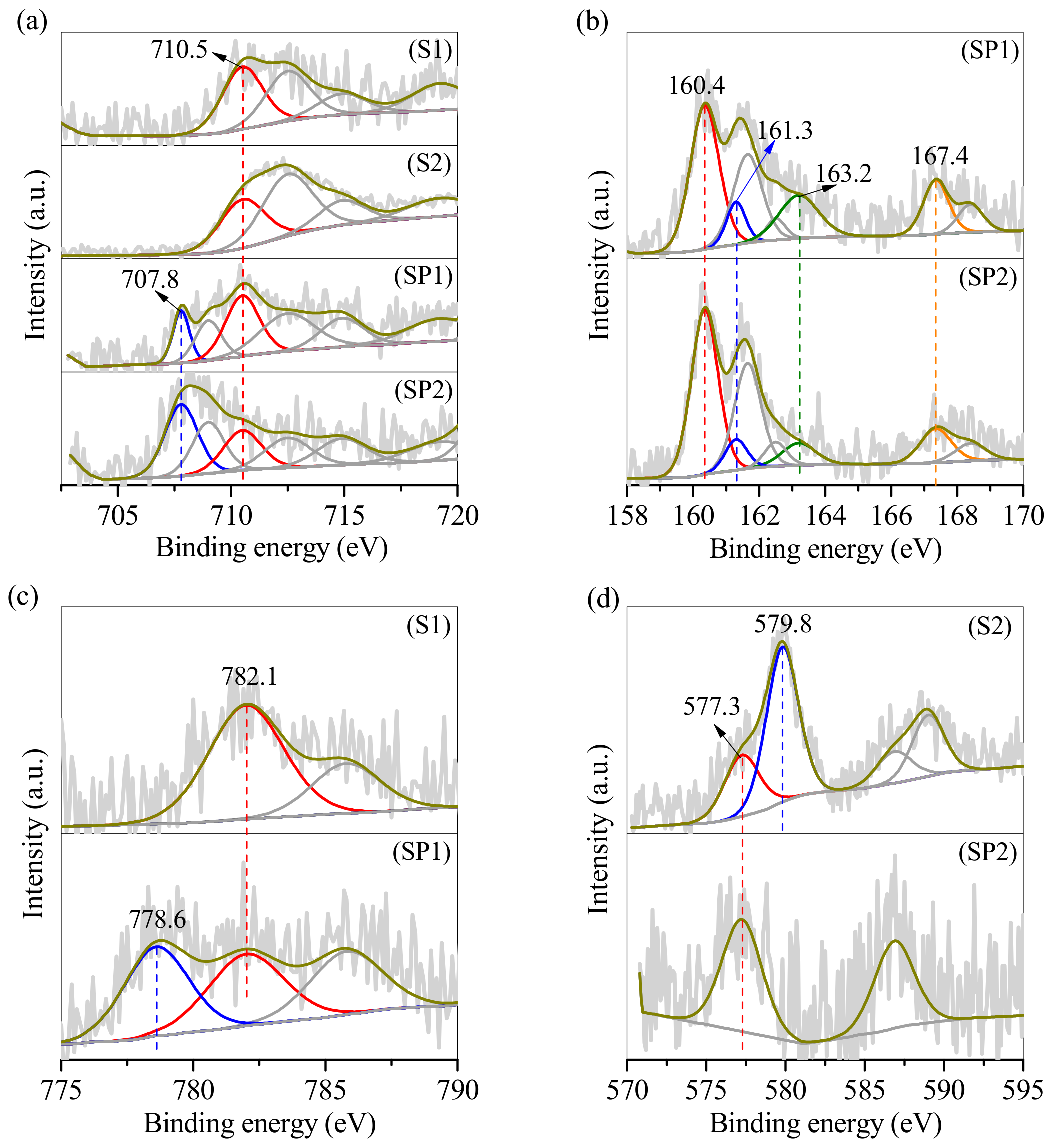
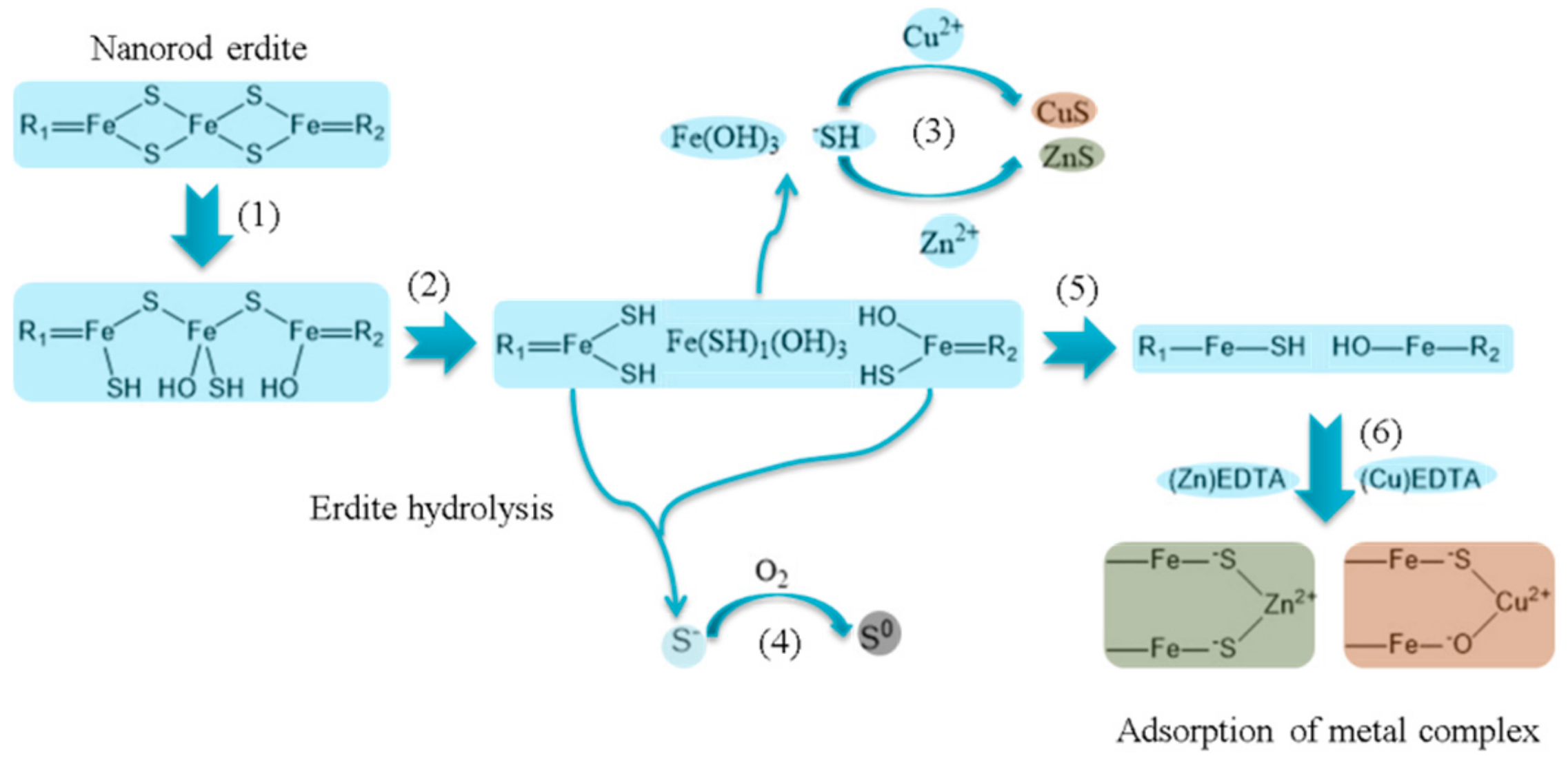
3.1. Conversion of the Two Types of Sludge to Erdite-Bearing Particles
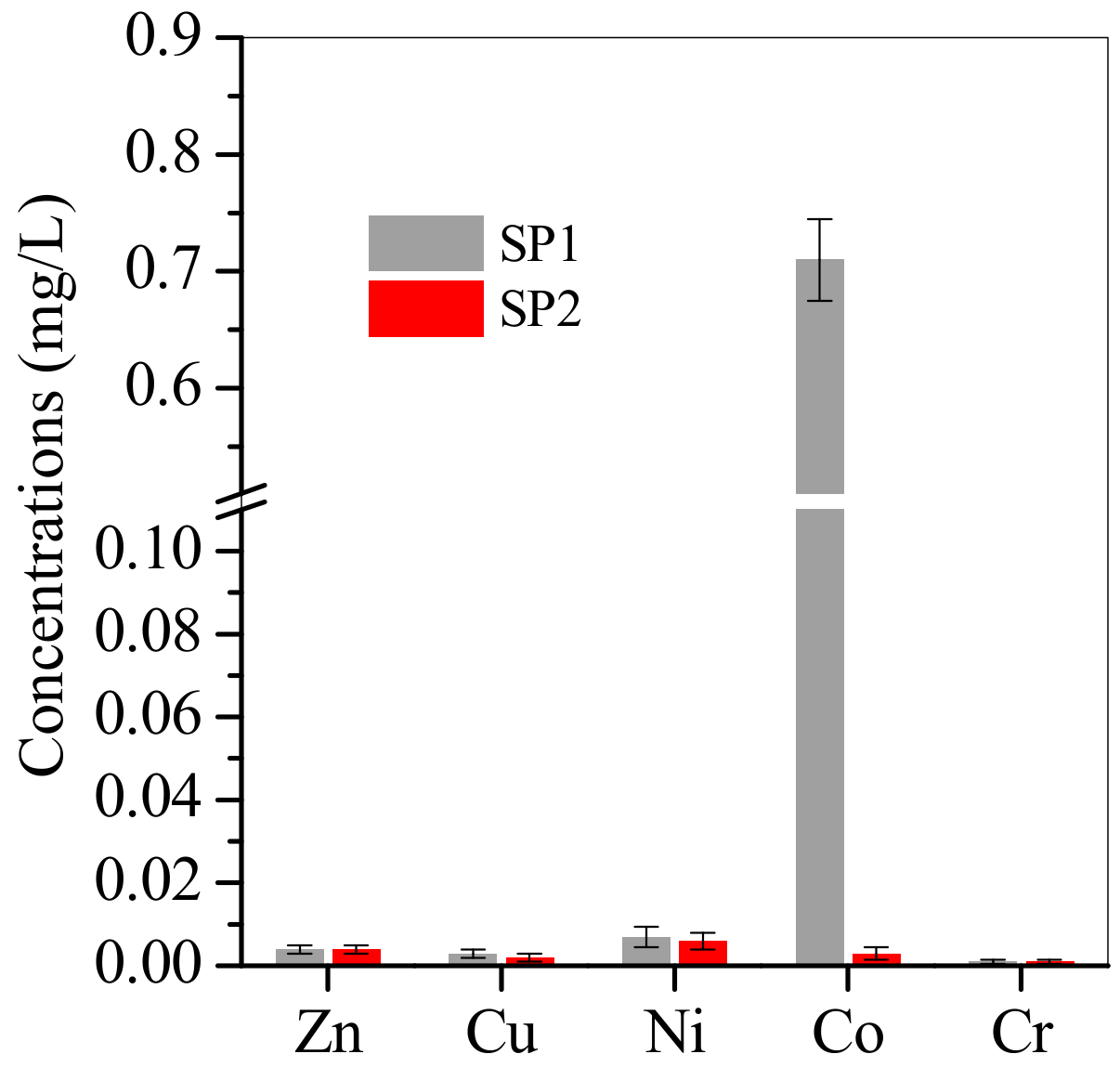
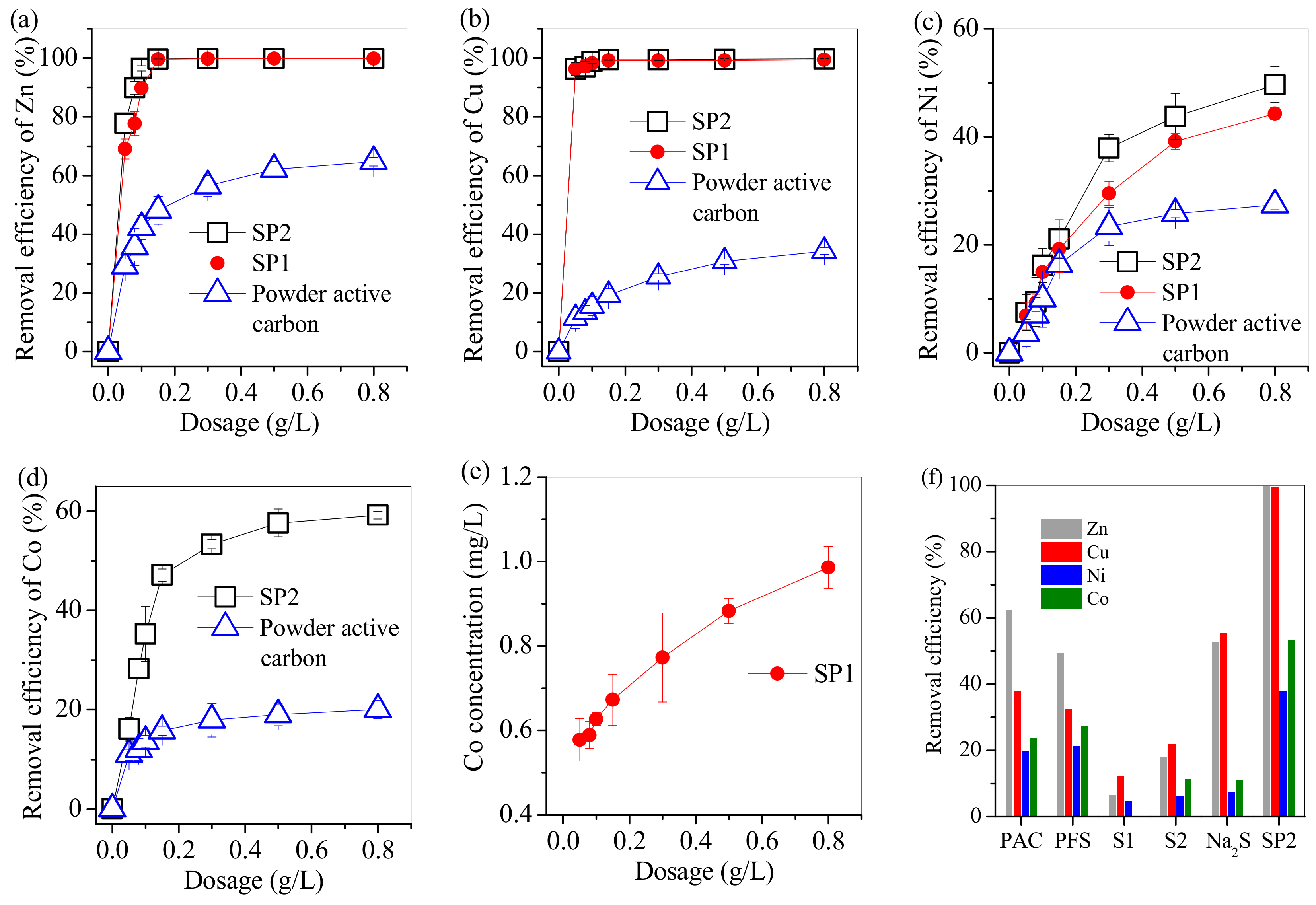
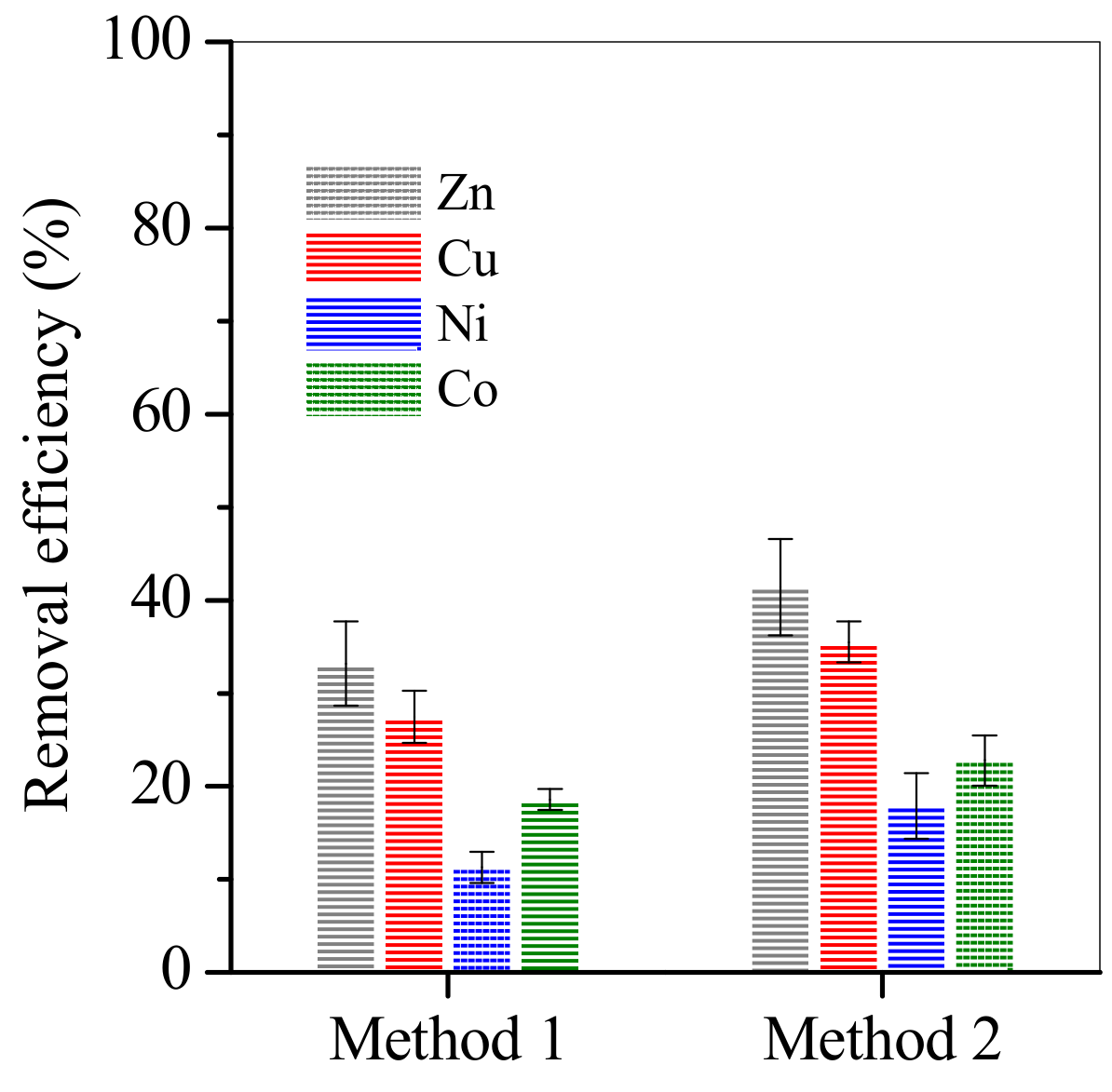
3.2. Adsorption of Cu, Zn, Ni and Co from Electroplating Wastewater Effluent
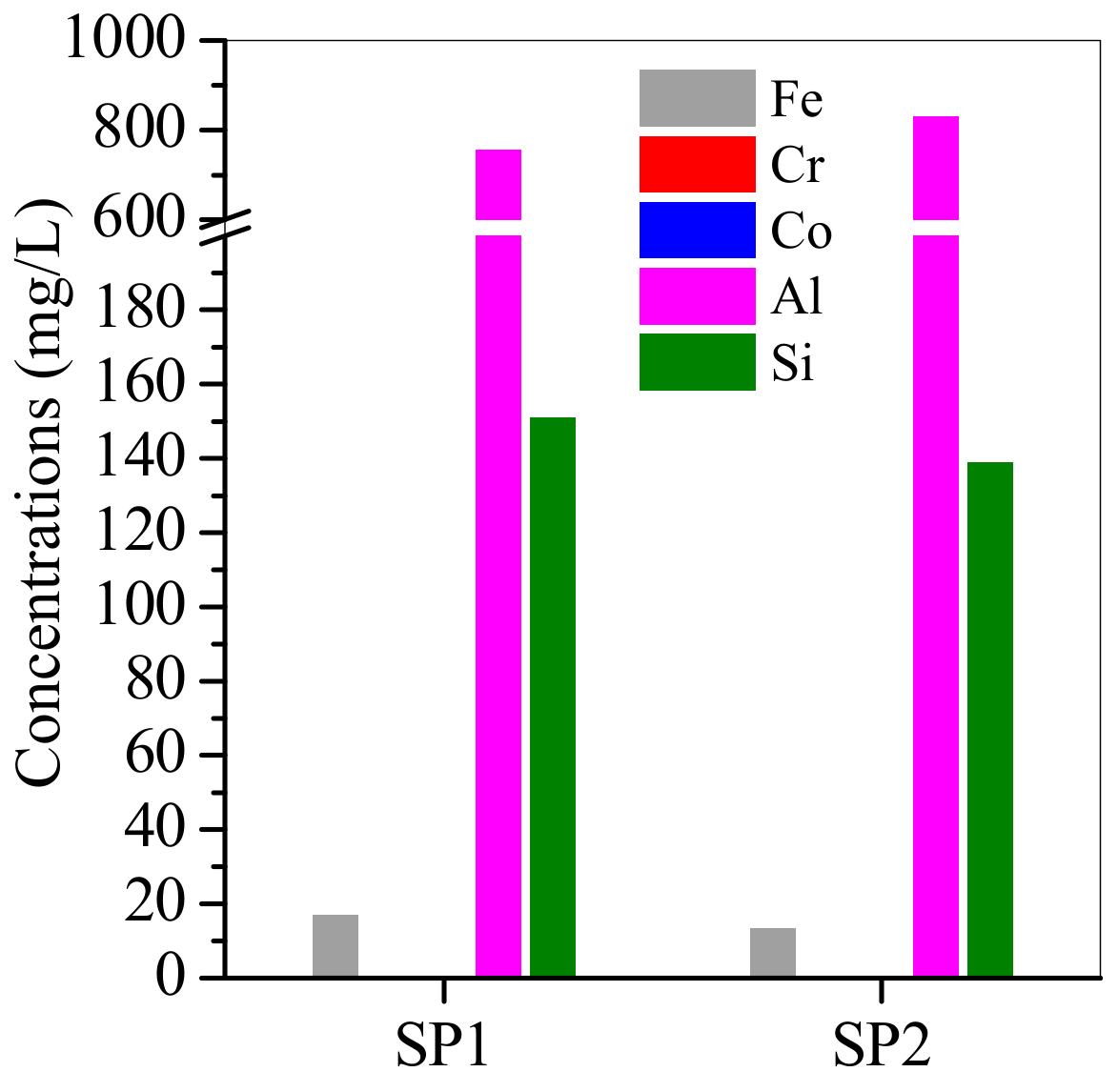
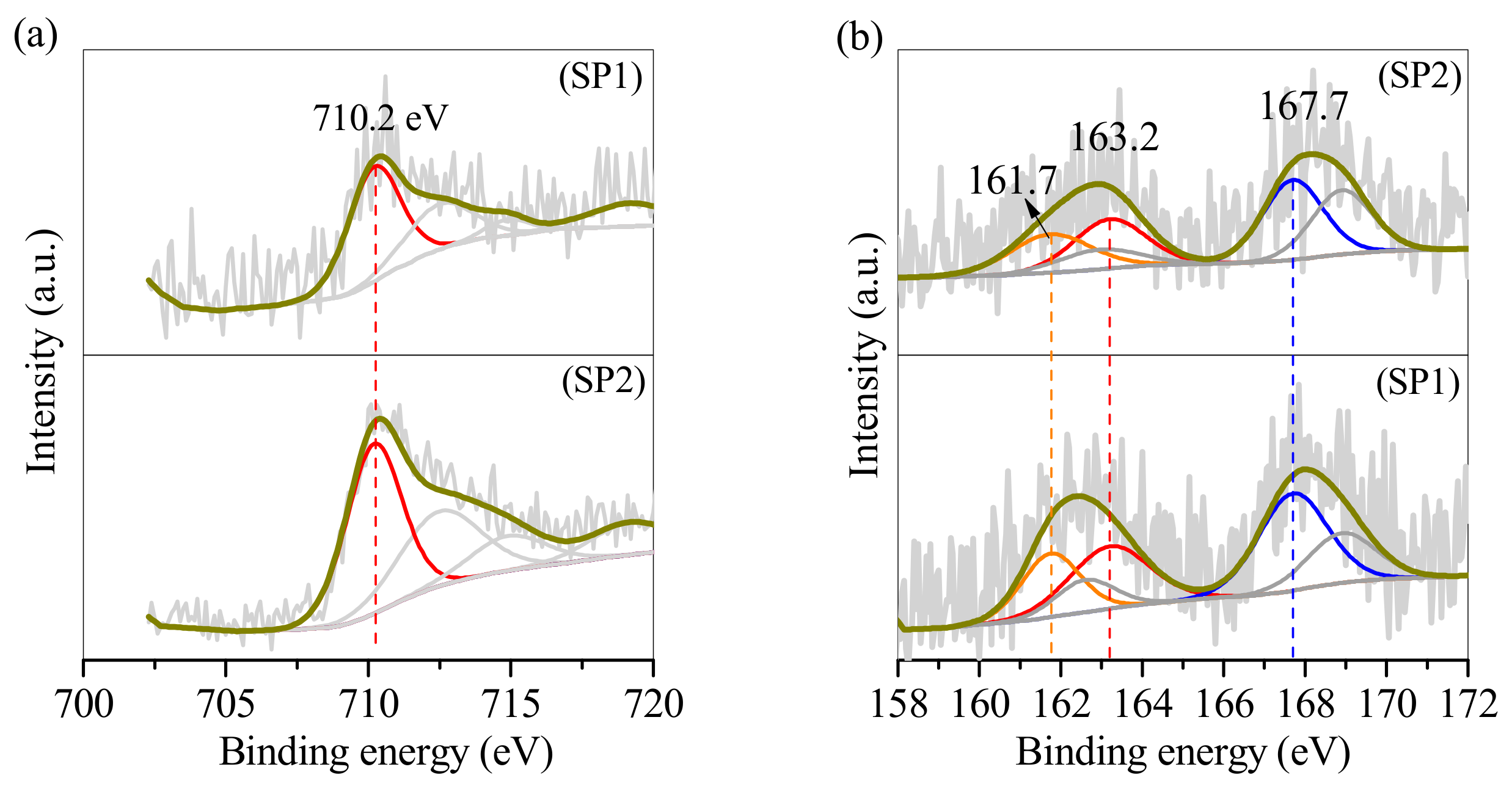
3.3. SP1 and SP2 Characterisation after Electroplating Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mizushima, I.; Tang, P.T.; Hansen, H.N.; Somers, M.A. Development of a new electroplating process for Ni–W alloy deposits. Electrochim. Acta 2005, 51, 888–896. [Google Scholar] [CrossRef]
- Wang, G.; Sui, J.; Wang, C.; Cai, G. Treatment of electroplating synthetical wastewater by combined process of oxidation and reduction, coagulation and sedimentation. China Water Wastewater 2007, 23, 57. [Google Scholar]
- Zhang, Z.; Li, L.; Zhu, H.; Wang, F.; Hua, J. Removing chromium from electroplating wastewater by chemical precipitation. Environ. Sci. Technol. 2008, 31, 96–97. [Google Scholar]
- Lin, Y.; Yen, S. Effects of additives and chelating agents on electroless copper plating. Appl. Surf. Sci. 2001, 178, 116–126. [Google Scholar] [CrossRef]
- Celary, P.; Sobik-Szołtysek, J. Vitrification as an alternative to landfilling of tannery sewage sludge. Waste Manag. 2014, 34, 2520–2527. [Google Scholar] [CrossRef]
