Overlapping Water and Nutrient Use Efficiencies and Carbon Assimilation between Coexisting Simple- and Compound-Leaved Trees from a Valley Savanna
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Leaf Mass Per Area
2.3. Leaf Gas Exchange
2.4. Leaf Nutrients
2.5. Leaf Water and Nutrient Use Efficiencies
2.6. Data Analysis
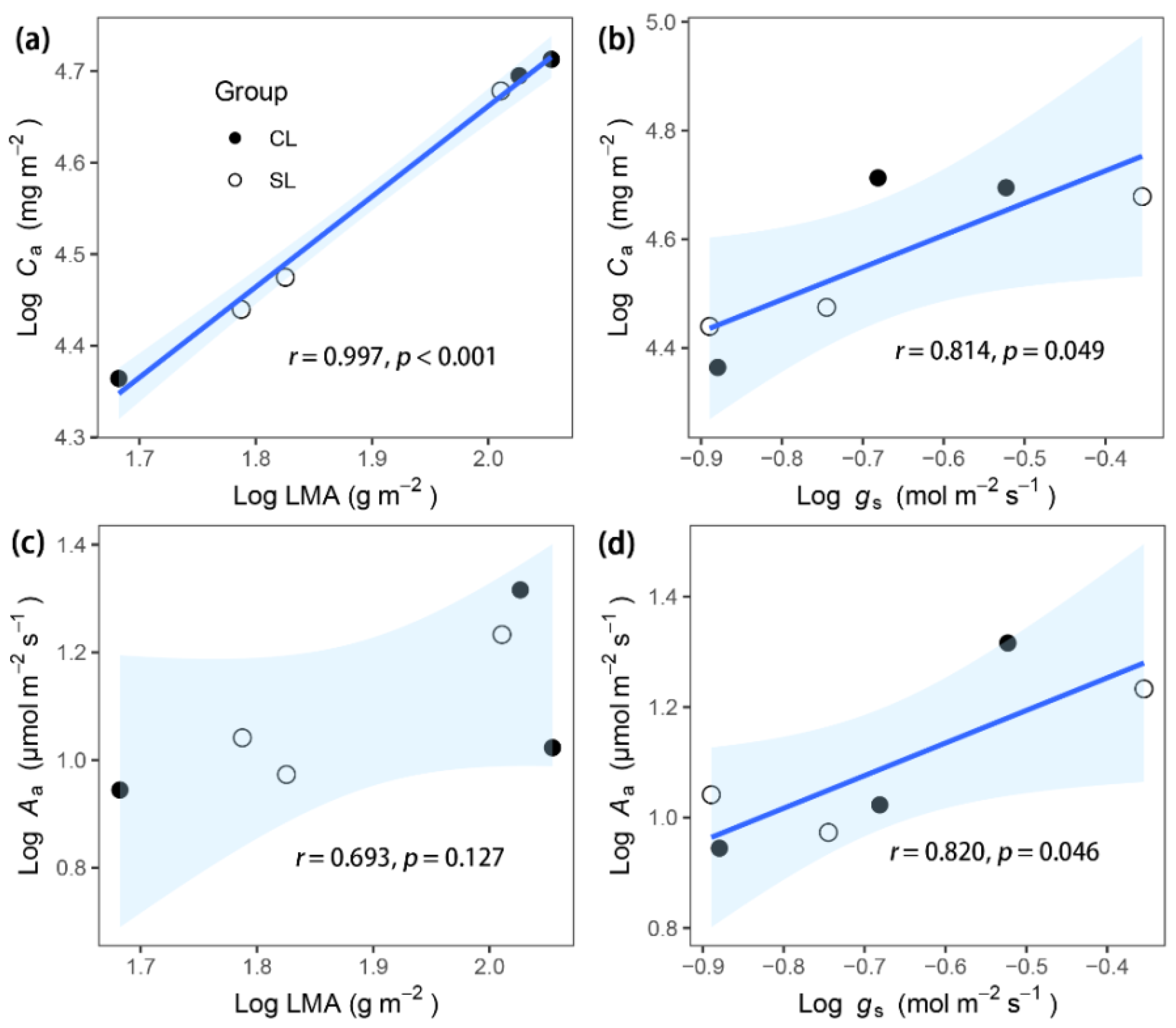
3. Results
4. Discussion
4.1. Comparable Photosynthetic Capacity between Simple- and Compound-Leaved Species
4.2. Convergence in Photosynthetic Nutrient Use Strategy across Simple- and Compound-Leaved Species
4.3. Water Use Efficiency across Simple- and Compound-Leaved Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hareven, D.; Gutfinger, T.; Parnis, A.; Eshed, Y.; Lifschitz, E. The making of a compound leaf: Genetic manipulation of leaf architecture in tomato. Cell 1996, 84, 735–744. [Google Scholar] [CrossRef]
- Malhado, A.C.M.; Whittaker, R.J.; Malhi, Y.; Ladle, R.J.; Ter Steege, H.; Phillips, O.; Aragao, L.E.O.C.; Baker, T.R.; Arroyo, L.; Almeida, S.; et al. Are compound leaves an adaptation to seasonal drought or to rapid growth? Evidence from the Amazon rain forest. Glob. Ecol. Biogeogr. 2010, 19, 852–862. [Google Scholar] [CrossRef]
- Ogden, M.S.; Lacroix, C.R. Comparative development of simple and compound leaves in the genus Cecropia. Botany 2017, 95, 185–193. [Google Scholar] [CrossRef]
- Bharathan, G.; Goliber, T.E.; Moore, C.; Kessler, S.; Pham, T.; Sinha, N.R. Homologies in leaf form inferred from KNOXI gene expression during development. Science 2002, 296, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, Y.L. Keeping leaves in shape. Nat. Plants 2020, 6, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U. Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs. Plant Ecol. 1998, 134, 1–11. [Google Scholar] [CrossRef]
