1. Introduction
Advanced oxidation processes (AOPs) is a cost-effective solution for removal of harmful and refractory pollutants from water. This method is preferred and ideal in the field of water treatment and air purification. The hydroxyl radical produced in AOPs, which is the most aggressive substance without choice, has a strong oxidation potential of 2.80 v. UV-based AOP technology has been widely used in the field of drinking water disinfection and has made important progress and achieved commercialization. However, in the field of wastewater treatment, cases of applying this method are few, because the reaction system needs a long reaction time and satisfies the basic conditions, such as sufficient reaction among the components in the reactor. In addition, the structural design of this reaction system also needs the design technology of chemical reaction mechanism and kinetic rate constant for optimization. At present, most large-scale photocatalytic reactors use the air lift method to enhance the mixing effect among the components and reduce the dead zone in the reactor [
1,
2]. The air lift internal circulation photoreactor uses air as the driving force to realize the internal circulation flow of water in the reactor. This photoreactor can realize the full mixing of substances in the fluid without mechanical stirring and pump lifting. It is widely used in various fields, such as in biology, environment, and chemical industry, because of its simple structure and low energy consumption [
3,
4].
Photochemical AOPs use UV radiation in combination with a chemical species (such as hydrogen peroxide, ozone, persulfate) to generate the hydroxyl radicals [
5]. In many AOPs technologies, UV/H
2O
2 process is one of the more promising technologies, that in H
2O
2 absorbs light energy and breaks the O–O bond to form a strong oxidant OH. The reaction rate constant of · OH with most organic compounds can reach 10
6–10
10 M
−1s
−1, which can oxidize and decompose toxic and refractory organic pollutants effectively. The UV/H
2O
2 system is most often used to degrade organic compounds by the combined mechanisms of direct UV photolysis and hydroxyl radical reactions [
5]. Therefore, UV/H
2O
2 process has been widely investigated and applied because of its strong oxidation capacity and clean environmental protection advantages. It has gradually become the preferred method for treating refractory organics and is a promising water treatment technology [
6,
7]. However, different target pollutants, water quality, concentration of H
2O
2, and intensity of ultraviolet light have great differences on the degradation performance of organic compounds. In addition, the geometry of the reactor, the hydrodynamic characteristics in the reactor, and the working conditions of the UV system determine the distribution of UV light.
Numerical simulation based on computational fluid dynamics (CFD) has been widely used in various reactor flow field research because of the development of computer technology, numerical method, and hydrodynamics theory. However, due to the complexity of the multiphase flow process, the research mainly focuses on the Reynolds averaged hydrodynamics of the reactor, which is very limited for understanding the local hydrodynamic conditions. The application of UV/AOPs technology in airlift reactor can increase the contact area of gas and liquid reactants and increase the effective light area in the reactor. Therefore, it can greatly improve the kinetic process of photocatalytic reaction, and has the advantages of strong mass transfer ability and controllable hydraulic retention time. Minghan Luo et al. used the multiphase flow method to simulate the hydrodynamics of an internal airlift circulation photocatalytic reactor, and obtained the information of phase holdup, fluid flow characteristics, and mixing effect [
8]. However, the application of UV/AOPs technology combined with chemical reaction kinetics model, radiation model, and pollutant transport model has not been reported. In addition, only few authors have explored the degradation characteristics of the airlift internal circulation photocatalytic reactor through experiments [
9,
10]. However, when the catalyst is added to the catalytic oxidation reaction process of the internal circulation photocatalytic reactor, the hydrodynamic and chemical reaction situations are still unclear.
