Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
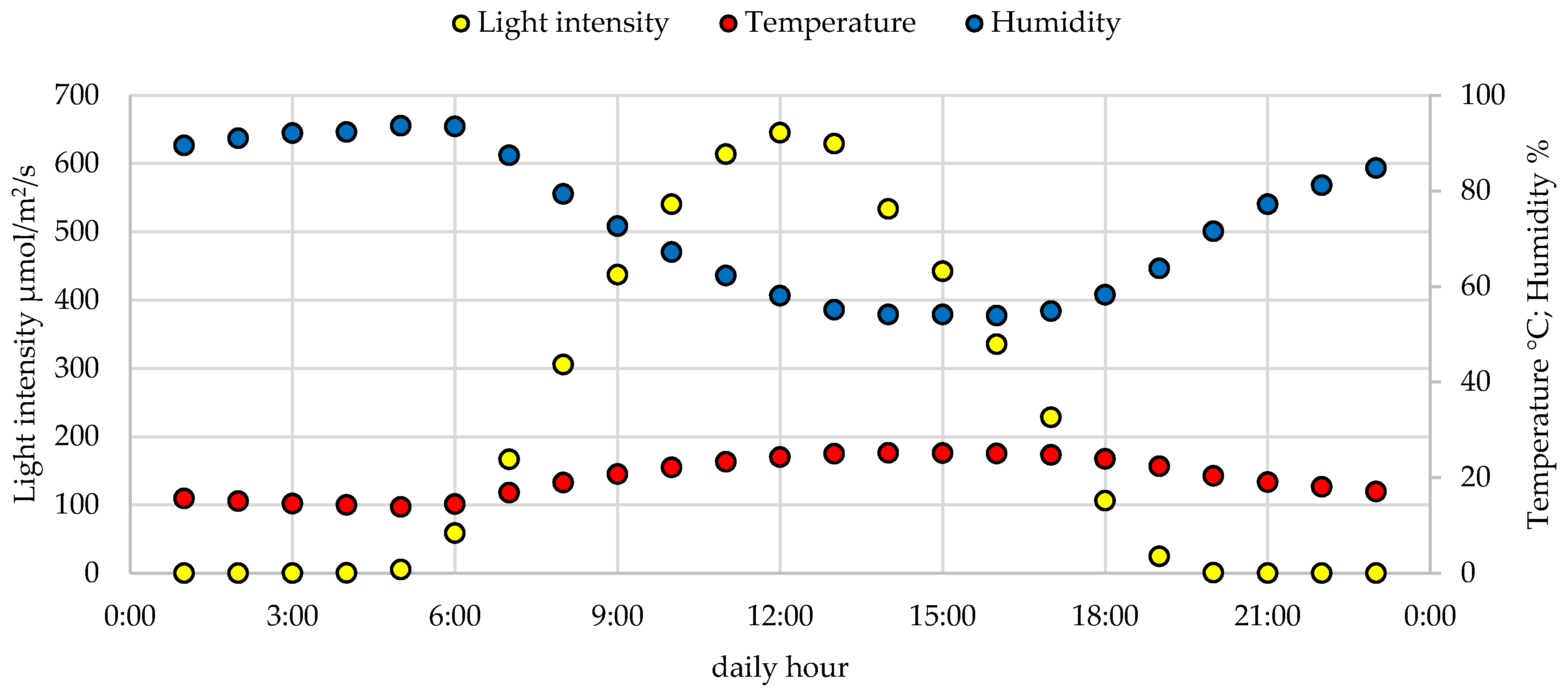
2.1. Plant Materials, Cultivation and Growth Conditions
2.2. PAW Preparation
2.3. Measurement of Selected Growth Parameters
2.3.1. Chlorophyll Content (N-Tester Value)
2.3.2. Chlorophyll Fluorescence Parameters
2.3.3. Root Electrical Capacitance
2.3.4. Plant Analysis
2.4. Data Analysis
3. Results and Discussion
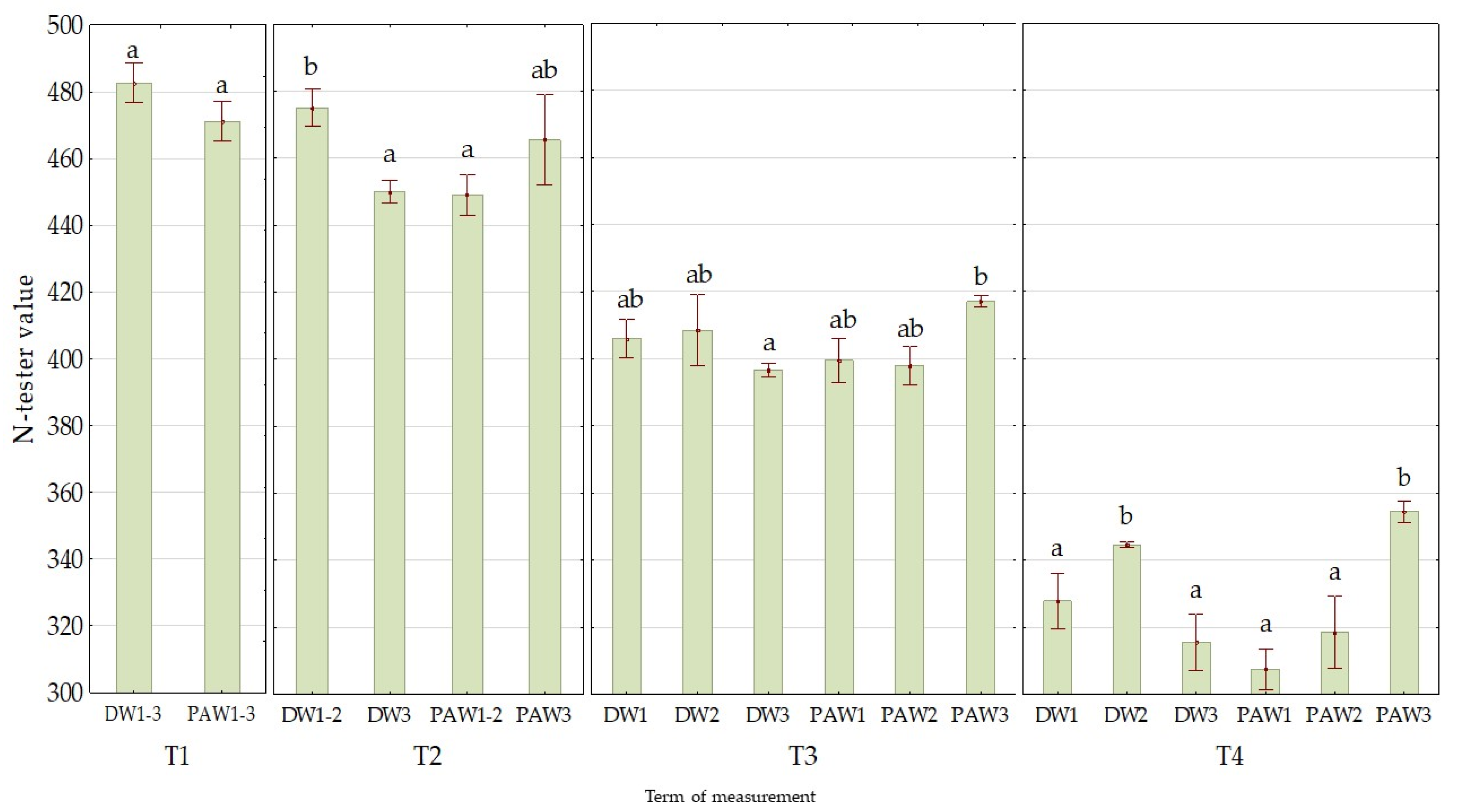
3.1. The Effect of PAW on Chlorophyll Contents (N-Tester Value)
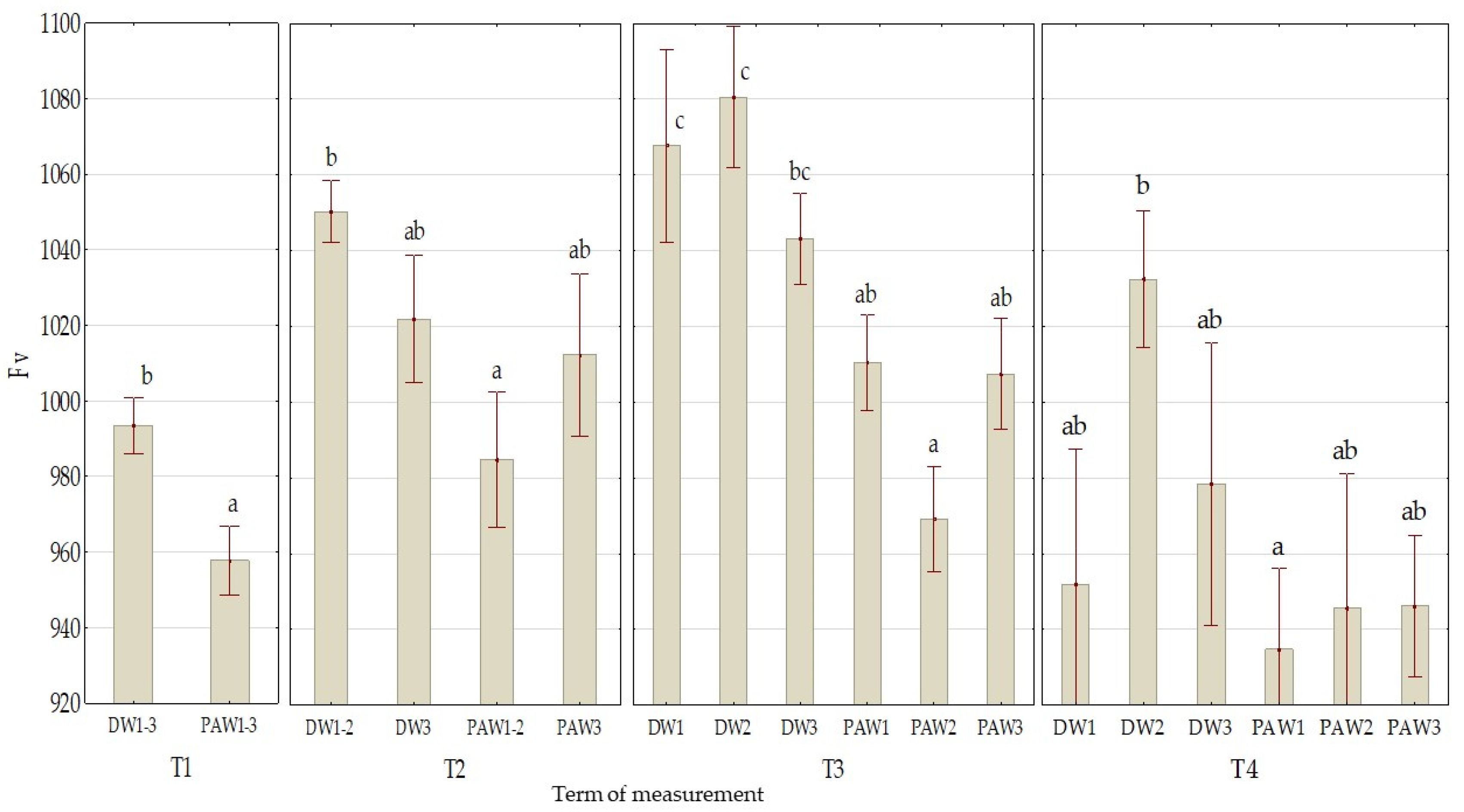
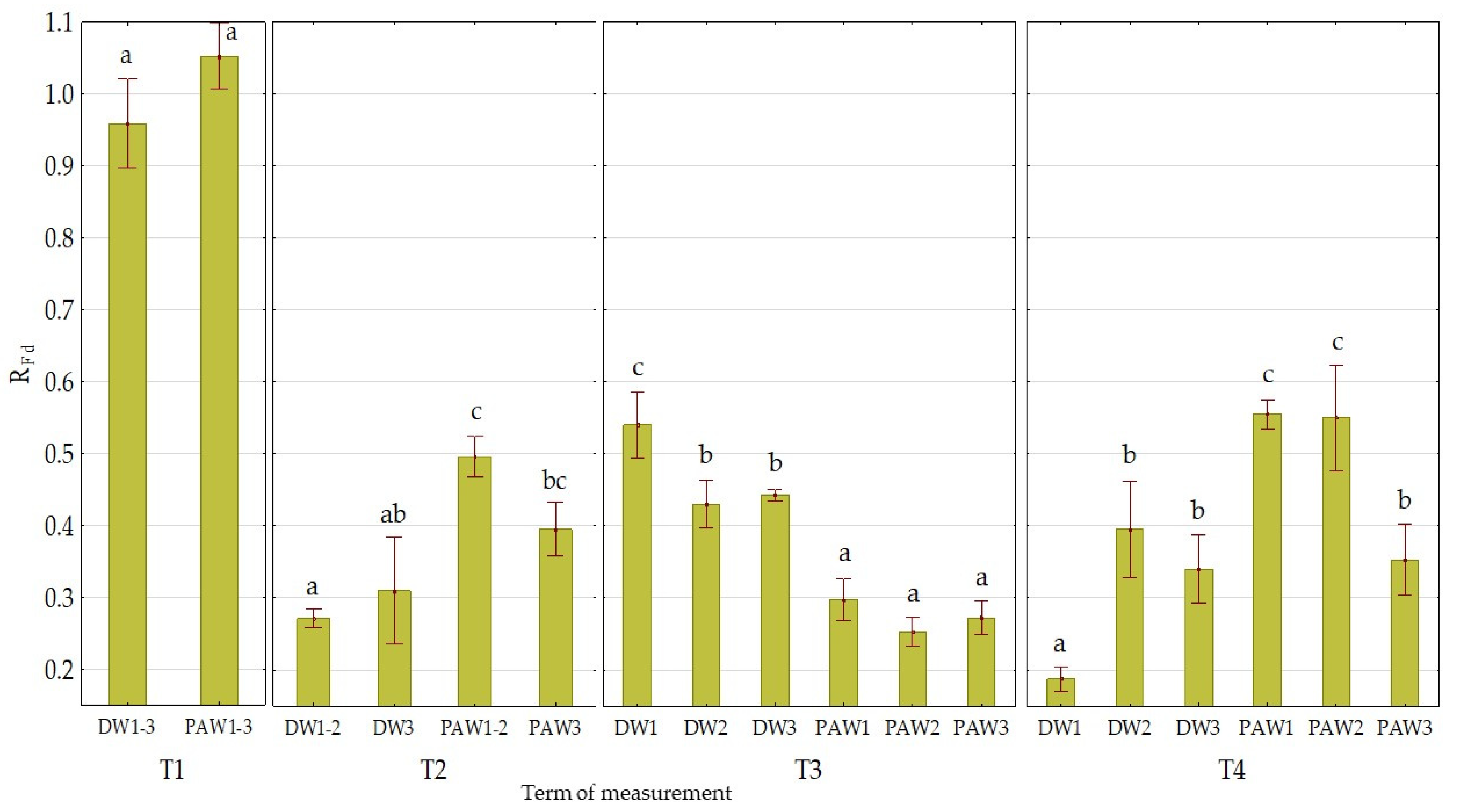
3.2. The Effect of PAW on Chlorophyll Fluorescence Parameters
3.3. The Effect of PAW on Root Electrical Capacitance (CR)
3.4. The Effect of PAW on Dry Matter Weight
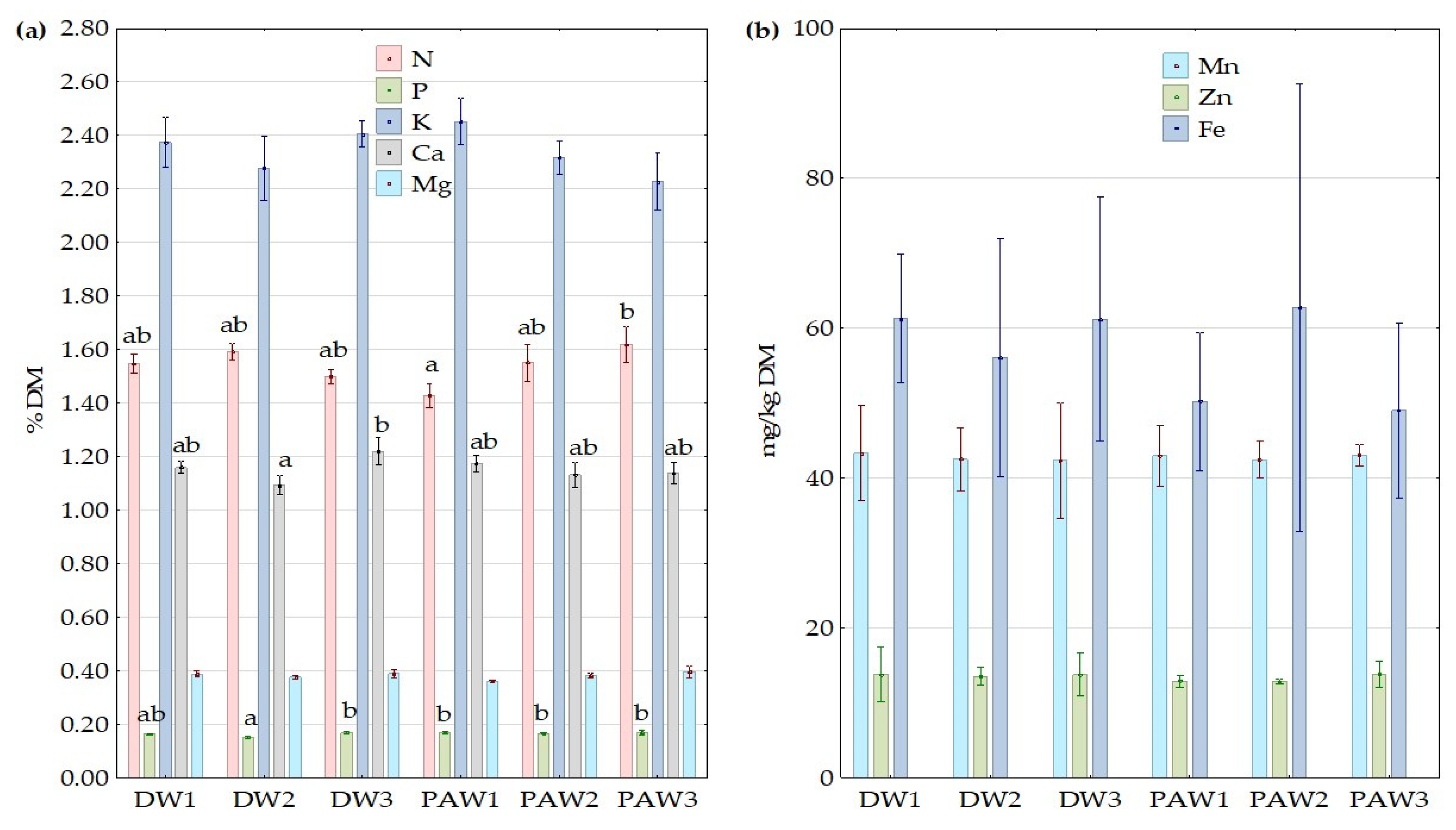
3.5. The Effect of PAW on Nutrients Content in Plant Tissue