- Niinemets, U.; Portsmuth, A.; Tobias, M. Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol. 2006, 171, 91–104. [Google Scholar] [CrossRef]
- Givnish, T. Ecological aspects of plant morphology: Leaf form in relation to environment. Acta Biotheor. 1978, 27, 83–142. [Google Scholar]
- Givnish, T.J. Leaf and canopy adaptations in tropical forests. In Physiological Ecology of Plants of the Wet Tropics, 1st ed.; Medina, E., Mooney, H.A., Vázquez-Yánes, C., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 12, pp. 51–84. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.J.; Song, J.; Niu, C.Y.; Hao, G.Y. Compound leaves are associated with high hydraulic conductance and photosynthetic capacity: Evidence from trees in Northeast China. Tree Physiol. 2019, 39, 729–739. [Google Scholar] [CrossRef]
- Zhao, W.L.; Zhang, J.L.; Zhang, Y.J.; Cao, K.F. Analysis of photosynthesis–water relationship between simple- and compound-leafed leguminous trees. Plant Sci. J. 2019, 37, 628–636, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Stowe, L.G.; Brown, J.L. A geographic perspective on the ecology of compound leaves. Evolution 1981, 35, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Falster, D.S.; Pickup, M.; Westoby, M. Cross-species patterns in the coordination between leaf and stem traits, and their implications for plant hydraulics. Physiol. Plant. 2006, 127, 445–456. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Portsmuth, A.; Tena, D.; Tobias, M.; Matesanz, S.; Valladares, F. Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann. Bot. 2007, 100, 283–303. [Google Scholar] [CrossRef]
