Groundwater Circulation in the Xianshui River Fault Region: A Hydrogeochemical Study
Abstract
:1. Introduction
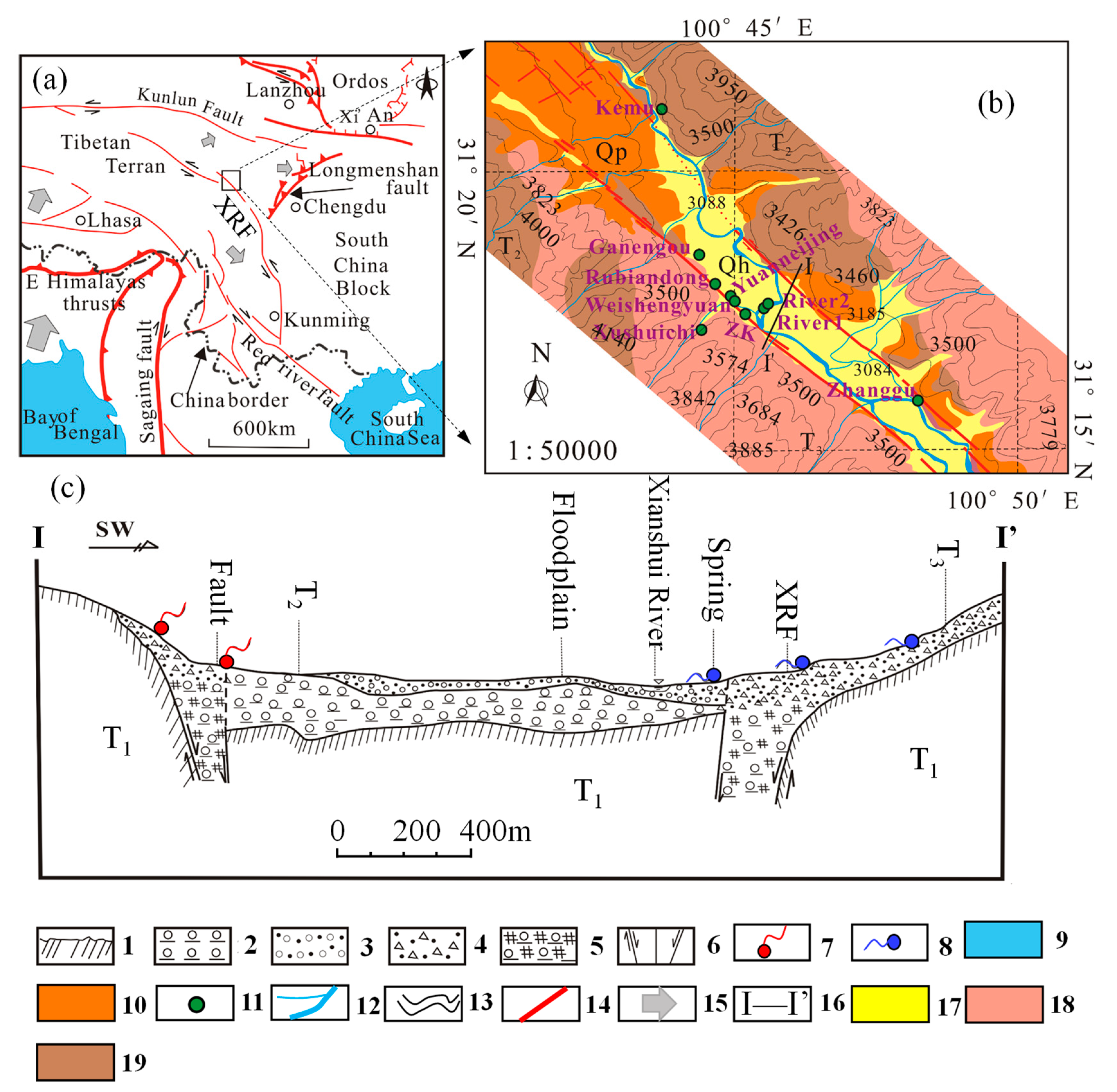
2. Study Area and its Hydrogeological Setting
3. Sampling and Analysis Methods
4. Results
4.1. Hydrochemical Characteristics
4.2. Stable Isotopic Compositions
4.3. Radium Isotopes
5. Discussion
5.1. Isotopic Characteristics Associated with Different Groups