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boliko, M.C. FAO and the Situation of Food Security and Nutrition in the World. J. Nutr. Sci. Vitaminol. 2019, 65, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Cavendish, H. Experiments on air. Philos. Trans. 1785, 75, 372–384. [Google Scholar]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Mohades, S.; Laroussi, M.; Sears, J.; Barekzi, N.; Razavi, H. Evaluation of the effects of a plasma activated medium on cancer cells. Phys. Plasmas 2015, 22, 122001. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plewa, J.M.; Yousfi, M.; Frongia, C.; Eichwald, O.; Ducommun, B.; Merbahi, N.; Lobjois, V. Low-temperature plasma-induced antiproliferative effects on multi-cellular tumor spheroids. New J. Phys. 2014, 16, 043027. [Google Scholar] [CrossRef]
- Stoffels, E. Biomedical applications of electric gas discharges. High Temp. Mater. Process 2011, 15, 241–251. [Google Scholar] [CrossRef]
- Park, D.P.; Davis, K.; Gilani, S.; Alonzo, C.A.; Dobrynin, D.; Friedman, G.; Fridman, A.; Rabinovich, A.; Fridman, G. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr. Appl. Phys. 2013, 13, 19–29. [Google Scholar] [CrossRef]
- Sarinont, T.; Katayama, R.; Wada, Y.; Koga, K.; Shiratani, M. Plant Growth enhancement of seeds immersed in plasma activated water. MRS Adv. 2017, 2, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review: Non-thermal plasma treatment of agricultural seeds. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Kitazaki, S.; Koga, K.; Shiratani, M.; Hayashi, N. Growth Enhancement of Radish Sprouts Induced by Low Pressure O2 Radio Frequency Discharge Plasma Irradiation. Jpn. J. Appl. Phys. 2012, 51, 01AE01. [Google Scholar] [CrossRef]