- Huntley, B.J.; Walker, B.H. Ecology of Tropical Savannas, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Silva, J.M.C.; Bates, J.M. Biogeographic patterns and conservation in the South American Cerrado: A tropical savanna hotspot. BioScience 2002, 52, 225–234. [Google Scholar] [CrossRef]
- Furley, P. Tropical savannas. Prog. Phys. Geogr. 2006, 30, 105–121. [Google Scholar] [CrossRef]
- Jin, Z.Z.; Ou, X.K. Yuanjiang, Nujiang, Jinshajiang, Lancangjiang: Vegetation of Dry–Hot Valley, 1st ed.; Yunnan University Press & Yunnan Science and Technology Press: Kunming, China, 2000. (In Chinese) [Google Scholar]
- Gates, D.M.; Schmerl, R.B. Perspectives of Biophysical Ecology, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1980; p. 611. [Google Scholar]
- Zhang, J.L.; Zhu, J.J.; Cao, K.F. Seasonal variation in photosynthesis in six woody species with different leaf phenology in a valley savanna in southwestern China. Trees 2007, 21, 631–643. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wu, H.D.; Yang, J.; Song, X.Y.; Yang, D.; He, F.L.; Zhang, J.L. Environmental filtering and spatial processes shape the beta diversity of liana communities in a valley savanna in southwest China. Appl. Veg. Sci. 2020. (Early View). [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Royer, D.L.; Wilf, P. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. Int. J. Plant Sci. 2006, 167, 11–18. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.C.; Wang, M.C.; Trabucco, A.; Li, Z.Q. Projected impact of climate change on the effectiveness of the existing protected area network for biodiversity conservation within Yunnan Province, China. Biol. Conserv. 2015, 184, 335–345. [Google Scholar] [CrossRef]
- Song, J.; Yang, D.; Niu, C.Y.; Zhang, W.W.; Wang, M.; Hao, G.Y. Correlation between leaf size and hydraulic architecture in five compound-leaved tree species of a temperate forest in NE China. For. Ecol. Manag. 2018, 418, 63–72. [Google Scholar] [CrossRef]
