The Process of Microbiological Remediation of the Polluted Słoneczko Reservoir in Poland: For Reduction of Water Pollution and Nutrients Management
Abstract
1. Introduction
2. Materials and Methods
2.1. The Characteristics of the Area
2.2. The Composition and the Application of the Microbiological Biopreparation
- ACS ODO—1 biopreparation: Water, a consortium of lactic acid bacteria, phototrophic bacteria, yeast, ecological molasses from sugar cane, fermented wheat bran, minerals. The additional ingredients of biopreparation at the micro-level: Phytosterols (sitosterol, taraxasterol), phytohormones, triterpenes (lupeol, betulin, betulinic acid), flavonoids (hyperoside, quercetin, kaempferol), ellagic acid, pyrocatechic acid, brevofolin (ellagic acid derivative), vitamins (C, PP, P, B3, B5, B8, B11, B1, B2, A, E, F), and tannins.
- ACS aqua 2 biopreparation: Water, sugar cane molasses, and effective microorganisms including the main strains of effective microorganisms: Lactobacillus casei, Lactobacillus plantarum—5.0 × 106 cfu·mL−1 and Saccharomycces cerevisiae—5.0 × 103 cfu·mL−1.
2.3. The Area Measurements
2.4. Statistical Analysis
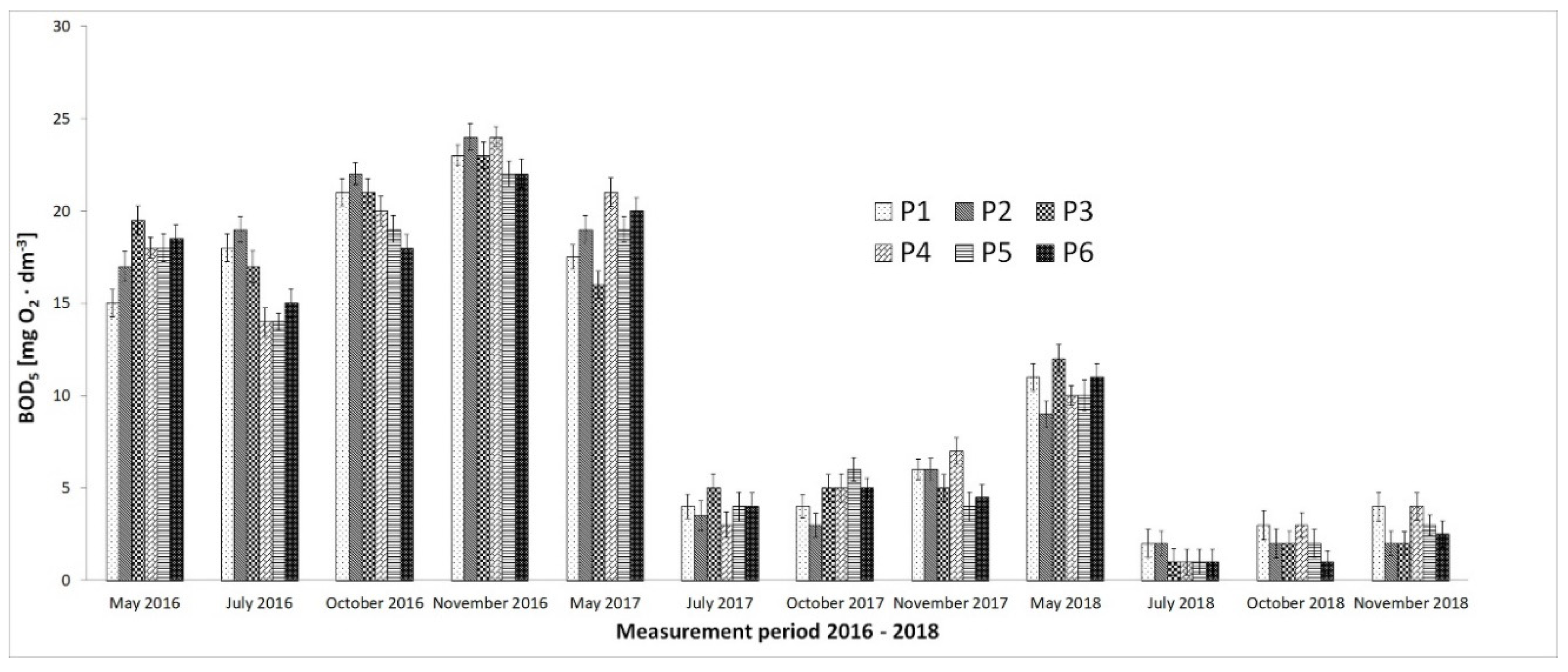
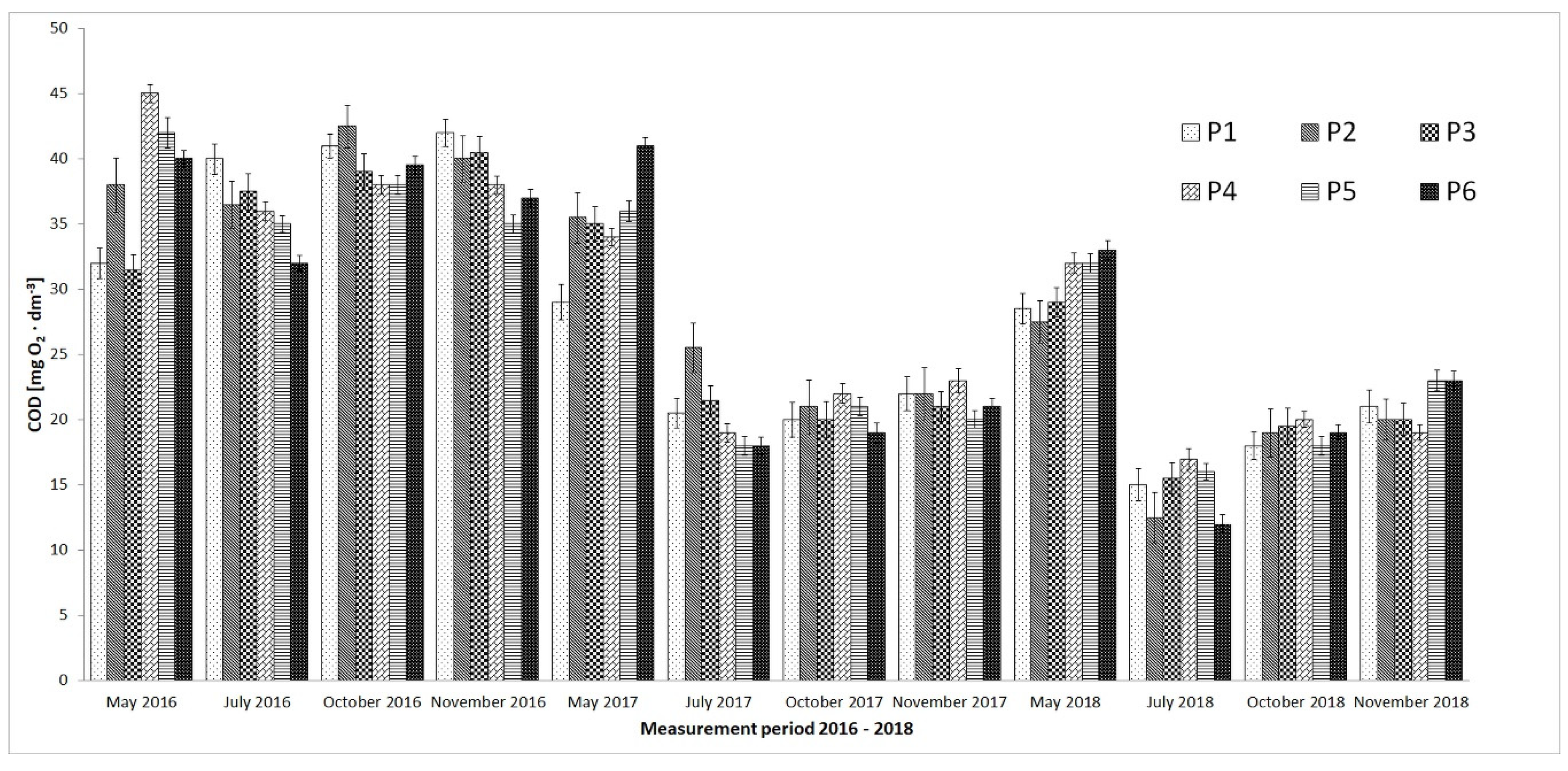
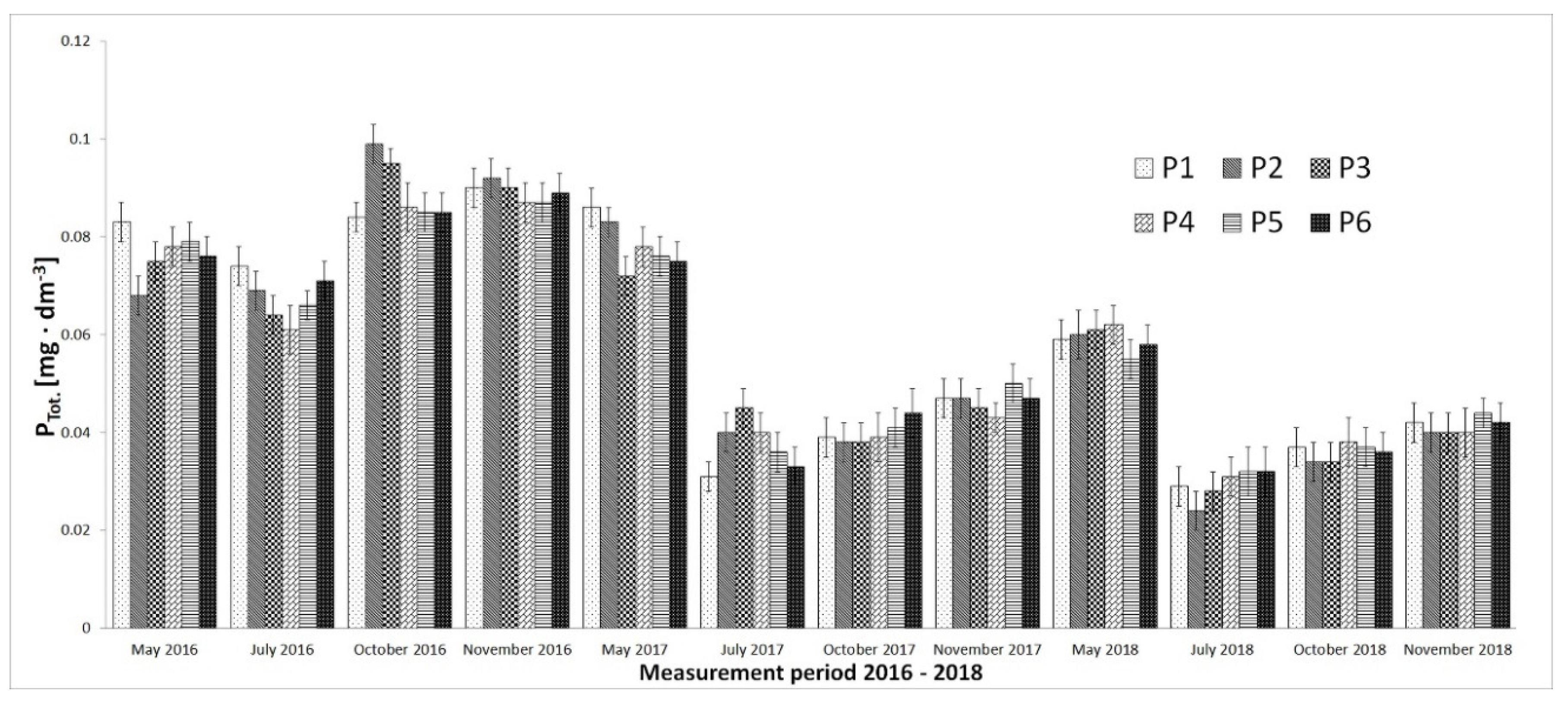