Although some numerical methods have been developed to study the performance of UV/AOPs technology for conventional reactors, they are limited by the need to combine the hydrodynamic model and the chemical kinetic model. Design of UV/H
2O
2 process requires knowledge about both system configuration and chemical kinetics [
11]. Therefore, when UV AOPs technology is applied to different reactors, more appropriate numerical tools are needed for the preliminary design and optimization. B.A. Wols et al. used the CFD model combined with ultraviolet radiation, transmission, and photochemical models to calculate the degradation of pollutants in the UV system, and evaluated the transport of pollutants by Lagrange method and Euler method, respectively. The research results show that these two methods can be used for the degradation process of UV/H
2O
2 system [
5]. Scott M. et al. [
11] used the CFD model of the UV/H
2O
2 system to compare CFD model characteristics (e.g., ultraviolet radiation submodel, turbulent submodel, and kinetic rate constant) with system parameters (e.g., inlet flow rate, hydrogen peroxide capacity, and radical scavenger sensitivity analysis). They found that the CFD model predicted the experimental percent removal of methylene blue. Moreover, its degradation was sensitive to the background dissolved organic carbon concentration. The reaction mechanisms for the degradation of organic contaminants by UV/AOPs processes typically comprises a complex chain of fast chemical reactions [
11]. Therefore, intermediates and products that resulted from these processes can be very sensitive to UV irradiation rates in the reactor, as well as turbulence and mixing levels [
8]. Wojciech et al. [
6] studied the radiation doses received in different zones of a reactor according to the spatial variability of the velocity field and radiation intensity.
Compared with the actual experiment, the numerical simulation has lower cost and shorter cycle. Thus, the fluid flow process is presented vividly, and data that are difficult to be observed in actual experiments are obtained. In recent years, researchers have performed the numerical simulation of fluid dynamics and the chemical reaction of various reaction devices [
6,
12,
13], whereas others have carried out corresponding experimental verification on the simulation results [
11], which proves that the numerical calculation method is very feasible in simulating the hydrodynamic characteristics in the reactor. In this study, UV/H
2O
2 photochemical reaction system was applied to the airlift internal circulation reactor. First, the velocity and volume fraction distribution of gas and liquid and the turbulence intensity were investigated. Second, the chemical reaction kinetic model was used to simulate the migration and transformation of chemical components in the air lift internal circulation photoreactor. Finally, the experimental data were compared to provide theoretical support for the design and application of this kind of reactor in the future.
2. Mathematical Methods and Materials
2.1. Hydrodynamic, Mass Transfer, and Turbulent Model
Based on the principles of conservation of mass and momentum, a two-dimensional CFD model was developed to calculate the local hydrodynamics in photoreactor, and the phasic volume fraction
must satisfy the relation:
where
is the total number of phases; the subscript
represents gas or liquid phase. The conservation equations are written by performing an ensemble average of the local instantaneous balance for each phase. The motion of each phase is governed by corresponding mass and momentum conservation equations.
Continuity equations:
where
and
stand for the volume fraction, density, and velocity vector, respectively.
Momentum equation:
where
,
, and
are the pressure, viscosity, and gravity acceleration, respectively.
is the interfacial force acting on phase
due to the presence of the other phase,
.
The turbulent dispersion force is the result of the turbulent fluctuations of liquid velocity. The standard
model for single-phase flows has been extended for the two-phase flows, which can be described as:
where
, and
are parameters in the standard
model and the following values are selected:
and
. In addition, the turbulent viscosities
can be computed by other equations [
8,
14].
2.2. Interphase Force Models
During momentum exchange between the different phases, only the drag and turbulent dispersion force was considered [
8,
15]. Accordingly, the interfacial force
is approximated to be the drag force and interphase turbulent dispersion force:
where
are the drag force and turbulent dispersion force, respectively.
The drag force between the gas and liquid phases is calculated by the following equation:
where
is the drag coefficient. The Grace relation is chosen for the drag force coefficient because bubbles are experimentally observed to be spherical and dispersed [
15].
The turbulent dispersion force,
, is calculated by the model of Lopez de Bertodano [
16]:
where
is the momentum transfer coefficient for the interphase drag force,
is the drag coefficient as described above,
stands for turbulent viscosity, and
is the liquid turbulent Schmidt number.
and
are the gas and liquid phase volume fractions, respectively.
2.3. Radiative Transfer Model
The radiative transfer equation (RTE) for an absorbing, emitting, and scattering medium at position
in the direction
is
where
and
are position and direction vectors, respectively. The
is the radiation intensity, which depends on position and direction,
is the refractive index,
is the Stefan–Boltzmann constant (5.67 × 10
−8 Wm
−2K
−4),
is the absorption coefficient,
is the scattering coefficient,
is the phase function, and
is the solid angle. Additionally,
is the optical thickness or opacity of the fluid. The refractive index
is important when considering radiation in semitransparent media [
17].
