Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia Demonstrate Morphological and Ecological Stasis
Abstract
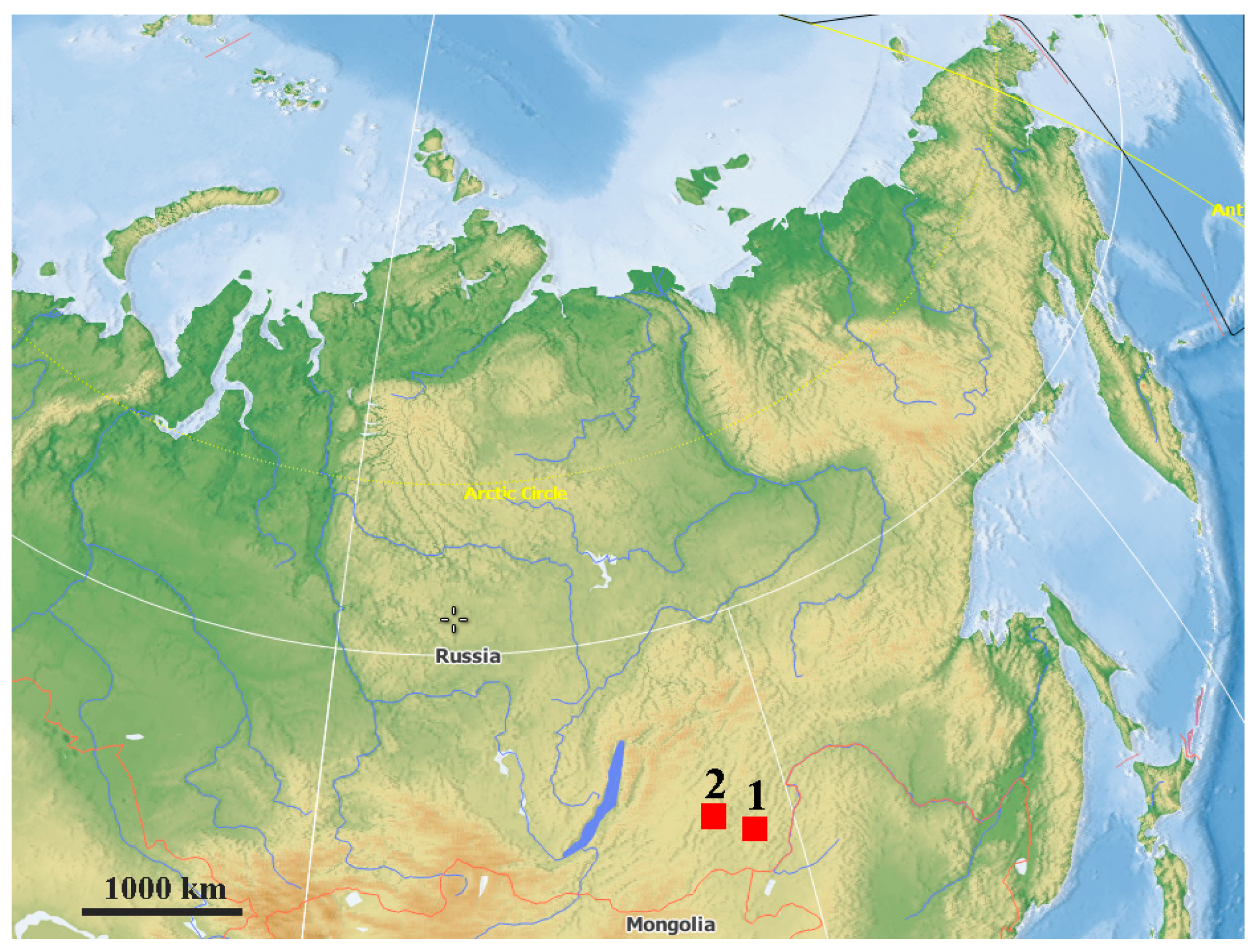
1. Introduction
2. Materials and Methods
3. Results
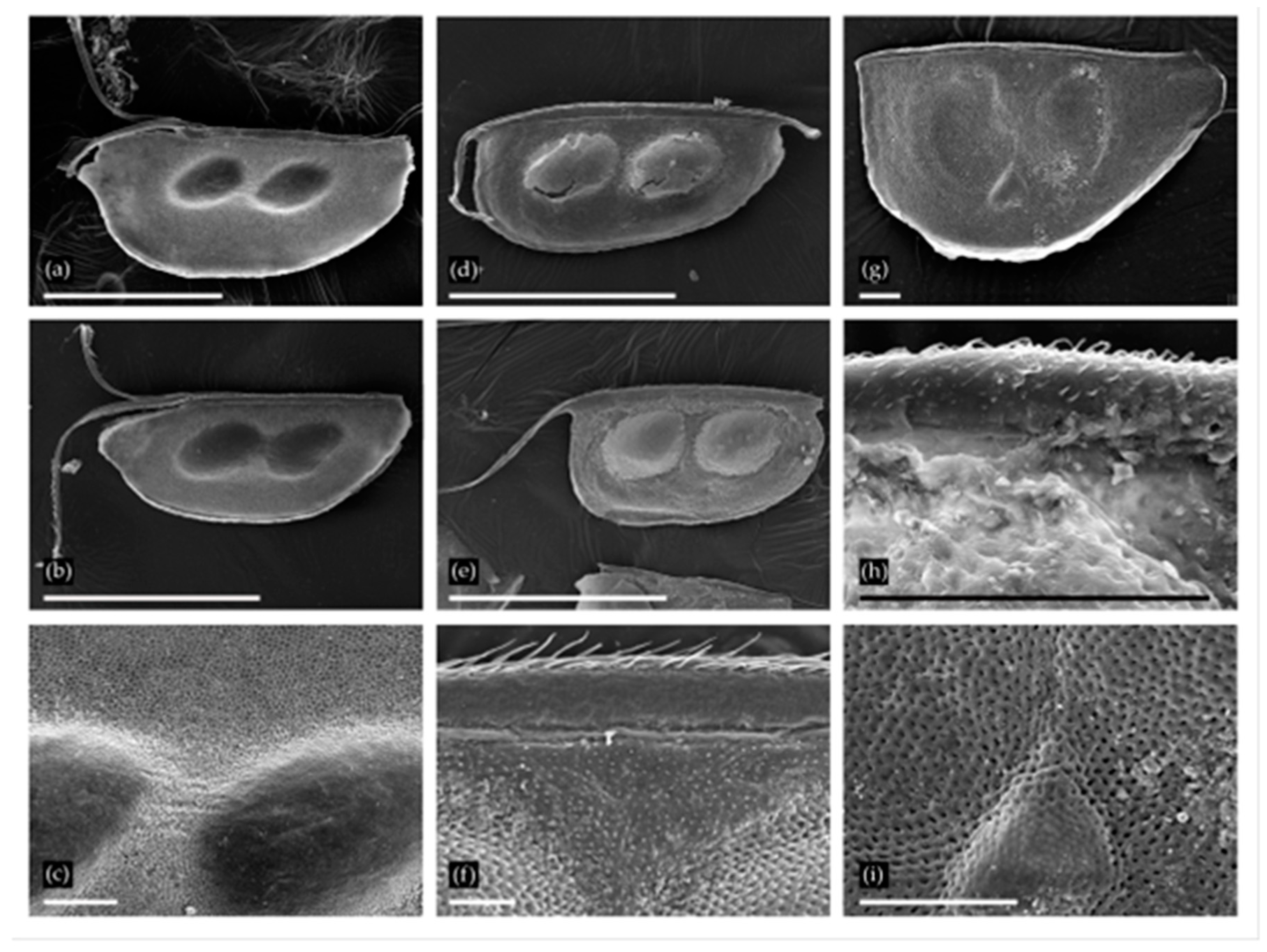
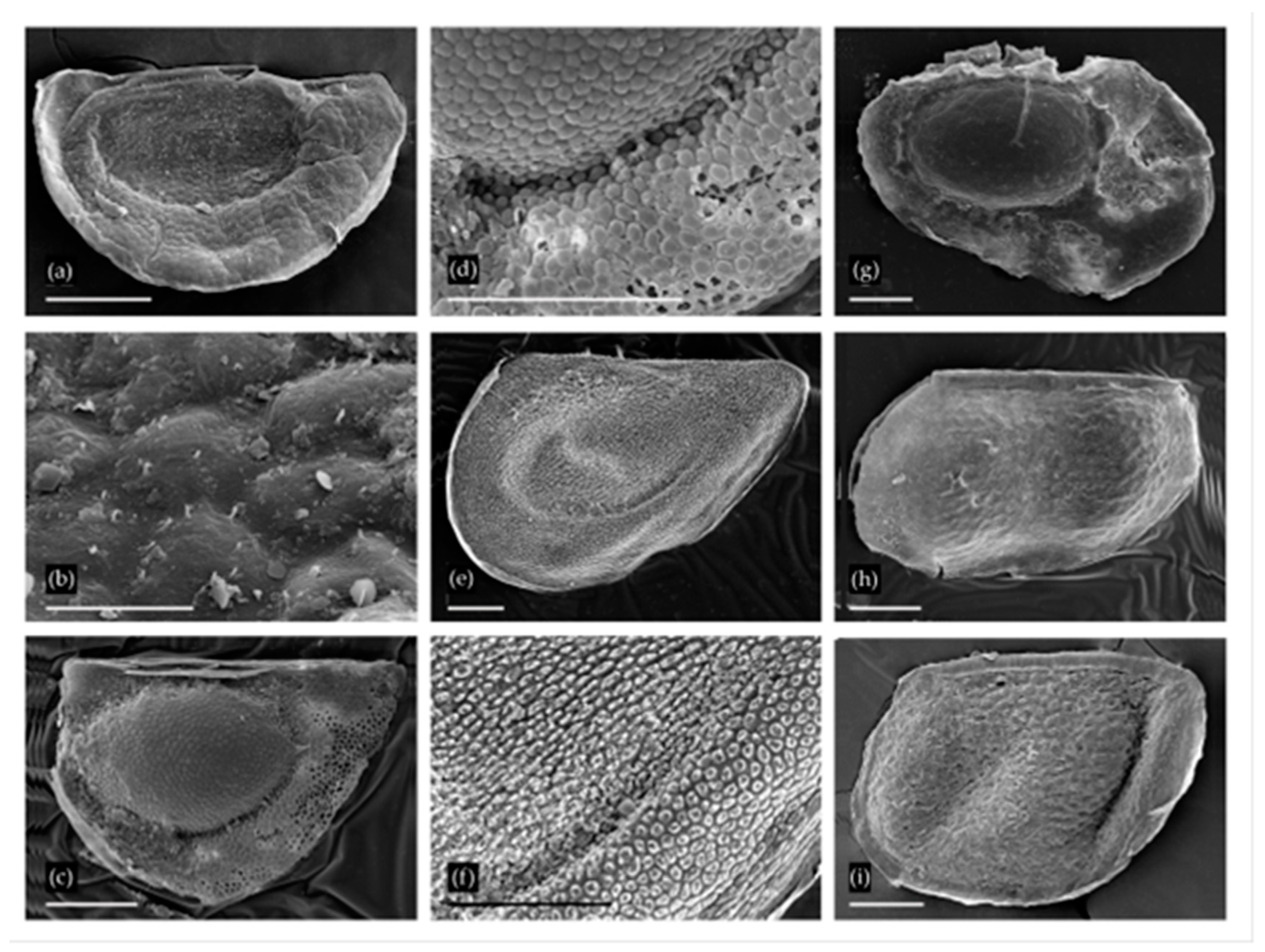
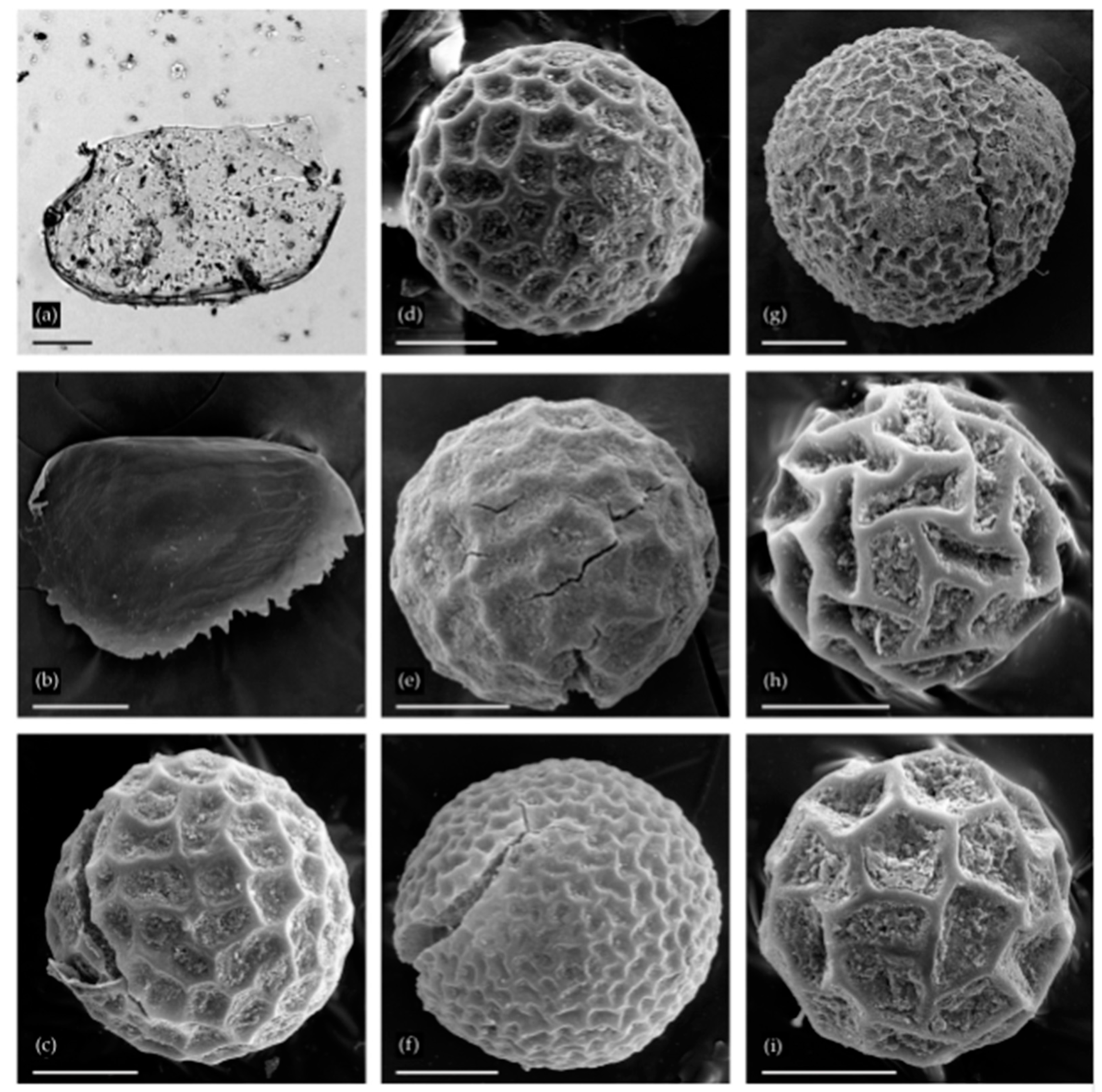
3.1. Cladocera
3.2. Anostraca
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harvey, T.H.P.; Vélez, M.I.; Butterfield, N.J. Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc. Natl. Acad. Sci. USA 2012, 109, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Lagebro, L.; Gueriau, P.; Hegna, T.A.; Rabet, N.; Butler, A.D.; Budd, G.E. The oldest notostracan (Upper Devonian Strud locality, Belgium). Palaeontology 2015, 58, 497–509. [Google Scholar] [CrossRef]
- Gueriau, P.; Rabet, N.; Du Tien Hat, E. The Strud crustacean fauna (Late Devonian, Belgium): Updated review and palaeoecology of an early continental ecosystem. Earth Environ. Sci. Trans. R. Soc. Edinb. 2016, 107, 79–90. [Google Scholar] [CrossRef]
- Gueriau, P.; Rabet, N.; Clément, G.; Lagebro, L.; Vannier, J.; Briggs, D.E.G.; Charbonnier, S.; Olive, S.; Béthoux, O. A 365-Million-Year-Old Freshwater Community Reveals Morphological and Ecological Stasis in Branchiopod Crustaceans. Curr. Biol. 2016, 26, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, K.; Kotov, A.A. The fossil record of the Cladocera (Crustacea: Branchiopoda): Evidence and hypotheses. Earth Sci. Rev. 2016, 163, 162–189. [Google Scholar] [CrossRef]
- Smirnov, N.N. Mesozoic Anomopoda (Crustacea) from Mongolia. Zool. J. Linn. Soc. 1992, 104, 97–116. [Google Scholar] [CrossRef]
- Kotov, A.A.; Korovchinsky, N.M. First record of fossil Mesozoic Ctenopoda (Crustacea, Cladocera). Zool. J. Linn. Soc. 2006, 146, 269–274. [Google Scholar] [CrossRef]
- Kotov, A.A. Jurassic Cladocera (Crustacea, Branchiopoda) with a description of an extinct Mesozoic order. J. Nat. Hist. 2007, 41, 13–37. [Google Scholar] [CrossRef]
- Kotov, A.A.; Taylor, D.J. Mesozoic fossils (145 Mya) suggest the antiquity of the subgenera of Daphnia and their coevolution with chaoborid predators. BMC Evol. Biol. 2011, 11, 129. [Google Scholar] [CrossRef]
