New Approach to Remove Heavy Metals from Wastewater by the Coagulation of Alginate-Rhamnolipid Solution with Aluminum Sulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
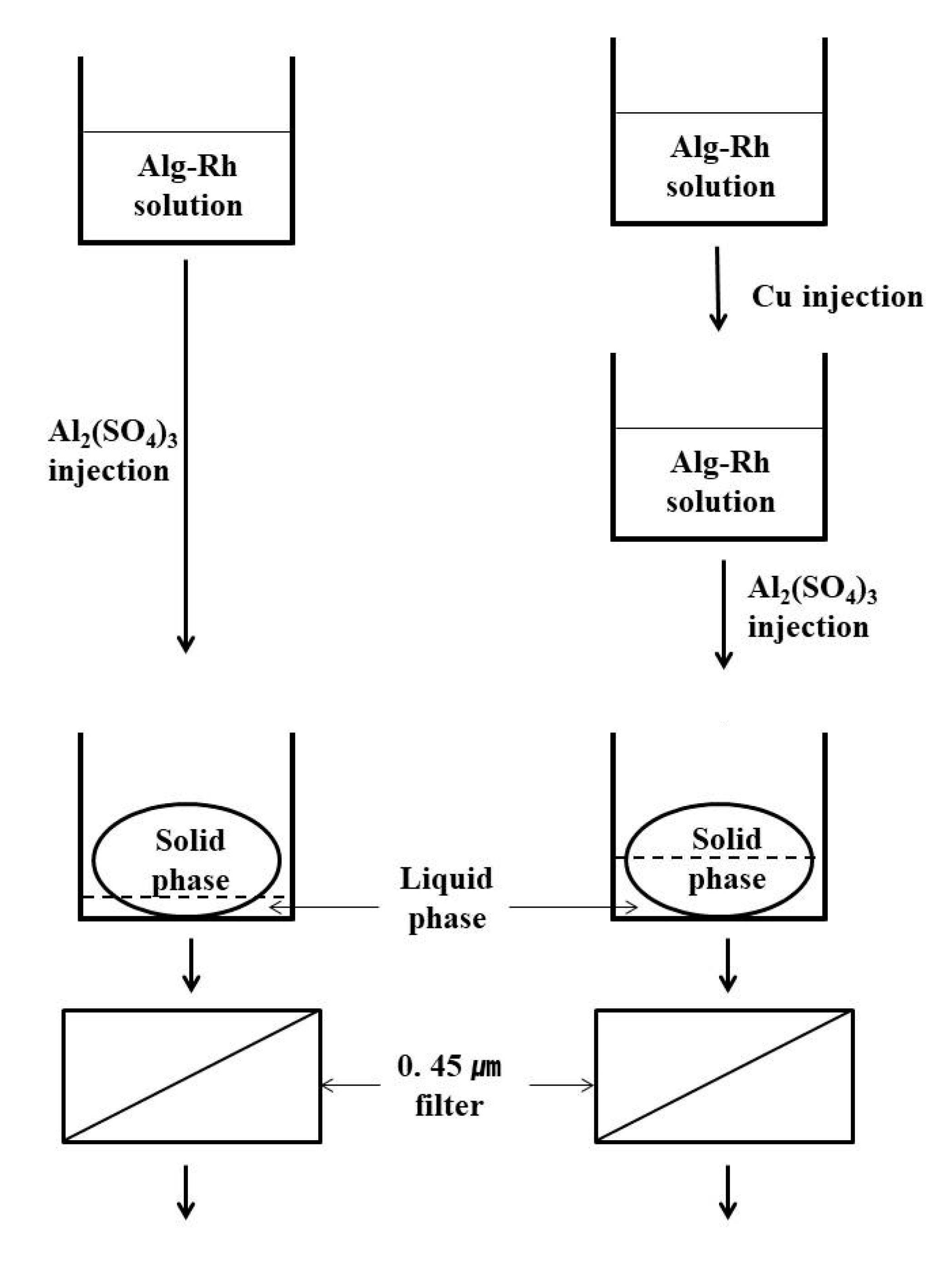
2.2. Experimental Methods
2.3. Analysis
3. Results and Discussion
3.1. Characterization of the Purified Water
3.2. Respective Correlation
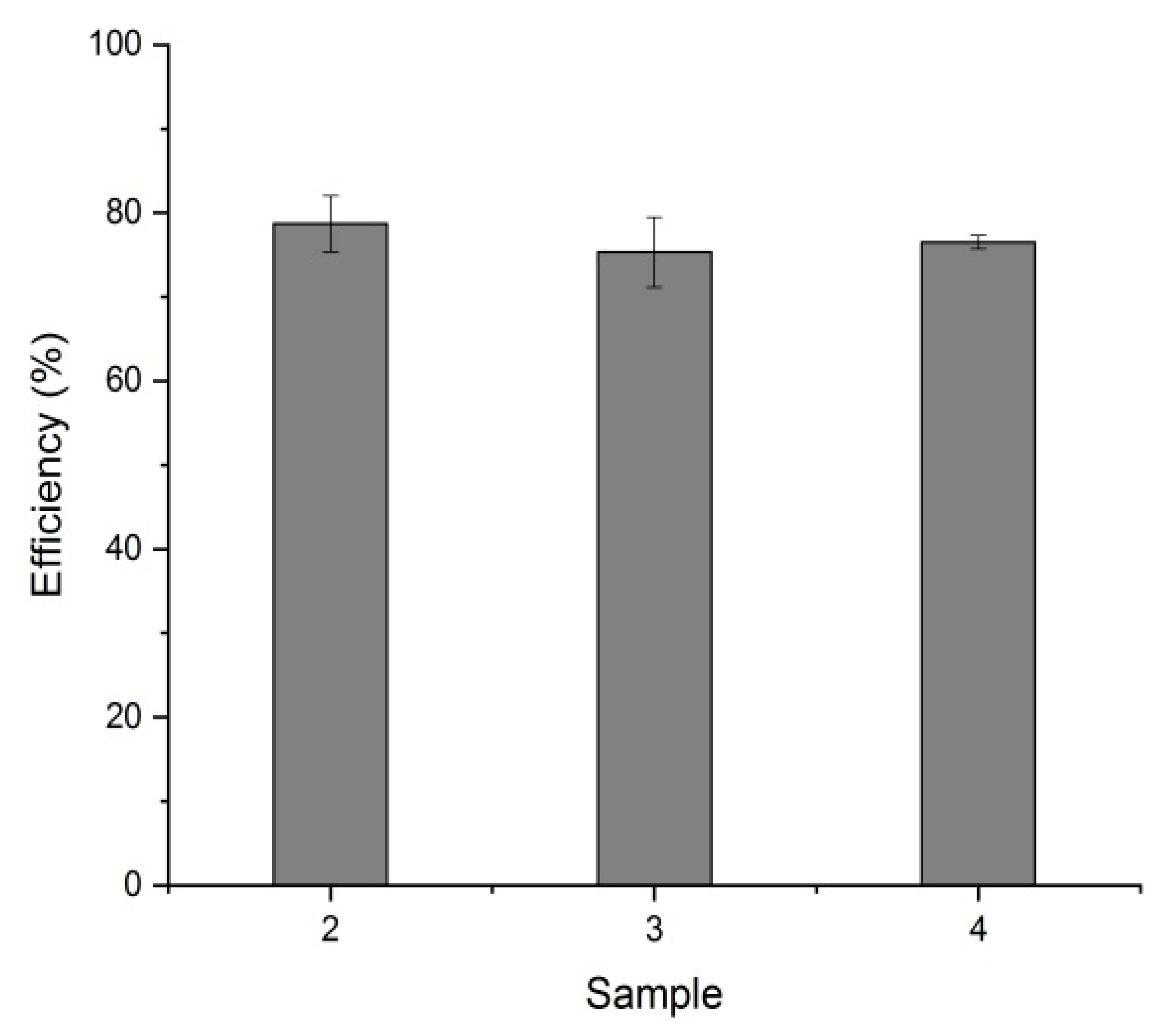
3.3. Efficiency of Heavy Metal Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.; Kim, S.; Sudha, P.N. Preparation and characterization of nano chitosan for treatment wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hidal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Do, V.; Pham, V.; Han, J. Return flow ion concentration polarization desalination: A new way to enhance electromembrane desalination. Water Res. 2019, 159, 501–510. [Google Scholar] [CrossRef]
- Yan, Z.; Pan, J.; Gao, F.; An, Z.; Liu, H.; Huang, Y.; Wang, X. Seawater quality criteria derivation and ecological risk assessment for oil pollution in China. Mar. Pollut. Bull. 2019, 142, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.; Baptista, A.; Cusioli, L.; Seibert, D.; Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative ofremoval by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Chu, Z.; Fan, X.; Wang, W.; Huang, W. Quantitative evaluation of heavy metals’ pollution hazards andestimation of heavy metals’ environmental costs in leachate during foodwaste composting. Waste Manag. 