2.4. Kinetic Model
In this study, photolysis of methylene blue (MB) was selected as a model reaction material. Since methylene blue is not directly photolyzed and does not react with H
2O
2 alone, MB is a dye that is good indicator for evaluation in the UV/AOPs study due to the decolorization upon reaction with the ·OH. The detailed kinetic model for the UV/H
2O
2 systems has been extensively verified by several previous studies and particularly in [
18] for continuous flow stirred tank reactors and by Scott M. Alpert et al. [
11].
Table 1 summarizes the system of kinetic rate equations describing the production of the ·OH from H
2O
2 and the corresponding degradation of MB, along with their rate constants. The photolysis of H
2O
2 is shown as reaction in
Table 1. In this study, the kinetic model was incorporated into CFD model using User-Defined Function (UDF) of FLUENT to prescribe the net rate of generation via
for each chemical species considered (Equations (1)–(7)).
2.5. Airlift Photoreactor Setup, Geometry, and Grid Generation
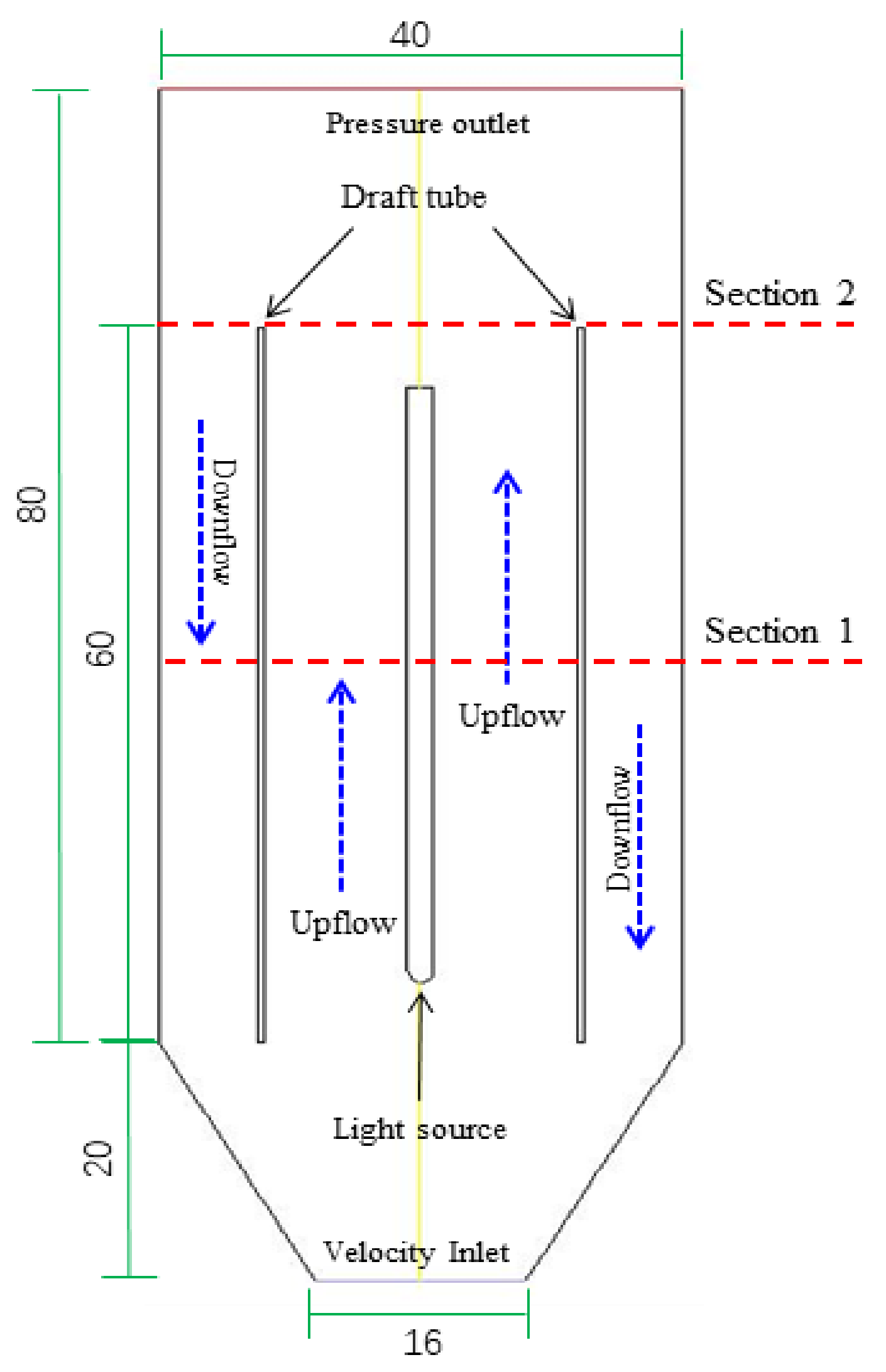
A flow through that continuously operated the airlift photoreactor was used to evaluate the CFD results experimentally. A simple two-dimensional geometry used for the internal airlift circulating photoreactor is shown in