- Bahar, N.H.A.; Ishida, F.Y.; Weerasinghe, L.K.; Guerrieri, R.; O’Sullivan, O.S.; Bloomfield, K.J.; Asner, G.P.; Martin, R.E.; Lloyd, J.; Malhi, Y.; et al. Leaf-level photosynthetic capacity in lowland Amazonian and high-elevation Andean tropical moist forests of Peru. New Phytol. 2017, 214, 1002–1018. [Google Scholar] [CrossRef]
- Norby, R.J.; Gu, L.H.; Haworth, I.C.; Jensen, A.M.; Turner, B.L.; Walker, A.P.; Warren, J.M.; Weston, D.J.; Xu, C.G.; Winter, K. Informing models through empirical relationships between foliar phosphorus, nitrogen and photosynthesis across diverse woody species in tropical forests of Panama. New Phytol. 2017, 215, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Coste, S.; Roggy, J.C.; Imbert, P.; Born, C.; Bonal, D.; Dreyer, E. Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiol. 2005, 25, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Domingues, T.F.; Ishida, F.Y.; Feldpausch, T.R.; Grace, J.; Meir, P.; Saiz, G.; Sene, O.; Schrodt, F.; Sonke, B.; Taedoumg, H.; et al. Biome-specific effects of nitrogen and phosphorus on the photosynthetic characteristics of trees at a forest-savanna boundary in Cameroon. Oecologia 2015, 178, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Gvozdevaite, A.; Oliveras, I.; Domingues, T.F.; Peprah, T.; Boakye, M.; Afriyie, L.; Peixoto, K.D.; de Farias, J.; de Oliveira, E.A.; Farias, C.C.A.; et al. Leaf-level photosynthetic capacity dynamics in relation to soil and foliar nutrients along forest-savanna boundaries in Ghana and Brazil. Tree Physiol. 2018, 38, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Prentice, I.C.; Dong, N.; Gleason, S.M.; Maire, V.; Wright, I.J. Balancing the costs of carbon gain and water transport: Testing a new theoretical framework for plant functional ecology. Ecol. Lett. 2014, 17, 82–91. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Wallin, G.; Gardesten, J.; Niyonzima, F.; Adolfsson, L.; Nsabimana, D.; Uddling, J. Photosynthetic capacity of tropical montane tree species in relation to leaf nutrients, successional strategy and growth temperature. Oecologia 2015, 177, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Milla-Moreno, E.A.; McKown, A.D.; Guy, R.D.; Soolanayakanahally, R.Y. Leaf mass per area predicts palisade structural properties linked to mesophyll conductance in balsam poplar (Populus balsamifera L.). Botany 2016, 94, 225–239. [Google Scholar] [CrossRef]
- John, G.P.; Scoffoni, C.; Buckley, T.N.; Villar, R.; Poorter, H.; Sack, L. The anatomical and compositional basis of leaf mass per area. Ecol. Lett. 2017, 20, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Zhang, J.L.; Poorter, L.; Cao, K.F. Productive leaf functional traits of Chinese savanna species. Plant Ecol. 2012, 213, 1449–1460. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Song, J.; Wang, M.; Li, N.; Niu, C.Y.; Hao, G.Y. Coordination of xylem hydraulics and stomatal regulation in keeping the integrity of xylem water transport in shoots of two compound-leaved tree species. Tree Physiol. 2015, 35, 1333–1342. [Google Scholar] [CrossRef]
- Domingues, T.F.; Meir, P.; Feldpausch, T.R.; Saiz, G.; Veenendaal, E.M.; Schrodt, F.; Bird, M.; Djagbletey, G.; Hien, F.; Compaore, H.; et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 2010, 33, 959–980. [Google Scholar] [CrossRef]
- Walker, A.P.; Quaife, T.; Van Bodegom, P.M.; De Kauwe, M.G.; Keenan, T.F.; Joiner, J.; Lomas, M.R.; MacBean, N.; Xu, C.G.; Yang, X.J.; et al. The impact of alternative trait-scaling hypotheses for the maximum photosynthetic carboxylation rate (V-cmax) on global gross primary production. New Phytol. 2017, 215, 1370–1386. [Google Scholar] [CrossRef]