5.2. Hydrogeochemical Characteristics Associated with Different Groups
5.3. Characteristics of the Radium Isotopes During the Circulation of Groundwater
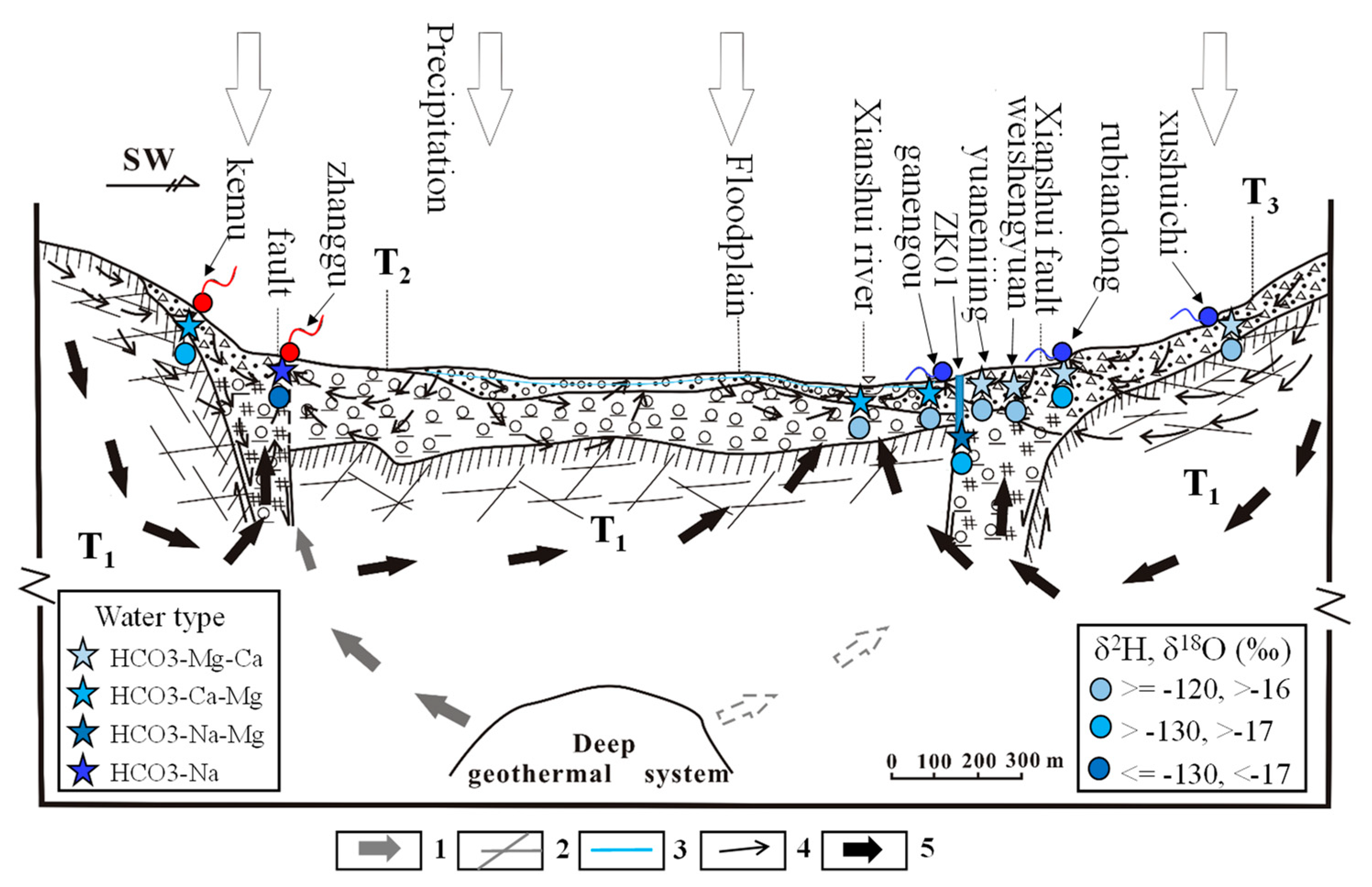
5.4. Groundwater Flow Systems Developed in the Study Area
6. Conclusions
- (1)
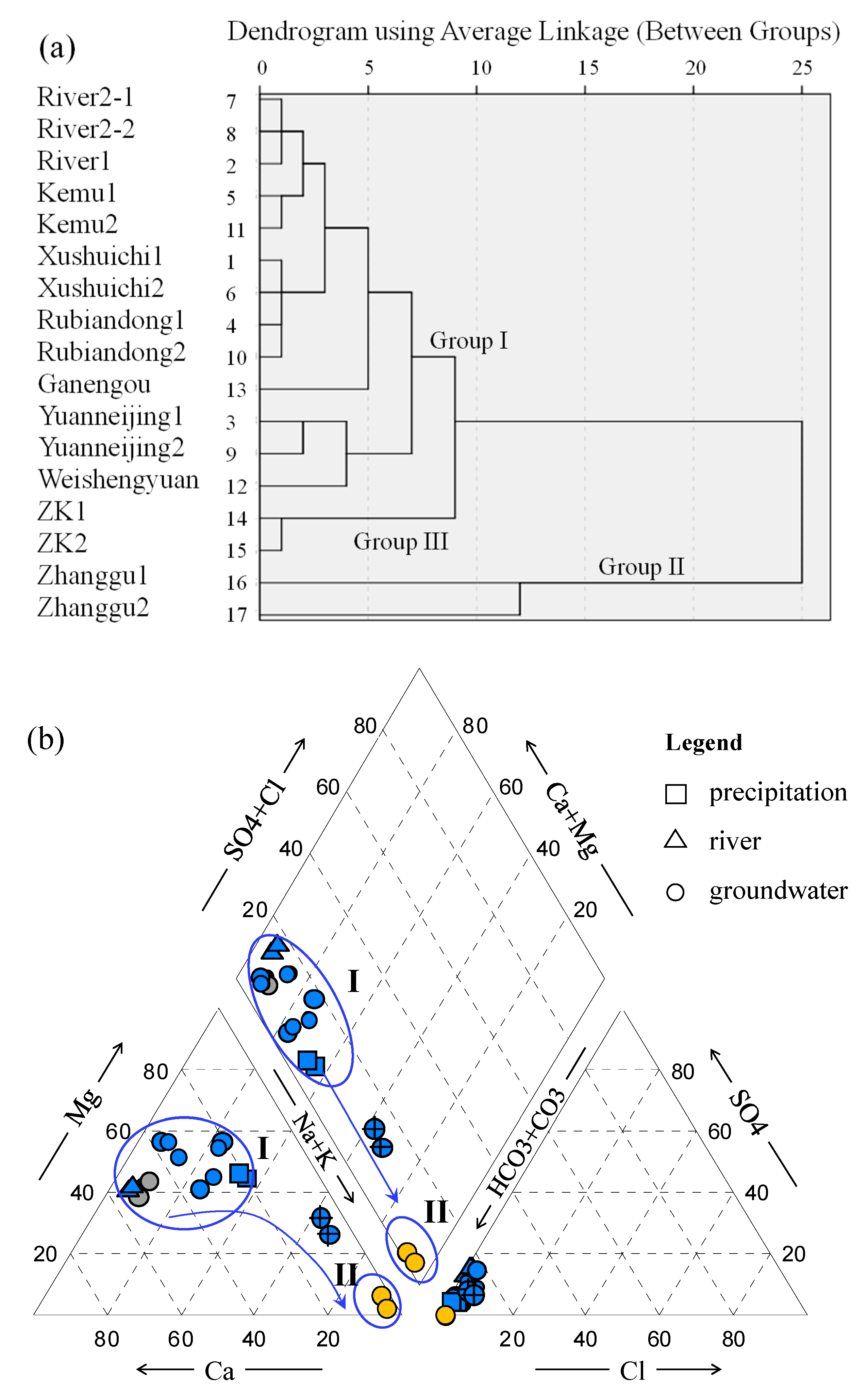
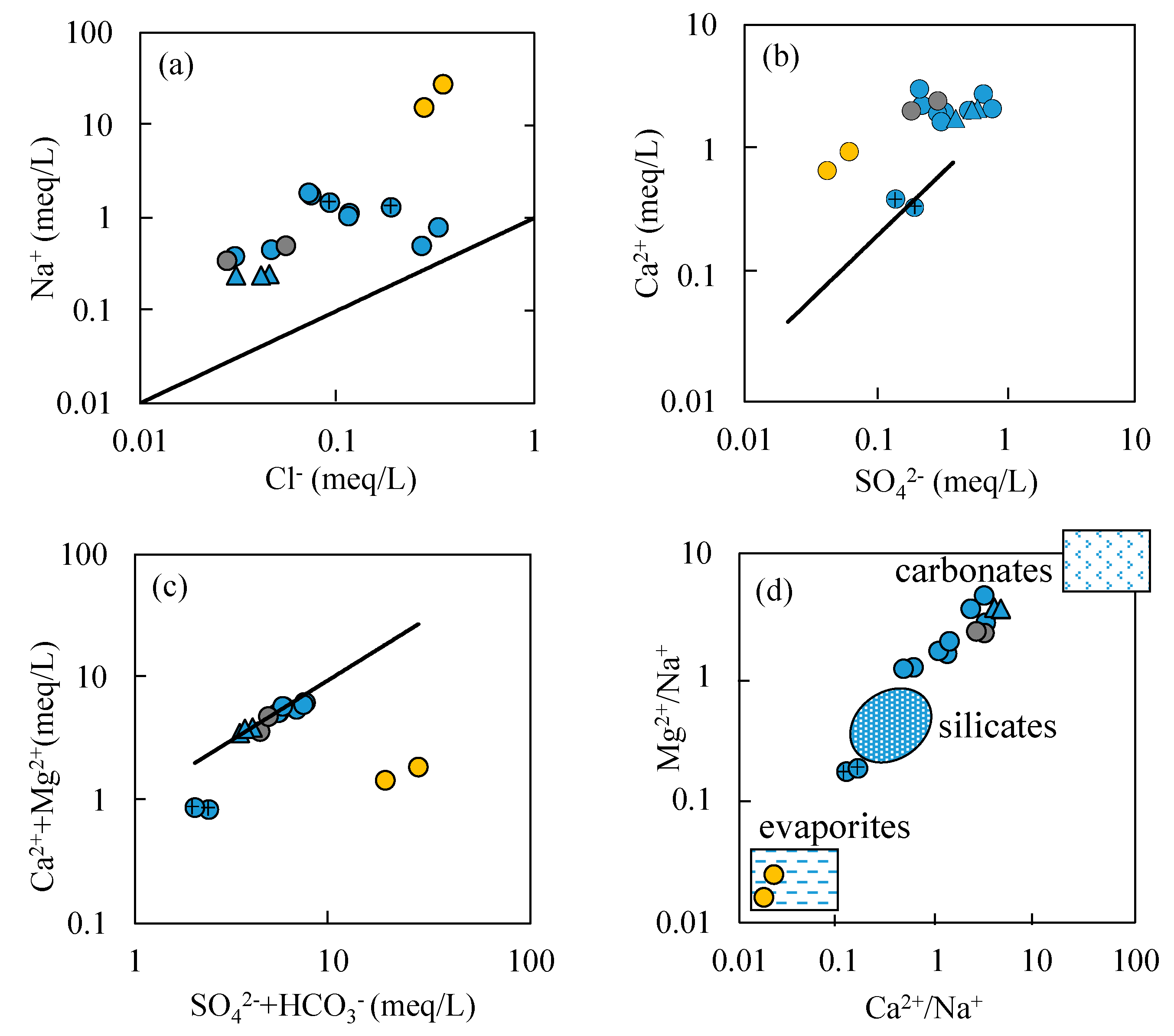
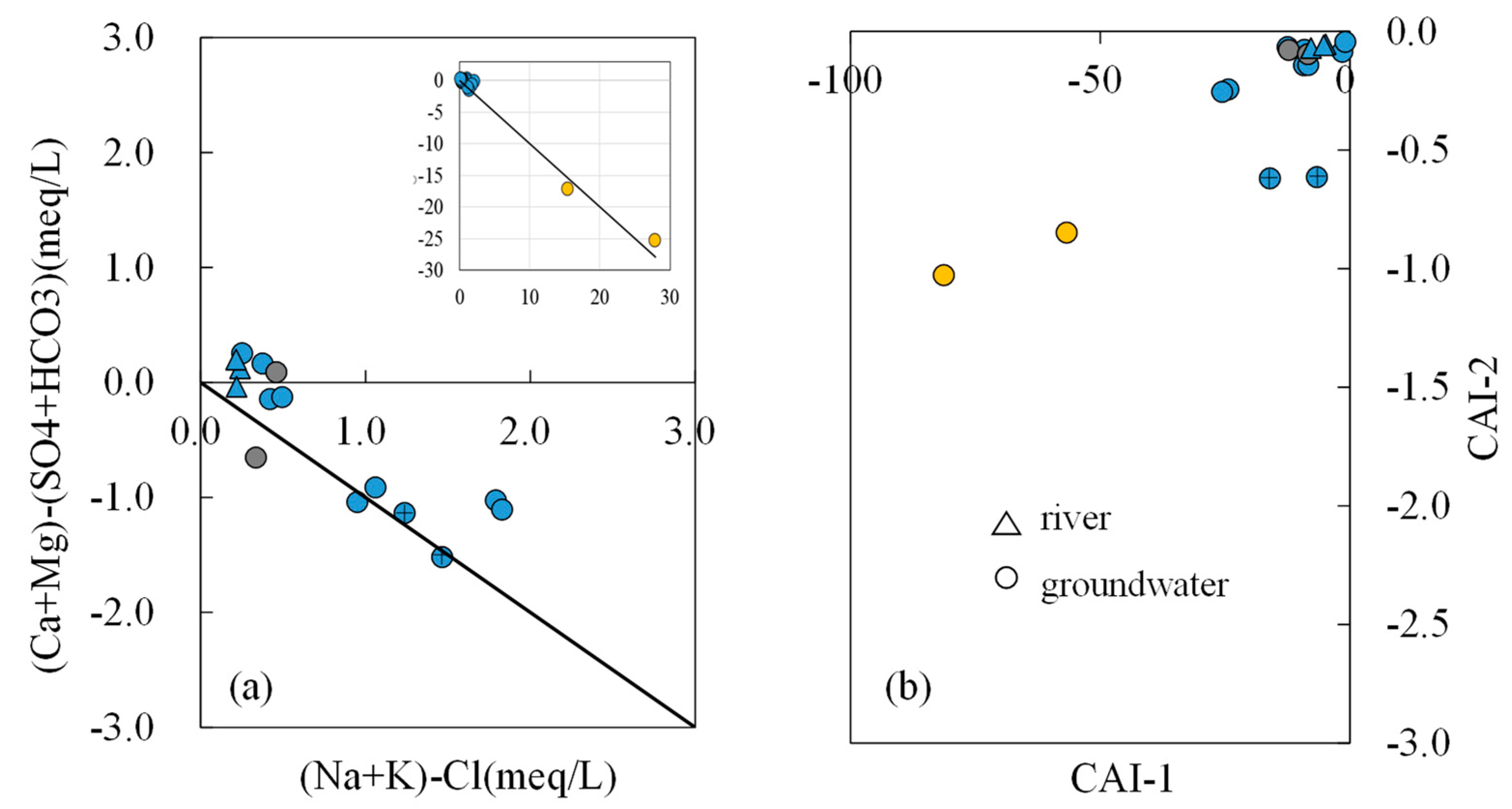
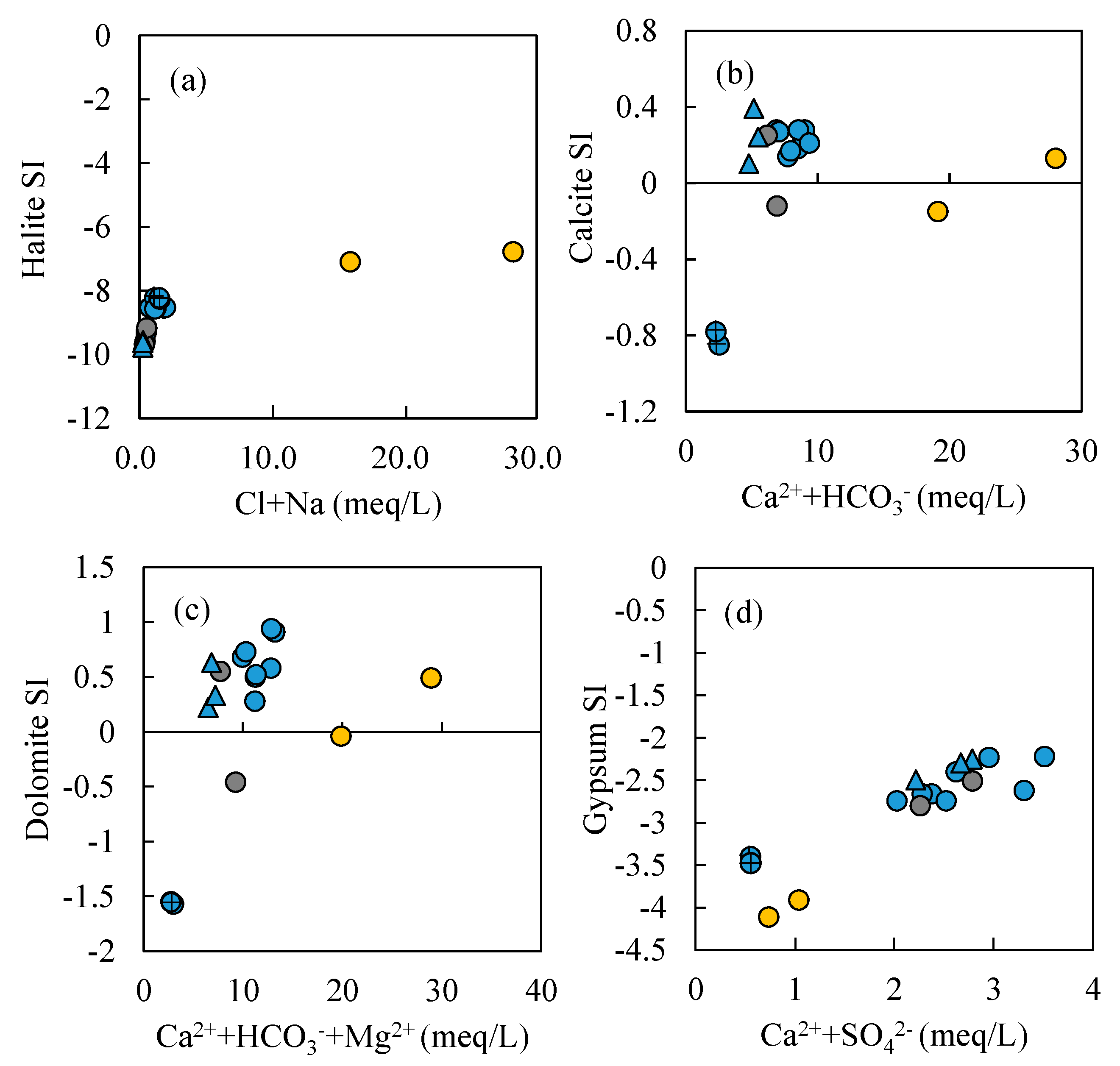
- Most water samples are characterized by HCO3-Ca-Mg or HCO3-Mg-Ca type, except for the borehole ZK which is HCO3-Na-Mg type and the hot spring Zhanggu which is HCO3-Na type. Chemical constituents of most water samples are mainly originated from the dissolution of carbonates, sulfates and cation exchange, while the chemical constituents of Zhanggu and ZK are mainly from the dissolution of evaporites and silicates and cation exchange.