Figure 1. It is conical bottomed, internally irradiated with the light source being placed in the central core axis, and has a draft tube with the internal circulation occurring around the light source. The reactor is divided into the central circulation area, the bottom air, and water distribution area. It is operated with a 54 W low-pressure mercury UV lamp (customized) that was approximately 2.3 cm in diameter and 50 cm in length and longitudinally placed at the axial center of the reactor. In this study, the position of the draft tube was set to 0.4 of r/R, where r is the distance between the reactor wall and the draft tube, and R is the distance between the reactor wall and the light source (R) [
8]. The inner part of the reactor is composed of a 60 cm long draft tube and a 50 cm long UV lamp. During the operation of the reactor, the solution with MB and H
2O
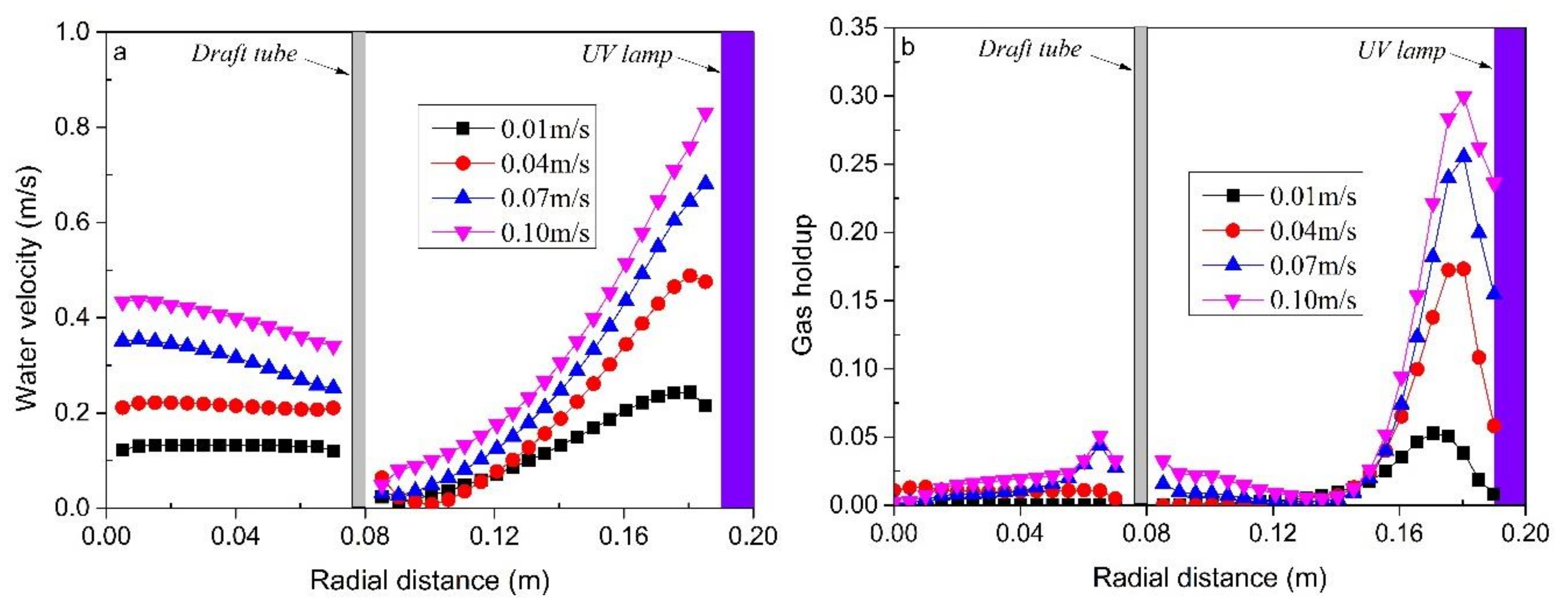
2 was injected from the bottom of the reactor at a rate of 0.3 m/s continuously. At the same time, the air was injected at flow velocity of 0.01, 0.04, 0.07, and 0.10 m/s, respectively. The reactor reached a stable condition after 120 min, then a total of six samples were taken at 30 min time intervals for MB determination. In addition, in order to further analyze the data of various physical elements in the reactor, the data used in this study are Section 1 in the middle of the reactor and Section 2 at the outlet of the reactor shown in
Figure 1.