- Bennike, O. Palaeoecology of two lake basins from Disko, West Greenland. J. Quat. Sci. 1995, 10, 149–155. [Google Scholar] [CrossRef]
- Knecht, R.J.; Benner, J.S.; Rogers, D.C.; Ridge, J.C. Surculichnus bifurcauda n. igen., n. isp., a trace fossil from Late Pleistocene glaciolacustrine varves of the Connecticut River Valley, USA, attributed to notostracan crustaceans based on neoichnological experimentation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 272, 232–239. [Google Scholar] [CrossRef]
- Frey, D.G. The Ecological Significance of Cladoceran Remains in Lake Sediments. Ecology 1960, 41, 684–699. [Google Scholar] [CrossRef]
- Polcyn, I. Application of Cladocera analysis in archaeology. Circaea 1996, 11, 41–48. [Google Scholar]
- Korhola, A.; Rautio, M. Cladocera and other branchiopod crustaceans. In Tracking Environmental Change Using Lake Sediments; Springer: Dordrecht, The Netherlands, 2001; pp. 5–41. [Google Scholar]
- Wojewódka, M.; Sinev, A.Y.; Zawisza, E.; Stańczak, J. A guide to the identification of subfossil chydorid Cladocera (Crustacea: Branchiopoda) from lake sediments of Central America and the Yucatan Peninsula, Mexico: Part II. J. Paleolimnol. 2020, 63, 37–64. [Google Scholar] [CrossRef]
- Frey, D.G. The Taxonomic and Phylogenetic significance of the head pores of the Chydoridae (Cladoceha). Int. Rev. Gesamten Hydrobiol. Hydrogr. 1959, 44, 27–50. [Google Scholar] [CrossRef]
- Szeroczyñska, K.; Sarmaja-Korjonen, K. Atlas of Subfossil Cladocera from Central and Northern Europe; Friends of the Lower Vistula Society: Świecie, Poland, 2007; ISBN 9788392491965. [Google Scholar]
- Kotov, A.A.; Ibragimova, A.G.; Neretina, A.N. Identification of Ceriodaphnia Dana, 1853 (Crustacea: Cladocera) taxa from European Russia based on ephippial morphology. Zootaxa 2018, 4527, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.G.; Cumming, B.F.; Smol, J.P. Cladocera and Anostraca from the Interior Plateau of British Columbia, Canada, as paleolimnological indicators of salinity and lake level. Hydrobiologia 1999, 392, 129–141. [Google Scholar] [CrossRef]
- Kirillova, I.V.; van der Plicht, J.; Gubin, S.V.; Zanina, O.G.; Chernova, O.F.; Lapteva, E.G.; Trofimova, S.S.; Zinovyev, E.V.; Zharov, A.A.; Fadeeva, E.O.; et al. Taphonomic phenomenon of ancient hair from Glacial Beringia: Perspectives for palaeoecological reconstructions. Boreas 2016, 45, 455–469. [Google Scholar] [CrossRef]
