Impact of Nutrients, Temperatures, and a Heat Wave on Zooplankton Community Structure: An Experimental Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
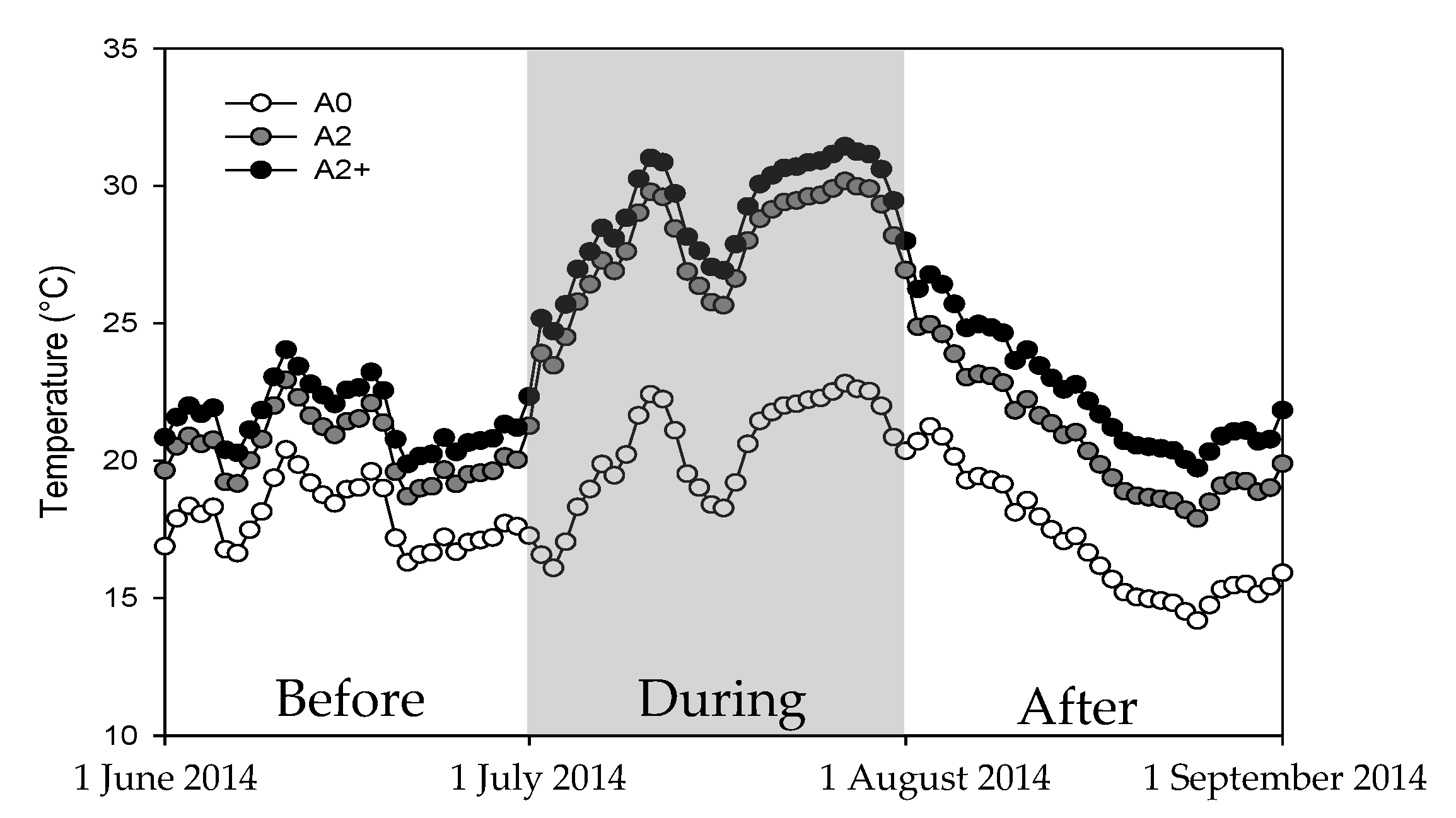
2.2. Heat Wave Treatment
2.3. Sampling and Laboratory Analysis
2.4. Stability Analyses
2.5. Statistical Analyses
3. Results
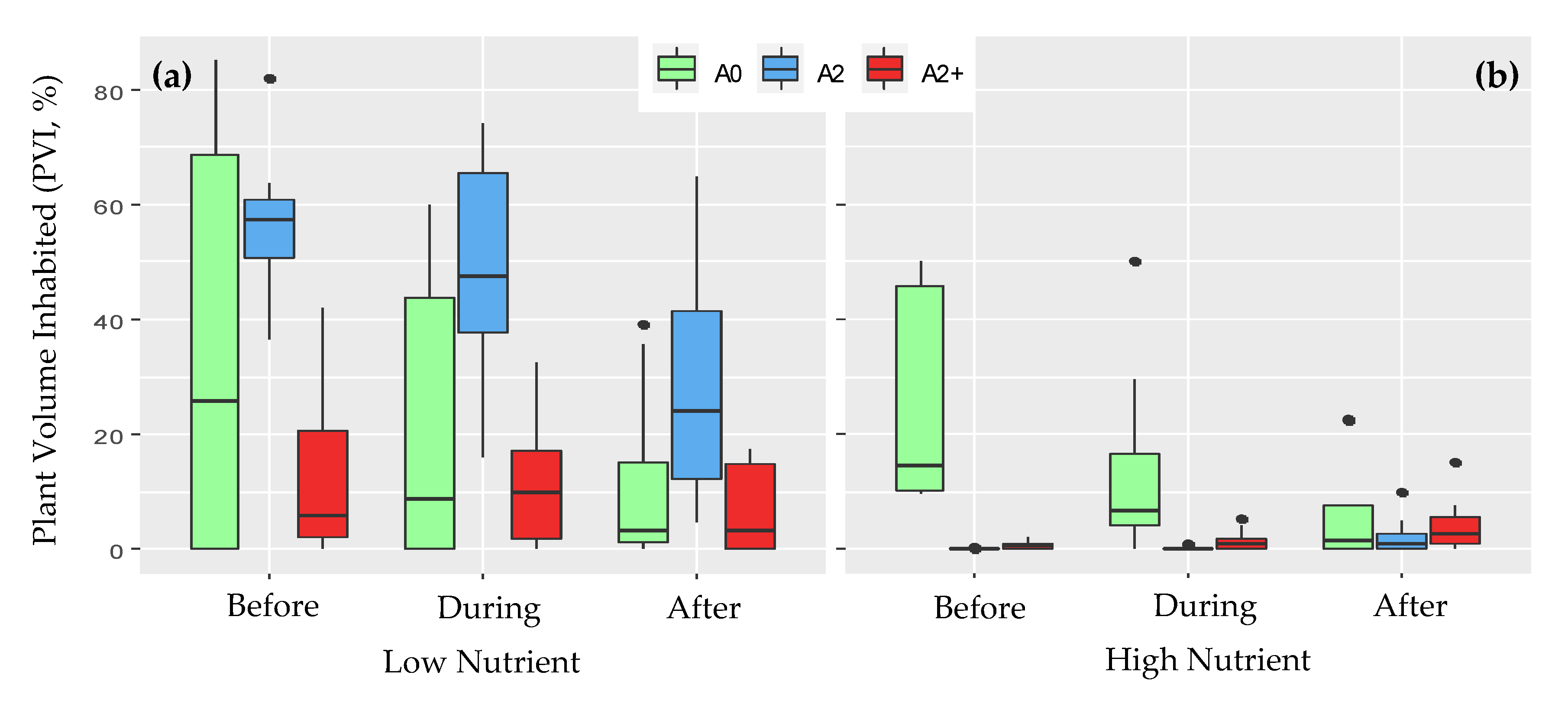
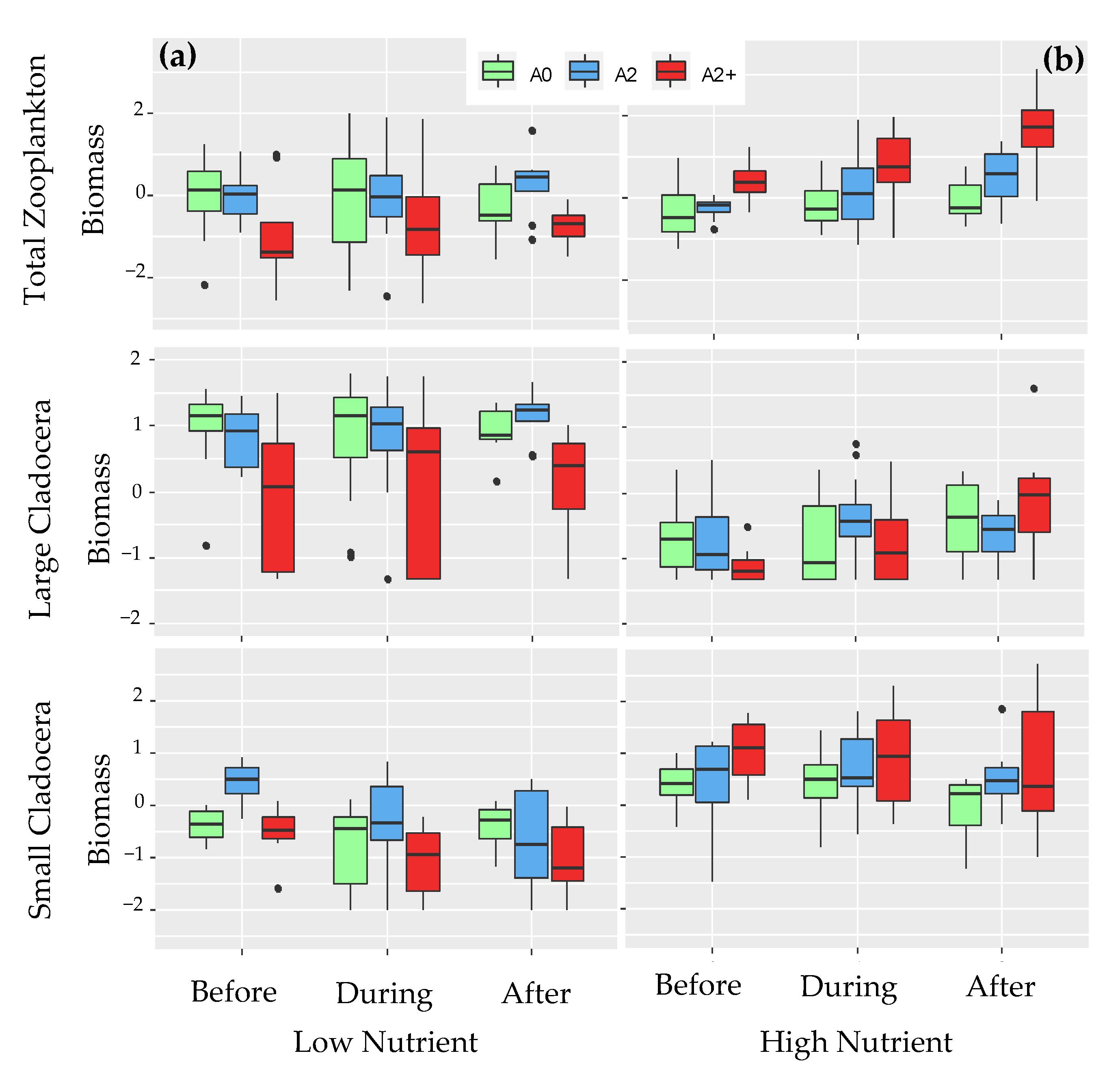
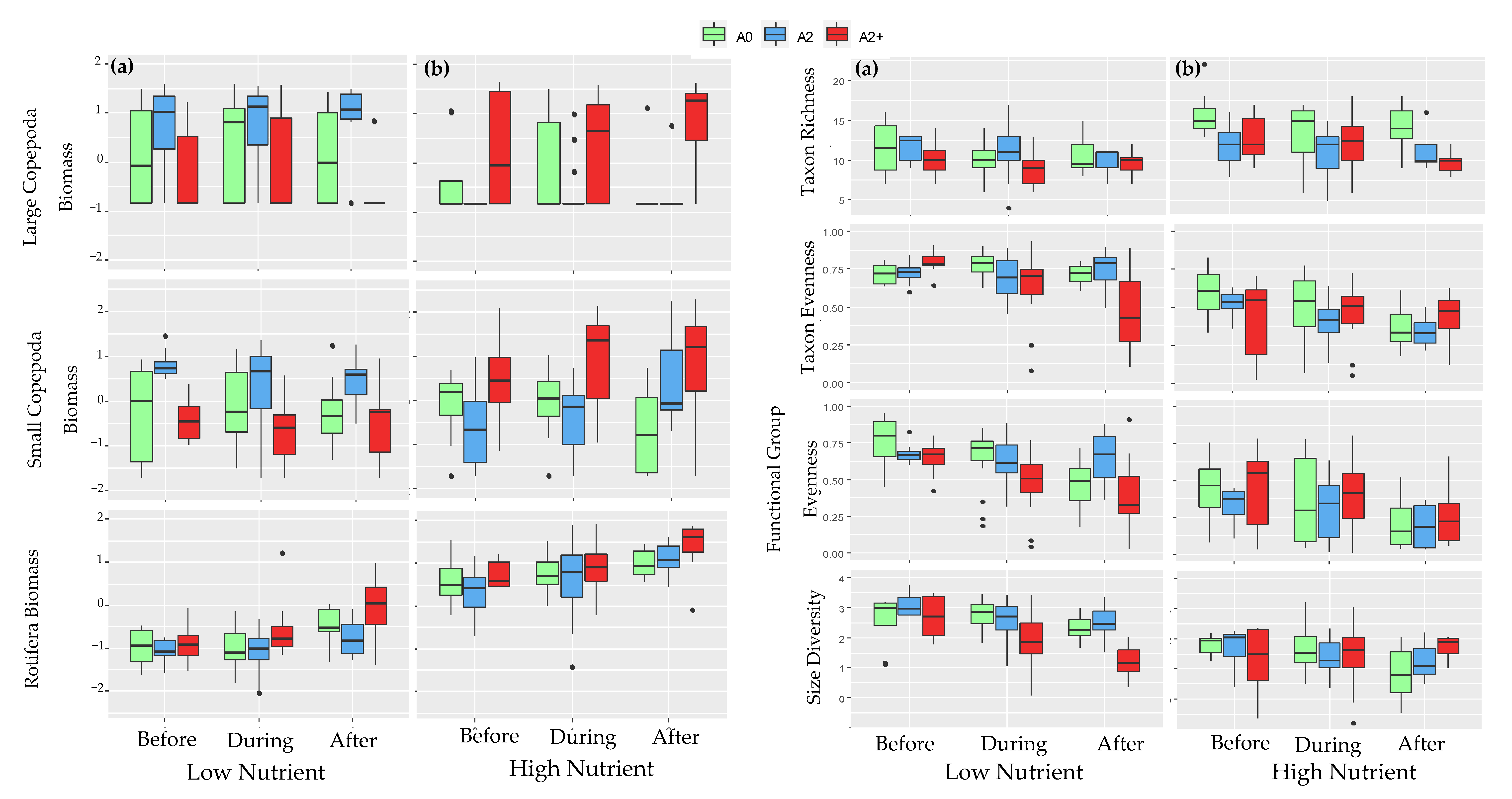
3.1. Community Structure before the Heat Wave
3.2. Community Structure during and after the Heat Wave
3.3. Co-Inertia Analysis
3.4. Stability Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiklomanov, I. World Fresh Water Resources. In Water in Crisis a Guide to the World’s Fresh Water Resources; Gleick, P., Ed.; Oxford University Press: Oxford, NY, USA, 1993; pp. 13–24. [Google Scholar]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A Global Inventory of Lakes Based on High-Resolution Satellite Imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L.A. The Biology of Lakes and Ponds, 3rd ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioğlu, M.; Özen, A.; et al. Climate Change Effects on Runoff, Catchment Phosphorus Loading and Lake Ecological State, and Potential Adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef]
- Moss, B. Allied Attack: Climate Change and Eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2001: The Scientific Basis; Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate, Change; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., Van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Meehl, G.; Tebaldi, C. More Intense, More Frequent, and Longer Lasting Heat Waves in the 21st Century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Christidis, N.; Jones, G.S.; Stott, P.A. Dramatically Increasing Chance of Extremely Hot Summers since the 2003 European Heatwave. Nat. Clim. Chang. 2015, 5, 46–50. [Google Scholar] [CrossRef]
- Jankowski, T.; Livingstone, D.M.; Bührer, H.; Forster, R.; Niederhauser, P. Consequences of the 2003 European Heat Wave for Lake Temperature Profiles, Thermal Stability, and Hypolimnetic Oxygen Depletion: Implications for a Warmer World. Limnol. Oceanogr. 2006, 51, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Huber, V.; Adrian, R.; Gerten, D. A Matter of Timing: Heat Wave Impact on Crustacean Zooplankton. Freshw. Biol. 2010, 55, 1769–1779. [Google Scholar] [CrossRef]
- Cao, Y.; Neif, É.M.; Li, W.; Coppens, J.; Filiz, N.; Lauridsen, T.L.; Davidson, T.A.; Søndergaard, M.; Jeppesen, E. Heat Wave Effects on Biomass and Vegetative Growth of Macrophytes after Long-Term Adaptation to Different Temperatures: A Mesocosm Study. Clim. Res. 2015, 66, 265–274. [Google Scholar] [CrossRef]