- Andrus, M.E. A review of metal precipitation chemicals for metal-finishing applications. Met. Finish. 2000, 98, 20–23. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Tian, Q. Elemental behavior of multi-component metal powders from waste printed circuit board during low-temperature alkaline smelting. Chin. J. Nonferrous Met. 2013, 23, 1757–1763. [Google Scholar]
- Sze, Y.K.P.; Xue, L.Z.; Lee, M.Y. Treatment of alloy-electroplating wastewater by an automated solvent extraction technique. Environ. Technol. 2001, 22, 979–990. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J.; Rao, L.A.K. Removal and recovery of heavy metals from electroplating wastewater by using kynaite as an adsorbent. J. Hazard. Mater. 2001, 87, 127–137. [Google Scholar] [CrossRef]
- Zhonghe, M.; Zhumei, W.; Ming, J. Treatment of low concentration complex nickel Chromium(Cr6+) COpper mixed electroplating wastewater. Mod. Chem. Res. 2018, 11, 26–27. [Google Scholar]
- Zhang, H.; Sun, M.; Song, L.; Guo, J.; Zhang, L. Fate of NaClO and membrane foulants during in-situ cleaning of membrane bioreactors: Combined effect on thermodynamic properties of sludge. Biochem. Eng. J. 2019, 147, 146–152. [Google Scholar] [CrossRef]
- Sun, M.; Yan, L.; Zhang, L.; Song, L.; Guo, J.; Zhang, H. New insights into the rapid formation of initial membrane fouling after in-situ cleaning in a membrane bioreactor. Process Biochem. 2019, 78, 108–113. [Google Scholar] [CrossRef]