The geometry was created inDesignModeler software (ANSYS, Inc., Canonsburg, PA USA). Given the characteristics of the axially symmetric structure of the reactor, the flow field simulation can be simplified reasonably by a 2D axial symmetry model. An unstructured numerical grid was implemented with a total number of 14,462 elements, where the minimum size of the grid is 0.15 mm, and the maximum face size is 5.0 mm.
2.6. Initial and Boundary Conditions
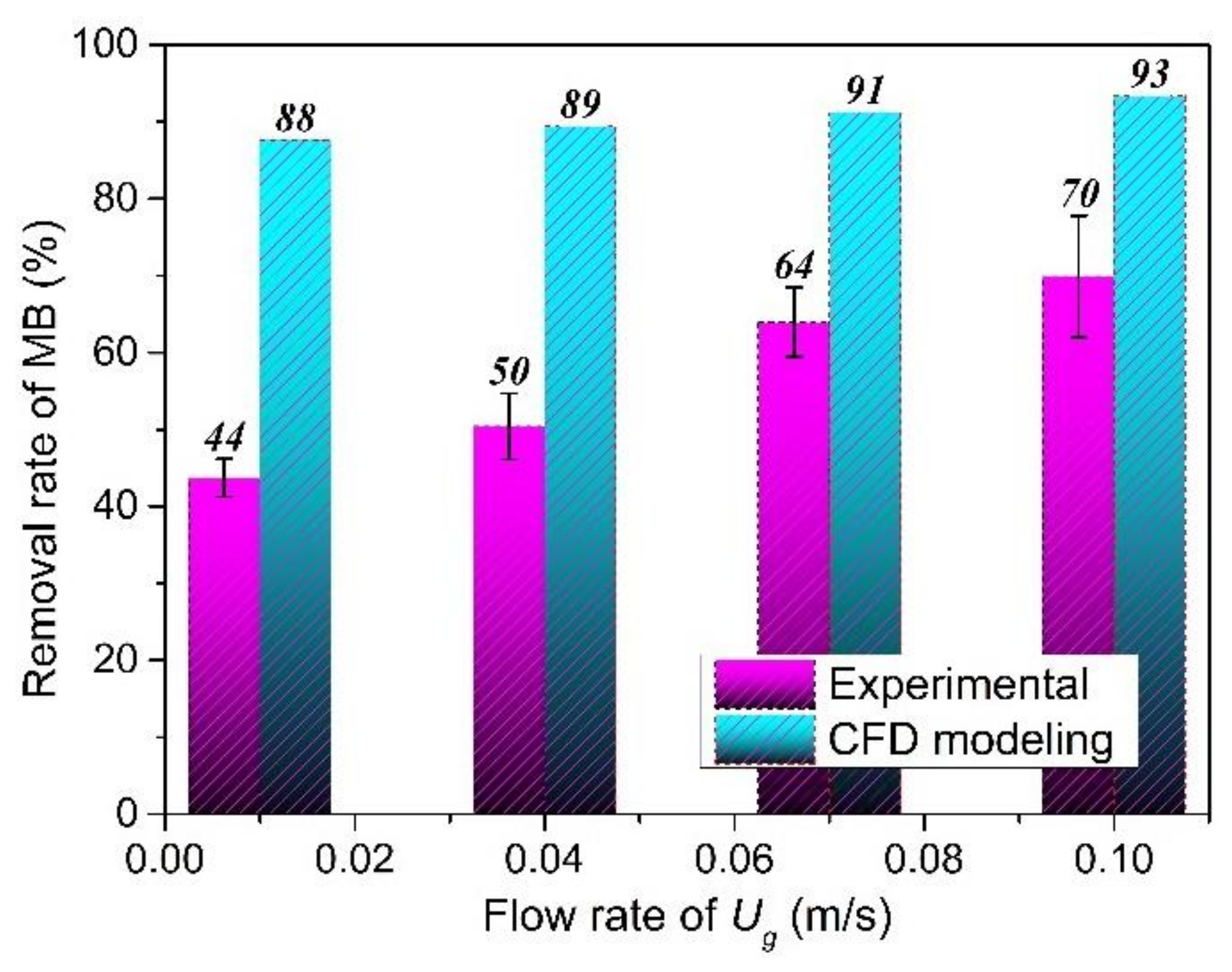
For the initial conditions, the liquid and gas phases enter the reactor from the bottom of the reactor at different flow rates. The gas and liquid phases were air and water in the standard state, respectively. A uniform air velocity Ug (0.01, 0.04, 0.07, and 0.10 m/s) was simulated from the gas inlet. Meanwhile, the liquid velocity was set at 0.3 m/s, the inlet MB concentration as model contaminant equaled 0.5 ppm, and the inlet H2O2 concentration was set equal to 10 ppm. The diameter and viscosity of air considered are 0.2 mm and 1.003 × 10−3 Pa, respectively. The refractive index and absorption coefficient were assigned at 1.376 and 12.78 (m−1, UVT = 88%), respectively.
A pressure boundary condition was applied to the top of the reactor with an average static reference pressure of 0 Pa. In addition, the periphery and inner area were defined as the opening and degassing boundary conditions, where the gas and liquid phases could leave the computation domain. For the reactor walls and the light source boundary, the gas phase was treated as free-slip, whereas a no-slip boundary condition was applied in the liquid phase [
19]. A no-slip boundary condition was imposed on the walls. In addition, the zero diffusive flux of species was specified at the walls. As per the radiation field boundary conditions, the lamp radiation was defined as a zero-thickness semitransparent wall and no-reflecting walls.