- Woodward, G.; Bonada, N.; Brown, L.E.; Death, R.G.; Durance, I.; Gray, C.; Hladyz, S.; Ledger, M.E.; Milner, A.M.; Ormerod, S.J.; et al. The Effects of Climatic Fluctuations and Extreme Events on Running Water Ecosystems. Philos. Trans. R. Soc. B 2016, 371, 20150274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, B. Engineering and Biological Approaches to the Restoration from Eutrophication of Shallow Lakes in Which Aquatic Plant Communities Are Important Components. Hydrobiologia 1990, 200–201, 367–377. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative Equilibria in Shallow Lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K. The Structuring Role of Submerged Macrophytes in Lakes; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Van De Waal, D.B.; Verschoor, A.M.; Verspagen, J.M.H.; Van Donk, E.; Huisman, J. Climate-Driven Changes in the Ecological Stoichiometry of Aquatic Ecosystems. Front. Ecol. Environ. 2010, 8, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Šorf, M.; Davidson, T.A.; Brucet, S.; Menezes, R.F.; Søndergaard, M.; Lauridsen, T.L.; Landkildehus, F.; Liboriussen, L.; Jeppesen, E. Zooplankton Response to Climate Warming: A Mesocosm Experiment at Contrasting Temperatures and Nutrient Levels. Hydrobiologia 2015, 742, 185–203. [Google Scholar] [CrossRef]
- Teixeira-De Mello, F.; Meerhoff, M.; Pekcan-Hekim, Z.; Jeppesen, E. Substantial Differences in Littoral Fish Community Structure and Dynamics in Subtropical and Temperate Shallow Lakes. Freshw. Biol. 2009, 54, 1202–1215. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.J.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of Climate Warming on Lake Fish Community Structure and Potential Effects on Ecosystem Function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Meerhoff, M.; Lacerot, G.; Clemente, J.M.; Scasso, F.; Kruk, C.; Goyenola, G.; García-Alonso, J.; Amsinck, S.L.; et al. High Predation Is of Key Importance for Dominance of Small-Bodied Zooplankton in Warm Shallow Lakes: Evidence from Lakes, Fish Exclosures and Surface Sediments. Hydrobiologia 2011, 667, 133–147. [Google Scholar] [CrossRef]
- Strecker, A.L.; Cobb, T.P.; Vinebrooke, R.D. Effects of Experimental Greenhouse Warming on Phytoplankton and Zooplankton Communities in Fishless Alpine Ponds. Limnol. Oceanogr. 2004, 49, 1182–1190. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; van Donk, E.; Hansson, L.A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer Climates Boost Cyanobacterial Dominance in Shallow Lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ü.N.; Šorf, M.; Stefanidis, K.; Brucet, S.; Türkan, S.; Agasild, H.; Baho, D.L.; Scharfenberger, U.; Hejzlar, J.; Papastergiadou, E.; et al. Effects of Nutrient and Water Level Changes on the Composition and Size Structure of Zooplankton Communities in Shallow Lakes under Different Climatic Conditions: A Pan-European Mesocosm Experiment. Aquat. Ecol. 2017, 1–17. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global Warming Benefits the Small in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining Body Size: A Third Universal Response to Warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Brucet, S.; Boix, D.; Quintana, X.D.; Jensen, E.; Nathansen, L.W.; Trochine, C.; Meerhoff, M.; Gascón, S.; Jeppesen, E.; Gascon, S.; et al. Factors Influencing Zooplankton Size Structure at Contrasting Temperatures in Coastal Shallow Lakes: Implications for Effects of Climate Change. Limnol. Oceanogr. 2010, 55, 1697–1711. [Google Scholar] [CrossRef] [Green Version]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-Induced Reductions in Body Size Are Greater in Aquatic than Terrestrial Species. Proc. Natl. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef] [Green Version]
- Havens, K.E.; Pinto-Coelho, R.M.; Beklioğlu, M.; Christoffersen, K.S.; Jeppesen, E.; Lauridsen, T.L.; Mazumder, A.; Méthot, G.; Alloul, B.P.; Tavşanoğlu, U.N.; et al. Temperature Effects on Body Size of Freshwater Crustacean Zooplankton from Greenland to the Tropics. Hydrobiologia 2015, 743, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of Multiple Stressors on Freshwater Biota across Spatial Scales and Ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- Moss, B.; Mckee, D.; Atkinson, D.; Collings, S.E.; Eaton, J.W.; Gill, A.B.; Harvey, I.; Hatton, K.; Heyes, T.; Wilson, D. How Important Is Climate? Effects of Warming, Nutrient Addition and Fish on Phytoplankton in Shallow Lake Microcosms. J. Appl. Ecol. 2003, 40, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Kronvang, B.; Jørgensen, T.B.; Larsen, S.E.; Andersen, H.E.; Søndergaard, M.; Liboriussen, L.; Bjerring, R.; Johansson, L.S.; Trolle, D.; et al. Recent Climate-Induced Changes in Freshwaters in Denmark. In Climatic Change and Global Warming of Inland Waters: Impacts and Mitigation for Ecosystems and Societies; Goldman, C.R., Kumagai, M., Robarts, R.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Schindler, D.W. The Cumulative Effects of Climate Warming and Other Human Stresses on Canadian Freshwaters in the New Millennium. Can. J. Fish. Aquat. Sci. 2001, 58, 18–29. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate Change and Freshwater Ecosystems: Impacts across Multiple Levels of Organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liboriussen, L.; Landkildehus, F.; Meerhoff, M.; Bramm, M.E.; Søndergaard, M.; Christoffersen, K.; Richardson, K.