Sustainable Exploitation of Dominant Fishes in the Largest Estuary in Southeastern China
Abstract
:1. Introduction
2. Material and Method
2.1. Ethical Statement
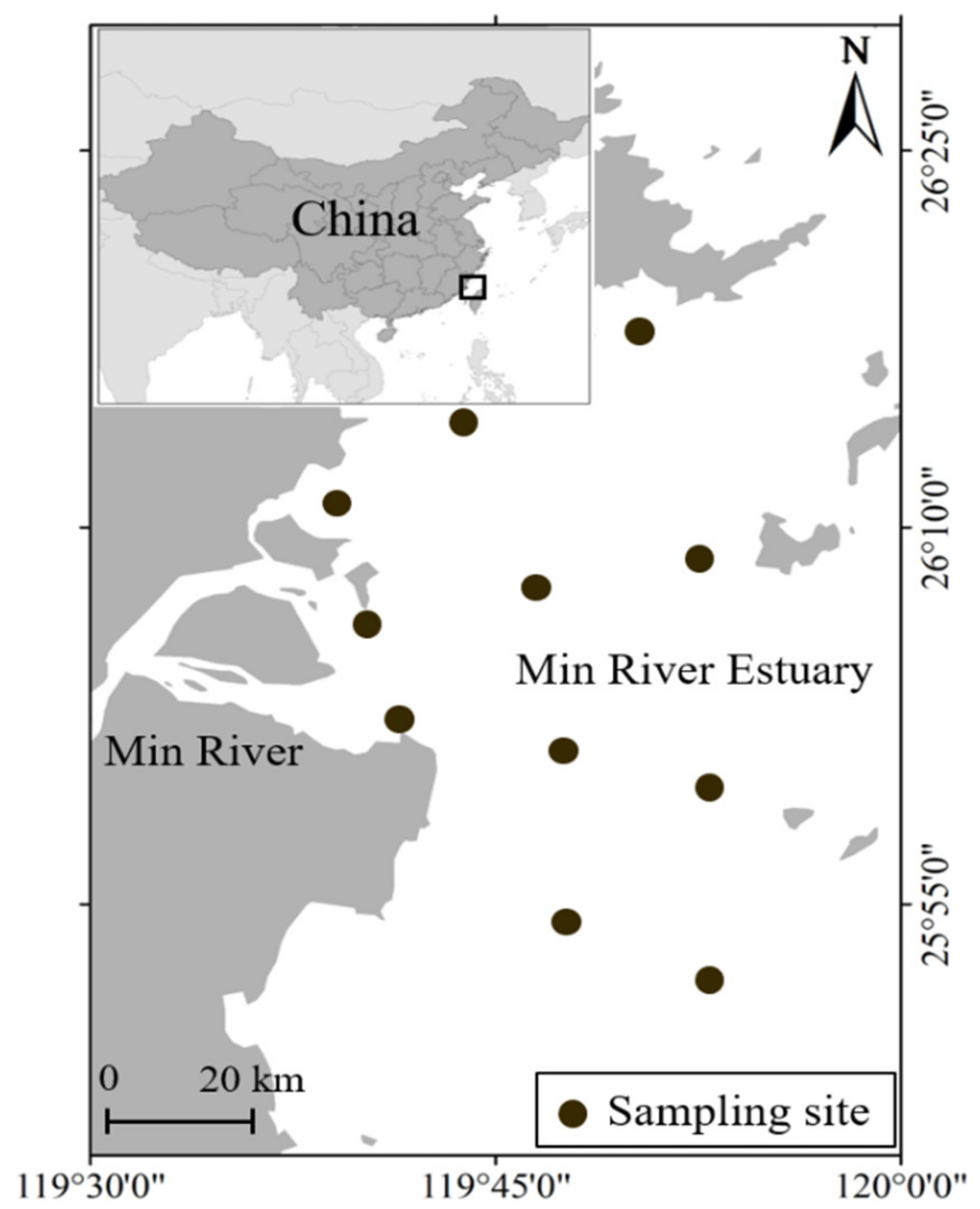
2.2. Study Area, Sampling, and Dominant Species
2.3. LBB Modeling
3. Results
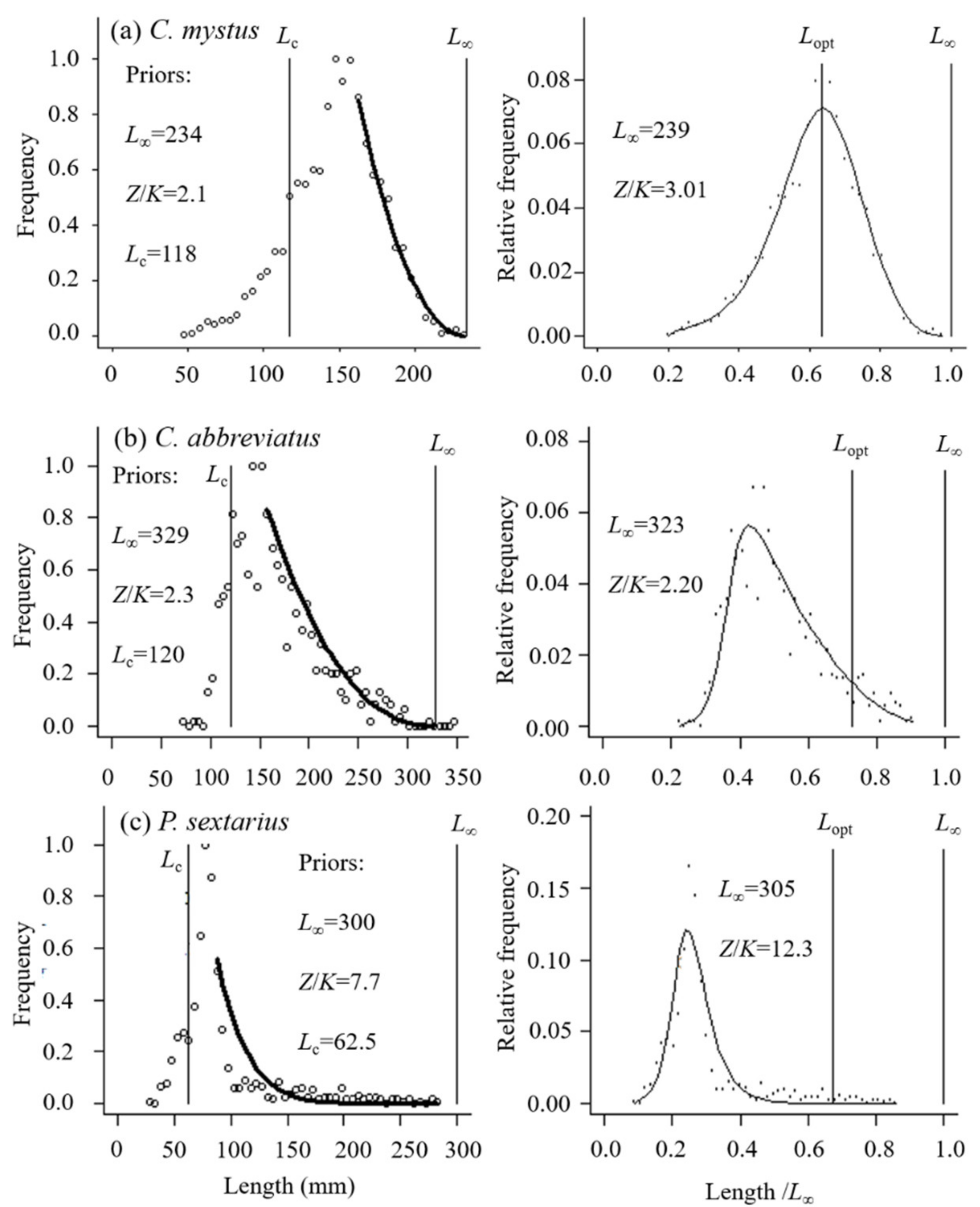
3.1. Relative Importance and Length Frequency of the Fishes
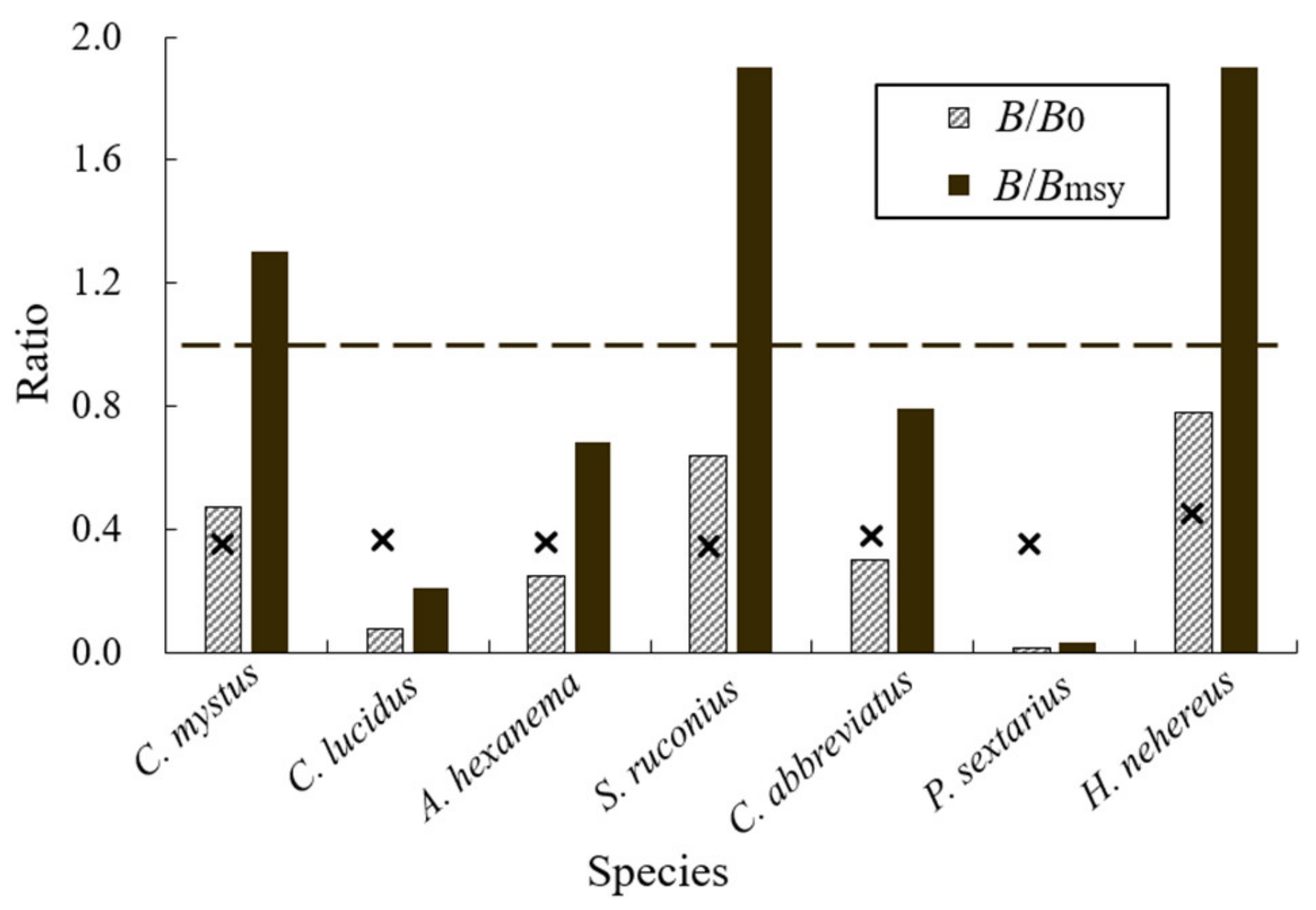
3.2. Current Exploitation Status of the Fishes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Watson, R.; Pauly, D. Systematic distortions in world fisheries catch trends. Nature 2001, 414, 534–536. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Quaas, M.; Matz-Lück, N. Status and rebuilding of European fisheries. Mar. Policy 2018, 93, 159–170. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Wiedenmann, J.; Deroba, J.J. The refined ORCS approach: A catch-based method for estimating stock status and catch limits for data-poor fish stocks. Fish. Res. 2017, 193, 60–70. [Google Scholar] [CrossRef]
- Martell, S.; Froese, R. A simple method for estimating MSY from catch and resilience. Fish Fish. 2013, 14, 504–514. [Google Scholar] [CrossRef]
- Zhou, S.; Punt, A.E.; Ye, Y.; Ellis, N.; Dichmont, C.M.; Haddon, M.; Smith, D.C.; Smith, A.D. Estimating stock depletion level from patterns of catch history. Fish Fish. 2017, 18, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish Fish. 2017, 18, 506–526. [Google Scholar] [CrossRef] [Green Version]