- (2)
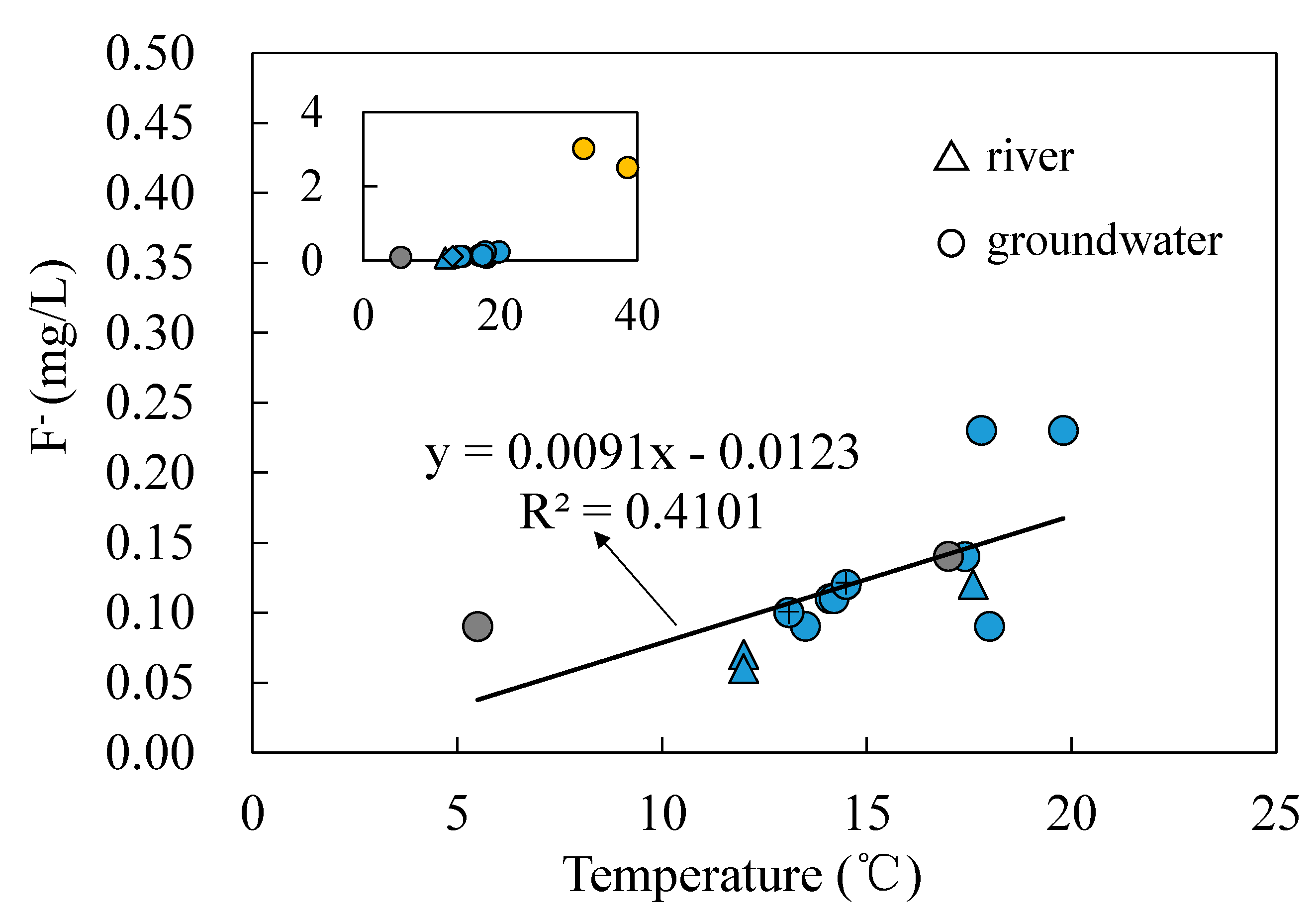
- Groundwater from the spring at Zhanggu may circulate at a deeper depth because of its high TDS and temperature as well as the high F concentration. The rest water samples excluding ZK may be circulated at a relatively shallow depth because of their similar hydrochemical compositions and lower TDS and temperature. Groundwater at ZK may be circulated at a depth between those two because of its mixed hydrochemical type, especially its concentrations of Na and Mg are between the above two groups (see Figure 2).
- (3)
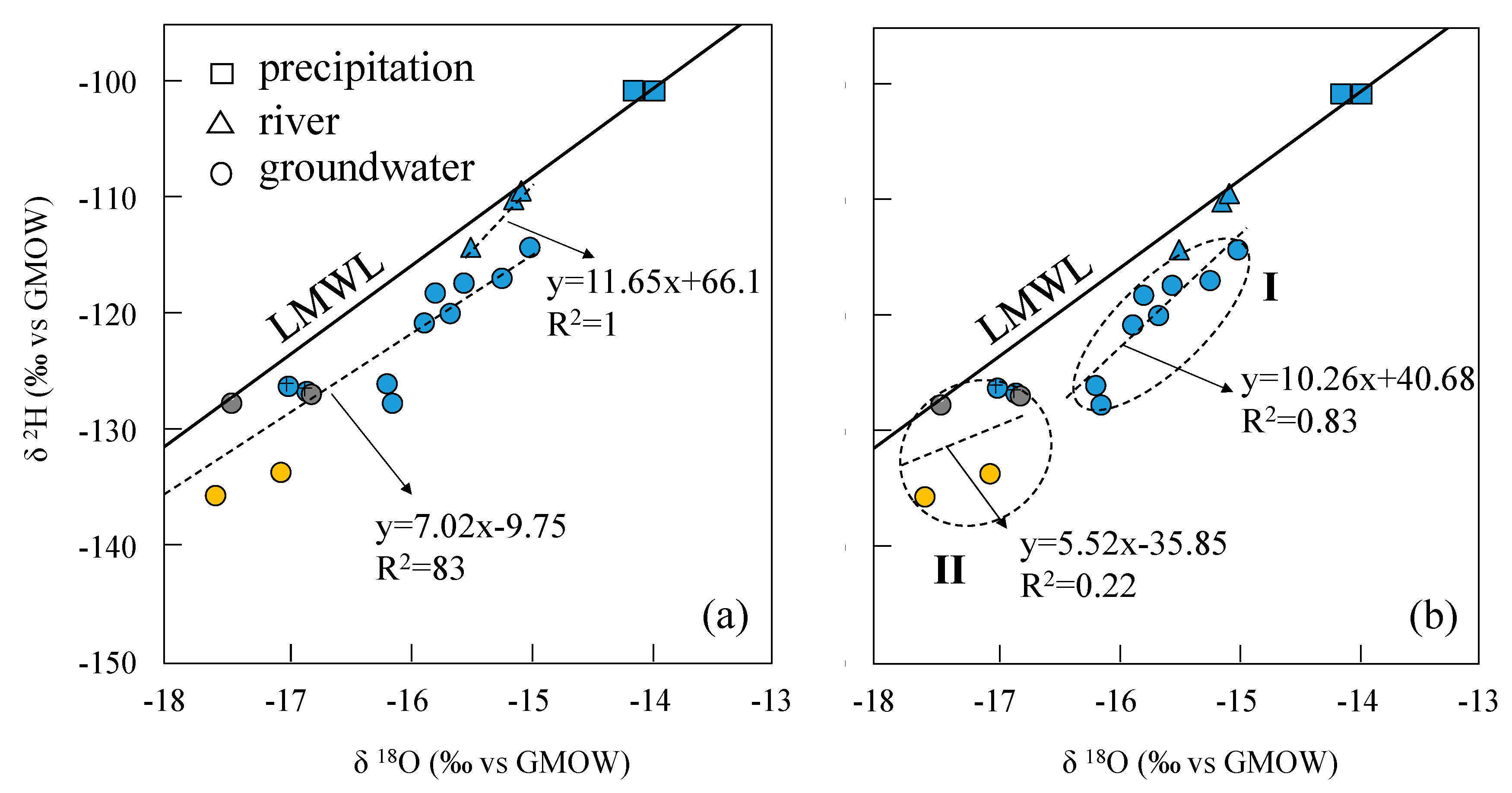
- The three groundwater circulation depths are supported by the stable isotope data. The 2H and 18O values of the water samples were distributed along the local meteoric water line, inferring that groundwater in the region is recharged by precipitation. The linear regression line of δ2H and δ18O at hot springs Zhanggu and Kemu intercepts with the LMWL at δ2H = −145.09‰ and δ18O = −19.79‰. The linear regression line of δ2H and δ18O of the remaining water samples, excluding ZK, intercepts with the LMWL at δ2H = −93.93‰ and δ18O = −13.12‰. The 2H and 18O values of ZK are between the two intercept points.