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Global Warming: Design of a Flow-through Shallow Lake Mesocosm Climate Experiment. Limnol. Oceanogr. Methods 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Jeppesen, E.; Moss, B.; Bennion, H.; Carvalho, L.; Demeester, L.; Feuchtmayr, H.; Friberg, N.; Gessner, M.O.; Hefting, M.; Lauridsen, T.L.; et al. Interaction of Climate Change and Eutrophication. In Climate Change Impacts on Freshwater Ecosystems; Kernan, M., Battarbee R., W., Moss, B., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2007: Synthesis Report; Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Core Writing Team; Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; 104p. [Google Scholar]
- Landkildehus, F.M.; Søndergaard, M.; Beklioglu, R.; Adrian, D.G.; Angeler, J.; Hejzlar, E.; Papastergiadou, P.; Zingel, A.; İdil Çakiroğlu, U.; Scharfenberger, S.; et al. Jeppesen. Climate change effects on shallow lakes: Design and preliminary results of a cross-European climate gradient mesocosm experiment. Estonian J. Ecol. 2014, 63, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Gran, G. Determination of the Equivalence Point in Potentiometric Titrations. Part II. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P.; Amsinck, S.L. Water Framework Directive: Ecological Classification of Danish Lakes. J. Appl. Ecol. 2005, 42, 616–629. [Google Scholar] [CrossRef]
- Jespersen, A.; Christoffersen, K. Measurements of Chlorophyll―A from Phytoplankton Using Ethanol as Extraction Solvent. Arch. Hydrobiol. 1987, 109, 445–454. [Google Scholar]
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The Dry Weight Estimate of Biomass in a Selection of Cladocera, Copepoda and Rotifera from the Plankton, Periphyton and Benthos of Continental Waters. Oecologia 1975, 19, 75–79. [Google Scholar] [CrossRef]
- Bottrell, H.H. A Review of Some Problems in Zooplankton Production Studies. Nor. J. Zool. 1976, 24, 419–456. [Google Scholar]
- McCauley, E. The Estimation of the Abundance and Biomass of Zooplankton in Samples. In A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters; Downing, J.A., Rigler, F.H., Eds.; Blackwell: Oxford, UK, 1984; pp. 228–265. [Google Scholar]
- Michaloudi, E. Dry Weights of the Zooplankton of Lake Mikri Prespa (Macedonia, Greece). Belgian J. Zool. 2005, 135, 223–227. [Google Scholar]
- Ruttner-Kolisko, A. Sugestions for Biomass Calculations of Plankton Rotifers. Arch. Hydrobiol. Belh. Ergebn. Limnol. 1977, 135, 223. [Google Scholar]
- Malley, D.F. Range of Variation in Estimates of Dry Weight for Planktonic Crustacea and Rotifera from Temperate North American Lakes; Canada Department of Fisheries and Oceans Central and Arctic Region: Sarnia, ON, USA, 1989.