- Xu, T.; Lei, X.; Sun, B.; Yu, G.; Zeng, Y. Highly efficient and energy-conserved flocculation of copper in wastewater by pulse-alternating current. Environ. Sci. Pollut. Res. Int. 2017, 24, 20577–20586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, G.; Yang, Y. Low-concentration electroplating wastewater treatment by heavy metal chelator. J. Zhejiang Univ. (Sci. Ed.) 2010, 37, 665–669. [Google Scholar] [CrossRef]
- Shih, Y.; Lin, C.; Huang, Y. Application of Fered-Fenton and chemical precipitation process for the treatment of electroless nickel plating wastewater. Sep. Purif. Technol. 2013, 104, 100–105. [Google Scholar] [CrossRef]
- Tembhekar, P.D.; Padoley, K.V.; Mudliar, S.L.; Mudliar, S.N. Kinetics of wet air oxidation pretreatment and biodegradability enhancement of a complex industrial wastewater. J. Environ. Chem. Eng. 2015, 3, 339–348. [Google Scholar] [CrossRef]
- Yong, L. Progress of electroplating wastewater treatment with micro-electrolysis method. Guangdong Chem. Ind. 2008, 35, 56. [Google Scholar]
- Li, Y.; Zeng, X.; Liu, Y.; Yan, S.; Hu, Z.; Ni, Y. Study on the treatment of copper-electroplating wastewater by chemical trapping and flocculation. Sep. Purif. Technol. 2003, 31, 91–95. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef]
- Fadel, D.; El-Bahy, S.; Abdelaziz, Y. Heavy metals removal using iminodiacetate chelating resin by batch and column techniques. Desalin. Water Treat. 2016, 57, 25718–25728. [Google Scholar] [CrossRef]