- Thorson, J.T.; Cope, J.M. Catch curve stock-reduction analysis: An alternative solution to the catch equations. Fish. Res. 2015, 171, 33–41. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. On the pile-up effect and priors for Linf and M/K: Response to a comment by Hordyk et al. on “A new approach for estimating stock status from length frequency data”. ICES J. Mar. Sci. 2019, 76, 461–465. [Google Scholar] [CrossRef]
- Ministry of Agriculture of China. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2005–2017. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics; Food and Agriculture Organization of the United Nation: Rome, Italy, 2016. [Google Scholar]
- Zhang, W.; Liu, M.; Sadovy De Mitcheson, Y.; Cao, L.; Leadbitter, D.; Newton, R.; Little, D.C.; Li, S.; Yang, Y.; Chen, X.; et al. Fishing for feed in China: Facts, impacts and implications. Fish Fish. 2019, 21, 47–62. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F.J. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Zhai, L.; Pauly, D. Yield-per-recruit, utility-per-recruit, and relative biomass of 21 exploited fish species in China’s coastal seas. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Li, J.; Kang, B. Dominant species drive seasonal dynamics of the fish community in the Min estuary, China. Oceanol. Hydrobiol. Stud. 2020, 49, 34–48. [Google Scholar] [CrossRef]