- Kitazaki, S.; Sarinont, T.; Koga, K.; Hayashi, N.; Shiratani, M. Plasma induced long-term growth enhancement of Raphanus sativus L. using combinatorial atmospheric air dielectric barrier discharge plasmas. Curr. Appl. Phys. 2014, 14, 149–153. [Google Scholar] [CrossRef]
- Koga, K.; Thapanut, S.; Amano, T.; Seo, H.; Itagaki, N.; Hayashi, N.; Shiratani, M. Simple method of improving harvest by nonthermal air plasma irradiation of seeds of Arabidopsis thaliana L. Appl. Phys. Express 2015, 9, 016201. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhour, R.; Zhang, X.; Yang, S. Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, e1700174. [Google Scholar] [CrossRef]
- Schlüter, O.; Ehlbeck, J.; Hertel, C.; Habermeyer, M.; Roth, A.; Engel, K.H.; Eisenbrand, G. Opinion on the use of plasma processes for treatment of foods. Mol. Nutr. Food Res. 2013, 57, 920–927. [Google Scholar] [CrossRef]
- Świecimska, M.; Tulik, M.; Sera, B.; Golinska, P.; Tomeková, J.; Medvecká, V.; Bujdakova, H.; Oszako, T.; Zahoranová, A.; Sery, M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum. Forests 2020, 11, 837. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R.; Brisset, J.L. Aqueous-Phase Chemistry of Electrical Discharge Plasma in Water and in Gas–Liquid Environments. In Plasma Chemistry and Catalysis in Gases and Liquids, 1st ed.; Parvulescu, V.I., Magureanu, M., Lukes, P., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2012; pp. 243–308. [Google Scholar]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Nagatomo, T.; Abiru, T.; Mitsugi, F.; Ebihara, K.; Kazuhiro, N. Study on ozone treatment of soil for agricultural application of surface dielectric barrier discharge. Jpn. J. Appl. Phys. 2016, 55, 01AB06. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Kutasi, K.; Popović, D.; Krstulović, N.; Milošević, S. Tuning the composition of plasma-activated water by a surface-wave microwave discharge and a kHz plasma jet. Plasma Sources Sci. Technol. 2019, 28, 095010. [Google Scholar] [CrossRef] [Green Version]
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357. [Google Scholar] [CrossRef]
- Eisenberg, G.M. Colorimetric determination of hydrogen peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Marczenko, Z. Spectrophotometric Determination of Elements, Ellis Horwood, Chichester; Prentice Hall Europe: Upper Saddle River, NJ, USA, 1976. [Google Scholar]
- Netto, A.L.; Campostrini, E.; Goncalves de Oliverira, J.; BressanSmith, R.E. Photosynthetic pigments, nitrogen, chlrophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 12. 2013. Available online: www.statsoft.com (accessed on 25 October 2020).