- Benouali, D.; Kherici, S.; Belabbassi, M.; Belkandouci, M.; Bennemra, A. Preliminary study of Zinc removal from cyanide-free alkaline electroplating effluent by precipitation using oxalis plants. Orient. J. Chem. 2014, 30, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Šćiban, M.; Radetić, B.; Kevrešan, Ž.; Klašnja, M. Adsorption of heavy metals from electroplating wastewater by wood sawdust. Bioresour. Technol. 2007, 98, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, F.; Ma, Y.; Cai, T.; Li, H.; Huang, Z.; Yuan, G. Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching. J. Hazard. Mater. 2010, 178, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.C.R.; Tenório, J.A.S. Thermal behavior of chromium electroplating sludge. Waste Manag. 2001, 21, 405–410. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, Y.; Zhu, S.; Yu, Y.; Huo, M.; Zhang, L.; Yang, J.; Bian, D.; Wang, Y. Green synthesis of magnetic adsorbent using groundwater treatment sludge for tetracycline adsorption. Engineering 2019, 5, 880–887. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Qu, Z.; Zhang, L.; Yu, Y.; Xie, X.; Huo, M.; Yang, J.; Bian, D.; Zhang, H. Green synthesis of magnetic sodalite sphere by using groundwater treatment sludge for tetracycline adsorption. J. Clean. Prod. 2020, 247, 119140. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2016, 307, 353–363. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, C.; Zeng, G.; He, H.; Luo, S. Treatment of vanadium smelting wastewater by means of coagulation-sand filtration-activated carbon filter-microfiltration-RO integration technology. Chin. J. Environ. Eng. 2014, 8, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z. Application and progress of PAC to industrial wastewater treatment. Shanghai Environ. Sci. 2003, 22, 353–357. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, K.; Liu, X.; Liu, P.; Huang, J. Experimental study of advanced treatment of coking wastewater using PFS coagulation-photocatalytic oxidation technology. Mod. Chem. Ind. 2015, 34, 487–494. [Google Scholar] [CrossRef]
- Chen, W.S.; Lin, H.S.; Lin, J.F. Study on treatment technology of waste water with copper in complex system. Adv. Mater. Res. 2011, 317, 1847–1851. [Google Scholar] [CrossRef]
- Glasauer, S.M.; Hug, P.; Weidler, P.G.; Gehring, A.U. Inhibition of sintering by Si during the conversion of Si-rich ferrihydrite to hematite. Clays Clay. Miner. 2000, 48, 51–56. [Google Scholar] [CrossRef]
- Ouyang, C.; Wang, X.; Wang, S. Phosphorus-doped CoS2 nanosheet arrays as ultra-efficient electrocatalysts for the hydrogen evolution reaction. Chem. Commun. (Camb.) 2015, 51, 14160–14163. [Google Scholar] [CrossRef]