- Wang, J.Q.; Huang, L.M.; Li, J.; Zhang, Y.Z.; Zhu, G.P.; Chen, X.J. Length-weight relationships of 45 fish species in the Min River Estuary, East China Sea. J. Appl. Ichthyol. 2016, 32, 131–133. [Google Scholar] [CrossRef]
- Zhu, D. Chinese Water Dictionary, 1st ed.; Qingdao Publishing Press: Qingdao, China, 2007; pp. 457–464. [Google Scholar]
- Feng, C.; He, X.B.; Zhao, C.X.; Li, J.; Kang, B. Functional diversity of fishes in the Minjiang Estuary, Southeast China. Chin. J. Appl. Ecol. 2019, 30, 3589–3595. (In Chinese) [Google Scholar]
- Xu, Z.L. Comparison of fish density between the Minjiang Estuary and Xinghua Bay during spring and summer. J. Fish. China 2010, 34, 1395–1403. (In Chinese) [Google Scholar]
- He, X.B.; Li, J.; Shen, C.; Shi, Y. The breadth and overlap of ecological niche of major fish species in the Minjiang River Estuary, China. Chin. J. Appl. Ecol. 2018, 29, 3085–3092. (In Chinese) [Google Scholar]
- He, X.; Li, J.; Shen, C.; Shi, Y.; Feng, C.; Guo, J.; Yan, Y.; Kang, B. Length-weight relationship and population dynamics of Bombay duck (Harpadon nehereus) in the Min River Estuary, East China Sea. Thalass. Int. J. Mar. Sci. 2018, 35, 253–261. [Google Scholar] [CrossRef]
- Guo, J.H. Research on Growth Characteristic and Resource Dynamic of Four Dominant Fishes in the Min River Estuary, East China Sea. Master’s Thesis, Jimei University, Xiamen, China, 2019. [Google Scholar]