- Neretina, A.N.; Gololobova, M.A.; Neplyukhina, A.A.; Zharov, A.A.; Rogers, C.D.; Horne, D.J.; Protopopov, A.V.; Kotov, A.A. Crustacean remains from the Yuka mammoth raise questions about non-analogue freshwater communities in the Beringian region during the Pleistocene. Sci. Rep. 2020, 10, 859. [Google Scholar] [CrossRef]
- Vereshchagin, N.K.; Baryshnikov, G.F. Paleoecology of the mammoth fauna in the Eurasian Arctic. In Paleoecology of Beringia; Elsevier: Amsterdam, The Netherlands, 1982; ISBN 9780123558602. [Google Scholar]
- Guthrie, R.D. Frozen Fauna of the Mammoth Steppe. The Story of Blue Babe; University of Chicago Press: Chicago, IL, USA; London, UK, 1990; ISBN 978-0226311234. [Google Scholar]
- Sinitsa, S.M.; Viliamova, E.S.; Yurgenson, G.A.; Reshetova, S.A.; Filenko, R.A. Geological Monuments of Transbaikalia: Cadastre of Stratigraphic and Paleontological Geological Natural Monuments; Nauka: Novosibirsk, Russia, 2014; ISBN 978-5-02-019120. [Google Scholar]
- Enikeev, F.I. Quaternary lacustrine morpholithogenesis and development of hydrolaccolites (southern Transbaikalia). Geomorphology 2018, 1, 45–53. [Google Scholar] [CrossRef]
- Karasev, E.V. Cenozoic fruits and seeds from the Urtuisky coal pit, Zabaikalie. In Proceedings of the 41st International Student Conference, Novosibirsk, Russia, 2003. [Google Scholar]
- Karasev, V.V. Caenozoic of Transbaikalia; ChitaGeolSjomka: Chita, Russia, 2002. [Google Scholar]
- Gibbard, P.L. The Quaternary System/Period and its major subdivisions. Russ. Geol. Geophys. 2015, 56, 686–688. [Google Scholar] [CrossRef]
- Bezrukova, E.V.; Kulagina, N.V.; Letunova, P.P.; Karabanov, E.V.; Wiliams, D.F.; Kuzmin, M.I.; Krapivina, S.M.; Vershinin, K.E.; Shestakova, O.N. Pliocene-quaternary vegetation and climate history of the Lake Baikal area, Eastern Siberia. In Long Continental Records from Lake Baikal; Kashiwaya, K., Ed.; Springer: Tokyo, Japan, 2003; pp. 111–135. ISBN 978-4-431-67981-3. [Google Scholar]
- Benzie, J.A.H. The Genus Daphnia (including Daphniopsis) (Anomopoda. Daphniidae); Kenobi Productions: Ghent, Belgium, 2005; ISBN 90-5782-151-6. [Google Scholar]
