Grapevine Sap Flow in Response to Physio-Environmental Factors under Solar Greenhouse Conditions
Abstract
1. Introduction
2. Materials and Methods
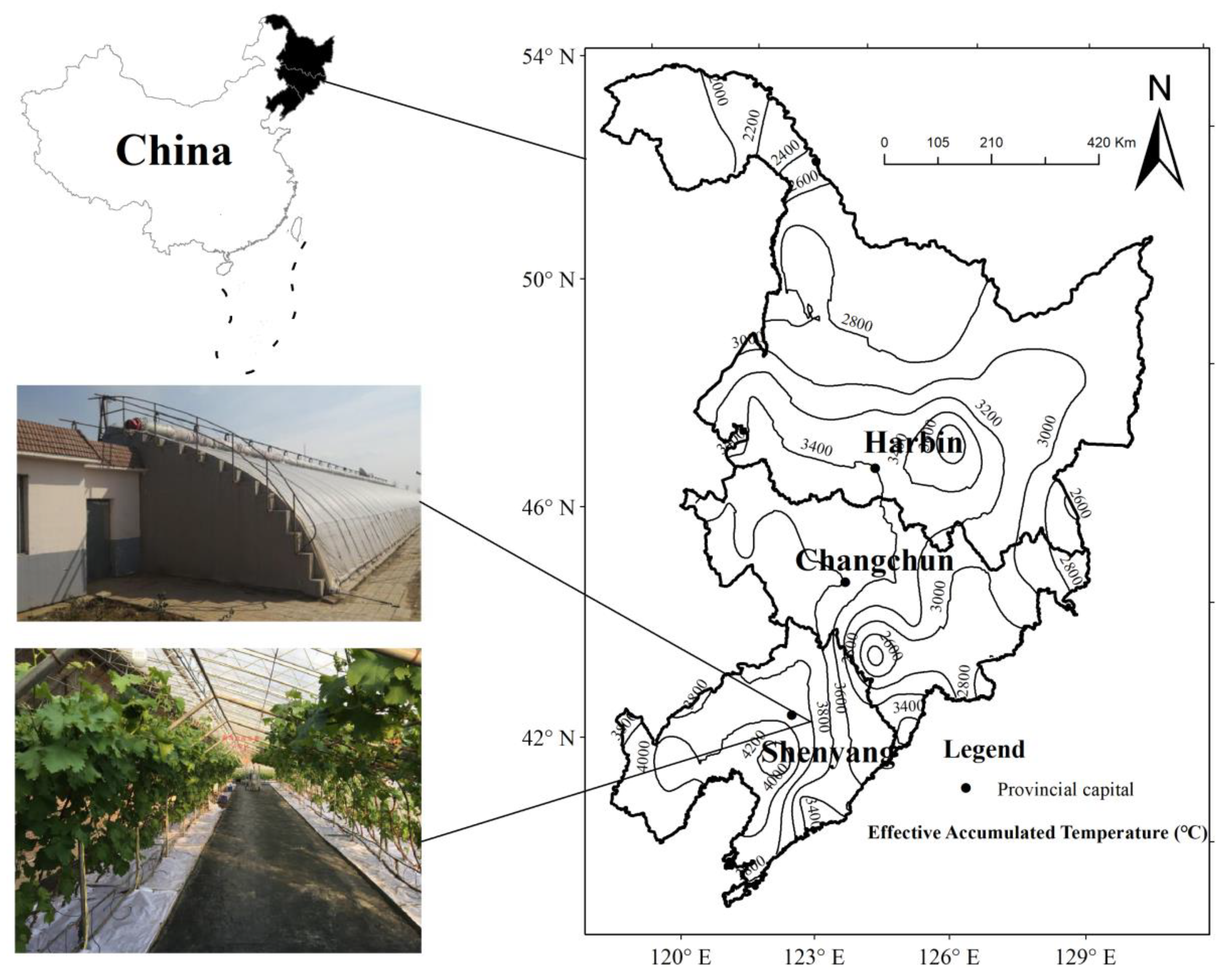
2.1. Study Site
2.2. Data Collection
2.2.1. Sap Flow
2.2.2. Leaf Area Index (LAI)
2.2.3. Soil Water Content
2.2.4. Meteorology
2.3. Data Analysis
3. Results
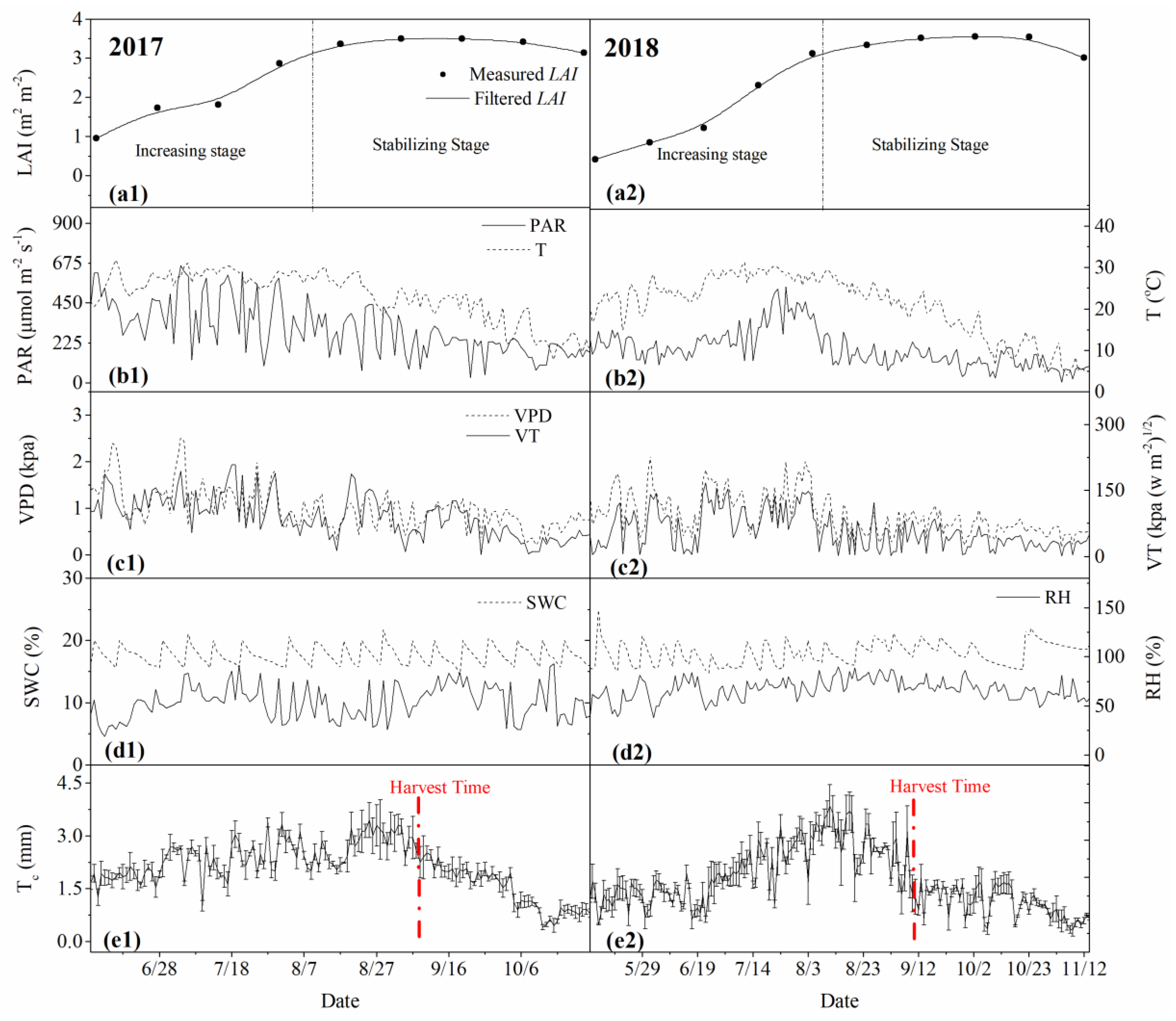
3.1. Dynamics of Physiological and Environmental Factors
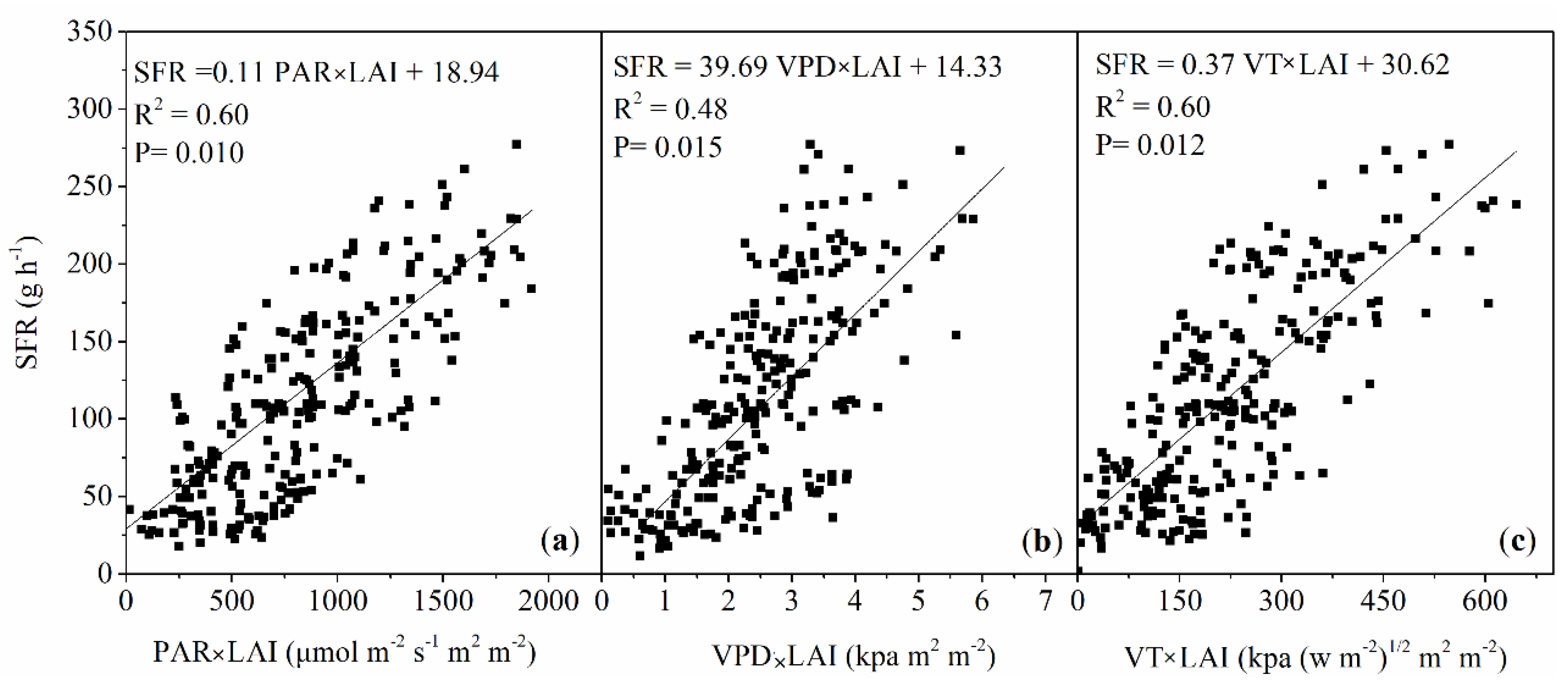
3.2. Control of Leaf Area Index on Sap Flow Rate
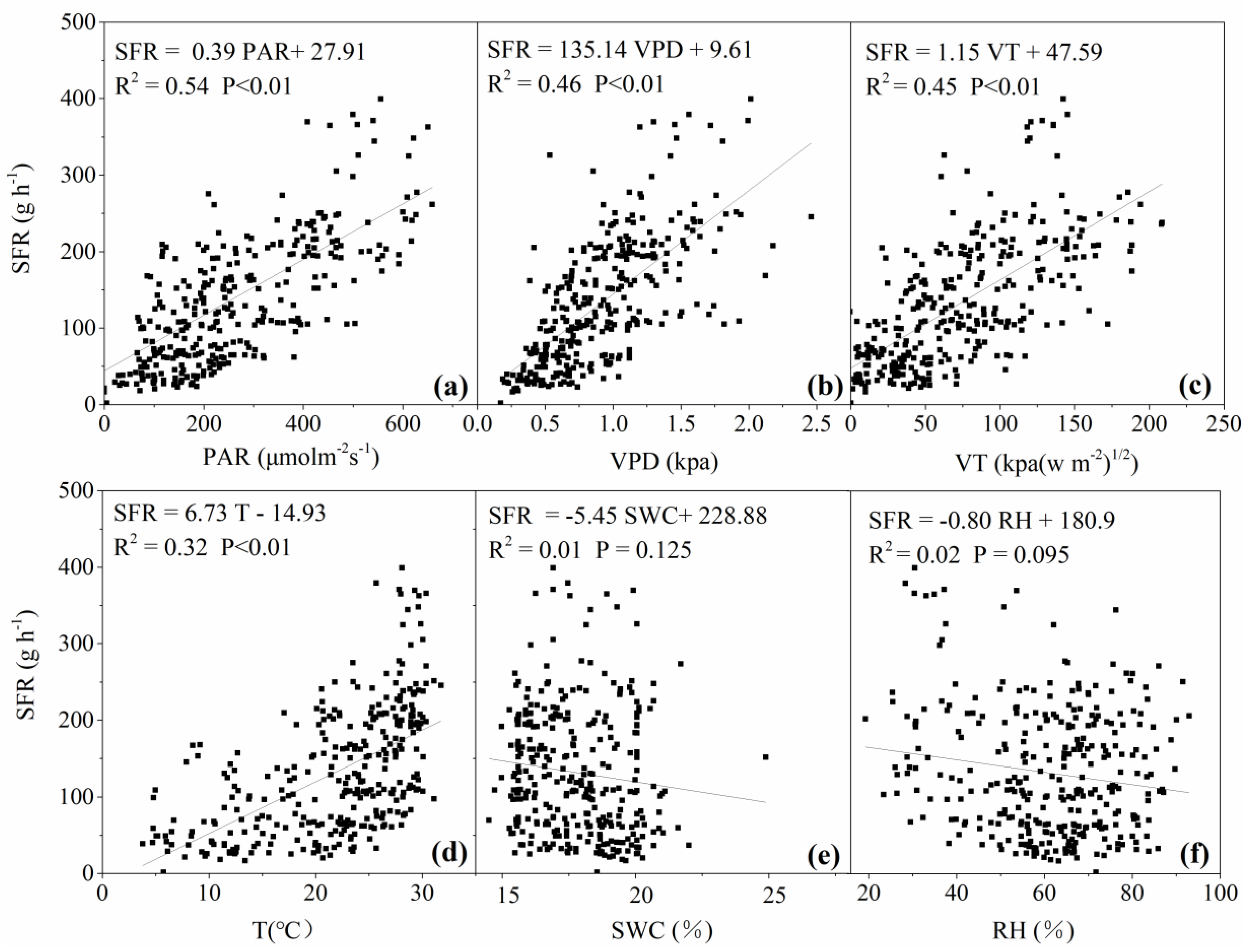
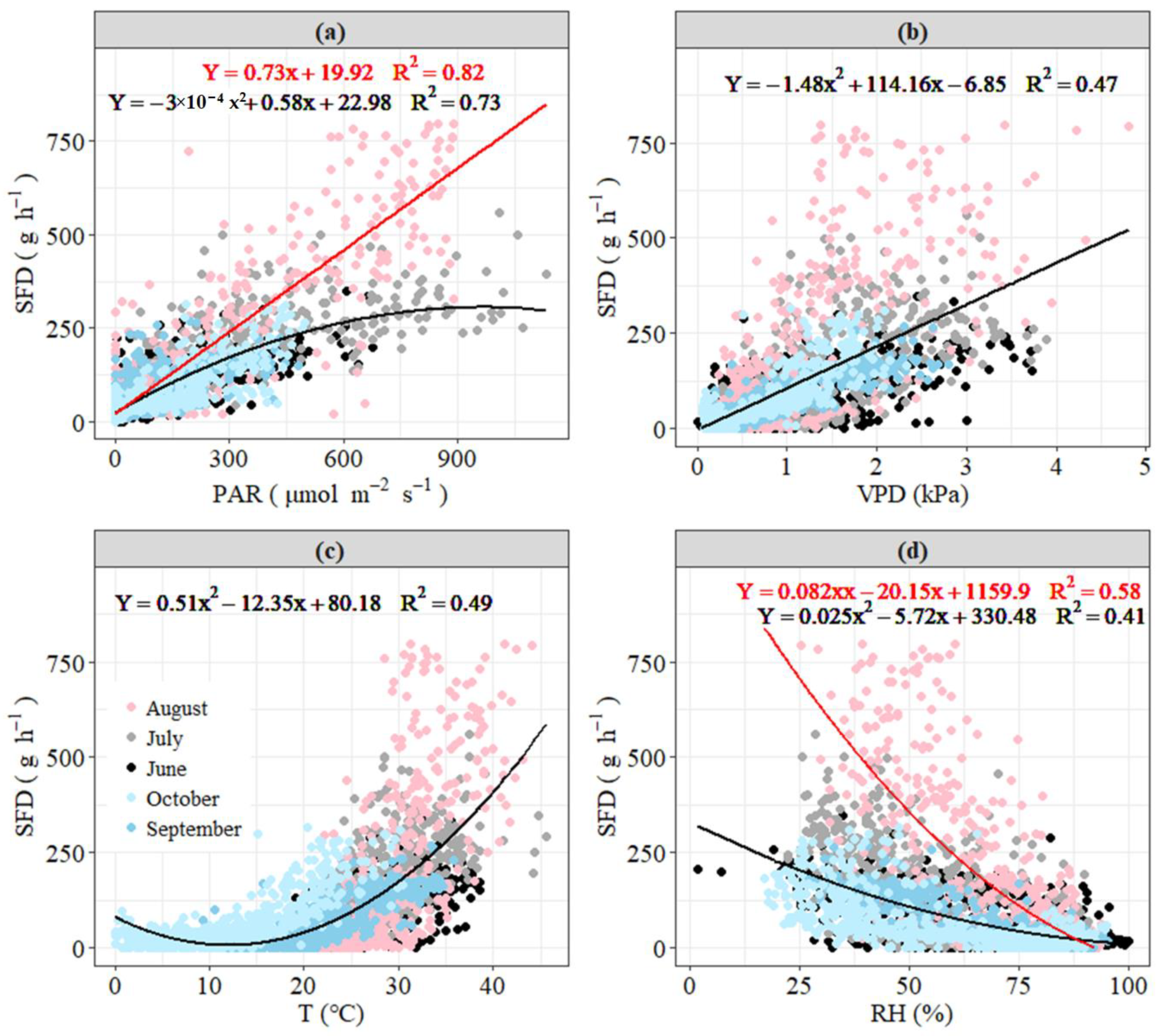
3.3. Response of Daily Sap Flow to Main Environmental Factors
3.4. Response of Hourly Sap Flow to Climatic Factors and Seasonal Variability
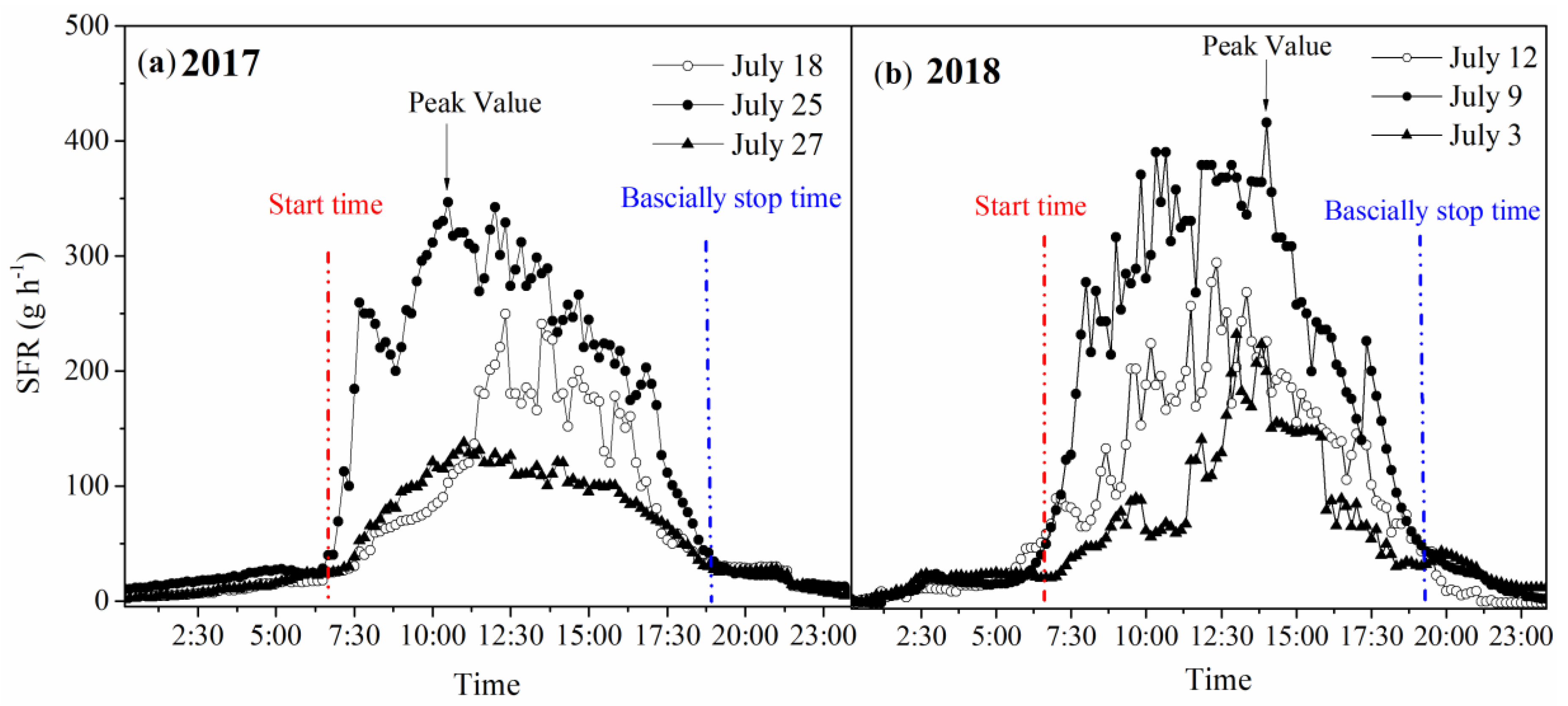
3.5. Diurnal Variations in SFR under Different Weather Conditions
3.6. Lags and Hysteresis of Sap Flow
4. Discussion
4.1. Influence of Physiological Factors on Sap Flow
4.2. Influence of Environmental Factors on Sap Flow
4.2.1. Daily Scale
4.2.2. Hourly Scale
4.3. Diurnal Variations of Sap Flow and Hysteresis Loops
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jasechko, S.; Sharp, Z.D.; Gibson, J.J.; Birks, S.J.; Yi, Y.; Fawcett, P.J. Terrestrial water fluxes dominated by transpiration. Nat. Cell Biol. 2013, 496, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.V.; Mendes, K.; Mutti, P.; Medeiros, S.; Silva, L.; Perez-Marin, A.M.; Campos, S.; Lúcio, P.S.; Lima, K.; Dos Reis, J.; et al. Environmental and biophysical controls of evapotranspiration from Seasonally Dry Tropical Forests (Caatinga) in the Brazilian Semiarid. Agric. For. Meteorol. 2020, 287, 107957. [Google Scholar] [CrossRef]
- Ma, C.; Luo, Y.; Shao, M.; Li, X.; Sun, L.; Jia, X. Environmental controls on sap flow in black locust forest in Loess Plateau, China. Sci. Rep. 2017, 7, 13160. [Google Scholar] [CrossRef] [PubMed]
- Miner, G.L.; Ham, J.M.; Kluitenberg, G.J. A heat-pulse method for measuring sap flow in corn and sunflower using 3D-printed sensor bodies and low-cost electronics. Agric. For. Meteorol. 2017. [Google Scholar] [CrossRef]