- Huang, L.M. Study on Fishery Resources and Fish Diversity in Minjiang River Estuary and Jiulong River Estuary and Their Adjacent Waters. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2011. [Google Scholar]
- The Editorial Board of Fujian Local Chronicles. Fujian Province Annals: Water Conservancy; China Social Sciences Publishing House: Beijing, China, 1999. [Google Scholar]
- Fishes of Fujian Province Editorial Committee. The Fishes of Fujian Province; Fujian Science and Technology Press: Fuzhou, China, 1984. [Google Scholar]
- Pianka, E.R. Ecology of the Agamid lizard Amphibolurus isolepis in Western Australia. Copeia 1971, 527–536. [Google Scholar] [CrossRef]
- Cheng, J.S. The structure and diversity of demersal fish communities in winter in the East China Sea and the Yellow Sea. Mar. Fish. Res. 2000, 21, 1–8. (In Chinese) [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Baldé, B.S.; Fall, M.; Kantoussan, J.; Sow, F.N.; Diouf, M.; Brehmer, P. Fish-length based indicators for improved management of the sardinella fisheries in Senegal. Reg. Stud. Mar. Sci. 2019, 31, 100801. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Holt, S.J. The evaluation of fisheries resources by the dynamic analysis stocks, and notes on the time factors involved. Int. Comm. Northwest Atl. Fish. Spec. Publ. 1958, 1, 77–95. [Google Scholar]
- Froese, R.; Winker, H.; Gascuel, D.; Sumaila, U.R.; Pauly, D. Minimizing the impact of fishing. Fish Fish. 2016, 17, 785–802. [Google Scholar] [CrossRef]