2019, 84, 119–128. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, G.; Ge, X.; Xu, G.; Guan, Y. Novel insights into heavy metal pollution of farmland based on reactiveheavy metals (RHMs): Pollution characteristics, predictive models, andquantitative source apportionment. J. Hazard. Mater. 2018, 360, 32–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Lai, Y.; Wang, L.; Wang, F.; Chen, Z. Predicting future contents of soil heavy metals and related health risks by combining the models of source apportionment, soil metal accumulation and industrial economic theory. Ecotoxicol. Environ. Saf. 2019, 171, 211–221. [Google Scholar] [CrossRef]
- Rai, P.; Lee, S.; Zhang, M.; Tsang, Y.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquaticenvironment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Peterson, B.; Tietböhl, T.; Marqueze, A. Interference of heavy metals present in the water of the Lagoa Tramandaí/RS on the carbohydrate metabolism of the GURI Sea Catfish (G. genidens) and Bay whiff (C. spilopterus). Comp. Biochem. Physiol. Part C 2019, 219, 42–49. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Zahab, A.; Osta, B.; Romanos, D.; Haddad, G. Heavy Metals Accumulation Effects on the Photosynthetic Performance of Geophytes in Mediterranean Reserve. J. King Saud Univ. 2019. [Google Scholar] [CrossRef]