- Hudec, I. A comparison of populations from the Daphnia similis group (Cladocera: Daphniidae). In Biology of Cladocera; Kořínek, V., Frey, D.G., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 9–22. ISBN 978-90-481-4101-2. [Google Scholar]
- Popova, E.V.; Petrusek, A.; Kořínek, V.; Mergeay, J.; Bekker, E.I.; Karabanov, D.P.; Galimov, Y.R.; Neretina, T.V.; Taylor, D.J.; Kotov, A.A. Revision of the Old World Daphnia (Ctenodaphnia) similis group Cladocera: Daphniidae). Zootaxa 2016, 4161, 1–40. [Google Scholar] [CrossRef]
- Krivenkova, I.F. Zooplankton of Lake Nozhiy. Estestv. Tekhnich. Nauki 2009, 5, 123–124. [Google Scholar]
- Afonina, E.Y.; Tashlykova, N.A. Plankton community and the relationship with the environment in saline lakes of Onon-Torey plain, Northeastern Mongolia. Saudi J. Biol. Sci. 2018, 25, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Afonina, E.Y.; Tashlykova, N.A. Plankton of Saline Lakes in Southeastern Transbaikalia: Transformation and Environmental Factors. Contemp. Probl. Ecol. 2019, 12, 155–170. [Google Scholar] [CrossRef]
- Alonso, M. Branchiopoda and Copepoda (Crustacea) in Mongolian saline lakes. Mongol. J. Biol. Sci. 2010, 8, 9–16. [Google Scholar]
- Crease, T.J.; Omilian, A.R.; Costanzo, K.S.; Taylor, D.J. Transcontinental phylogeography of the Daphnia pulex species complex. PLoS ONE 2012, 7, e46620. [Google Scholar] [CrossRef]
- Rogers, D.C.; Kotov, A.A.; Sinev, A.Y.; Glagolev, S.M.; Korovchinsky, N.M.; Smirnov, N.N.; Bekker, E.I. Chapter 16.2. Arthropoda: Class branchiopoda. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: London, UK, 2019; ISBN 9780123850249. [Google Scholar]
- Orlova-Bienkowskaja, M.Y. Cladocera. Anomopoda: Daphniidae, Genus Simocephalus; Backhuys: Leiden, The Netherlands, 2001; ISBN 90-5782-090-0. [Google Scholar]
- Smirnov, N.N. Macrothricidae and Moinidae of the World Fauna; Nauka: Moscow, Russia, 1976. [Google Scholar]
- Alonso, M. Crustacea: Branchiopoda, Fauna Iberica; Consejo Superior de Investigaciones Cientificas: Madrid, pain, 1996; ISBN 978-8400075712. [Google Scholar]
- Bekker, E.I.; Karabanov, D.P.; Galimov, Y.R.; Haag, C.R.; Neretina, T.V.; Kotov, A.A. Phylogeography of Daphnia magna Straus (Crustacea: Cladocera) in Northern Eurasia: Evidence for a deep longitudinal split between mitochondrial lineages. PLoS ONE 2018, 13, e0194045. [Google Scholar] [CrossRef]
