Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and MP Sample Preparation
2.2. NR-P Method Procedure
2.3. MPs Analyses
2.4. Image and Statistical Analyses
3. Results
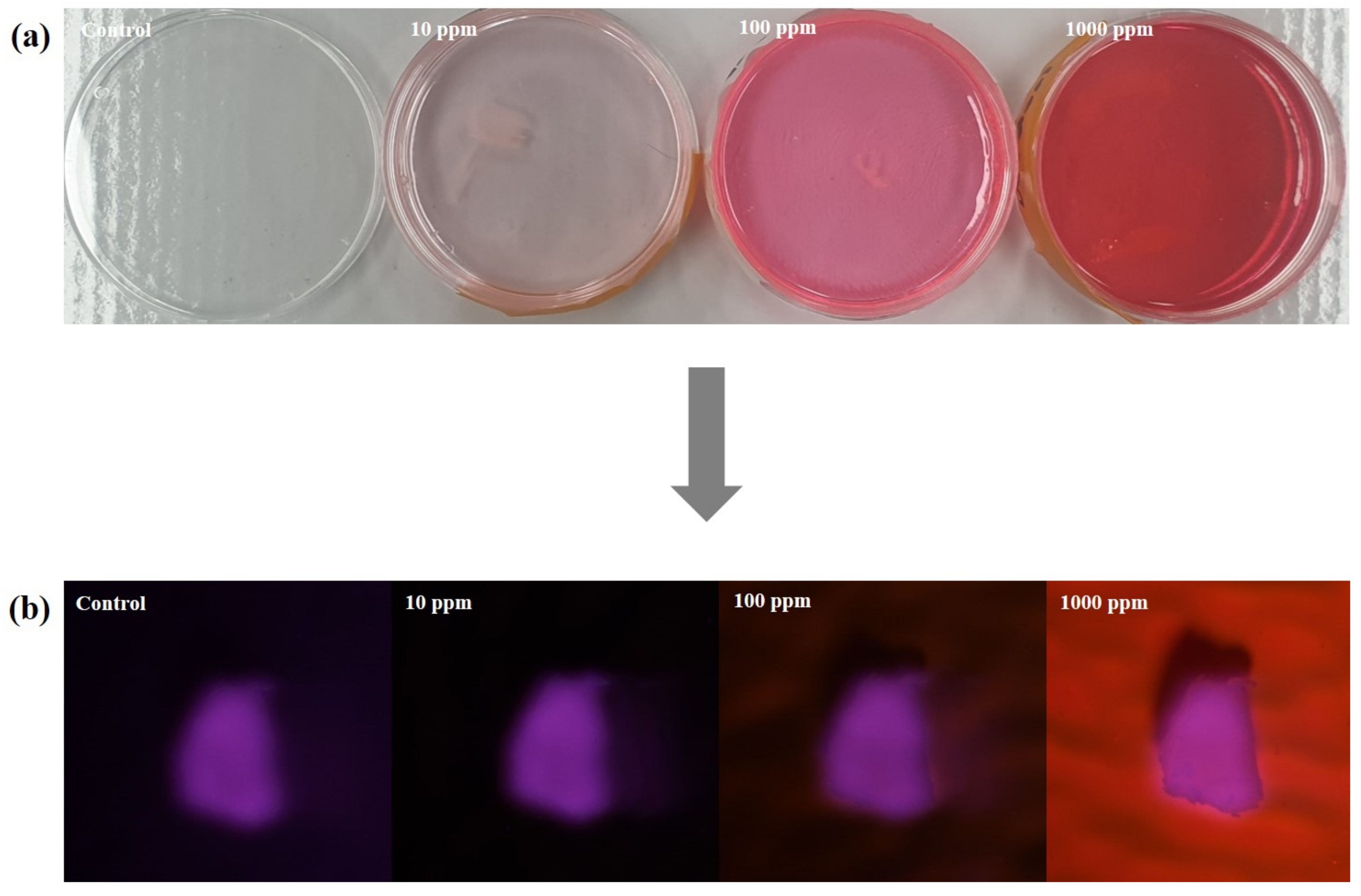
3.1. Optimization of NR-P Method
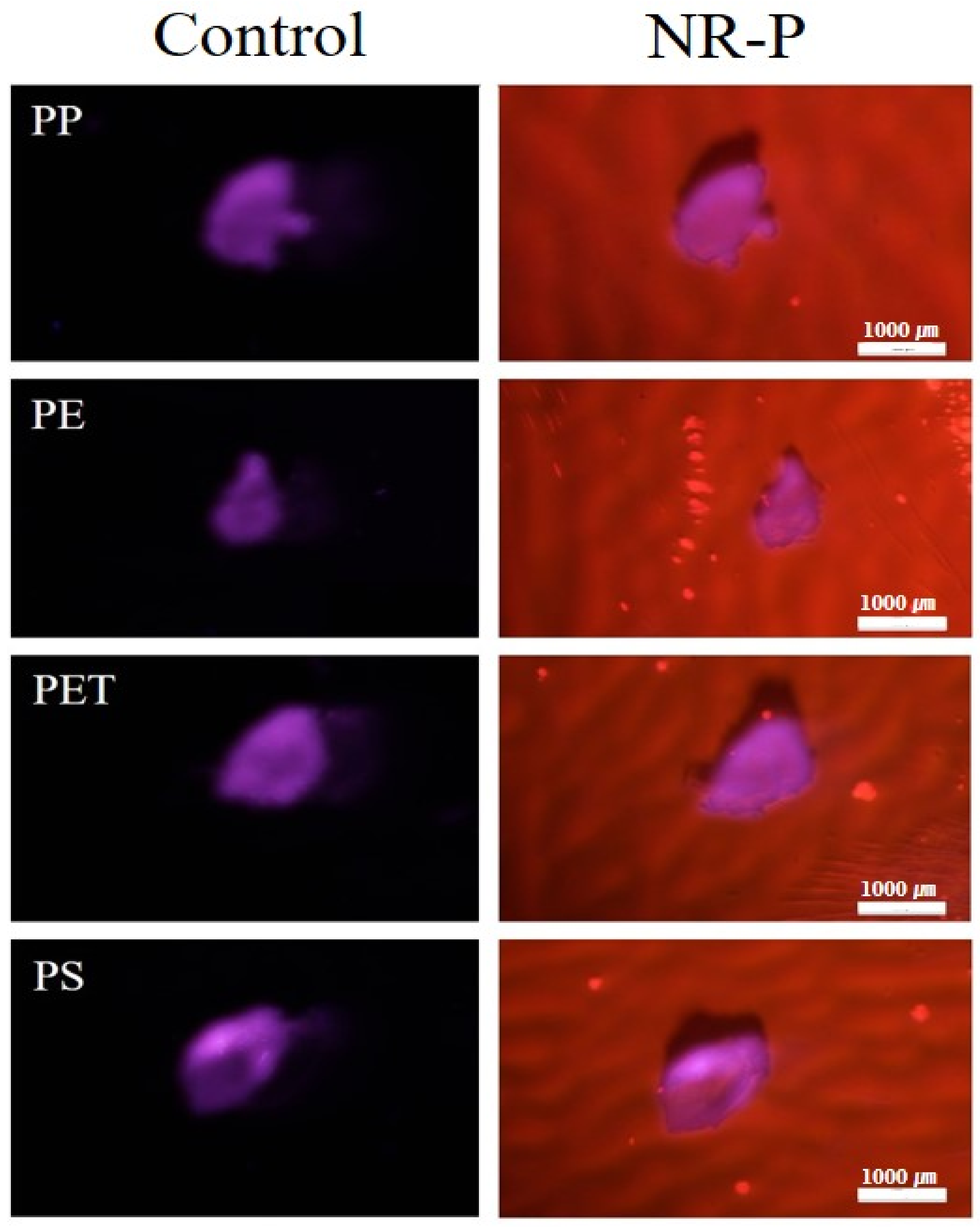
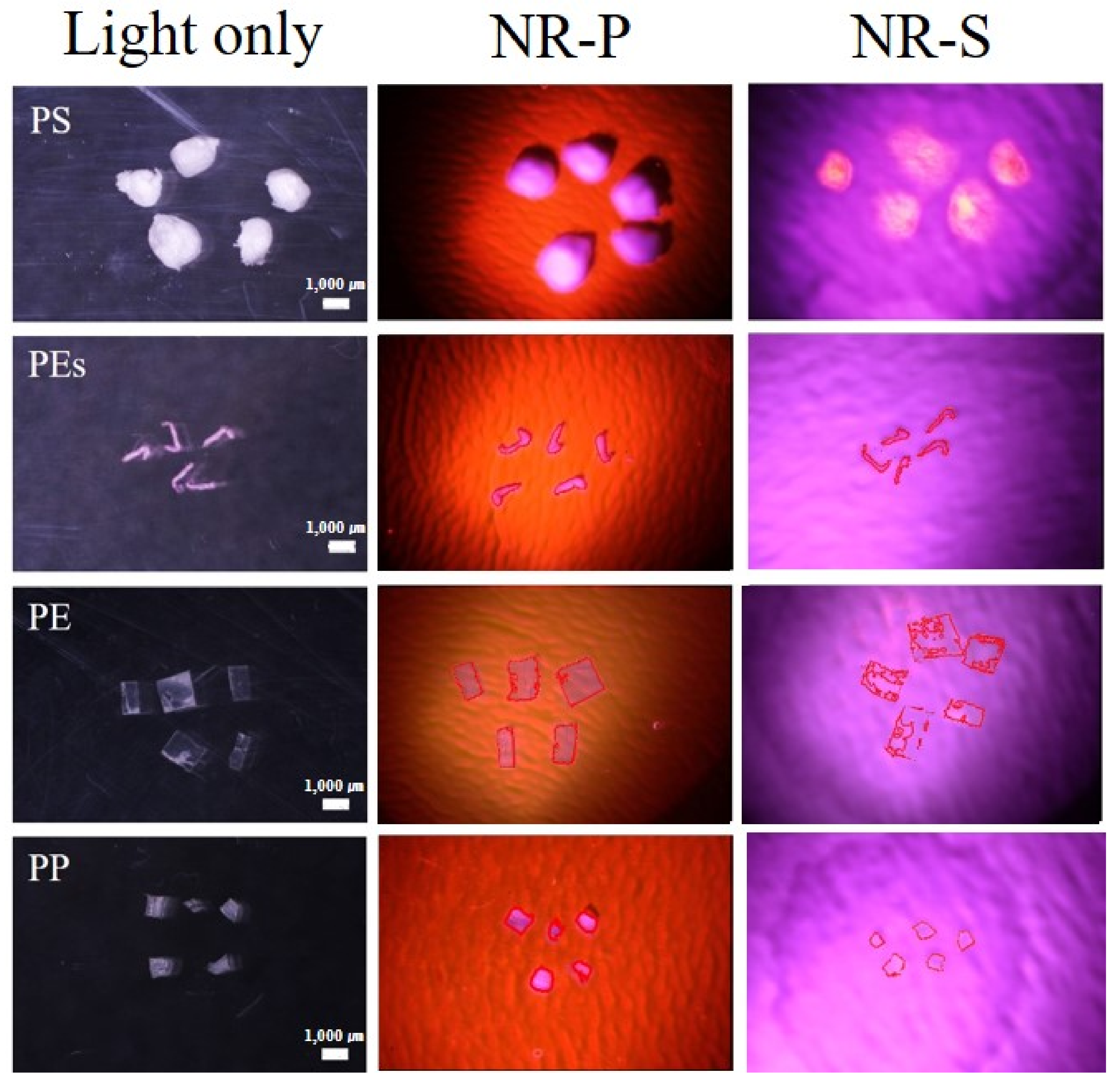
3.2. Validation of the NR-P Method in MP Analysis Using Diverse MPs
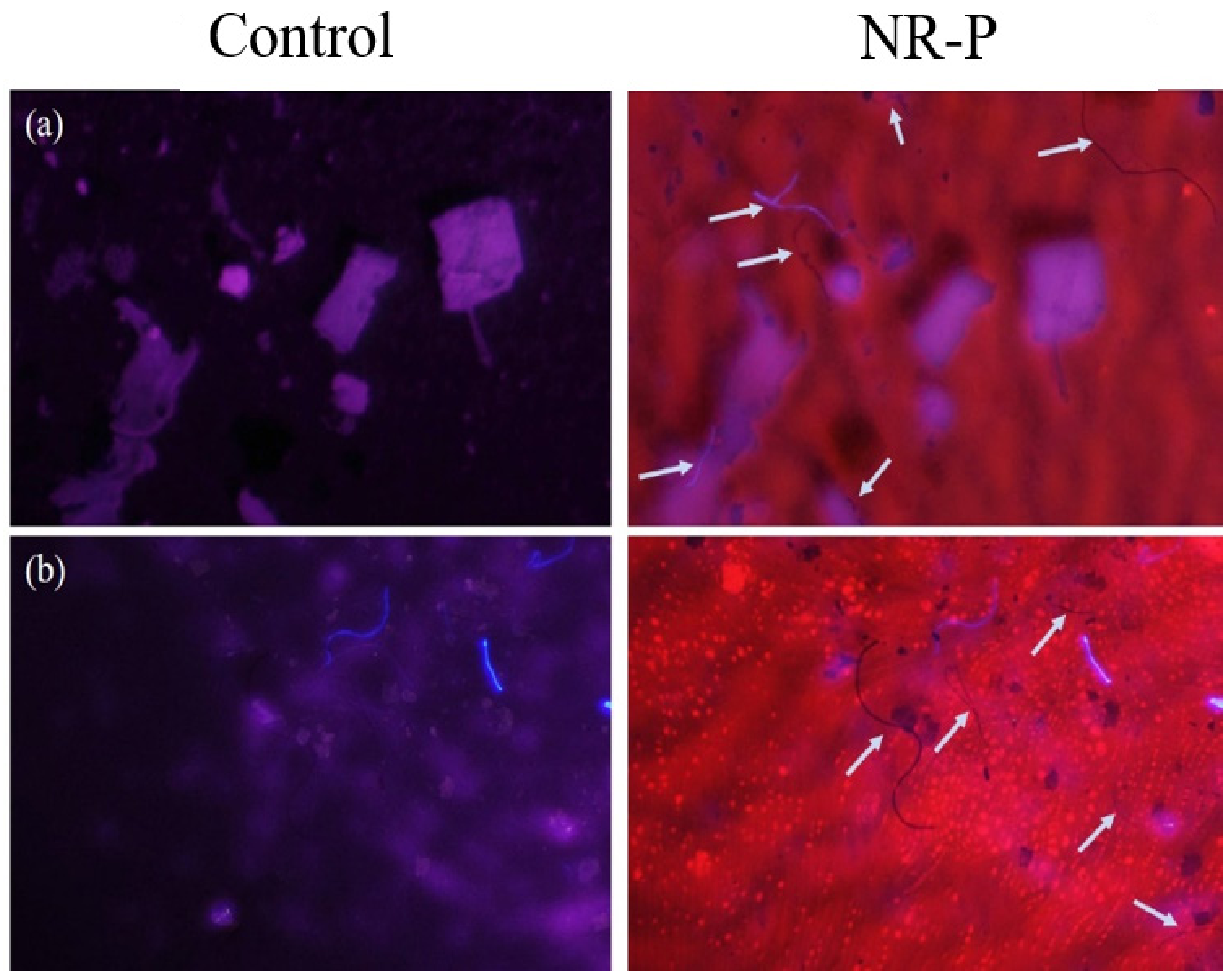
3.3. Effect of Organic Materials on MP Analysis with NR-P Method
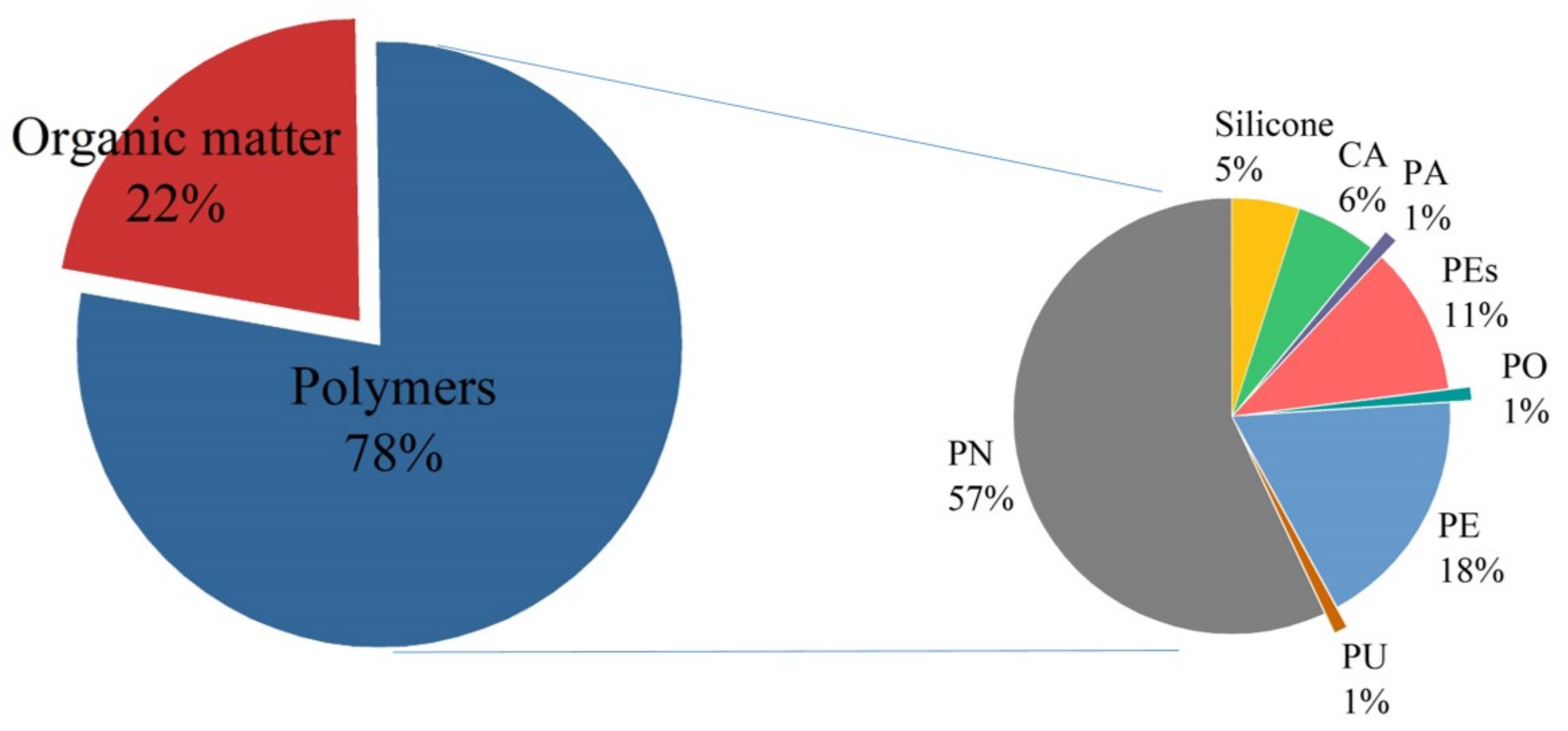
3.4. Application of NR-P Method in Field Sample Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2017; 43p. [Google Scholar]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G.; Moore, C.J.; Van Franeker, J.A.; Moloney, C.L. Monitoring the abundance of plastic debris in the marine environment. Phil. Trans. R Soc. B 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C. Plastic Debris in the Marine Environment: Consequences