- Meena, R.; Kannah, Y.; Sindhu, J.; Ragavi, J.; Kumar, G.; Gunasekaran, M.; Rajesh, J. Trends and resource recovery in biological wastewater treatment system. Bioresour. Technol. Rep. 2019, 7, 100235. [Google Scholar] [CrossRef]
- Bora, A.; Dutta, R. Removal of metals (Pb, Cd, Cu, Cr, Ni, and Co) from drinking water by oxidation-coagulation-absorption at optimized pH. J. Water Process Eng. 2019, 31, 100839. [Google Scholar] [CrossRef]
- Yun, Y.; Lee, E.; Kim, K.; Han, J. Sulfate reducing bacteria-based wastewater treatment system integrated with sulfide fuel cell for simultaneous wastewater treatment and electricity generation. Chemosphere 2019. [Google Scholar] [CrossRef]
- Kazi, C.; Yamamoto, O. Effectiveness of the sodium alginate as surgical sealant materials. Wound Med. 2019, 24, 18–23. [Google Scholar] [CrossRef]
- Bang, S.; Choi, J.; Cho, K.; Chung, C.; Kang, H.; Hong, S. Simultaneous reduction of copper and toxicity in semiconductor wastewater using protonated alginate beads. Chem. Eng. J. 2016, 288, 525–531. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochem. 2009, 44, 296–301. [Google Scholar] [CrossRef]