- Filiz, N.; Işkın, U.; Beklioǧlu, M.; Öğlü, B.; Cao, Y.; Davidson, T.A.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Phytoplankton Community Response to Nutrients, Temperature, and a Heat Wave in Shallow Lakes: An Experimental Approach. Water 2020, 12, 3394. [Google Scholar] [CrossRef]
- Davidson, T.A.; Audet, J.; Svenning, J.C.; Lauridsen, T.L.; Søndergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication Effects on Greenhouse Gas Fluxes from Shallow-Lake Mesocosms Override Those of Climate Warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Langenheder, S.; Lebret, K.; Lindström, E.; Östman, Ö.; Striebel, M. Decomposing Multiple Dimensions of Stability in Global Change Experiments. Ecol. Lett. 2018, 21, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. The Comprehensive R Archive Network. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 2 December 2020).
- Quintana, X.D.; Brucet, S.; Boix, D.; López-Flores, R.; Gascón, S.; Badosa, A.; Sala, J.; Moreno-Amich, R.; Egozcue, J.J. A Nonparametric Method for the Measurement of Size Diversitywith Emphasis on Data Standardization. Limnol. Oceanogr. Methods 2008, 6, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Brucet, S.; Boix, D.; López-Flores, R.; Badosa, A.; Quintana, X.D. Size and Species Diversity of Zooplankton Communities in Fluctuating Mediterranean Salt Marshes. Estuar. Coast. Shelf Sci. 2006, 67, 424–432. [Google Scholar] [CrossRef]
- Quintana, X.D.; Arim, M.; Badosa, A.; Blanco, J.M.; Boix, D.; Brucet, S.; Compte, J.; Egozcue, J.J.; de Eyto, E.; Gaedke, U.; et al. Predation and Competition Effects on the Size Diversity of Aquatic Communities. Aquat. Sci. 2014, 77, 45–57. [Google Scholar] [CrossRef]
- Ye, L.; Chang, C.Y.; García-Comas, C.; Gong, G.C.; Hsieh, C. Increasing Zooplankton Size Diversity Enhances the Strength of Top-down Control on Phytoplankton through Diet Niche Partitioning. J. Anim. Ecol. 2013, 82, 1052–1061. [Google Scholar] [CrossRef]
- Barnett, A.J.; Finlay, K.; Beisner, B.E. Functional Diversity of Crustacean Zooplankton Communities: Towards a Trait-Based Classification. Freshw. Biol. 2007, 52, 796–813. [Google Scholar] [CrossRef]
- Obertegger, U.; Manca, M. Response of Rotifer Functional Groups to Changing Trophic State and Crustacean Community. J. Limnol. 2011, 70, 231–238. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Tavşanoğlu, Ü.N.; Vidal, N.; Yu, J.; Teixeira-de Mello, F.; Çakiroğlu, A.I.; He, H.; Liu, Z.; Jeppesen, E. Salinity Shapes Zooplankton Communities and Functional Diversity and Has Complex Effects on Size Structure in Lakes. Hydrobiologia 2018, 813, 237–255. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. CAR—An R Companion to Applied Regression; Sage Publications: Texas, CA, USA, 2019. [Google Scholar]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analyzing Ecological Data; Springer: NYC, USA, 2007. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography (Cop.) 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D. Co-inertia Analysis: An Alternative Method for Studying Species–Environment Relationships. Freshw. Biol. 1994, 31, 277–294. [Google Scholar] [CrossRef]
- Öğlü, B.; Bhele, U.; Järvalt, A.; Tuvikene, L.; Timm, H.; Seller, S.; Haberman, J.; Agasild, H.; Nõges, P.; Silm, M.; et al. Is Fish Biomass Controlled by Abiotic or Biotic Factors? Results of Long-Term Monitoring in a Large Eutrophic Lake. J. Great Lakes Res. 2019, 46, 881–890. [Google Scholar] [CrossRef]
- Audet, J.; Neif, É.M.; Cao, Y.; Hoffmann, C.C.; Lauridsen, T.L.; Larsen, S.E.; Søndergaard, M.; Jeppesen, E.; Davidson, T.A. Heat-Wave Effects on Greenhouse Gas Emissions from Shallow Lake Mesocosms. Freshw. Biol. 2017, 62, 1130–1142. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Pedersen, L.J.; Jeppesen, E.; Søndergaard, M. The Importance of Macrophyte Bed Size for Cladoceran Composition and Horizontal Migration in a Shallow Lake. J. Plankton Res. 1996, 18, 2283–2294. [Google Scholar] [CrossRef] [Green Version]
- Burks, R.L.; Lodge, D.M.; Jeppesen, E.; Lauridsen, T.L. Diel Horizontal Migration of Zooplankton: Costs and Benefits of Inhabiting the Littoral. Freshw. Biol. 2002, 47, 343–365. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic Structure, Species Richness and Diversity in Danish Lakes: Changes along a Phosphorus Gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Gyllström, M.; Hansson, L.A.; Jeppesen, E.; García-Criado, F.; Gross, E.; Irvine, K.; Kairesalo, T.; Kornijow, R.; Miracle, M.R.; Nykänen, M.; et al. The Role of Climate in Shaping Zooplankton Communities of Shallow Lakes. Limnol. Oceanogr. 2005, 50, 2008–2021. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Goldman, C.R. Experimental Separation of the Direct and Indirect Effects of Herbivorous Zooplankton on Phytoplankton in a Subalpine Lake. Int. Ver. Theor. Angew. Limnol. Verh. 1990, 24, 493–498. [Google Scholar] [CrossRef]