- Poyatos, R.; Villagarcía, L.; Domingo, F.; Piñol, J.; Llorens, P. Modelling evapotranspiration in a Scots pine stand under Mediterranean mountain climate using the GLUE methodology. Agric. For. Meteorol. 2007, 146, 13–28. [Google Scholar] [CrossRef]
- Rana, G.; Ferrara, R.M.; Mazza, G. A model for estimating transpiration of rainfed urban trees in Mediterranean environment. Theor. Appl. Clim. 2019, 138, 683–699. [Google Scholar] [CrossRef]
- O’Grady, A.P.; Worledge, D.; Battaglia, M. Constraints on transpiration of Eucalyptus globulus in southern Tasmania, Australia. Agric. For. Meteorol. 2008, 148, 453–465. [Google Scholar] [CrossRef]
- Komatsu, H.; Kang, Y.; Kume, T.; Yoshifuji, N.; Hotta, N. Transpiration from aCryptomeria japonica plantation, part 1: Aerodynamic control of transpiration. Hydrol. Process. 2006, 20, 1309–1320. [Google Scholar] [CrossRef]
- Tie, Q.; Hu, H.; Tian, F.; Guan, H.; Lin, H. Environmental and physiological controls on sap flow in a subhumid mountainous catchment in North China. Agric. For. Meteorol. 2017, 46–57. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; Zeppel, M.; Palmer, A.; Eamus, D. Seasonal variations in tree water use and physiology correlate with soil salinity and soil water content in remnant woodlands on saline soils. J. Arid. Environ. 2016, 129, 102–110. [Google Scholar] [CrossRef]
- Gong, X.; Liu, H.; Sun, J.; Gao, Y.; Zhang, H. Comparison of Shuttleworth-Wallace model and dual crop coefficient method for estimating evapotranspiration of tomato cultivated in a solar greenhouse. Agric. Water Manag. 2019, 217, 141–153. [Google Scholar] [CrossRef]
- Yan, H.; Acquah, S.J.; Zhang, C.; Wang, G.; Huang, S.; Zhang, H.; Zhao, B.; Wu, H. Energy partitioning of greenhouse cucumber based on the application of Penman-Monteith and Bulk Transfer models. Agric. Water Manag. 2019, 217, 201–211. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Modelling the transpiration of a greenhouse zucchini crop grown under a Mediterranean climate using the Penman-Monteith equation and its simplified version. Aust. J. Agric. Res. 2004, 55, 931. [Google Scholar] [CrossRef]