- Ji, M.; Su, X.; Zhao, Y.; Qi, W.; Wang, Y.; Chen, G.; Zhang, Z. Effective adsorption of Cr (VI) on mesoporous Fe-functionalized Akadama clay: Optimization, selectivity, and mechanism. Appl. Surf. Sci. 2015, 344, 128–136. [Google Scholar] [CrossRef]
- Diakonov, I.I.; Schott, J.; Martin, F.; Harrichourry, J.-C.; Escalier, J. Iron (III) solubility and speciation in aqueous solutions. Experimental study and modelling: Part 1. hematite solubility from 60 to 300 °C in NaOH–NaCl solutions and thermodynamic properties of Fe(OH)4−(aq). Geochim. Cosmochim. Acta 1999, 63, 2247–2261. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Huo, Y.; Chen, Y.; Qu, Z.; Yu, Y.; Wang, Z.; Fan, W.; Peng, J.; Wang, Z. Addition of MnO2 in synthesis of nano-rod erdite promoted tetracycline adsorption. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, X.; Dong, G.; Yu, Y.; Yu, H.; Bian, D.; Zhang, L.; Yang, J.; Wang, X.; Huo, M. Valorization of manganese-containing groundwater treatment sludge by preparing magnetic adsorbent for Cu (II) adsorption. J. Environ. Manag. 2019, 236, 446–454. [Google Scholar] [CrossRef]
- Ibrado, A.S.; Fuerstenau, D.W. Adsorption of the cyano complexes of Ag(I), Cu(I), Hg(II), Cd(II) and Zn(II) on activated carbon. Min. Metall. Explor. 1989, 6, 23–28. [Google Scholar] [CrossRef]
- Shi, T.; Tang, B. Adsorption characteristic study of Zn(Ⅱ)and Zn(Ⅱ)—EDTA at the activated carbon solution interface. Ind. Water Treat. 2000, 20, 12–15. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Kang, J.; Fan, M.; Qu, J. Removal of tetracycline from water by Fe-Mn binary oxide. J. Environ. Sci. 2012, 24, 242–247. [Google Scholar] [CrossRef]