- Di Palma, L.; Petrucci, E.; Pietrangeli, B. Environmental effects of using chelating agents in polluted sediment remediation. Bull. Environ. Contam. Toxicol. 2015, 94, 340–344. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Chen, W.; Qu, Y.; Xu, Z.; He, F.; Chen, Z.; Huang, S.; Li, Y. Heavy metal (Cu, Cd, Pb, Cr) washing from river sediment using biosurfactant rhamnolipid. Environ. Sci. Pollut. Res. 2017, 24, 16344–16350. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Seo, Y.; Kim, I.; Han, J. Co-production of biodiesel and alginate from Laminaria japonica. Sci. Total Environ. 2019, 673, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Zeftawy, M.A.; Mulligan, C. Use of rhamnolipid to remove heavy metals from wastewater by micellar-enhanced ultrafiltration (MEUF). Sep. Purif. Technol. 2011, 77, 120–127. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Uzaşçı, S.; Erim, F.B. Removal of Fluoride from Aqueous Solution Using Aluminum Alginate Beads. Clean-Soil Air Water 2014, 43, 724–730. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Zhao, X.; Wang, Z. Highly efficient removal of copper ions from water by using a novel alginate-polyethyleneimine hybrid aerogel. Int. J. Biol. Macromol. 2019, 138, 1079–1086. [Google Scholar] [CrossRef]