- Ali, H.B.; Bournet, P.-E.; Cannavo, P.; Chantoiseau, E. Development of a CFD crop submodel for simulating microclimate and transpiration of ornamental plants grown in a greenhouse under water restriction. Comput. Electron. Agric. 2018, 149, 26–40. [Google Scholar] [CrossRef]
- Van Herk, I.G.; Gower, S.T.; Bronson, D.R.; Tanner, M.S. Effects of climate warming on canopy water dynamics of a boreal black spruce plantation. Can. J. For. Res. 2011, 41, 217–227. [Google Scholar] [CrossRef]
- Abdelfatah, A.; Aranda, X.; Savé, R.; De Herralde, F.; Biel, C. Evaluation of the response of maximum daily shrinkage in young cherry trees submitted to water stress cycles in a greenhouse. Agric. Water Manag. 2013, 118, 150–158. [Google Scholar] [CrossRef]
- Alarcón, J.; Domingo, R.; Green, S.; Sánchez-Blanco, M.; Rodríguez, P.; Torrecillas, A. Sap flow as an indicator of transpiration and the water status of young apricot trees. Plant Soil 2000, 227, 77–85. [Google Scholar] [CrossRef]
- Li, T.L. Science and Technology and Industrial Development Status and Trends of China’s Facilities Vegetable. China Rural Sci. Technol. 2016, 5, 77–79. [Google Scholar]
- Shackel, K.A.; Johnson, R.S.; Medawar, C.K.; Phene, C.J. Substantial errors in estimates of sap flow using the heat balance technique on woody stems under field conditions. J. Am. Soc. Hortic. Sci. 1992, 117, 351–356. [Google Scholar] [CrossRef]
- Solie, R.B.T.N. An Introduction to Environmental Biophysicsby Gaylon S. Campbell. Am. Sci. 1978, 66, 237–238. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, S.; Wei, X.; Wang, X. Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid Northwest China. Agric. Water Manag. 2014, 136, 23–33. [Google Scholar] [CrossRef]