- (4)
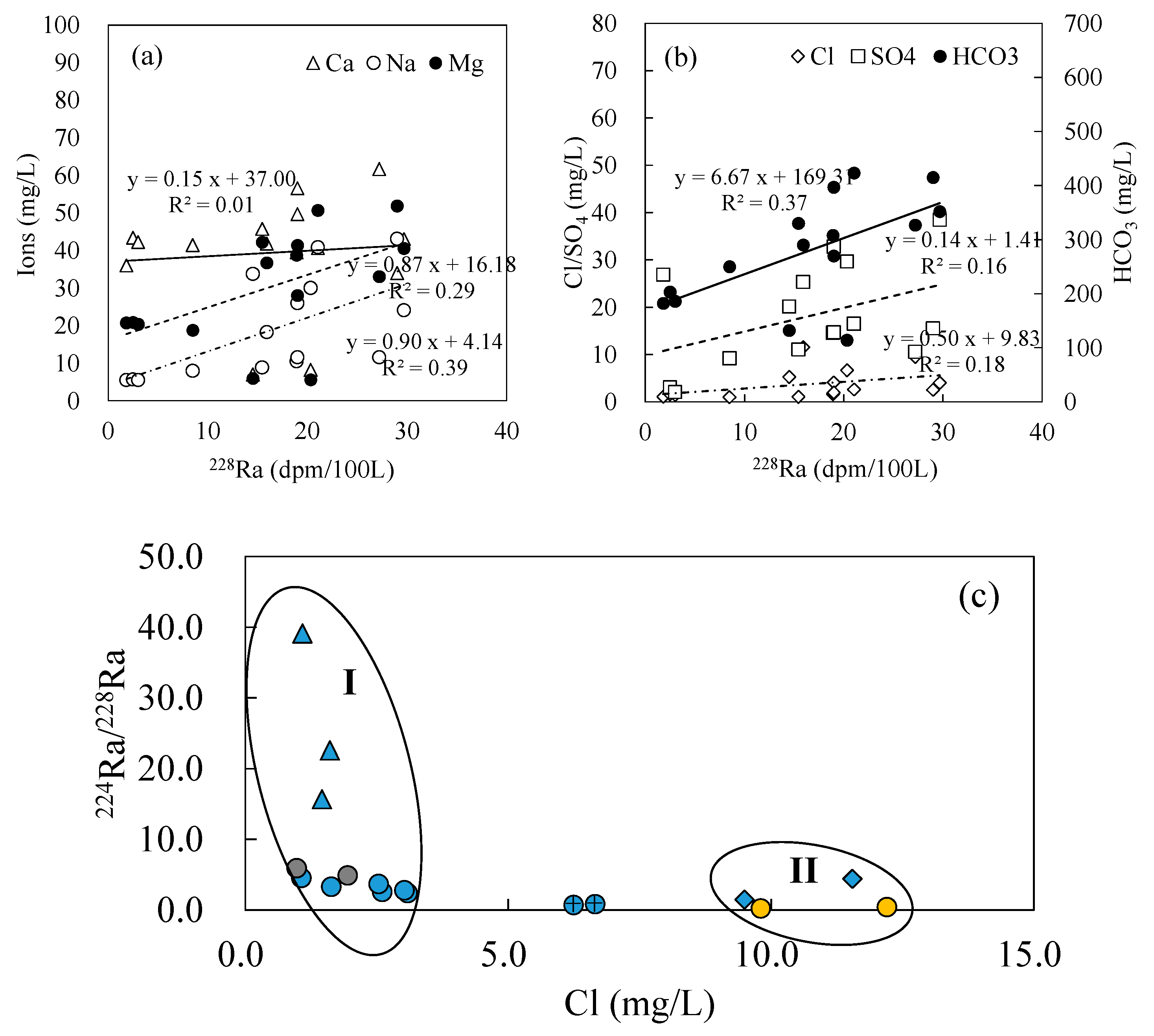
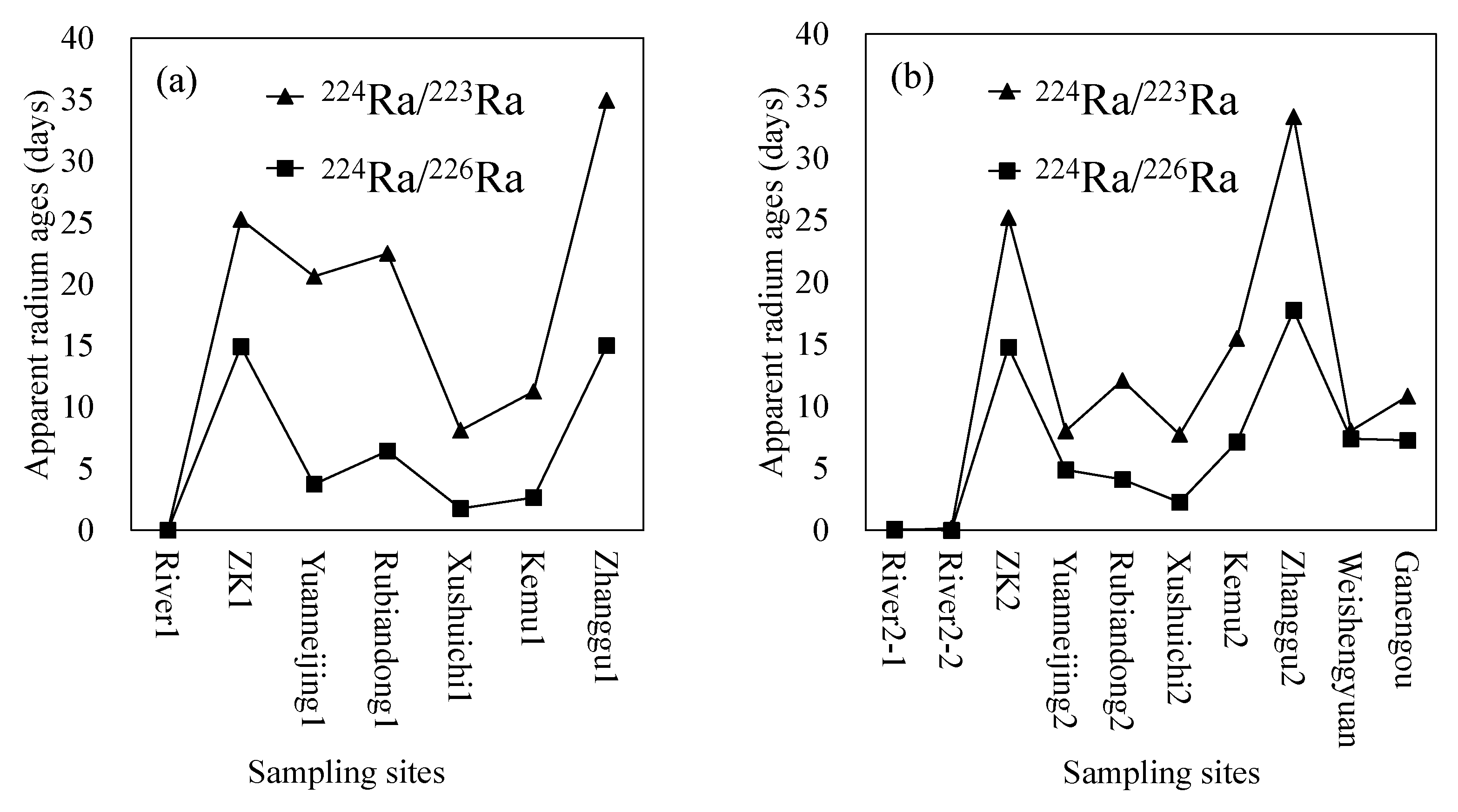
- The three groundwater circulation depths are also supported by the apparent radium age data. The hot spring at Zhanggu with high temperature has longer apparent radium age than other water samples, except for the borehole ZK, inferring that the spring water may have a longer resident time or that it is circulated at a deeper depth. The apparent radium ages of the two cold springs at Rubiandong and Ganengou, and the two domestic wells at Weishengyuan and Ganengou are similar but less than that of the hot spring at Zhanggu, indicating they may have traveled at a shorter distance or circulated at a relatively shallow depth. Groundwater at ZK has the longest apparent radium ages but may be circulated at a depth between the above two due to the relatively low hydraulic conductivity of the formation around the borehole.