- Koning, L.A.; Veste, M.; Freese, D.; Lebzien, S. Effects of nitrogen and phosphate fertilization on leaf nutrient content, photosynthesis, and growth of the novel bioenergy crop Fallopia sachalinensis cv. ‘Igniscum Candy’. J. Appl. Bot. Food Qual. 2015, 88, 22–28. [Google Scholar]
- Ortuzar-Iragorri, M.A.; Alonso, A.; Castellón, A.; Besga, G.; Estavillo, J.M.; Aizpurua, A. N-Tester® use in soft winter wheat: Evaluation of nitrogen status and grain yield prediction. Agron. J. 2005, 97, 1380–1389. [Google Scholar] [CrossRef]
- Takaki, K.; Takahata, J.; Watanabe, S.; Satta, N.; Yamada, O.; Fujio, T.; Sasaki, Y. Improvements in plant growth rate using underwater discharge. J. Phys. Conf. Ser. 2013, 418, 012140. [Google Scholar]
- Lindsay, A.; Byrns, B.; King, W.; Andhvarapou, A.; Fields, J.; Knappe, D.; Foneto, W.; Shannon, S. Fertilization of radishes, tomatoes, and marigolds using a large-volume atmospheric glow discharge. Plasma Chem. Plasma Process. 2014, 34, 1271–1290. [Google Scholar]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene expression in Tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryer, M.J.; Andrews, J.R.; Oxborough, K.; Blowers, D.A.; Baker, N.R. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998, 116, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Kučerová, K.; Henselová, M.; Slováková, L.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, e1800131. [Google Scholar] [CrossRef]
- Ndiffo Yemeli, G.B.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The effect of water activated by nonthermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process. Polym. 2020, e2000205. [Google Scholar]
- Stirbet, A.; Govindjee, R. Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J-I-P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of Potato (Solanum Tuberosum L.) Plants to Spraying by Hydrogen Peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef] [Green Version]
- Haitz, M.; Lichtenthaler, H.K. The measurement of Rfd-values as plant vitality indices with the portable field fluorometer and the PAM-fluorometer. In Applications of Chlorophyll Fluorescence; Lichtenthaler, H.K., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 249–254. [Google Scholar]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Takács, T.; Végh, R.K.; Anton, A.; Rajkai, K. Electrical impedance and capacitance method: A new approach for detection of functional aspects of arbuscular mycorrhizal colonization in maize. Eur. J. Soil Biol. 2013, 54, 25–31. [Google Scholar] [CrossRef]
- Fan, L.M.; Liu, X.F.; Ma, Y.F.; Xiang, Q.S. Effects of plasma-activated water treatment on seed germination and growth of mung bean sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, J.; Hong, E.J.; Kim, S.B. Plasma-activated water regulates root hairs and cotyledon size dependent on cell elongation in Nicotiana tabacum L. Plant Biotechnol. Rep. 2020, 14, 663–672. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Tan, X.W.; Ikeda, H.; Oda, M. Absorption, translocation, and assimilation of foliar-applied urea compared with nitrate and ammonium in tomato plants. Soil Sci. Plant Nutr. 1999, 45, 609–616. [Google Scholar] [CrossRef]
- Uscola, M.; Villar-Salvador, P.; Oliet, J.; Warren, C.R. Foliar absorption and root translocation of nitrogen from different chemical forms in seedlings of two Mediterranean trees. Environ. Exp. Bot. 2014, 104, 34–43. [Google Scholar] [CrossRef]
- Peuke, A.D.; Jeschke, W.D.; Dietz, K.J.; Schreiber, L.; Hartung, W. Foliar Application of Nitrate or Ammonium as Sole Nitrogen Supply in Ricinus communis. I. Carbon and Nitrogen Uptake and Inflows. New Phytol. 1998, 138, 675–687. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Beverly, R.B. The Dilution Effect in Plant Nutrition Studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar]