2.7. Numerical Solution
ANSYS Fluent 16.2 was used to read the mesh and perform the CFD computations. The segregated steady state solver was used to solve the governing equations. Second-order upwind discretization scheme was applied, except for pressure, for which the standard scheme was selected. The SIMPLE algorithm was selected for the pressure–velocity coupling. The variation of velocity magnitude, model contaminant concentration, and irradiation flux at several points of the computational domain were used as indicators of convergence (at least 20 iterations). Additionally, the convergence of the numerical solution was assured by monitoring the scaled residuals to a criterion of at least 10−4 for the MB concentration. Although simulation is always tracked with time, the solution algorithm has been run with steady and transient flow simulations.
2.8. Chemicals and Analytical Methods
For the airlift photoreactor experiments, the chemicals used for experiments were reagent grade or higher and supplied by Sigma–Aldrich. MB powder and H
2O
2 solution (30%
w/
v) were used as purchased without further purification. The MB and H
2O
2 were diluted separately with ultrapure laboratory water. Distilled water was used in all experiments and analytical determinations. The MB concentration in the airlift photoreactor effluent was determined spectrophotometrically by following the peak at 664 nm using a UV spectrophotometric probe (UV1800, Shimadzu Co., Kyoto, Japan spectrophotometer). The relationship between absorbance and concentration of MB solution is y = 0.189x + 0.003, where R
2 = 0.9998. The hydrogen peroxide concentration was determined via UV spectrophotometry by using
method [
20].