- Köstner, B.M.M.; Schulze, E.-D.; Kelliher, F.M.; Hollinger, D.Y.; Byers, J.N.; Hunt, J.E.; McSeveny, T.M.; Meserth, R.; Weir, P.L. Transpiration and canopy conductance in a pristine broad-leaved forest of Nothofagus: An analysis of xylem sap flow and eddy correlation measurements. Oecologia 1992, 91, 350–359. [Google Scholar] [CrossRef]
- De León, M.A.P.; Bailey, B.N. Evaluating the use of Beer’s law for estimating light interception in canopy architectures with varying heterogeneity and anisotropy. Ecol. Model. 2019, 406, 133–143. [Google Scholar] [CrossRef]
- Liu, C.; Du, T.; Li, F.; Kang, S.; Li, S.; Tong, L. Trunk sap flow characteristics during two growth stages of apple tree and its relationships with affecting factors in an arid region of northwest China. Agric. Water Manag. 2012, 104, 193–202. [Google Scholar] [CrossRef]
- Kropp, H.; Loranty, M.M.; Alexander, H.D.; Berner, L.T.; Natali, S.M.; Spawn, S.A. Environmental constraints on transpiration and stomatal conductance in a Siberian Arctic boreal forest. J. Geophys. Res. Biogeosciences 2017, 122, 487–497. [Google Scholar] [CrossRef]
- Massoud, E.C.; Purdy, A.; Christoffersen, B.; Santiago, L.; Xu, C. Bayesian inference of hydraulic properties in and around a white fir using a process-based ecohydrologic model. Environ. Model. Softw. 2019, 115, 76–85. [Google Scholar] [CrossRef]
- Nagler, P.L.; Glenn, E.P.; Thompson, T.L. Comparison of transpiration rates among saltcedar, cottonwood and willow trees by sap flow and canopy temperature methods. Agric. For. Meteorol. 2003, 116, 73–89. [Google Scholar] [CrossRef]
- Du, T.S.; Kang, S.Z.; Zhang, B.Z.; Li, S.E.; Yang, X.Y. Stem sap flow of grape under different drip irrigation patterns and its relationships with environmental factors in arid oasis region of Shiyang River basin. Chin. J. Appl. Ecol. 2008, 19, 299–305. [Google Scholar] [CrossRef]
- Li, B.; Shi, B.; Yao, Z.; Shukla, M.K.; Du, T. Energy partitioning and microclimate of solar greenhouse under drip and furrow irrigation systems. Agric. Water Manag. 2020, 234, 106096. [Google Scholar] [CrossRef]