- Qu, C.M.; Han, X.G.; Su, B.; Huang, J.H.; Jiang, G.M. The characteristics of foliar δ13C values of plants and plant water use efficiency indicated by δ13C values in two fragmented rainforests in Xishuangbanna, Yunnan. Acta. Bot. Sin. 2001, 43, 186–192, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef]

| Species | Family | Leaf Type |
|---|---|---|
| Bauhinia brachycarpa | Leguminosae | SL |
| Strophioblachia fimbricalyx | Euphorbiaceae | SL |
| Trigonostemon tuberculatus | Euphorbiaceae | SL |
| Bischofia polycarpa | Euphorbiaceae | CL |
| Cipadessa baccifera | Meliaceae | CL |
| Campylotropis delavayi | Leguminosae | CL |
| Trait Name | Abbreviation | Unit | CL | SL | t-Values | p-Values |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | |||||
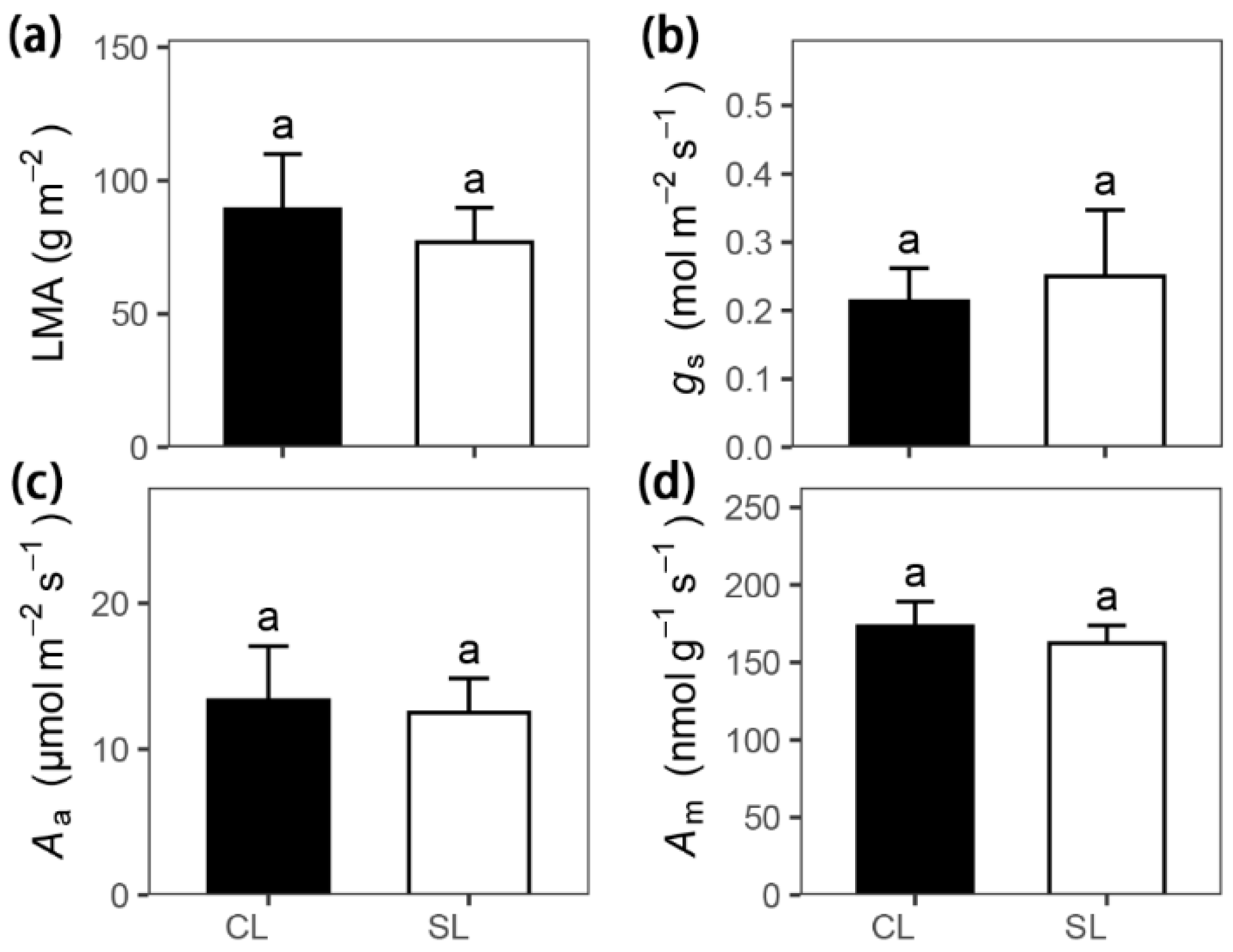
| Leaf mass per area | LMA | g m−2 | 89.23 ± 20.66 | 76.90 ± 12.90 | 0.34 | 0.76 |
| Maximum photosynthetic rate per mass | Am | nmol g–1 s–1 | 173.17 ± 16.04 | 162.26 ± 11.47 | 0.50 | 0.64 |
| Maximum photosynthetic rate per area | Aa | μmol m–2 s–1 | 13.35 ± 3.71 | 12.5 ± 2.35 | 0.09 | 0.94 |
| Stomatal conductance per area | gs | mol m–2 s–1 | 0.21 ± 0.05 | 0.25 ± 0.10 | −0.17 | 0.88 |
| Intrinsic water use efficiency | WUEi | μmol mol–1 | 62.09 ± 5.78 | 58.73 ± 13.84 | 0.40 | 0.72 |
| Stable carbon isotopic composition | δ13C | −‰ | 26.93 ± 0.48 | 27.01 ± 0.65 | −0.09 | 0.94 |
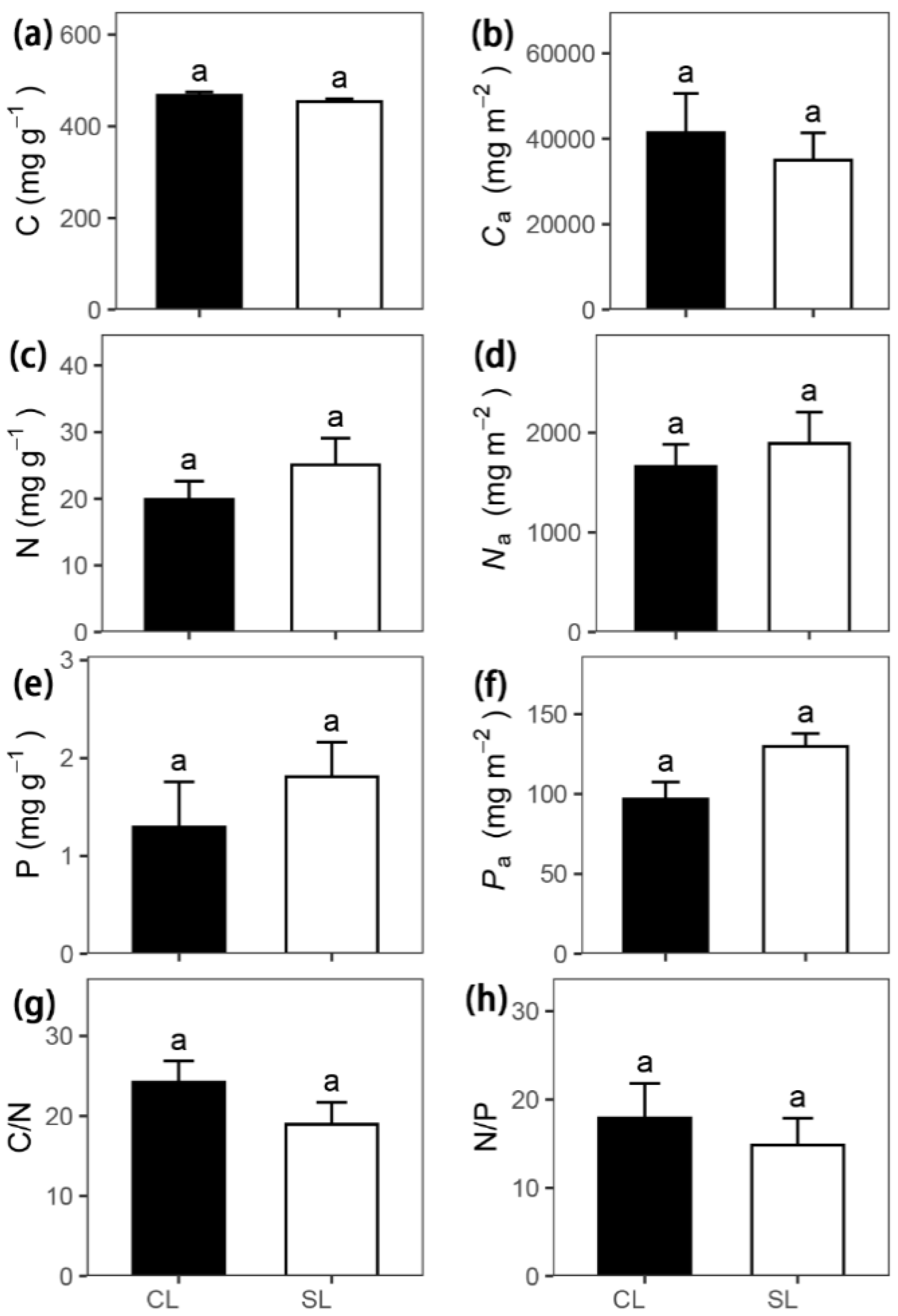
| Leaf carbon concentration per mass | C | mg g−1 | 467.56 ± 7.35 | 453.44 ± 6.01 | 1.50 | 0.21 |
| Leaf nitrogen concentration per mass | N | mg g−1 | 19.90 ± 2.75 | 25.06 ± 4.02 | −1.12 | 0.33 |