and Solutions. In Proceedings of the Symposium Marine Nature Conservation in Europe, Straslund, Germany, 8–12 May 2006; pp. 107–115. [Google Scholar]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic-an emerging contaminant of potential concern?: Learned Discourses. Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Cozar, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Cai, H.; Du, F.; Li, L.; Li, B.; Li, J.; Shi, H. A practical approach based on FT-IR spectroscopy for identification of semi-synthetic and natural celluloses in microplastic investigation. Sci. Total Environ. 2019, 669, 692–701. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, M.; Scholz-Böttcher, B.M.; Oberbeckmann, S.; Labrenz, M.; Fischer, D.; Eichhorn, K.-J.; Voit, B. Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 2018, 410, 5313–5327. [Google Scholar] [CrossRef]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2016, 5, 17988. [Google Scholar] [CrossRef]
- Munno, K.; De Frond, H.; O’Donnell, B.; Rochman, C.M. Increasing the Accessibility for Characterizing Microplastics: Introducing New Application-Based and Spectral Libraries of Plastic Particles (SLoPP and SLoPP-E). Anal. Chem. 2020, 92, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603–604, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Nel, H.A.; Froneman, P.W. A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar. Pollut. Bull. 2015, 101, 274–279. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Scircle, A.; Cizdziel, J.V.; Missling, K.; Li, L.; Vianello, A. Single-Pot Method for the Collection and Preparation of Natural Water for Microplastic Analyses: Microplastics in the Mississippi River System during and after Historic Flooding. Environ. Toxicol. Chem. 2020, 39, 986–995. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the Efficacy of Nile Red in Microplastic Quantification: A Containing Approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Fischer, E.K. Nile