- Filstrup, C.T.; Hillebrand, H.; Heathcote, A.J.; Harpole, W.S.; Downing, J.A. Cyanobacteria Dominance Influences Resource Use Efficiency and Community Turnover in Phytoplankton and Zooplankton Communities. Ecol. Lett. 2014, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, A.; Filstrup, C.; Kendall, D.; Downing, J. Biomass Pyramids in Lake Plankton: Influence of Cyanobacteria Size and Abundance. Inland Waters 2016, 6, 250–257. [Google Scholar] [CrossRef]
- Moran, R.; Harvey, I.; Moss, B.; Feuchtmayr, H.; Hatton, K.; Heyes, T.; Atkinson, D. Influence of Simulated Climate Change and Eutrophication on Three-Spined Stickleback Populations: A Large Scale Mesocosm Experiment. Freshw. Biol. 2010, 55, 315–325. [Google Scholar] [CrossRef]
- Šorf, M.; Cábová, M.; Rychtecký, P. Environmentally-Induced Plasticity of Life History Parameters in the Cladoceran Simocephalus Vetulus. Limnologica 2017, 66, 40–43. [Google Scholar] [CrossRef]
- Mehlis, M.; Bakker, T.C.M. The Influence of Ambient Water Temperature on Sperm Performance and Fertilization Success in Three-Spined Sticklebacks (Gasterosteus Aculeatus). Evol. Ecol. 2014, 28, 655–667. [Google Scholar] [CrossRef]
- Mckee, D.; Hatton, K.; Eaton, J.W.; Atkinson, D.; Atherton, A.; Harvey, I.; Moss, B. Effects of Simulated Climate Warming on Macrophytes in Freshwater Microcosm Communities. Aquat. Bot. 2002, 74, 71–83. [Google Scholar] [CrossRef]
- Van Doorslaer, W.; Stoks, R.; Jeppesen, E.; De Meester, L. Adaptive Microevolutionary Responses to Simulated Global Warming in Simocephalus Vetulus: A Mesocosm Study. Glob. Chang. Biol. 2007, 13, 878–886. [Google Scholar] [CrossRef]
- Moore, M.V.; Folt, C.L.; Stemberger, R.S. Consequences of Elevated Temperatures for Zoo-Plankton Assemblages in Temperate Lakes. Arch. Hydrobiol. 1996, 135, 289–319. [Google Scholar]
- Whiteside, M.C. Danish Chydorid Cladocera: Modern Ecology and Core Studies. Ecol. Monogr. 1970, 40, 79–118. [Google Scholar] [CrossRef]
- Amoros, C.; Jacquet, C. The Dead-Arm Evolution of River Systems: A Comparison between the Information Provided by Living Copepoda and Cladocera Populations and by Bosminidae and Chydroidae Remains. In Cladocera; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar] [CrossRef]
- Frey, D.G. On the Plurality of Chydorus Sphaericus (O. F. Müller) (Cladocera, Chydoridae), and Designation of a Neotype from Sjaelsø, Denmark. Hydrobiologia 1980, 69, 82–123. [Google Scholar] [CrossRef]
- Kutikova, L.A.; Fernando, C.H. Brachionus Calyciflorus Pallas (Rotatoria) in Inland Waters of Tropical Latitudes Brachionus Calyciflorus Tropical in Waters. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1995, 80, 429–441. [Google Scholar] [CrossRef]
- May, L. Rotifer Occurrence in Relation to Water Temperature in Loch Leven, Scotland. In Biology of Rotifers; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar] [CrossRef]
- De Eyto, E.; Irvine, K. The Response of Three Chydorid Species to Temperature, PH and Food. Hydrobiologia 2001, 459, 165–172. [Google Scholar] [CrossRef]
- Paul, R.J.; Lamkemeyer, T.; Maurer, J.; Pinkhaus, O.; Pirow, R.; Seidl, M.; Zeis, B. Thermal Acclimation in the Microcrustacean Daphnia: A Survey of Behavioural, Physiological and Biochemical Mechanisms. J. Therm. Biol. 2004, 29, 655–662. [Google Scholar] [CrossRef]
- Yampolsky, L.Y.; Schaer, T.M.M.; Ebert, D. Adaptive Phenotypic Plasticity and Local Adaptation for Temperature Tolerance in Freshwater Zooplankton. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132744. [Google Scholar] [CrossRef] [Green Version]
- Donze, M. Measurements of the Effect of Heating on Survival and Growth of Natural Plankton Populations. Int. Ver. Theor. Angew. Limnol. Verh. 1978, 20, 1822–1826. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The Evolutionary and Ecological Role of Heat Shock Proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [Green Version]
- Zingel, P.; Cremona, F.; Nõges, T.; Cao, Y.; Neif, É.M.; Coppens, J.; Işkın, U.; Lauridsen, T.L.; Davidson, T.A.; Søndergaard, M.; et al. Effects of Warming and Nutrients on the Microbial Food Web in Shallow Lake Mesocosms. Eur. J. Protistol. 2018, 64, 1–12. [Google Scholar] [CrossRef]
- Jeppesen, E.; Audet, J.; Davidson, T.A.; Neif, É.M.; Cao, Y.; Filiz, N.; Lauridsen, T.L.; Larsen, S.E.; Beklioğlu, M.; Søndergaard, M. Heat Wave Effects on Nutrients, Oxygen Metabolism and Bacterioplankton Production in Shallow Lake Mesocosms after 11 Years of Differential Warming at Contrasting Nutrient Loadings. Water. under review.