- Duursma, R.A.; Blackman, C.J.; Lopéz, R.; Martin-StPaul, N.; Cochard, H.; Medlyn, B.E. On the minimum leaf conductance: Its role in models of plant water use, and ecological and environmental controls. New Phytol. 2018, 221, 693–705. [Google Scholar] [CrossRef]
- Frank, D.C.; Poulter, B.; Saurer, M.; Esper, J.; Huntingford, C.; Helle, G.; Treydte, K.; Zimmermann, N.E.; Schleser, G.H.; Ahlström, A.; et al. Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Chang. 2015, 5, 579–583. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Medlyn, B.E.; Duursma, R.A.; Prentice, I.C.; Wang, H.; Baig, S.; Eamus, D.; De Dios, V.R.; Mitchell, P.J.; Ellsworth, D.S.; et al. Optimal stomatal behaviour around the world. Nat. Clim. Chang. 2015, 5, 459–464. [Google Scholar] [CrossRef]
- E Ewers, B.; Gower, S.T.; Bond-Lamberty, B.; Wang, C.K. Effects of stand age and tree species on canopy transpiration and average stomatal conductance of boreal forests. Plant Cell Environ. 2005, 28, 660–678. [Google Scholar] [CrossRef]
- Bernier, P.; Bartlett, P.; Black, T.; Barr, A.; Kljun, N.; McCaughey, J. Drought constraints on transpiration and canopy conductance in mature aspen and jack pine stands. Agric. For. Meteorol. 2006, 140, 64–78. [Google Scholar] [CrossRef]
- Limousin, J.M.; Rambal, S.; Ourcival, J.M.; Rocheteau, A.; Joffre, R.; Rodriguezcortina, R. Long-term transpiration change with rainfall decline in a MediterraneanQuercus ilexforest. Glob. Chang. Biol. 2009, 15, 2163–2175. [Google Scholar] [CrossRef]
- Cinnirella, S.; Magnani, F.; Saracino, A.; Borghetti, M. Response of a mature Pinus laricio plantation to a three-year restriction of water supply: Structural and functional acclimation to drought. Tree Physiol. 2002, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Depante, M.; Morison, M.Q.; Petrone, R.M.; DeVito, K.J.; Kettridge, N.; Waddington, J.M. Hydraulic redistribution and hydrological controls on aspen transpiration and establishment in peatlands following wildfire. Hydrol. Process. 2019, 33, 2714–2728. [Google Scholar] [CrossRef]