- (5)
- Therefore, there may exist three groundwater flow systems in the study area based on the above analyses: the deep groundwater system recharged by the regional groundwater flow; the shallow groundwater system recharged by precipitations and local groundwater flow; and the intermediate groundwater system recharged by mixed local and regional groundwater flow.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press LLC: New York, NY, USA, 1997; pp. 36–61. [Google Scholar]
- Farid, I.; Trabelsi, R.; Zouari, K.; Beji, R. Geochemical and isotopic study of surface and groundwater in Ain Bou Mourra basin, central Tunisia. Quat. Int. 2013, 303, 210–227. [Google Scholar] [CrossRef]
- Farid, I.; Zouari, K.; Rigane, A.; Beji, R. Origin of the groundwater salinity and geochemical processes in detrital and carbonate aquifers: Case of Chougafiya basin (Central Tunisia). J. Hydrol. 2015, 530, 508–532. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, G.; Wang, X.; Wan, L.; Shi, Z.M.; Wanke, H.K.; Uugulu, S.; Uahengo, C.-I. Groundwater quality and associated hydrogeochemical processes in Northwest Namibia. J. Geochem. Explor. 2018, 186, 202–214. [Google Scholar] [CrossRef]
- Pan, G.; Li, X.; Zhang, J.; Liu, Y.; Liang, H. Groundwater-flow-system characterization with hydrogeochemistry: A case in the lakes discharge area of the Ordos Plateau, China. Hydrogeol. J. 2019, 27, 669–683. [Google Scholar] [CrossRef]
- Hagedorn, B.; Whittier, R.B. Solute sources and water mixing in a flashy mountainous stream (Pahsimeroi River, U.S. Rocky Mountains): Implications on chemical weathering rate and groundwater–surface water interaction. Chem. Geol. 2015, 391, 123–137. [Google Scholar] [CrossRef]
- Tóth, J. Groundwater as a geologic agent: An overview of the causes, processes, and manifestations. Hydrogeol. J. 1999, 7, 1–14. [Google Scholar] [CrossRef]
- Yin, L.; Hou, G.; Su, X.; Dong, W.; Dong, J.Q.; Hao, Y.H.; Wang, X.Y. Isotopes (δD and δ18O) in precipitation, groundwater and surface water in the Ordos Plateau, China: Implications with respect to groundwater recharge and circulation. Hydrogeol. J. 2011, 19, 429–443. [Google Scholar] [CrossRef]
- Bai, M.; Chevalier, M.L.; Pan, J.; Replumaz, A.; Hervé, L.P.; Métois, M.; Li, H. Southeastward increase of the late Quaternary slip-rate of the Xianshuihe fault, eastern Tibet. Geodynamic and seismic hazard implications. Earth Planet. Sci. Lett. 2018, 485, 19–31. [Google Scholar] [CrossRef]
- Du, Y.; Guo, C.; Zhang, Y.; Zhang, G.; Zhang, M. Luhuo fault distribution characteristics and Quaternary activity along the Xianshui River Fault, East Tibetan Plateau. In Proceedings of the Conference on Chanllenges and Countermeasures for the Construction of Sichuan-Tibet Railway, Chengdu, China, 14 October 2016; pp. 54–65. (In Chinese). [Google Scholar]
- Guo, J.; Zhou, X.; Wang, L.; Zhang, Y.; Shen, X.; Zhou, H.; Ye, S.; Fang, B. Hydrogeochemical characteristics and sources of salinity of the springs near Wenquanzhen in the eastern Sichuan Basin, China. Hydrogeol. J. 2018, 26, 1137–1151. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.J.; Huang, X. Chemical and isotopic characteristics of hot water in the Kangding-Daofu section of Xianshuihe fault zone, Sichuan, China. J. Chengdu Univ. Technol. Sci. Technol. Ed. 2018, 45, 733–745. (In Chinese) [Google Scholar]
- Zhang, X.J. Preliminary study on characteristics of Xialatuo Basin in Xianshui River fault zone. Sichuan Earthq. 1988, 2, 29–34. (In Chinese) [Google Scholar]
- Li, T.; Du, Q. Activity Fault Zone of Xianshui River and Risk Assessment of Strong Earthquake; Chengdu Map Publishing House: Chengdu, China, 1997. (In Chinese) [Google Scholar]
- Tang, X.; Zhang, J.; Pang, Z.; Hu, S.; Tian, J.; Bao, S. The eastern Tibetan Plateau geothermal belt, western China: Geology, geophysics, genesis, and hydrothermal system. Teconophysics 2017, 717, 433–448. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Tang, X.; Tian, J.; Wang, Y.; Guo, Q.; Pang, Z. Geothermal data analysis at the high-temperature hydrothermal area in Western Sichuan. Sci. China Earth Sci. 2017, 60, 1507–1521. [Google Scholar] [CrossRef]
- Zhao, Q.S. The Hydrogeochemcial Characteristics and Forming Model of Hot Water in the Xian Shui-He Fracture Zone. J. Chengdu Univ. Sci. Technol. 1984, 2, 77–88. (In Chinese) [Google Scholar]
- Moore, W.S.; Reid, D.F. Extraction of radium from natural waters using manganese-impregnated acrylic fibers. J. Geophys. Res. 1973, 78, 8880–8886. [Google Scholar] [CrossRef]
- Moore, W.S.; Cook, L.M. Radium removal from drinking water. Nature 1974, 253, 262–263. [Google Scholar] [CrossRef]
- Luo, X.; Jiao, J.J.; Wang, X.; Liu, K.; Lian, E.; Yang, S. Groundwater discharge and hydrologic partition of the lakes in desert environment: Insights from sTable 18O/2H and radium isotopes. J. Hydrol. 2017, 546, 189–203. [Google Scholar] [CrossRef]
- Moore, W.S.; Arnold, R. Measurement of 223Ra and 224Ra in coastal waters using a delayed coincidence counter. J. Geophys. Res. 1996, 101, 1321–1329. [Google Scholar] [CrossRef]
- Garcia-Solsona, E.; Garcia-Orellana, J.; Masque, P.; Dulaiova, H. Uncertainties associated with 223Ra and 224Ra measurements in water via a Delayed Coincidence Counter (RaDeCC). Mar. Chem. 2008, 109, 198–219. [Google Scholar] [CrossRef]
- Kim, G.; Burnett, W.C.; Dulaiova, H.; Swarzenski, P.W.; Moore, W.S. Measurement of 224Ra and 226Ra activities in natural waters using a radon-in-air monitor. Environ. Sci. Technol. 2001, 35, 4680–4683. [Google Scholar] [CrossRef]
- Todd, D. Groundwater Hydrology, 2nd ed.; John Wiley and Sons. Inc.: New York, NY, USA, 1980. [Google Scholar]
- WHO (World Health Organization). Guidelines for Drinking Water Quality, Health Criteria and Other Sup-Porting Information; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Villegas, P.; Paredes, V.; Betancur, T.; Taupin, J.D.; Toro, L.E. Groundwater evolution and mean water age inferred from hydrochemical and isotopic tracers in a tropical confined aquifer. Hydrol. Process. 2018, 32, 2158–2175. [Google Scholar] [CrossRef]
- Afsin, M.; Allen, D.M.; Kirste, D.; Durukan, U.G.; Gurel, A.; Oruc, O. Mixing processes in hydrothermal spring systems and implications for interpreting geochemical data: A case study in the Cappadocia region of Turkey. Hydrogeol. J. 2014, 22, 7–23. [Google Scholar] [CrossRef]