- Montoliu-Elena, L.; Elías-Gutiérrez, M.; Silva-Briano, M. Moina macrocopa (Straus, 1820): A species complex of a common Cladocera, highlighted by morphology and DNA barcodes. Limnetica 2019, 38, 253–277. [Google Scholar]
- Forró, L. Moina kaszabi sp.n. (Crustacea, Cladocera), its separation from M. belli Gurney by multivariative analyses. Acta Zool. Hungar. 1988, 34, 203–214. [Google Scholar]
- Kotov, A.A.; Karabanov, D.P.; Bekker, E.I.; Neretina, T.V.; Taylor, D.J. Phylogeography of the Chydorus sphaericus Group (Cladocera: Chydoridae) in the Northern Palearctic. PLoS ONE 2016, 11, e0168711. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, M.; Taylor, D.J. Cryptic species within the Chydorus sphaericus species complex (Crustacea: Cladocera) revealed by molecular markers and sexual stage morphology. Mol. Phylogenet. Evol. 2009, 50, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, N.N. Chydoridae Fauni Mira; Nauka: Moscow, Russia, 1971. [Google Scholar]
- Thiéry, A.; Brtek, J.; Gasc, C. Cyst morphology of European branchiopods (Crustacea: Anostraca, Notostraca, Spinicaudata, Laevicaudata). Bull. Mus. Nat. Hist. Natur. A. 1995, 17, 107–139. [Google Scholar]
- Mura, G.; Thierry, A. Taxonomical significance of scanning electron microscopic morphology of the Euphyllopod’s resting eggs from Morocco, Part, I. Anostraca. Vie Milieu 1986, 36, 125–131. [Google Scholar]
- Brendonck, L.; Rogers, D.C.; Olesen, J.; Weeks, S.; Hoeh, W.R. Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater. Hydrobiologia 2008, 198, 167–176. [Google Scholar] [CrossRef]
- Rogers, D.C. Branchiopoda (Anostraca, Notostraca, Laevicaudata, Spinicaudata, Cyclestherida). In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780123706263. [Google Scholar]
- Rogers, D.C.; Padhye, S. A new species of Streptocephalus (Crustacea: Anostraca: Streptocephalidae) from the Western Ghats, India, with a key to the Asian species. Zootaxa 2014, 75–84. [Google Scholar] [CrossRef]
- Shu, S.-S.; Rogers, D.C.; Chen, X.-Y.; Sanoamuang, L.-O. Streptocephalus diversity in Myanmar, with description of a new species (Branchiopoda, Anostraca). Zookeys 2018, 734, 1–12. [Google Scholar] [CrossRef]