- Brown, S.M.; Petrone, R.M.; Chasmer, L.; Mendoza, C.A.; Lazerjan, M.S.; Landhäusser, S.M.; Silins, U.; Leach, J.; DeVito, K.J. Atmospheric and soil moisture controls on evapotranspiration from above and within a Western Boreal Plain aspen forest. Hydrol. Process. 2013, 28, 4449–4462. [Google Scholar] [CrossRef]
- Mackay, D.S.; Ewers, B.E.; Cook, B.D.; Davis, K.J. Environmental drivers of evapotranspiration in a shrub wetland and an upland forest in northern Wisconsin. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Oberbauer, S.F.; Clark, D.B. Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant Cell Environ. 2004, 27, 551–567. [Google Scholar] [CrossRef]
- Wilson, T.G.; Kustas, W.; Alfieri, J.G.; Anderson, M.C.; Gao, F.; Prueger, J.H.; McKee, L.G.; Alsina, M.M.; Sanchez, L.A.; Alstad, K.P. Relationships between soil water content, evapotranspiration, and irrigation measurements in a California drip-irrigated Pinot noir vineyard. Agric. Water Manag. 2020, 237, 106186. [Google Scholar] [CrossRef]
- Hölscher, D.; Koch, O.; Korn, S.; Leuschner, C. Sap flux of five co-occurring tree species in a temperate broad-leaved forest during seasonal soil drought. Trees 2005, 19, 628–637. [Google Scholar] [CrossRef]
- Mutti, P.R.; Da Silva, L.L.; Medeiros, S.D.S.; Dubreuil, V.; Mendes, K.R.; Marques, T.V.; Lúcio, P.S.; e Silva, C.M.S.; Bezerra, B.G. Basin scale rainfall-evapotranspiration dynamics in a tropical semiarid environment during dry and wet years. Int. J. Appl. Earth Obs. Geoinf. 2019, 75, 29–43. [Google Scholar] [CrossRef]
- Petousi, I.; Daskalakis, G.; Fountoulakis, M.; Lydakis, D.; Fletcher, L.; Stentiford, E.; Manios, T. Effects of treated wastewater irrigation on the establishment of young grapevines. Sci. Total. Environ. 2019, 658, 485–492. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; Arbonès, A.; Alegre, S.; Rufat, J.; Marsal, J. Peach Tree Response to Single and Combined Regulated Deficit Irrigation Regimes under Shallow Soils. J. Am. Soc. Hortic. Sci. 2003, 128, 432–440. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Liu, S.; Wang, Y.; Gao, Z.; Zhang, L.; Wei, X.; Wei, X. Using Bayesian analysis to compare the performance of three evapotranspiration models for rainfed jujube (Ziziphus jujuba Mill.) plantations in the Loess Plateau. Agric. Water Manag. 2015, 159, 341–357. [Google Scholar] [CrossRef]