- Lewis, A.E. ChemInform abstract: Review of metal sulfide precipitation. Cheminform 2012, 43, 222–234. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A. Adsorption of copper (II) and EDTA-chelated copper (II) onto granular activated carbons. J. Chem. Technol. Biotechnol. 2000, 75, 1054–1060. [Google Scholar] [CrossRef]
- Campi, E.; Ostacoli, G.; Meirone, M.; Saini, G. Stability of the complexes of tricarballylic and citric acids with bivalent metal ions in aqueous solution. J. Inorg. Nucl. Chem. 1964, 26, 553–564. [Google Scholar] [CrossRef]
- Legrand, D.L.; Nesbitt, H.W.; Bancroft, G.M. X-ray photoelectron spectroscopic study of a pristine millerite (NiS) surface and the effect of air and water oxidation. Am. Min. 1998, 83, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhou, J.; Shang, C.; Kikkert, G.A. Removal of aqueous hydrogen sulfide by granular ferric hydroxide—Kinetics, capacity and reuse. Chemosphere 2014, 117, 324–329. [Google Scholar] [CrossRef]
- Schlosser, C.; Streu, P.; Frank, M.; Lavik, G.; Croot, P.L.; Dengler, M.; Achterberg, E.P. H2S events in the Peruvian oxygen minimum zone facilitate enhanced dissolved Fe concentrations. Sci. Rep. 2018, 8, 12642. [Google Scholar] [CrossRef]