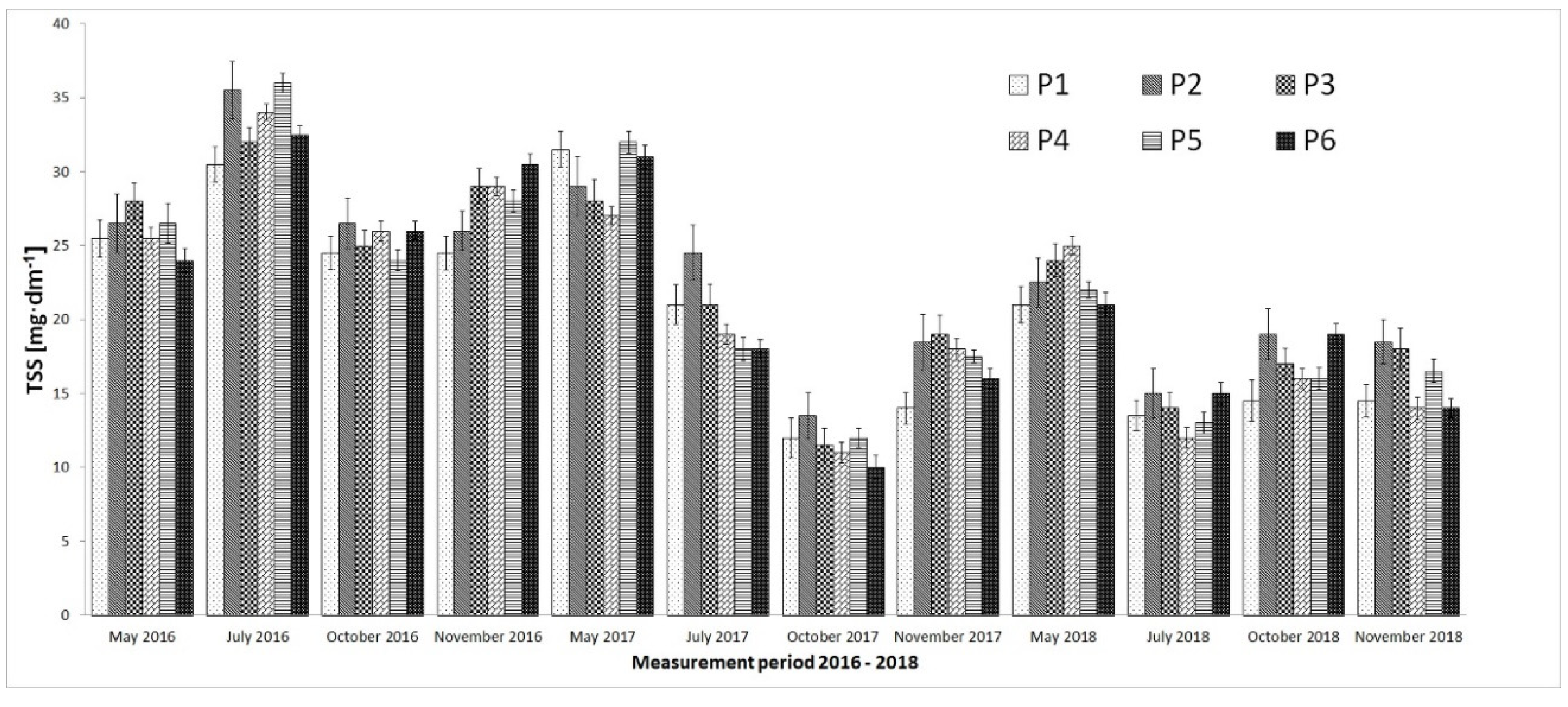
3. Results
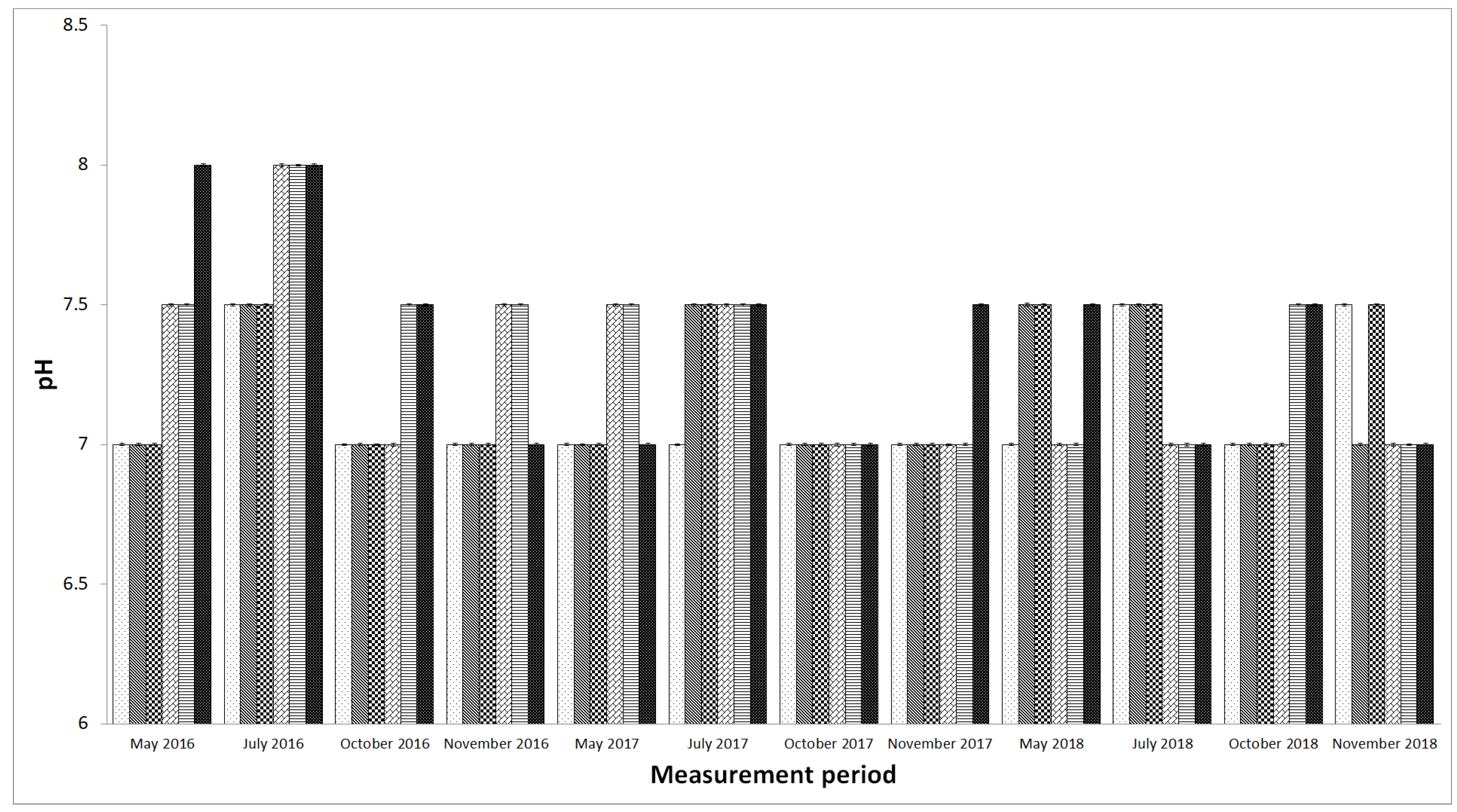
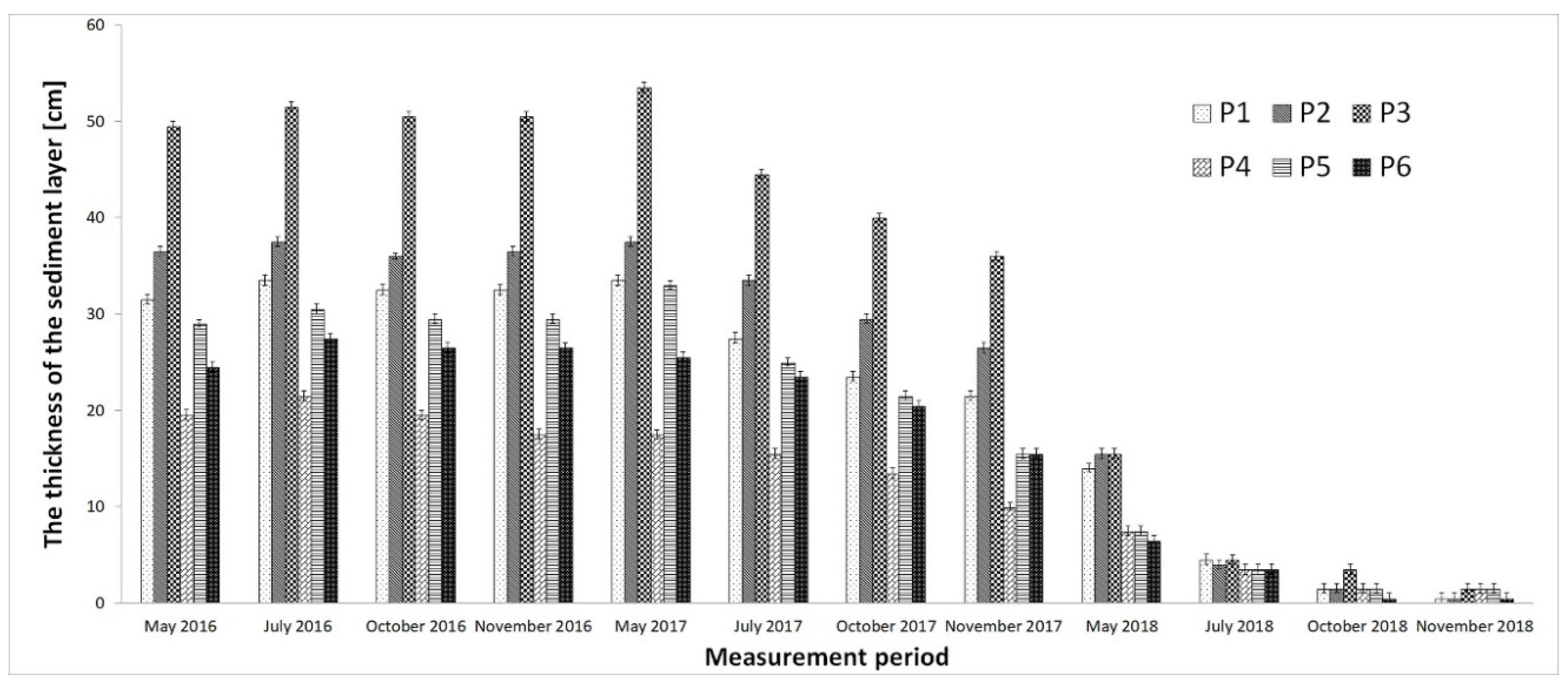
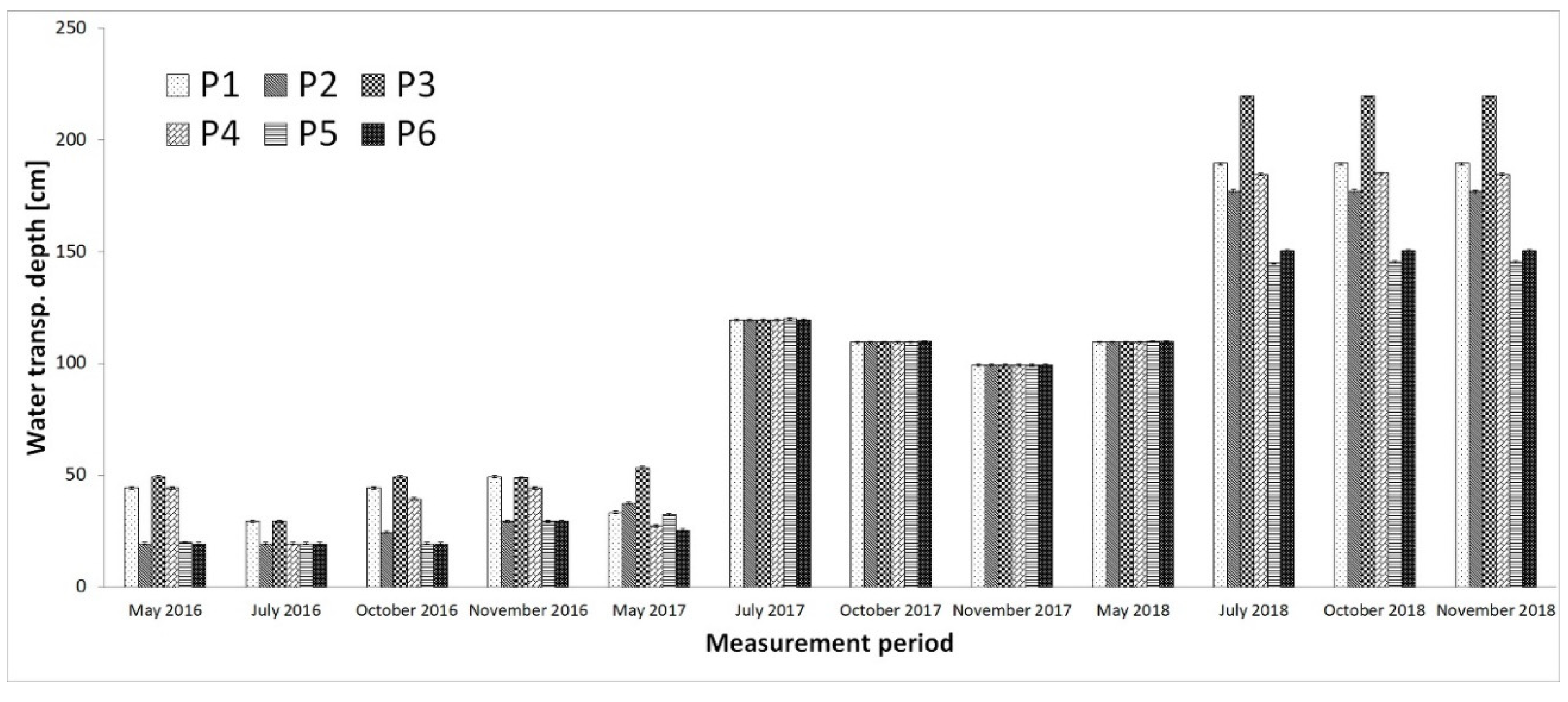
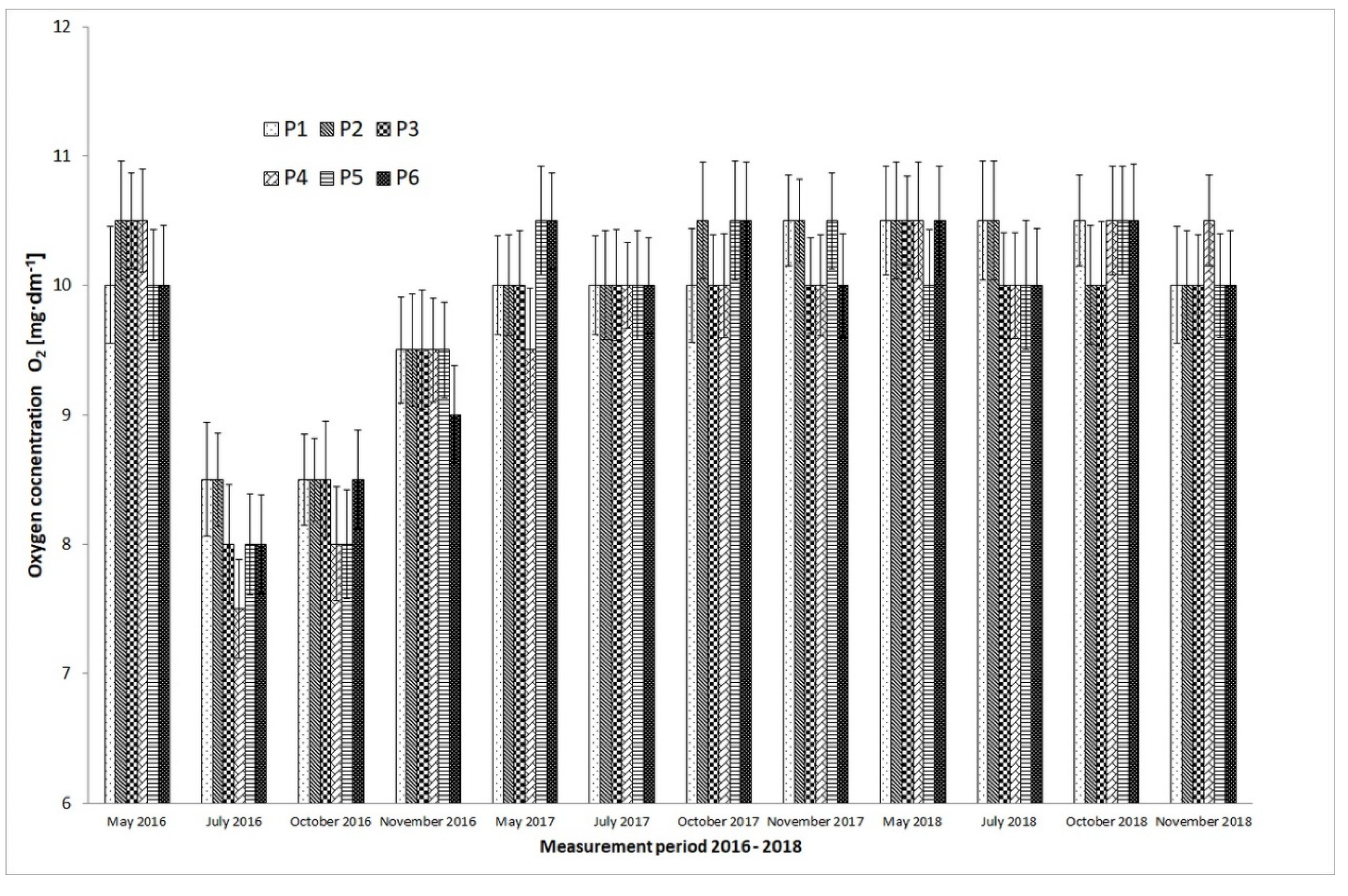
4. Discussion
5. Conclusions
- The microbiological revitalization process showed a positive effect on improving the quality parameters of the Słoneczko reservoir waters.