- Beverton, R.J.; Holt, S.J. Manual of Methods for Fish Stock Assessment: Part 2-Tables of Yield Functions; (Fisheries Report No. 38); FAO: Rome, Italy, 1966. [Google Scholar]
- Xue, L.J.; Zhou, Y.D.; Xu, K.D.; Zhang, H.D. Estimation of biological parameter, biomass, sustaining yield for Coilia mystus in the offshore of Zhoushan. J. Fujian Fish. 2011, 33, 18–23. (In Chinese) [Google Scholar]
- Masuda, H.; Amaoka, K.; Arage, C.; Uyeno, T.; Yoshino, T. The Fishes of the Japanese Archipelago; Tokai University Press: Tokyo, Japan, 1984; p. 437. [Google Scholar]
- Wang, L.J.; You, F.; Wang, Q.X.; Wu, Z.H.; Liu, M.X. Length-weight and length-length relationships of 11 fish species from Zhimai River estuary, China. J. Appl. Ichthyol. 2015, 31, 435–436. [Google Scholar] [CrossRef]
- James, P.S.; Fisher, W.; Bianchi, G. FAO Species Identification Sheets for Fishery Purposes; Western Indian Ocean (Fishing Area 51); FAO: Rome, Italy, 1984; Volume 2. [Google Scholar]
- Sommer, C.; Schneider, W.; Poutiers, J.M. FAO Species Identification Field Guide for Fishery Purposes. The Living Marine Resources of Somalia; FAO: Rome, Italy, 1996; Volume 376. [Google Scholar]
- Palomares, M.L.D.; Froese, R.; Derrick, B.; Noel, S.L.; Tsui, G.; Woroniak, J.; Pauly, D. A Preliminary Global Assessment of the Status of Exploited Marine Fish and Invertebrate Populations. 2018. Available online: https://oceanrep.geomar.de/43547/1/OceanaReportFinal.pdf (accessed on 10 September 2020).