- Shu, S.; Maeda-Martinez, A.M.; Rogers, D.C.; Yang, J.; Chen, X. Morphological characterization of Streptocephalus sirindhornae (Branchiopoda: Anostraca) from South East Asia: First record of the Streptocephalidae from China. Zootaxa 2015, 3911, 447–450. [Google Scholar] [CrossRef]
- Rogers, D.C. Two new cryptic anostracan (Branchiopoda: Streptocephalidae, Chirocephalidae) species. J. Crust. Biol. 2014, 34, 862–874. [Google Scholar] [CrossRef][Green Version]
- Mura, G. Pattern of Egg Shell Morphology in Thamnocephalids and Streptocephalids of the New World (Anostraca). Crustaceana 1992, 62, 300–311. [Google Scholar] [CrossRef]
- Brendonck, L.; Coomans, A. Egg morphology in African Streptocephalidae (Crustacea: Branchiopoda: Anostraca) Part 1: North of Zambezi and Kunene rivers, and Madagascar. Arch. Hydrobiol. Suppl. 1994, 3, 335–356. [Google Scholar]
- Brendonck, L.; Coomans, A. Egg morphology in African Streptocephalidae (Crustacea: Branchiopoda: Anostraca) Part 2: South of Zambezi and Kunene rivers. Arch. Hydrobiol. 1994, 3, 313–334. [Google Scholar]
- Padhye, S.; Timms, B.; Ghate, H.V. Large branchiopod (Crustacea: Branchiopoda) egg morphology of Western Ghats, Maharashtra, India. Zootaxa 2016, 4079, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Munuswamy, N.; Subramoniam, T.; Mura, G. Ootaxonomic findings on anostracan eggs: A scanning electron microscopic study. Cytobios 1985, 42, 93–97. [Google Scholar]
- Velu, C.S.; Munuswamy, N. Updated diagnoses for the Indian species of Streptocephalus (Crustacea: Branchiopoda: Anostraca). Zootaxa 2005, 1049, 33–48. [Google Scholar] [CrossRef]
- Vekhov, N.V. Branchiopod crustaceans (Anostraca and Notostraca) of ephemeral water bodies of the steppe zone. Zool. Zh. 1989, 68, 241–250. [Google Scholar]
- Yevdokimov, N.A.; Yermokhin, M.V. Crustaceans of zooplankton in temporary waterbodies of Saratov District on territory of different natural zones. Biol. Vnutr. Vod 2009, 1, 62–69. [Google Scholar]
- Frey, D.G. Questions concerning cosmopolitanism in Cladocera. Arch. Hydrobiol. 1982, 93, 484–502. [Google Scholar]
- Fryer, G. Structure and habits of living branchiopod crustaceans and their bearing on the interpretation of fossil forms. Earth Environ. Sci. Trans. R. Soc. Edinb. 1985, 76, 103–113. [Google Scholar] [CrossRef]