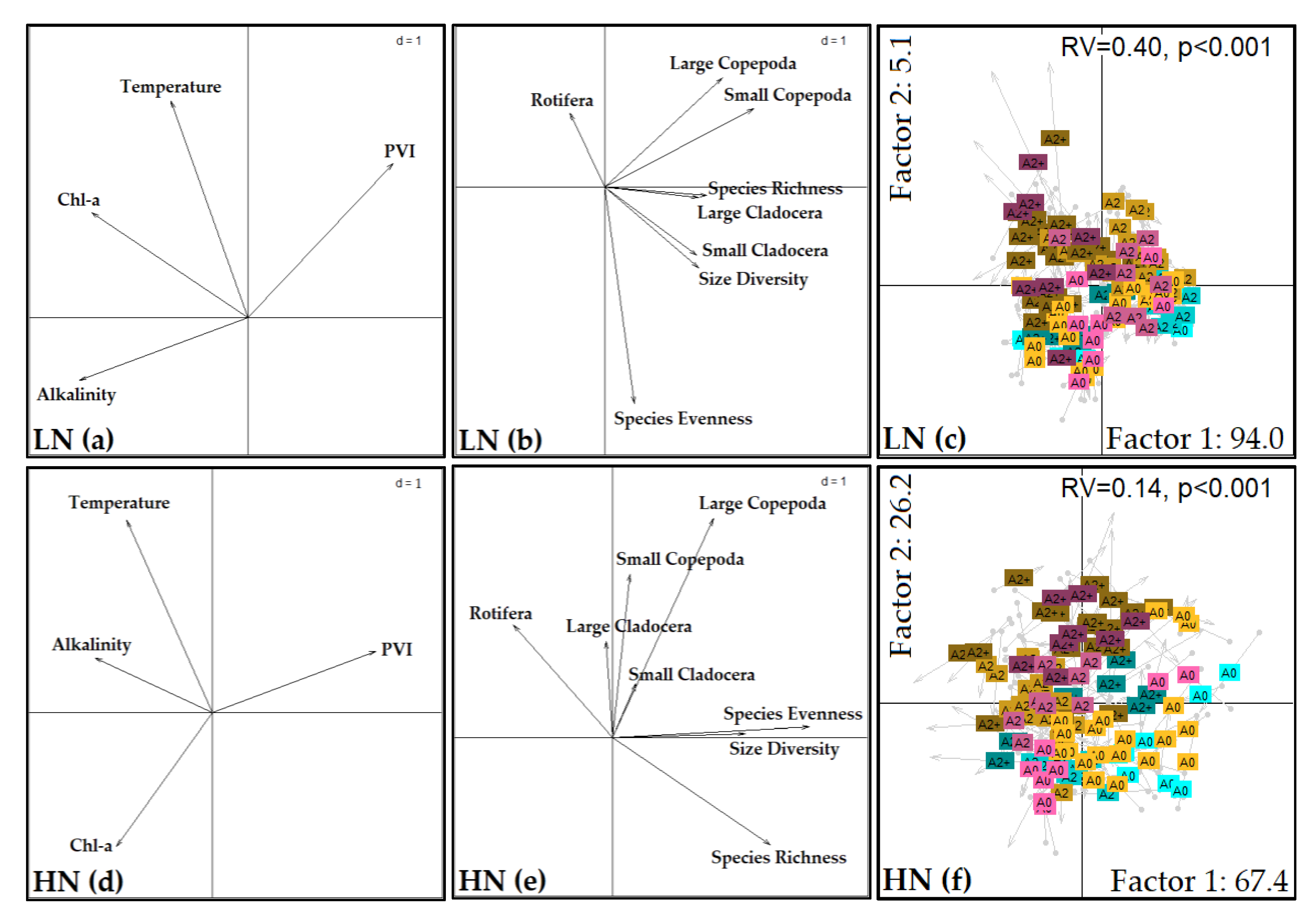
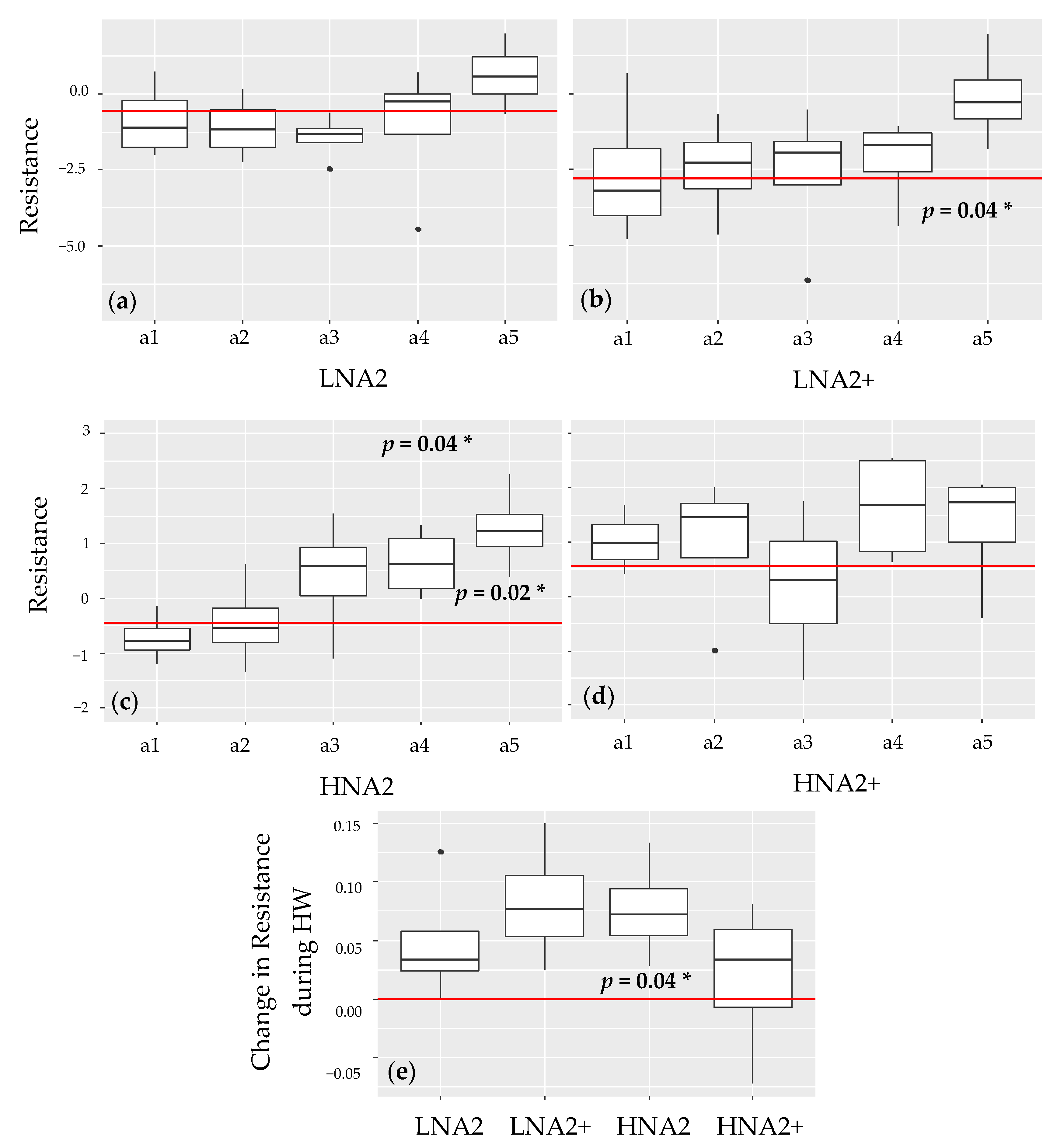
| Low Nutrient (LN) | High Nutrient (HN) | |||||
|---|---|---|---|---|---|---|
| A0 | A2 | A2+ | A0 | A2 | A2+ | |
| TP (mg/L) | 0.013 ± 0.002 | 0.009 ± 0.0003 | 0.014 ± 0.002 | 0.306 ± 0.06 | 0.570 ± 0.06 | 0.338 ± 0.06 |
| TN (mg/L) | 0.31 ± 0.05 | 0.19 ± 0.02 | 0.35 ± 0.06 | 2.83 ± 0.48 | 3.43 ± 0.37 | 2.13 ± 0.25 |
| Model | Total Zoo-Plankton | Large Cladocera | Small Cladocera | Large Copepoda | Small Copepoda | Rotifera | Size Diversity | Zoo/Phyto | Taxon Richness | Taxon Evenness | Taxon Diversity | Functional Group Diversity | Functional Group Evenness | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEFORE | LNA0vsHNA0 (Nutrient effect) | ns | 0.001 (−) | ns | ns | ns | <0.001 (+) | ns | 0.02 (−) | 0.01 (+) | ns | ns | 0.01 (−) | 0.01 (−) |
| LNA0vsLNA2 | ns | ns | 0.001 (+) | ns | 0.03 (+) | ns | ns | ns | ns | ns | ns | ns | ns | |
| LNA0vsLNA2+ | ns | ns | ns | ns | ns | ns | ns | ns | ns | 0.048 (+) | ns | ns | ns | |
| HNA0vsHNA2 | ns | ns | ns | ns | ns | ns | ns | 0.09 (−) | 0.04 (−) | ns | ns | ns | ns | |
| HNA0vsHNA2+ | 0.07 (+) | ns | ns | ns | ns | ns | ns | ns | 0.08 (−) | ns | ns | ns | ns | |
| A2 * Nutrient | ns | NA | ns | ns | 0.04 (−) | NA | NA | ns | NA | NA | NA | NA | NA | |
| A2+ * Nutrient | 0.04 (+) | NA | ns | ns | ns | NA | NA | ns | NA | NA | NA | NA | NA | |
| DURING | LNA0vsHNA0 (Nutrient Effect) | ns | <0.001 (−) | 0.002 (+) | ns | ns | <0.001 (+) | <0.001 (−) | 0.03 (−) | 0.001 (+) | 0.001 (−) | 0.02 (−) | 0.01 (−) | 0.004 (−) |
| LNA0vsLNA2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| LNA0vsLNA2+ | ns | ns | ns | ns | 0.07 (−) | 0.01 (+) | <0.001 (−) | 0.045(−) | ns | 0.04 (−) | 0.02 (−) | 0.03 (−) | 0.03 (−) | |
| HNA0vsHNA2 | ns | ns | ns | ns | ns | ns | ns | ns | 0.06 (−) | ns | ns | ns | ns | |
| HNA0vsHNA2+ | 0.004 (+) | ns | ns | ns | 0.03 (+) | ns | ns | ns | ns | ns | ns | ns | ns | |
| A2 * Nutrient | ns | NA | NA | ns | ns | NA | ns | ns | 0.02 (−) | NA | NA | ns | NA | |
| A2+ * Nutrient | 0.01 (+) | NA | NA | 0.04 (+) | 0.004 (+) | NA | ns | 0.03 (+) | ns | NA | NA | 0.03 (+) | NA | |