- Zhang, C.; Seo, Y.; Kang, H.; Lim, J. Exploitable carrying capacity and potential biomass yield of sectors in the East China Sea, Yellow Sea, and East Sea/Sea of Japan large marine ecosystems. Deep Sea Res. Top. Stud. Oceanogr. 2019, 163, 16–28. [Google Scholar] [CrossRef]
- Kang, B.; Liu, M.; Huang, X.; Li, J.; Yan, Y.; Han, C.; Chen, S. Fisheries in Chinese seas: What can we learn from controversial official fisheries statistics? Rev. Fish Biol. Fish. 2018, 28, 503–519. [Google Scholar] [CrossRef]
- Shan, X.; Sun, P.; Jin, X.; Li, X.; Dai, F. Long-term changes in fish assemblage structure in the Yellow River Estuary ecosystem, China. Mar. Coast. Fish. 2013, 5, 65–78. [Google Scholar] [CrossRef]
- Cheng, J.S.; Yu, L.F. The change of structure and diversity of demersal fish communities in the Yellow Sea and East China Sea in winter. J. Fish. China 2004, 1, 29–35. (In Chinese) [Google Scholar]
- Li, Z.; Shan, X.; Jin, X.; Dai, F. Long-term variations in body length and age at maturity of the small yellow croaker (Larimichthys polyactis Bleeker, 1877) in the Bohai Sea and the Yellow Sea, China. Fish. Res. 2011, 110, 67–74. [Google Scholar] [CrossRef]
- Guan, W.B.; Chen, H.H.; He, W.H. Reproductive characteristics and condition status of Coilia mystus (Linnaeus) in the Changjiang River Estuary. Process Fish. Sci. 2011, 32, 1–9. [Google Scholar]
- Xie, Y.; Huang, L.M.; Li, J.; Li, W.W.; Zhang, Y.Z. Resource assessment of Clupeiformes fishes in Fujian coastal waters. Mar. Fish. 2012, 34, 285–294. (In Chinese) [Google Scholar]
- Ghosh, S. Fishery, reproductive biology and diet characteristics of Bombay duck Harpadon nehereus from the Saurashtra coast. Indian J. Mar. Sci. 2014, 43, 418–426. [Google Scholar]
- Bapat, S.; Banerji, S.; Bal, D. Observations on the biology of Harpodon nehereus (Hamilton). J. Zool. Soc. India 1951, 3, 341–356. [Google Scholar]
- Bapat, S.V. The Bombay duck, Harpodon nehereus (Ham.). Bull. Cent. Mar. Fish. Res. Inst. 1970, 21, 1–87. [Google Scholar]
- Laga, A.; Affandi, R.; Muchsin, I.; Kamal, M.M. Growth and exploitation rate of the Bombay duck (Harpodon nehereus Hamilton, 1822) (Fish: Synodontidae) in Tarakan Island waters, Indonesia. Int. J. Sci. Basic Appl. Res. 2015, 22, 341–353. [Google Scholar]
- Lin, L.S. Spatial distribution and environmental characteristics of Harpodon nehereus in the East China Sea region. J. Shanghai Ocean Univ. 2009, 18, 66–71. (In Chinese) [Google Scholar]
- Luo, H.D. Study of Main Biology Character and Analysis of Resources Status on the Harpodon nehereus. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2012. [Google Scholar]
- Alverson, D.L.; Pereyra, W.T. Demersal fish explorations in the northeastern Pacific Ocean-an evaluation of exploratory fishing methods and analytical approaches to stock size and yield forecasts. J. Fish. Res. Can. 1969, 26, 1985–2001. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Gascuel, D. Size still matters. A response to Svedäng (2013): Size matters: Ne quid nimis. Fish. Res. 2015, 164, 329–330. [Google Scholar] [CrossRef]