Red Staining as a Subsidiary Method for Microplastic Quantification: A Comparison of Three Solvents and Factors Influencing Application Reliability. J. Earth Sci. Environ. Stud. 2017, 2472–6397. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR. Imaging Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment; NOAA Technical Memorandum; National Oceanic and Atmospheric Administration, U.S. Department of Commerce: Washington, DC, USA, 2015.
- Fischer, E.K.; Paglialonga, L.; Czech, E.; Tamminga, M. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef]
- Hengstmann, E.; Tamminga, M.; vom Bruch, C.; Fischer, E.K. Microplastic in beach sediments of the Isle of Rügen (Baltic Sea)—Implementing a novel glass elutriation column. Mar. Pollut. Bull. 2018, 126, 263–274. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). Sci. Total Environ. 2020, 719, 137498. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I.P.; Esiukova, E.E.; Bagaev, A.V.; Bagaeva, M.A.; Grave, A.N. Three-dimensional distribution of anthropogenic microparticles in the body of sandy beaches. Sci. Total Environ. 2018, 628–629, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Grove, N.R.; Kohl, P.A.; Allen, S.A.B.; Jayaraman, S.; Shick, R. Functionalized polynorbornene dielectric polymers: Adhesion and mechanical properties. J. Polym. Sci. B Polym. Phys. 1999, 37, 3003–3010. [Google Scholar] [CrossRef]
- Haselwander, T.F.A.; Heitz, W.; Krügel, S.A.; Wendorff, J.H. Polynorbornene: Synthesis, properties and simulations. Macromol. Chem. Phys. 1996, 197, 3435–3453. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.-P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef]


| Size (μm) | Accuracy (%) | p-Value | |
|---|---|---|---|
| Control | NR-P | ||
| 1000 | 86 (±8.5) | 96 (±3.1) | 0.20 |
| 700–1000 | 87 (±5.4) | 93 (±4.3) | 0.18 |
| 400–700 | 76 (±12.8) | 93 (±3.5) | 0.11 |
| 100–400 | 53 (±15) | 84 (±21) | 0.04 |
| Shape | Recovery Rates (%) | p-Value | |
|---|---|---|---|
| NR-S | NR-P | ||
| Sphere | - | 90 (±2.7) | - |
| Fiber | 89 (±6.4) | 100 | 0.0004 |
| Film | 37 (±14.9) | 98 (±3.8) | 0.0146 |
| Flake | 58 (±4.5) | 92 (±14.3) | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Park, S.; Lee, B.; Ahn, J.; Kim, S. Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method. Water 2020, 12, 3251. https://doi.org/10.3390/w12113251
Kang H, Park S, Lee B, Ahn J, Kim S. Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method. Water. 2020; 12(11):3251. https://doi.org/10.3390/w12113251
Chicago/Turabian StyleKang, Heejun, Saerom Park, Bokjin Lee, Jaehwan Ahn, and Seogku Kim. 2020. "Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method" Water 12, no. 11: 3251. https://doi.org/10.3390/w12113251
APA StyleKang, H., Park, S., Lee, B., Ahn, J., & Kim, S. (2020). Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method. Water, 12(11), 3251. https://doi.org/10.3390/w12113251