| AFTER | LNA0vsHNA0 (Nutrient Effect) | ns | <0.001 (−) | ns | ns | ns | <0.001(+) | <0.001 (−) | 0.005 (−) | <0.001 (+) | <0.001 (−) | 0.002 (−) | 0.02 (−) | 0.02 (−) |
| LNA0vsLNA2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | 0.07 (+) | |
| LNA0vsLNA2+ | ns | 0.03 (−) | ns | ns | ns | ns | 0.004 (−) | 0.02 (−) | ns | 0.02 (−) | 0.01 (−) | ns | ns | |
| HNA0vsHNA2 | ns | ns | ns | ns | ns | ns | ns | ns | 0.03 (−) | ns | ns | ns | ns | |
| HNA0vsHNA2+ | 0.002 (+) | ns | ns | 0.01 (+) | 0.05 (+) | ns | 0.07 (+) | 0.003 (+) | 0.005 (−) | ns | ns | ns | ns | |
| A2 * Nutrient | ns | ns | ns | ns | ns | NA | ns | ns | 0.06 (−) | ns | ns | ns | ns | |
| A2+ * Nutrient | 0.001(+) | 0.009 (+) | 0.04 (+) | 0.002 (+) | 0.04 (+) | NA | 0.001 (+) | <0.001 (+) | 0.02(−) | 0.02 (+) | 0.04 (+) | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Işkın, U.; Filiz, N.; Cao, Y.; Neif, É.M.; Öğlü, B.; Lauridsen, T.L.; Davidson, T.A.; Søndergaard, M.; Tavşanoğlu, Ü.N.; Beklioğlu, M.; et al. Impact of Nutrients, Temperatures, and a Heat Wave on Zooplankton Community Structure: An Experimental Approach. Water 2020, 12, 3416. https://doi.org/10.3390/w12123416
Işkın U, Filiz N, Cao Y, Neif ÉM, Öğlü B, Lauridsen TL, Davidson TA, Søndergaard M, Tavşanoğlu ÜN, Beklioğlu M, et al. Impact of Nutrients, Temperatures, and a Heat Wave on Zooplankton Community Structure: An Experimental Approach. Water. 2020; 12(12):3416. https://doi.org/10.3390/w12123416
Chicago/Turabian StyleIşkın, Uğur, Nur Filiz, Yu Cao, Érika M. Neif, Burak Öğlü, Torben L. Lauridsen, Thomas A. Davidson, Martin Søndergaard, Ülkü Nihan Tavşanoğlu, Meryem Beklioğlu, and et al. 2020. "Impact of Nutrients, Temperatures, and a Heat Wave on Zooplankton Community Structure: An Experimental Approach" Water 12, no. 12: 3416. https://doi.org/10.3390/w12123416
APA StyleIşkın, U., Filiz, N., Cao, Y., Neif, É. M., Öğlü, B., Lauridsen, T. L., Davidson, T. A., Søndergaard, M., Tavşanoğlu, Ü. N., Beklioğlu, M., & Jeppesen, E. (2020). Impact of Nutrients, Temperatures, and a Heat Wave on Zooplankton Community Structure: An Experimental Approach. Water, 12(12), 3416. https://doi.org/10.3390/w12123416