- Fryer, G. Studies on the functional morphology and biology of the Notostraca (Crustacea: Branchiopoda). Phil. Trans. R. Soc. Lond. B 1988, 321, 27–124. [Google Scholar] [CrossRef]
- Kerfoot, W.C.; Lynch, M. Branchiopod communities: Associations with planktivorous fish in space and time. In Predation. Direct and Indirect Impacts on Aquatic Communities; Academic Press: Hanover, Germany; London, UK, 1987; pp. 367–378. [Google Scholar]
- Richter, G.; Baszio, S. Traces of a limnic food web in the Eocene Lake Messel—A preliminary report based on fish coprolite analyses. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 166, 345–368. [Google Scholar] [CrossRef]
- Daborn, G.R. Occurrence of an arctic fairy shrimp Polyartemiella hazeni (Murdoch) 1884 (Crustacea: Anostraca) in Alberta and Yukon Territory. Can. J. Zool. 1976, 54, 2026–2028. [Google Scholar] [CrossRef]
- Beladjal, L.; Dierckens, K.; Mertens, J. Dispersal of fairy shrimp Chirocephalus diaphanus (Branchiopoda: Anostraca) by the trout (Salmo trutta). J. Crust. Biol. 2007, 27, 71–73. [Google Scholar] [CrossRef]
- Hurka, H.; Friesen, N.; Bernhardt, K.-G.; Neuffer, B.; Smirnov, S.V.; Shmakov, A.I.; Blattner, F.R. The Eurasian steppe belt: Status quo, origin and evolutionary history. Turczaninowia 2019, 22, 5–71. [Google Scholar] [CrossRef]
- Vekhov, N.V.; Vekhova, T.P. Regional traits of the fauna of anostracans and notostracans of out-of-tundra territory of European Russia and Ukraine. Vetst. Zool. 1993, 5, 12–18. [Google Scholar]
- Barnes, I.; Shapiro, B.; Lister, A.; Kuznetsova, T.; Sher, A.; Guthrie, D.; Thomas, M.G. Genetic structure and extinction of the woolly mammoth, Mammuthus primigenius. Curr. Biol. 2007, 17, 1072–1075. [Google Scholar] [CrossRef]
- Řičánková, V.P.; Horsák, M.; Hais, M.; Robovský, J.; Chytrý, M. Environmental correlates of the Late Quaternary regional extinctions of large and small Palaearctic mammals. Ecography 2018, 41, 516–527. [Google Scholar] [CrossRef]
- Flössner, D. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas; Backhuys: Leiden, The Netherlands, 2000. [Google Scholar]