- Liu, D.Y.; Zhang, W.; Liu, Y.M.; Chen, X.P.; Zou, C.Q. Soil Application of Zinc Fertilizer Increases Maize Yield by Enhancing the Kernel Number and Kernel Weight of Inferior Grains. Front. Plant Sci. 2020, 11, 188. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of Zinc Nutrition for Increasing Zinc Availability, Uptake, Yield, and Quality of Maize (Zea Mays L.) Grains: An Overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar]
- Patel, K.; Kumar, A.; Durani, S. Analysis of the structural consensus of the zinc coordination centers of metalloprotein structures. Biochim. Biophys. Acta. 2007, 1774, 1247–1253. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]

| Soil Parameter | Value |
|---|---|
| pH (CaCl2) | 6.09 |
| Cox | 0.80% |
| Clay | 20% |
| Silt | 27% |
| Sand | 53% |
| Cation Exchange Capacity | 164 mmol/kg |
| N total | 0.19% |
| N-NH4+ (K2SO4) | 1.48 mg/kg |
| N-NO3− (K2SO4) | 17.2 mg/kg |
| P (Mehlich 3) | 36.4 mg/kg |
| K (Mehlich 3) | 400 mg/kg |
| Ca (Mehlich 3) | 2720 mg/kg |
| Mg (Mehlich 3) | 214 mg/kg |
| Chlorophyll Fluorescence Parameters | Pulse Type | Light Inetnsity (μmol/m2/s) | Phase | Duration (s) | 1st Pulse (s) | Pulse Interval (s) |
|---|---|---|---|---|---|---|
| ΦPSII, Fv | Saturation | 2400 | - | 1 pulse | ||
| RFd | Flash | 900 | L | 60 | 0.2 | 1 |
| DR | 88 | 1 | 1 | |||
| Saturation | 2400 | L | 60 | 7 | 12 | |
| DR | 88 | 11 | 26 | |||
| Actinic | 300 | L | 60 | - | - |
| Treatment | N-Tester Value | |
|---|---|---|
| Mean value of water type | DW | 420 a ± 11 |
| PAW | 414 a ± 10 | |
| Mean value of treatment variant | DW1 | 418 a ± 19 |
| DW2 | 429 ab ± 15 | |
| DW3 | 413 a ± 20 | |
| PAW1 | 404 a ± 19 | |
| PAW2 | 408 a ± 18 | |
| PAW3 | 431 b ± 14 | |
| Treatment | ΦPSII | Fv | RFd | |
|---|---|---|---|---|
| Mean value of water type | DW | 0.759 a ± 0.002 | 1022 b ± 8 | 0.51 a ± 0.04 |
| PAW | 0.755 a ± 0.003 | 973 a ± 7 | 0.57 b ± 0.05 | |
| Mean value of treatment variant | DW1 | 0.759 ab ± 0.003 | 1022 c ± 16 | 0.44 a ± 0.07 |
| DW2 | 0.758 ab ± 0.003 | 1033 c ± 12 | 0.53 bc ± 0.08 | |
| DW3 | 0.758 ab ± 0.003 | 1009 bc ± 12 | 0.54 bc ± 0.10 | |
| PAW1 | 0.749 a ± 0.006 | 980 ab ± 13 | 0.61 c ± 0.08 | |
| PAW2 | 0.760 b ± 0.002 | 959 a ± 11 | 0.60 c ± 0.08 | |
| PAW3 | 0.756 ab ± 0.004 | 978 a ± 11 | 0.49 ab ± 0.09 | |
| Treatment | T2 | T4 | Mean CR | |
|---|---|---|---|---|
| Mean value of water type | DW | - | - | 0.192 a ± 0.003 |
| PAW | - | - | 0.220 b ± 0.006 | |
| Mean value of treatment variant | DW1 | 0.20 a ± 0.01 | 0.18 a ± 0.01 | 0.188 a ± 0.004 |
| DW2 | 0.19 ab ± 0.01 | 0.188 a ± 0.005 | ||
| DW3 | 0.20 a ± 0.00 | 0.20 ab ± 0.01 | 0.201 ab ± 0.005 | |
| PAW1 | 0.19 a ± 0.01 | 0.21 bc ± 0.01 | 0.198 ab ± 0.006 | |
| PAW2 | 0.23 b ± 0.01 | 0.23 c ± 0.01 | 0.212 b ± 0.009 | |
| PAW3 | 0.27 d ± 0.02 | 0.251 c ± 0.011 | ||
| Treatment | DM (g/plant) | |
|---|---|---|
| Mean value of water type | DW | 0.546 ± 0.007 |
| PAW | 0.529 ± 0.010 | |
| Mean value of treatment variant | DW1 | 0.534 ± 0.010 |
| DW2 | 0.544 ± 0.027 | |
| DW3 | 0.559 ± 0.016 | |
| PAW1 | 0.548 ± 0.020 | |
| PAW2 | 0.521 ± 0.015 | |
| PAW3 | 0.519 ± 0.015 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545. https://doi.org/10.3390/w12123545
Škarpa P, Klofáč D, Krčma F, Šimečková J, Kozáková Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water. 2020; 12(12):3545. https://doi.org/10.3390/w12123545
Chicago/Turabian StyleŠkarpa, Petr, Daniel Klofáč, František Krčma, Jana Šimečková, and Zdenka Kozáková. 2020. "Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.)" Water 12, no. 12: 3545. https://doi.org/10.3390/w12123545
APA StyleŠkarpa, P., Klofáč, D., Krčma, F., Šimečková, J., & Kozáková, Z. (2020). Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water, 12(12), 3545. https://doi.org/10.3390/w12123545