- Arantes, G.M.; Field, M.J. Ferric–thiolate bond dissociation studied with electronic structure calculations. J. Phys. Chem. A 2015, 119, 10084–10090. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.-N.; Cheng, C. Preparation of new spongy adsorbent for removal of EDTA–Cu(II) and EDTA–Ni(II) from water. Chin. Chem. Lett. 2013, 24, 383–385. [Google Scholar] [CrossRef]




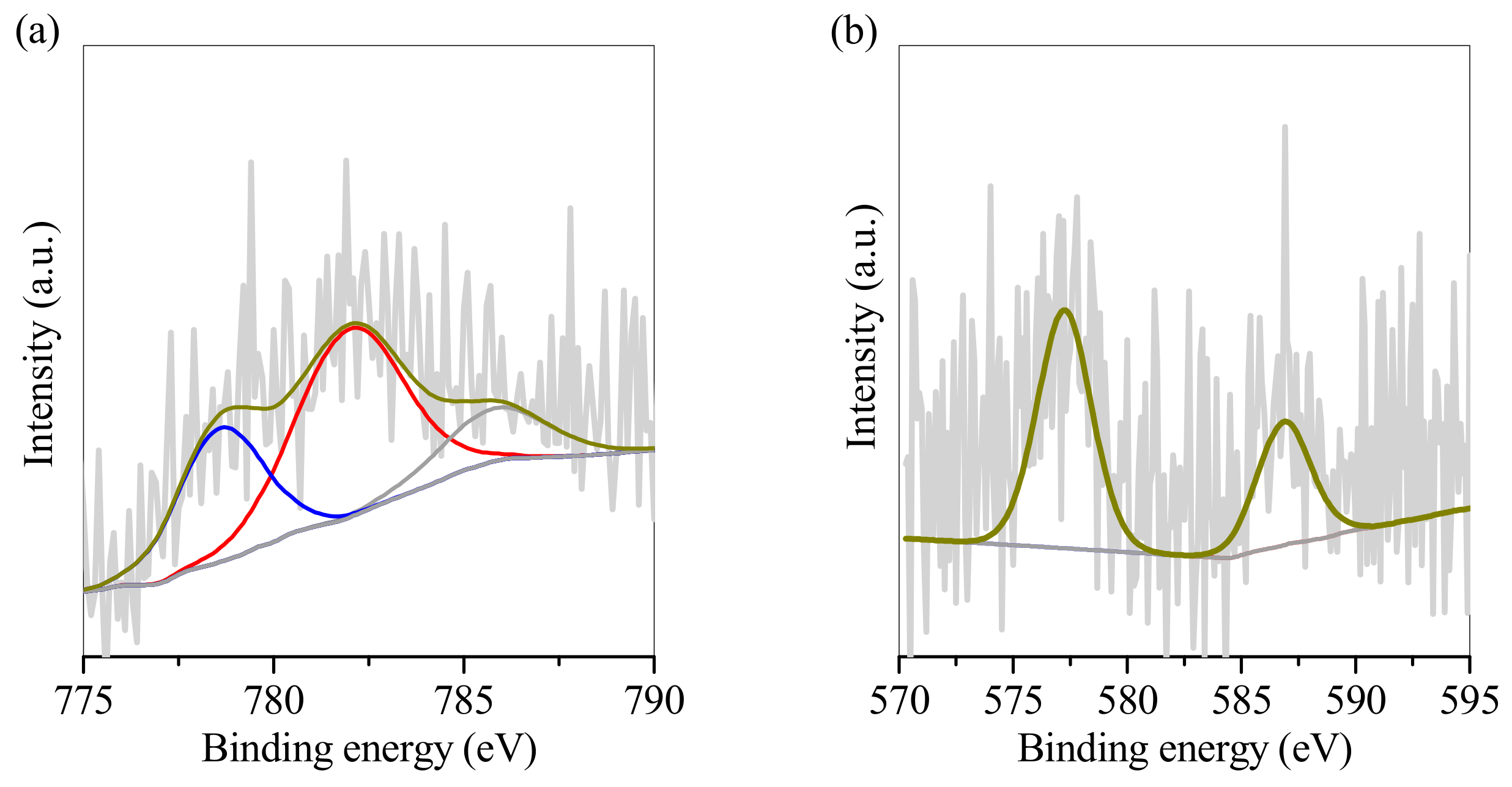
| Element | Relative Weight Percentage (wt.%) | |
|---|---|---|
| S1 | S2 | |
| Fe | 25.6 | 36.8 |
| Cr | 0.06 | 7.8 |
| Co | 5.5 | 0.04 |
| Ca | 0.5 | 1.3 |
| Si | 0.9 | 1.1 |
| Al | 2.05 | 2.78 |
| Na | 1.7 | 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Khan, A.; Wang, Z.; Chen, Y.; Zhu, S.; Sun, T.; Liang, D.; Yu, H. Upcycling of Electroplating Sludge to Prepare Erdite-Bearing Nanorods for the Adsorption of Heavy Metals from Electroplating Wastewater Effluent. Water 2020, 12, 1027. https://doi.org/10.3390/w12041027
Liu Y, Khan A, Wang Z, Chen Y, Zhu S, Sun T, Liang D, Yu H. Upcycling of Electroplating Sludge to Prepare Erdite-Bearing Nanorods for the Adsorption of Heavy Metals from Electroplating Wastewater Effluent. Water. 2020; 12(4):1027. https://doi.org/10.3390/w12041027
Chicago/Turabian StyleLiu, Yanwen, Asghar Khan, Zhihua Wang, Yu Chen, Suiyi Zhu, Tong Sun, Dongxu Liang, and Hongbin Yu. 2020. "Upcycling of Electroplating Sludge to Prepare Erdite-Bearing Nanorods for the Adsorption of Heavy Metals from Electroplating Wastewater Effluent" Water 12, no. 4: 1027. https://doi.org/10.3390/w12041027