| Leaf phosphorus concentration per mass | P | mg g−1 | 1.29 ± 0.46 | 1.81 ± 0.35 | −1.01 | 0.38 |
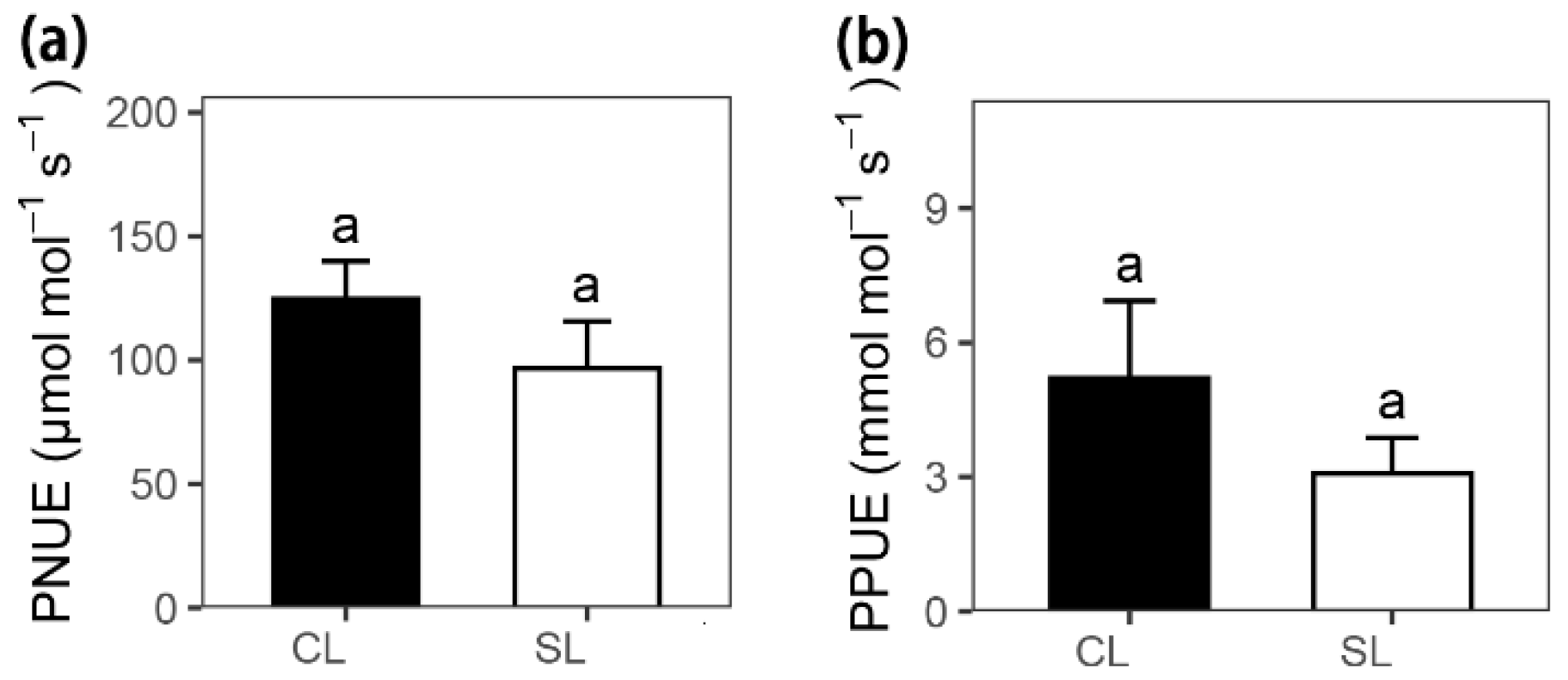
| Photosynthetic N use efficiency | PNUE | μmol mol–1 s–1 | 124.72 ± 15.16 | 96.66 ± 18.99 | 1.13 | 0.34 |
| Photosynthetic P use efficiency | PPUE | mmol mol–1 s–1 | 5.20 ± 1.73 | 3.08 ± 0.80 | 1.12 | 0.33 |
| Leaf carbon to nitrogen ratio | C/N | 24.23 ± 2.66 | 18.97 ± 2.75 | 1.30 | 0.27 | |
| Leaf nitrogen to phosphorus ratio | N/P | 17.90 ± 3.91 | 14.86 ± 3.01 | 0.58 | 0.60 | |
| Leaf carbon concentration per area | Ca | mg m−2 | 41,432.06 ± 9167.82 | 35,019.26 ± 6373.80 | 0.44 | 0.69 |
| Leaf nitrogen concentration per area | Na | mg m−2 | 1662.08 ± 221.70 | 1893.22 ± 314.62 | −0.50 | 0.65 |
| Leaf phosphorus concentration per area | Pa | mg m−2 | 96.91 ± 10.57 | 129.83 ± 8.03 | −2.27 | 0.11 |
| LMA | Am | Aa | gs | WUEi | δ13C | C | N | P | PNUE | PPUE | C/N | N/P | Ca | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am | −0.200 | ||||||||||||||
| Aa | 0.693 | 0.475 | |||||||||||||
| gs | 0.796 | 0.014 | 0.820* | ||||||||||||
| WUEi | −0.511 | 0.565 | −0.172 | −0.705 | |||||||||||
| δ13C | −0.051 | 0.520 | 0.409 | 0.490 | −0.338 | ||||||||||
| C | −0.150 | 0.585 | 0.167 | 0.135 | −0.026 | 0.743 | |||||||||
| N | −0.659 | −0.279 | −0.512 | −0.309 | −0.102 | 0.060 | −0.142 | ||||||||
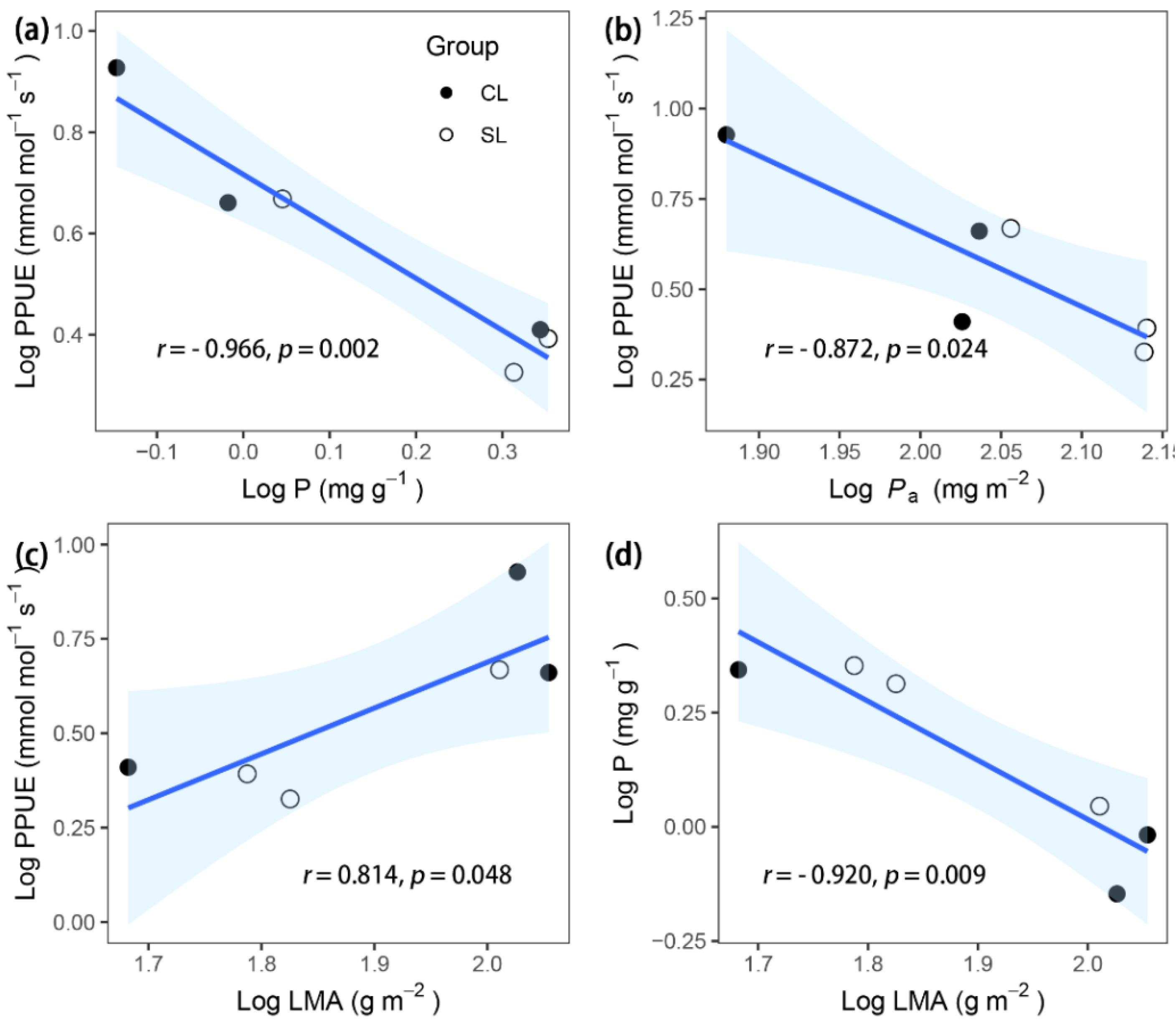
| P | −0.920 ** | −0.095 | −0.789 | −0.764 | 0.338 | −0.091 | −0.158 | 0.714 | |||||||
| PNUE | 0.435 | 0.650 | 0.609 | 0.251 | 0.323 | 0.176 | 0.364 | −0.911 * | −0.605 | ||||||
| PPUE | 0.814* | 0.349 | 0.866 * | 0.723 | −0.171 | 0.220 | 0.300 | −0.744 | −0.966 ** | 0.739 | |||||
| C/N | 0.629 | 0.337 | 0.519 | 0.318 | 0.097 | 0.022 | 0.248 | −0.994 *** | −0.716 | 0.931 ** | 0.761 | ||||