- Neves, L.H.; Santos, R.I.N.; Teixeira, G.I.D.S.; De Araujo, D.G.; Silvestre, W.V.D.; Pinheiro, H.A. Leaf gas exchange, photochemical responses and oxidative damages in assai (Euterpe oleracea Mart.) seedlings subjected to high temperature stress. Sci. Hortic. 2019, 257, 257. [Google Scholar] [CrossRef]
- Mendes, K.R.; Granja, J.A.A.; Ometto, J.; Antonino, A.C.D.; Menezes, R.S.C.; Pereira, E.C.; Pompelli, M.F. Croton blanchetianus modulates its morphophysiological responses to tolerate drought in a tropical dry forest. Funct. Plant Biol. 2017, 44, 1039. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, D.; Lakso, A.; Piccioni, R. Transpiration of apple trees in a humid climate using heat pulse sap flow gauges calibrated with whole-canopy gas exchange chambers. Agric. For. Meteorol. 2005, 130, 85–94. [Google Scholar] [CrossRef]
- Shao, G.; Huang, D.; Cheng, X.; Cui, J.; Zhang, Z. Path analysis of sap flow of tomato under rain shelters in response to drought stress. Int. J. Agric. Biol. Eng. 2016, 9, 54–62. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Li, Z.; Tang, J.; Caldwell, P.; Zhang, W. Biophysical control of whole tree transpiration under an urban environment in Northern China. J. Hydrol. 2011, 402, 388–400. [Google Scholar] [CrossRef]
- Juhász, Á.; Sepsi, P.; Nagy, Z.; Tőkei, L.; Hrotkó, K. Water consumption of sweet cherry trees estimated by sap flow measurement. Sci. Hortic. 2013, 164, 41–49. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Chen, X.; Zhou, G.; Cheng, J.; Zhang, D.; Meng, Z.; Zhang, Q. Partitioning evapotranspiration in an intact forested watershed in southern China. Ecohydrology 2014, 8, 1037–1047. [Google Scholar] [CrossRef]
- Farrer, E.C.; Goldberg, D.E.; King, A.A. Time Lags and the Balance of Positive and Negative Interactions in Driving Grassland Community Dynamics. Am. Nat. 2010, 175, 160–173. [Google Scholar] [CrossRef]