- Li, Y.; Xia, B.; Zhao, Q.; Liu, F.; Zhang, P.; Du, Q.; Wang, D.; Li, D.; Wang, Z.; Zia, Y. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. J. Environ. Sci. 2011, 23, 404–411. [Google Scholar] [CrossRef]
- Papageorgiou, S.; Katsaros, F.; Kouvelos, E.; Nolan, J.; Deit, H.; Kanellopoulos, N. Heavy metal sorption by calcium alginate beads from Laminaria digitate. J. Hazard. Mater. B 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Blando, A.; Macias, M.; Cantero, D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- He, X.; Abdoli, L.; Li, H. Participation of copper ions in formation of alginate conditioning layer: Evolved structure and regulated microbial adhesion. Colloids Surf. B Biointerfaces 2018, 162, 220–227. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Wang, J.; Zhu, P.; Zhao, J.; Zhang, C.; Guo, Y.; Jin, X. Thermal degradation and pyrolysis behavior of aluminum alginate investigated by TG-FTIR-MS and Py-GC-MS. Polym. Degrad. Stab. 2015, 118, 59–68. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiaoyan, L.; Bin, L.; Xuegang, L. Fluoride adsorption from aqueous solution by aluminum alginate particles prepared via electrostatic spinning device. Chem. Eng. J. 2014, 256, 306–315. [Google Scholar] [CrossRef]

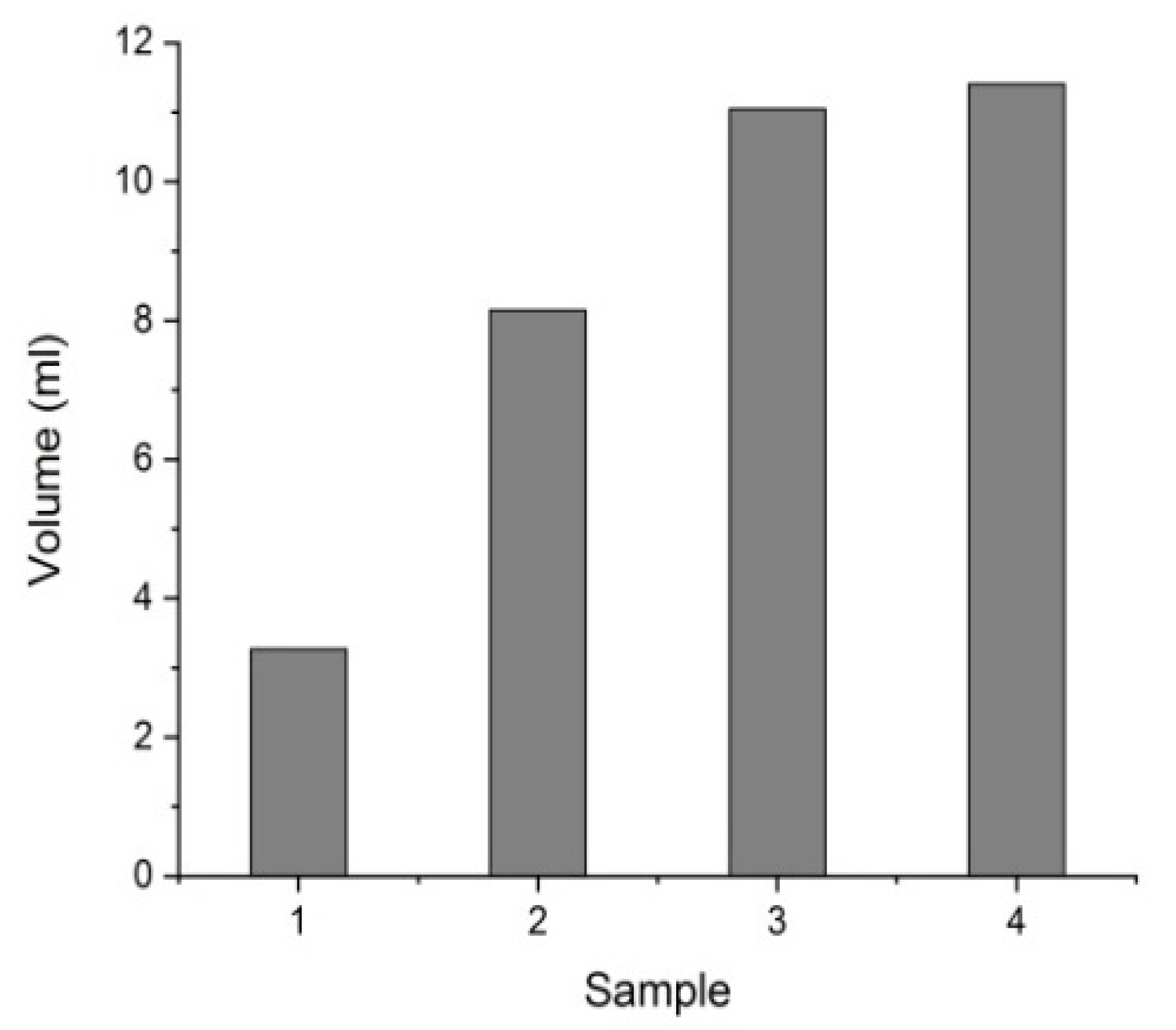

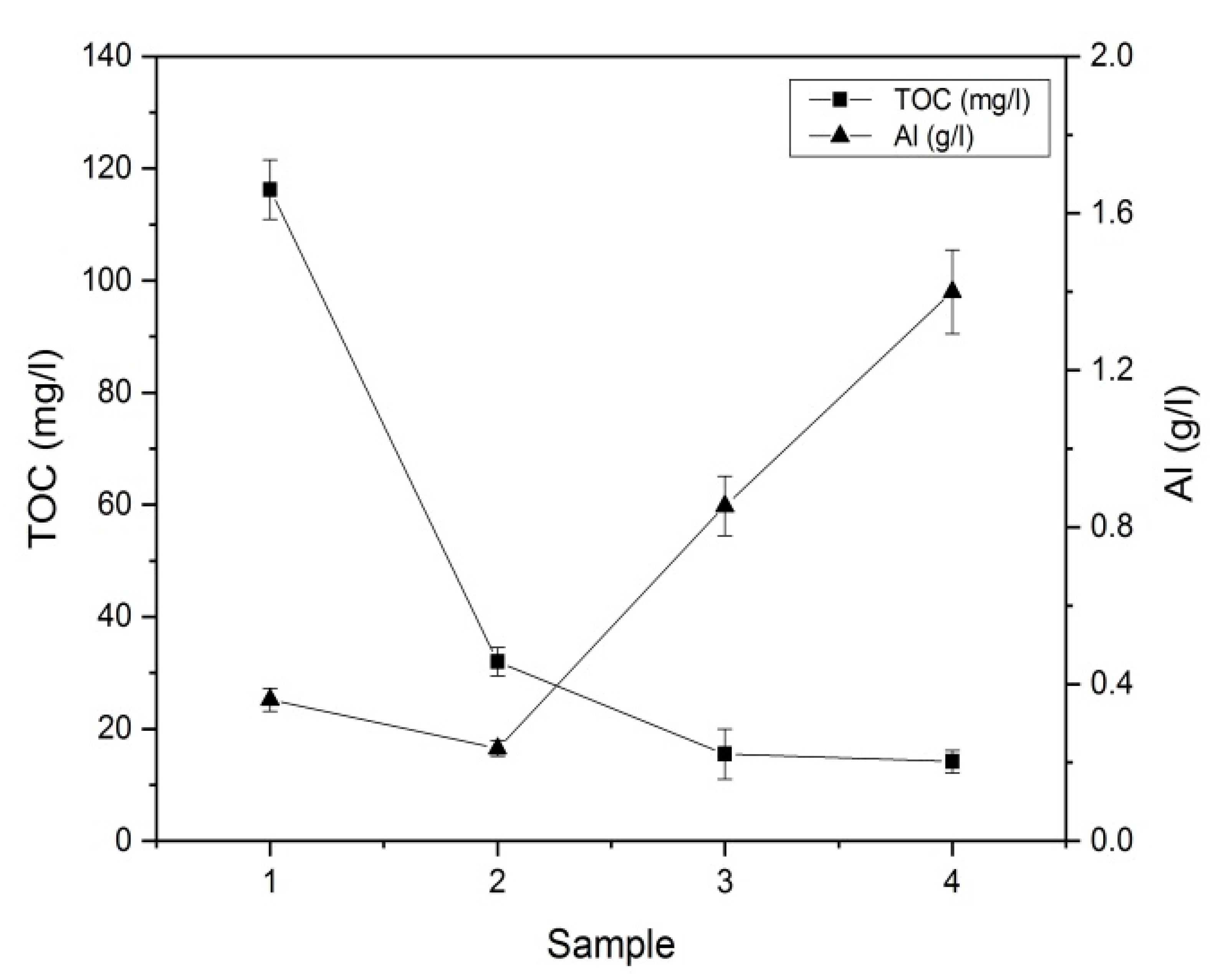