- Katsanou, K.; Siavalas, G.; Lambrakis, N. Geochemical controls on fluoriferous groundwaters of the Pliocene and the more recent aquifers: The case of Aigion region, Greece. J. Contam. Hydrol. 2013, 155, 55–68. [Google Scholar] [CrossRef]
- Bershaw, J.; Penny, S.M.; Garzione, C.N. Stable isotopes of modern water across the Himalaya and eastern Tibetan Plateau: Implications for estimates of paleoelevation and paleoclimate. J. Geophys. Res. Atmos. 2012, 117, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.H.; Kong, Y.; Li, J.; Tian, J. An isotopic geoindicator in the hydrological cycle. Procedia Earth Planet. Sci. 2017, 17, 534–537. [Google Scholar] [CrossRef]
- Clayton, R.N.; Steiner, A. Oxygen isotope studies of the geothermal system at Wairakei, New Zealand. Geochim. Cosmochim. Acta 1975, 39, 1179–1186. [Google Scholar] [CrossRef]
- Luo, S.D.; Ku, T.L.; Roback, R.; Murrell, M.; Mcling, T.L. In-situ radionuclide transport and preferential groundwater flows at INEEL (Idaho): Decay-series disequilibrium studies. Geochim. Cosmochim. Acta 2000, 64, 867–881. [Google Scholar] [CrossRef]
- Luo, X. Groundwater Discharge Quantification in Marine, and Desert Environments Using Radium Quartet, Radon-222 and Stable Isotopes. Ph.D. Thesis, The University of Hong Kong, Hong Kong, China, 2014. [Google Scholar]
- Szabo, Z.; DePaul, V.T.; Fischer, J.M.; Kraemer, T.F.; Jacobsen, E. Occurrence and geochemistry of radium in water from principal drinking-water aquifer systems of the United States. Appl. Geochem. 2012, 27, 729–752. [Google Scholar] [CrossRef] [Green Version]
- Vesterbacka, P.; Turtiainen, T.; Heinavaara, S.; Arvela, H. Activity concentrations of 226Ra and 228Ra in drilled well water in Finland. Radiat. Prot. Dosim. 2006, 121, 406–412. [Google Scholar] [CrossRef]
- Vinson, D.S.; Vengosh, A.; Hirschfeld, D.; Dwyer, G.S. Relationships between radium and radon occurrence and hydrochemistry in fresh groundwater from fractured crystalline rocks, North Carolina (USA). Chem. Geol. 2009, 260, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Aikainen, M. Radium content and the 226Ra/228Ra activity ratio in groundwater from bedrock. Geochem. Cosmochim. Acta 1981, 45, 1375–1381. [Google Scholar] [CrossRef]
- Khalil, M.M.; Tokunaga, T.; Yousef, A.F. Insights from stable isotopes and hydrochemistry to the Quaternary groundwater system, sossuth of the Ismailia Canal, Egypt. J. Hydrol. 2015, 527, 555–564. [Google Scholar] [CrossRef]
- Prada, S.J.; Cruz, V.; Figueira, C. Using stable isotopes to characterize groundwater recharge sources in the volcanic island of Madeira, Portugal. J. Hydrol. 2016, 536, 409–425. [Google Scholar] [CrossRef]
- Seraphin, P.; Vallet-Coulomb, C.; Goncalves, J. Partitioning groundwater recharge between rainfall infiltration and irrigation return flow using stable isotopes: The Crau aquifer. J. Hydrol. 2016, 542, 241–253. [Google Scholar] [CrossRef]
- Winter, T.C.; Rosenberry, D.O.; Labaugh, J.W. Where does the groundwater in small watersheds come from? Ground Water 2010, 41, 989–1000. [Google Scholar] [CrossRef]
- Fu, C.; Li, X.; Ma, J.; Liu, L.; Gao, M.; Bai, Z. A hydrochemistry and multi-isotopic study of groundwater origin and hydrochemical evolution in the middle reaches of the Kuye River basin. Appl. Geochem. 2018, 98, 82–93. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, X.; Rioual, P.; Qin, X.; Liu, Z.; Xiong, H.; Yu, J. Hydrogeochemistry of three watersheds (the Erlqis, Zhungarer and Yili) in northern Xinjiang, NW China. Appl. Geochem. 2011, 26, 1535–1548. [Google Scholar] [CrossRef]
- Schoeller, H. Geochemistry of Groundwater; UNESCO Studies and Reports in Hydrology 7; UNESCO: Paris, France, 1984. [Google Scholar]
- Drever, J. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Dickson, B.L. Radium in ground water. In The Environmental Behavior of Radium; International Atomic Energy Agency: Vienna, Austria, 1990; Volume 1, pp. 335–372. [Google Scholar]
- Sturchio, N.C.; Banner, J.L.; Binz, C.M.; Heraty, L.B.; Musgrove, M. Radium geochemistry of ground waters in Paleozoic carbonate aquifers, midcontinent, USA. Appl. Chem. 2001, 16, 109–122. [Google Scholar] [CrossRef]
- Luders, V.; Plessen, B.; Romer, R.L.; Weise, S.M.; Banks, D.A.; Hoth, P.; Dulski, P.; Schettler, G. Chemistry and isotopic composition of Rotliegend and upper carboniferous formation waters from the north German Basin. Chem. Geol. 2010, 276, 198–208. [Google Scholar] [CrossRef]
- Moore, W.S. Ages of continental shelf waters determined from 223Ra and 224Ra. J. Geophys. Res. Oceans 2000, 105, 22117–22122. [Google Scholar] [CrossRef]
- Dulaiova, H.; Burnett, W.C.; Wattayakorn, G.; Sojisuporn, P. Are groundwater inputs into river-dominated areas important? The Chao Phraya River-Gulf of Thailand. Limnol. Oceanogr. 2006, 51, 2232–2247. [Google Scholar] [CrossRef] [Green Version]
- Knee, K.L.; Garcia-Solsona, E.; Garcia-Orellana, J.; Boehm, A.B.; Paytan, A. Using radium isotopes to cahracterize water ages and coastal mixing rates: A sensitive analysis. Limnol. Oceanogr. Methods 2011, 9, 380–395. [Google Scholar] [CrossRef] [Green Version]

| First Sampling Campaign (2 August 2017) | ||||||||||||||||
| Sites | Note | elev/m | T (°C) | pH | Na+ | K+ | Mg2+ | Ca2+ | Cl− | NO3− | SO42− | HCO3− | F− | TDS | E (%) | Water Type |
| Xushuichi1 | spring | 3262 | 18.0 | 7.54 | 8.880 | 0.720 | 42.20 | 45.75 | 1.07 | 0.62 | 11.06 | 330.0 | 0.19 | 275.31 | 3.9 | HCO3-Mg-Ca |
| River1 | river | 3112 | 17.6 | 7.83 | 5.500 | 1.080 | 20.71 | 36.00 | 1.09 | 1.55 | 20.13 | 182.0 | 0.09 | 177.06 | 3.8 | HCO3-Ca-Mg |
| Yuanneijing1 | well | 3148 | 19.8 | 7.44 | 25.99 | 1.65 | 41.40 | 56.56 | 4.09 | 14.36 | 32.78 | 397.0 | 0.23 | 375.33 | −2.5 | HCO3-Mg-Ca |
| Rubiandong1 | spring | 3180 | 17.8 | 7.65 | 40.88 | 3.1 | 50.68 | 40.60 | 2.61 | 2.57 | 16.51 | 423.0 | 0.23 | 368.45 | 3.9 | HCO3-Mg-Ca |
| Kemu1 | spring | 3192 | 28.0 | 7.64 | 7.98 | 0.62 | 18.78 | 41.43 | 0.98 | 1.49 | 9.20 | 250.0 | 0.14 | 205.48 | −4.8 | HCO3-Ca-Mg |
| Zhanggu1 | spring | 3110 | 38.6 | 7.11 | 640.8 | 15.42 | 10.71 | 19.54 | 12.21 | 4.99 | 3.06 | 1650.0 | 2.51 | 1531.7 | 4.1 | HCO3-Na |
| ZK1 | well | 3140 | 14.5 | 7.72 | 33.77 | 3.19 | 5.94 | 7.03 | 5.25 | 0.06 | 9.71 | 132.0 | 0.07 | 128.95 | −1.8 | HCO3-Na-Mg |