- Kume, T.; Komatsu, H.; Kuraji, K.; Suzuki, M. Less than 20-min time lags between transpiration and stem sap flow in emergent trees in a Bornean tropical rainforest. Agric. For. Meteorol. 2008, 148, 1181–1189. [Google Scholar] [CrossRef]


| Grapevine Number | Trunk Length (m) | Average Length of Lateral Branch (m) | Number of Fruit Bearing Branches | Trunk Diameter (cm) | ||||
|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | |
| 1 | 3.81 | 3.83 | 0.65 | 0.61 | 14 | 13 | 1.09 | 1.15 |
| 2 | 3.82 | 3.89 | 0.59 | 0.65 | 14 | 15 | 1.03 | 1.09 |
| 3 | 3.85 | 3.88 | 0.61 | 0.70 | 15 | 15 | 1.09 | 1.18 |
| 4 | 3.84 | 3.81 | 0.64 | 0.60 | 15 | 14 | 1.04 | 1.08 |
| 5 | 4.01 | 4.17 | 0.59 | 0.53 | 15 | 15 | 1.07 | 1.12 |
| 6 | 3.84 | 3.82 | 0.61 | 0.62 | 15 | 14 | 1.09 | 1.17 |
| 7 | 3.87 | 3.88 | 0.66 | 0.6 | 15 | 13 | 1.01 | 1.06 |
| 8 | 3.82 | 3.83 | 0.62 | 0.64 | 14 | 14 | 1.06 | 1.12 |
| 9 | 3.89 | 3.93 | 0.58 | 0.62 | 14 | 15 | 1.05 | 1.13 |
| 10 | 3.89 | 3.81 | 0.61 | 0.59 | 15 | 15 | 1.04 | 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Fu, S.; Chen, D.; Zheng, S.; Wang, T.; Bai, Y. Grapevine Sap Flow in Response to Physio-Environmental Factors under Solar Greenhouse Conditions. Water 2020, 12, 3081. https://doi.org/10.3390/w12113081
Wei X, Fu S, Chen D, Zheng S, Wang T, Bai Y. Grapevine Sap Flow in Response to Physio-Environmental Factors under Solar Greenhouse Conditions. Water. 2020; 12(11):3081. https://doi.org/10.3390/w12113081
Chicago/Turabian StyleWei, Xinguang, Shining Fu, Dianyu Chen, Siyu Zheng, Tieliang Wang, and Yikui Bai. 2020. "Grapevine Sap Flow in Response to Physio-Environmental Factors under Solar Greenhouse Conditions" Water 12, no. 11: 3081. https://doi.org/10.3390/w12113081
APA StyleWei, X., Fu, S., Chen, D., Zheng, S., Wang, T., & Bai, Y. (2020). Grapevine Sap Flow in Response to Physio-Environmental Factors under Solar Greenhouse Conditions. Water, 12(11), 3081. https://doi.org/10.3390/w12113081