| N/P | 0.802 | −0.063 | 0.724 | 0.831 * | −0.533 | 0.166 | 0.117 | −0.282 | −0.874 * | 0.196 | 0.806 | 0.289 | |||
| Ca | 0.997 *** | −0.155 | 0.712 | 0.814 * | −0.518 | 0.008 | −0.072 | −0.676 | −0.941 ** | 0.468 | 0.846 * | 0.654 | 0.819 * | ||
| Na | 0.703 | −0.529 | 0.435 | 0.763 | −0.774 | −0.011 | −0.334 | 0.071 | −0.546 | −0.284 | 0.377 | −0.106 | 0.797 | 0.683 | |
| Pa | −0.471 | −0.536 | −0.667 | −0.446 | −0.058 | −0.287 | −0.597 | 0.551 | 0.778 | −0.666 | −0.872 * | −0.605 | −0.683 | −0.523 | −0.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-S.-D.; Yang, D.; Wu, H.-D.; Zhang, Y.-B.; Zhang, S.-B.; Zhang, Y.-J.; Zhang, J.-L. Overlapping Water and Nutrient Use Efficiencies and Carbon Assimilation between Coexisting Simple- and Compound-Leaved Trees from a Valley Savanna. Water 2020, 12, 3037. https://doi.org/10.3390/w12113037
Wang Y-S-D, Yang D, Wu H-D, Zhang Y-B, Zhang S-B, Zhang Y-J, Zhang J-L. Overlapping Water and Nutrient Use Efficiencies and Carbon Assimilation between Coexisting Simple- and Compound-Leaved Trees from a Valley Savanna. Water. 2020; 12(11):3037. https://doi.org/10.3390/w12113037
Chicago/Turabian StyleWang, Yang-Si-Ding, Da Yang, Huai-Dong Wu, Yun-Bing Zhang, Shu-Bin Zhang, Yong-Jiang Zhang, and Jiao-Lin Zhang. 2020. "Overlapping Water and Nutrient Use Efficiencies and Carbon Assimilation between Coexisting Simple- and Compound-Leaved Trees from a Valley Savanna" Water 12, no. 11: 3037. https://doi.org/10.3390/w12113037
APA StyleWang, Y.-S.-D., Yang, D., Wu, H.-D., Zhang, Y.-B., Zhang, S.-B., Zhang, Y.-J., & Zhang, J.-L. (2020). Overlapping Water and Nutrient Use Efficiencies and Carbon Assimilation between Coexisting Simple- and Compound-Leaved Trees from a Valley Savanna. Water, 12(11), 3037. https://doi.org/10.3390/w12113037