| Yushui1 | rain | 2978 | 19.5 | 7.21 | 2.45 | 1.01 | 0.37 | 0.97 | 0.21 | 0.25 | 0.11 | 13.0 | 0.06 | 11.87 | −4.1 | HCO3-Na-Ca |
| Yushui(ce)1 | rain | 3148 | 18.0 | 7.34 | 2.60 | 1.25 | 0.60 | 1.41 | 0.10 | 0.48 | 0.10 | 15.0 | 0.08 | 14.04 | −3.8 | HCO3-Na-Ca |
| Second Sampling Campaign (16 November 2017) | ||||||||||||||||
| Sites | Note | elev/m | T (°C) | pH | Na+ | K+ | Mg2+ | Ca2+ | Cl− | NO3− | SO42− | HCO3− | F− | TDS | E (%) | Water Type |
| Xushuichi2 | spring | 3162 | 14.1 | 7.82 | 10.5 | 0.38 | 38.80 | 39.50 | 1.64 | 0.66 | 14.58 | 308.0 | 0.11 | 260.08 | 1.9 | HCO3-Mg-Ca |
| River2-1 | river | 3112 | 12.0 | 7.94 | 5.68 | 0.62 | 20.87 | 43.38 | 1.61 | 2.64 | 29.66 | 203.0 | 0.13 | 205.96 | 0.8 | HCO3-Ca-Mg |
| River2-2 | river | 3115 | 12.0 | 8.14 | 5.5 | 0.62 | 20.35 | 42.23 | 1.46 | 2.67 | 26.85 | 186.0 | 0.11 | 192.68 | 3.8 | HCO3-Ca-Mg |
| Yuanneijing2 | well | 3148 | 14.4 | 7.64 | 24.10 | 0.59 | 40.58 | 43.00 | 4.03 | 6.66 | 38.48 | 352.0 | 0.16 | 353.44 | −2.7 | HCO3-Mg-Ca |
| Rubiandong2 | spring | 3180 | 14.2 | 7.79 | 43.10 | 0.94 | 51.86 | 34.01 | 2.54 | 2.16 | 15.53 | 415.0 | 0.10 | 357.64 | 3.9 | HCO3-Mg-Ca |
| Kemu2 | spring | 3192 | 5.5 | 7.50 | 11.55 | 0.44 | 28.04 | 49.66 | 1.95 | 2.39 | 14.69 | 270.0 | 0.11 | 243.72 | 4.2 | HCO3-Ca-Mg |
| Zhanggu2 | spring | 3110 | 32.2 | 7.15 | 359.54 | 1.15 | 9.19 | 13.85 | 9.81 | 1.24 | 2.07 | 1123.0 | 3.02 | 958.35 | −5.0 | HCO3-Na |
| ZK2 | well | 3140 | 13.1 | 7.80 | 30.01 | 4.29 | 5.61 | 8.19 | 6.66 | 0.11 | 6.95 | 114.0 | 0.03 | 115.82 | 1.2 | HCO3-Na-Mg |
| Weishengyuan | well | 3151 | 13.5 | 7.84 | 18.25 | 0.98 | 36.75 | 41.84 | 11.55 | 26.42 | 25.34 | 290.0 | 0.09 | 306.13 | −4.3 | HCO3-Mg-Ca |
| Ganengou | spring | 3166 | 14.5 | 7.50 | 11.55 | 0.61 | 33.02 | 61.68 | 9.50 | 5.90 | 10.59 | 327.0 | 0.20 | 296.35 | 2.1 | HCO3-Ca-Mg |
| First Sampling Campaign (2 August 2017) | ||||
| Sites | δ2H | StDev(δ2H) | δ18O | StDev(δ18O) |
| Yushui1 | −100.68 | 0.189 | −14.14 | 0.056 |
| Yushui (ce)1 | −110.72 | 0.602 | −13.96 | 0.114 |
| River1 | −114.18 | 0.300 | −15.48 | 0.099 |
| Yuanneijing1 | −114.19 | 0.356 | −14.99 | 0.055 |
| Xushuichi1 | −119.88 | 0.484 | −15.65 | 0.077 |
| ZK1 | −126.60 | 0.485 | −16.82 | 0.055 |
| Rubiandong1 | −127.61 | 0.216 | −16.12 | 0.097 |
| Kemu1 | −126.83 | 0.085 | −16.79 | 0.063 |
| Zhanggu1 | −133.56 | 0.363 | −17.04 | 0.131 |
| Second Sampling Campaign (16 November 2017) | ||||
| Sites | δ2H | StDev(δ2H) | δ18O | StDev(δ18O) |
| River2-1 | −109.32 | 0.310 | −15.06 | 0.054 |
| River2-2 | −110.06 | 0.204 | −15.12 | 0.111 |
| Yuanneijing2 | −120.69 | 0.521 | −15.86 | 0.049 |
| Xushuichi2 | −117.27 | 0.608 | −15.53 | 0.089 |
| ZK2 | −126.16 | 0.168 | −16.98 | 0.044 |
| Rubiandong2 | −125.94 | 0.611 | −16.16 | 0.070 |
| Kemu2 | −127.60 | 0.238 | −17.45 | 0.073 |
| Zhanggu2 | −135.56 | 0.345 | −17.58 | 0.048 |
| Weishengyuan | −118.11 | 0.147 | −15.77 | 0.089 |
| Ganengou | −116.85 | 0.199 | −15.22 | 0.074 |
| First Sampling Campaign (2 August 2017) | ||||||
| Sites | 223Ra(error) | 224Ra(error) | 226Ra(error) | 228Ra(error) | 224Ra/223Ra | 224Ra/226Ra |
| Xushuichi1 | 0.14(0.02) | 70.0(3.5) | 4.43(0.2) | 15.44(1.7) | 500.00 | 15.80 |
| River1 | 0.05(0.01) | 70.8(3.6) | 3.20(0.4) | 1.81(0.3) | 1416.00 | 22.13 |
| Yuanneijing1 | 0.45(0.05) | 45.3(2.3) | 4.16(0.5) | 19.00(1.5) | 100.67 | 10.89 |
| Rubiandong1 | 0.68(0.07) | 53.9(2.7) | 8.24(1.2) | 21.04(1.4) | 79.26 | 6.54 |
| Kemu1 | 0.15(0.02) | 50.2(2.51) | 3.74(0.5) | 8.48(1.3) | 333.33 | 13.37 |
| ZK1 | 0.18(0.02) | 10.5(0.53) | 7.61(1.1) | 14.51(1.1) | 55.56 | 1.31 |
| Zhanggu1 | 33.10(3.30) | 531.22(26.56) | 411.02(28.5) | 12,740.96(891.9) | 16.05 | 1.29 |
| Mean | 5.06 | 85.04 | 62.73 | 2017.47 | 357.27 | 10.19 |
| Second Sampling Campaign (16 November 2017) | ||||||
| Sites | 223Ra(error) | 224Ra(error) | 226Ra(error) | 228Ra(error) | 224Ra/223Ra | 224Ra/226Ra |
| Xushuichi2 | 0.21(0.02) | 62.4(3.1) | 3.24(0.2) | 18.91(1.2) | 297.14 | 19.26 |
| River2-1 | 0.07(0.01) | 56.0(2.8) | 1.93(0.1) | 2.48(1.4) | 800.00 | 29.02 |
| Yuanneijing2 | 0.36(0.04) | 103.1(5.2) | 8.77(0.3) | 29.69(1.3) | 286.39 | 11.76 |
| Rubiandong2 | 0.51(0.05) | 86.6(4.3) | 6.36(0.4) | 29.20(1.1) | 169.80 | 13.62 |
| Kemu2 | 0.66(0.07) | 72.6(3.6) | 9.43(0.5) | 19.94(1.5) | 110.00 | 7.70 |
| ZK2 | 0.38(0.02) | 12.2(0.6) | 6.61(0.4) | 20.32(1.1) | 31.58 | 1.82 |
| Zhanggu2 | 33.05(3.31) | 367.62(18.4) | 355.02(10.85) | 18,365.4(1285.6) | 11.12 | 1.04 |
| River2-2 | 0.06(0.01) | 47.0(2.4) | 1.59(0.11) | 3.18(0.8) | 783.33 | 29.56 |
| Weishengyuan | 0.28(0.03) | 79.8(4.0) | 10.96(0.42) | 15.92(1.6) | 285.71 | 7.30 |
| Ganengou | 0.30(0.02) | 60.6(3.0) | 8.04(0.56) | 27.20(1.2) | 200.00 | 7.50 |
| Mean | 3.59 | 94.73 | 41.19 | 200.21 | 297.51 | 12.86 |
| First Sampling Campaign (2 August 2017) | ||
| Sites | Equation (1) | Equation (2) |
| River1 | 0.00 | 0.00 |
| ZK1 | 25.26 | 14.91 |
| Yuanneijing1 | 20.62 | 3.74 |
| Rubiandong1 | 22.49 | 6.43 |
| Xushuichi1 | 8.12 | 1.78 |
| Kemu1 | 11.28 | 2.66 |
| Zhanggu1 | 34.94 | 15.00 |
| Mean | 17.53 | 6.36 |
| Second Sampling Campaign (16 November 2017) | ||
| Sites | Equation (1) | Equation (2) |
| River2-1 | 0.00 | 0.10 |
| River2-2 | 0.16 | 0.00 |
| ZK2 | 25.21 | 14.76 |
| Yuanneijing2 | 8.01 | 4.88 |
| Rubiandong2 | 12.09 | 4.10 |
| Xushuichi2 | 7.73 | 2.27 |
| Kemu2 | 15.48 | 7.12 |
| Zhanggu2 | 33.35 | 17.73 |
| Ganengou | 10.81 | 7.26 |
| Weishengyuan | 8.03 | 7.40 |
| Mean | 12.09 | 6.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Y.-K.; Yang, Y.; Li, F.; Xiao, S. Groundwater Circulation in the Xianshui River Fault Region: A Hydrogeochemical Study. Water 2020, 12, 3310. https://doi.org/10.3390/w12123310
Zhao Y, Zhang Y-K, Yang Y, Li F, Xiao S. Groundwater Circulation in the Xianshui River Fault Region: A Hydrogeochemical Study. Water. 2020; 12(12):3310. https://doi.org/10.3390/w12123310
Chicago/Turabian StyleZhao, Yuqing, You-Kuan Zhang, Yonglin Yang, Feifei Li, and Sa Xiao. 2020. "Groundwater Circulation in the Xianshui River Fault Region: A Hydrogeochemical Study" Water 12, no. 12: 3310. https://doi.org/10.3390/w12123310
APA StyleZhao, Y., Zhang, Y.-K., Yang, Y., Li, F., & Xiao, S. (2020). Groundwater Circulation in the Xianshui River Fault Region: A Hydrogeochemical Study. Water, 12(12), 3310. https://doi.org/10.3390/w12123310