| Sample Number | TOC (mg/L) | SO42− (g/L) |
|---|---|---|
| Sample 1 | 116.3 (±5.3) | 2.244 (±0.190) |
| Sample 2 | 32.00 (±2.5) | 1.573 (±0.078) |
| Sample 3 | 15.50 (±4.4) | 5.340 (±0.184) |
| Sample 4 | 14.17 (±2.0) | 8.976 (±0.734) |
| Specification | TOC | SO42− | Al3+ |
|---|---|---|---|
| R2 | 0.948 | 0.840 | 0.859 |
| p value | 0.004 | 0.094 | 0.089 |
| F value | 0.026 | 0.262 | 0.245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Kim, K. New Approach to Remove Heavy Metals from Wastewater by the Coagulation of Alginate-Rhamnolipid Solution with Aluminum Sulfate. Water 2020, 12, 3406. https://doi.org/10.3390/w12123406
Lee A, Kim K. New Approach to Remove Heavy Metals from Wastewater by the Coagulation of Alginate-Rhamnolipid Solution with Aluminum Sulfate. Water. 2020; 12(12):3406. https://doi.org/10.3390/w12123406
Chicago/Turabian StyleLee, Aleum, and Kyoungrean Kim. 2020. "New Approach to Remove Heavy Metals from Wastewater by the Coagulation of Alginate-Rhamnolipid Solution with Aluminum Sulfate" Water 12, no. 12: 3406. https://doi.org/10.3390/w12123406
APA StyleLee, A., & Kim, K. (2020). New Approach to Remove Heavy Metals from Wastewater by the Coagulation of Alginate-Rhamnolipid Solution with Aluminum Sulfate. Water, 12(12), 3406. https://doi.org/10.3390/w12123406