- Rogers, D.C. Anostracan (Crustacea: Branchiopoda) zoogeography II. Relating distribution to geochemical substrate properties in the USA. Zootaxa 2014, 3856, 1–49. [Google Scholar] [CrossRef]
- Bredenkamp, G.J.; Spada, F.; Kazmierczak, E. On the origin of northern and southern hemisphere grasslands. Plant Ecol. 2002, 163, 209–229. [Google Scholar] [CrossRef]
- Fenzel, B.; Pécsel, M.; Velichko, A.A. Atlas of Paleoclimates and Paleoenvironments of the Northern Hemisphere, Late Pleistocene-Holocene; Gustav Fischer Verlag: Budapest, Hungary; Stuttgart, Germany, 1992. [Google Scholar]
| Taxon | Urtuy | Nozhiy | Recent Steppe Water Bodies in Transbaikalia and Mongolia |
|---|---|---|---|
| Cladocera | |||
| Daphnia (Daphnia) pulex group | 675 | - | + |
| Daphnia (Ctenodaphnia) magna Straus | 10 | - | + |
| D. (C.) similis Claus | - | 24 | + |
| Ceriodaphni pulchella-reticulata | 1 | - | + |
| C. laticaudata P.E. Müller | 3 | - | + |
| Simocephalus sp. | 6 | - | + |
| Moina sp. | 1 | 3 | + |
| Chydorus cf. sphaericus | 74 | - | + |
| Alona s.lat. | 1 | - | + |
| Anostraca | |||
| Streptocephalus (Streptocephalus) sp. | 6 | - | - |
| Branchinecta cf. paludosa Müller | - | 61 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharov, A.A.; Neretina, A.N.; Rogers, D.C.; Reshetova, S.A.; Sinitsa, S.M.; Kotov, A.A. Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia Demonstrate Morphological and Ecological Stasis. Water 2020, 12, 3063. https://doi.org/10.3390/w12113063
Zharov AA, Neretina AN, Rogers DC, Reshetova SA, Sinitsa SM, Kotov AA. Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia Demonstrate Morphological and Ecological Stasis. Water. 2020; 12(11):3063. https://doi.org/10.3390/w12113063
Chicago/Turabian StyleZharov, Anton A., Anna N. Neretina, D. Christopher Rogers, Svetlana A. Reshetova, Sofia M. Sinitsa, and Alexey A. Kotov. 2020. "Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia Demonstrate Morphological and Ecological Stasis" Water 12, no. 11: 3063. https://doi.org/10.3390/w12113063
APA StyleZharov, A. A., Neretina, A. N., Rogers, D. C., Reshetova, S. A., Sinitsa, S. M., & Kotov, A. A. (2020). Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia Demonstrate Morphological and Ecological Stasis. Water, 12(11), 3063. https://doi.org/10.3390/w12113063