- McConnaughey, R.A.; Hiddink, J.G.; Jennings, S.; Pitcher, C.R.; Kaiser, M.J.; Suuronen, P.; Sciberras, M.; Rijnsdorp, A.D.; Collie, J.S.; Mazor, T.; et al. Choosing best practices for managing impacts of trawl fishing on seabed habitats and biota. Fish Fish. 2019, 21, 319–337. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Ma, H.; Ma, L.; Cui, H.; Ma, Q. Development and characterization of 19 microsatellite markers for Collichthys lucidus. Conserv. Genet. Resour. 2011, 3, 503–506. [Google Scholar] [CrossRef]
- Liang, C.; Pauly, D. Growth and mortality of exploited fishes in China’s coastal seas and their uses for yield-per-recruit analyses. J. Appl. Ichthyol. 2017, 33, 746–756. [Google Scholar] [CrossRef]

| Species | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Coilia mystus | 516.0 | 6.9 | 506.2 | 6086.6 |
| Collichthys lucidus | 709.5 | 5.7 | 579.1 | 3693.1 |
| Amblychaeturichthys hexanema | 867.5 | 12.0 | 703.4 | 506.2 |
| Secutor ruconius | 1866.3 | 831.1 | 224.1 | 231.2 |
| Cynoglossus abbreviatus | 1440.1 | 223.8 | 334.3 | 1878.5 |
| Polydactylus sextarius | - | 6502.4 | 3208.8 | - |
| Harpodon nehereus | 155.2 | 2222.5 | 4211.9 | 2413.6 |
| Variables | Coilia mystus | Collichthys lucidus | Amblychaeturichthys hexanema | Secutor ruconius | Cynoglossus abbreviatus | Polydactylus sextarius | Harpodon nehereus |
|---|---|---|---|---|---|---|---|
| quantity | 2104 | 1355 | 1175 | 968 | 897 | 805 | 487 |
| length range | 47–235 | 22–205 | 36–190 | 21–107 | 72–350 | 29–284 | 50–350 |
| L∞ by user (mm) | 234.0 [16,35] | 213.0 [36] | 174.0 [37] | 101.0 [38] | 329.0 [16] | 300.0 [39] | 323.0 [16,21] |
| L∞ by LBB (mm) | 239.0 | 214.0 | 180.0 | 104.0 | 323.0 | 305.0 | 314.0 |
| Lc (mm) | 147.6 | 174.0 | 83.4 | 72.0 | 117.6 | 65.0 | 156.0 |
| M/K | 1.71 | 1.42 | 1.50 | 2.07 | 1.10 | 1.30 | 1.27 |
| F/K | 1.30 | 17.00 | 2.10 | 0.95 | 1.10 | 11.00 | 0.67 |
| Z/K | 3.01 | 18.40 | 3.60 | 3.02 | 2.20 | 12.30 | 1.94 |
| F/M | 0.78 | 12.00 | 1.40 | 0.48 | 0.98 | 7.40 | 0.55 |
| E (i.e., F/Z) | 0.43 | 0.92 | 0.58 | 0.31 | 0.50 | 0.89 | 0.35 |
| Lmean (mm) | 168.0 | 185.0 | 107.0 | 77.5 | 181.0 | 127.0 | 202.0 |
| Lopt (mm) | 152.0 | 144.0 | 119.0 | 62.0 | 235.0 | 205.0 | 222.0 |
| Lmean/Lopt | 1.10 | 1.30 | 0.88 | 1.30 | 0.72 | 0.47 | 0.89 |
| Lc_opt (mm) | 123.0 | 140.0 | 103.0 | 48.0 | 196.0 | 197.0 | 174.0 |
| Lc/Lc_opt | 1.20 | 1.20 | 0.81 | 1.50 | 0.60 | 0.33 | 0.90 |
| B/B0 | 0.47 | 0.08 | 0.25 | 0.64 | 0.30 | 0.01 | 0.51 |
| B/B0 (when F = M) | 0.357 | 0.367 | 0.364 | 0.345 | 0.383 | 0.368 | 0.377 |
| B/Bmsy | 1.30 | 0.21 | 0.68 | 1.90 | 0.79 | 0.03 | 1.40 |
| Y′/R | 0.0300 | 0.0180 | 0.0440 | 0.0140 | 0.0640 | 0.0007 | 0.0480 |
| status [40] | healthy | collapsed | overfished | healthy | overfished | collapsed | healthy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lin, L.; Li, Y.; Xing, Y.; Kang, B. Sustainable Exploitation of Dominant Fishes in the Largest Estuary in Southeastern China. Water 2020, 12, 3390. https://doi.org/10.3390/w12123390
Wang L, Lin L, Li Y, Xing Y, Kang B. Sustainable Exploitation of Dominant Fishes in the Largest Estuary in Southeastern China. Water. 2020; 12(12):3390. https://doi.org/10.3390/w12123390
Chicago/Turabian StyleWang, Linlong, Li Lin, Yuan Li, Yankuo Xing, and Bin Kang. 2020. "Sustainable Exploitation of Dominant Fishes in the Largest Estuary in Southeastern China" Water 12, no. 12: 3390. https://doi.org/10.3390/w12123390