- Bottom sediments (soft organic fractions) have been virtually eliminated to result in a sandy bottom.
- Water clarity has improved significantly, and in 2018 the bottom of the reservoir was visible at all measuring points.
- Microorganisms from biomass mixtures introduced into the reservoir water also reduced the level of organic pollution to a safe state for aquatic ecosystems.
- The eutrophication process was eliminated, which had a positive effect on the oxygen concentration in the reservoir water in summer.
- Natural plant formation (emerging and submerged macrophytes) in the coastal zone and littoral allowed for effective nutrient uptake, therefore, their level despite intensive nitrification processes was also reduced.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Furgała-Selezniow, G.; Jankun-Woźnicka, M.; Mika, M. Lake regions under human pressure in the context of socio-economic transition in Central-Eastern Europe: The case study of Olsztyn Lakeland, Poland. Land Use Policy 2020, 90. [Google Scholar] [CrossRef]
- Wacnik, A.; Kupryjanowicz, M.; Mueller-Bieniek, A.; Karczewski, M.; Cywa, K. The environmental and cultural contexts of the late Iron age and medieval settlement in the Mazurian Lake District, NE Poland: Combined palaeobotanical and archaeological data. Veg. Hist. Archaeobotany 2014, 23, 439–459. [Google Scholar] [CrossRef]
- Tuohino, A. In Search of the Sense of Finnish Lakes. A Geographical Approach to Lake Tourism Marketing. Nord. Geogr. Publ. 2019, 44, 139. [Google Scholar]
- Dynowski, P.; Senetra, A.; Źróbek-Sokolnik, A.; Kozłowski, J. The impact of recreational activities on aquatic vegetation in Alpine Lakes. Water 2019, 11, 173. [Google Scholar] [CrossRef]
- Dustin, D.L.; Jacobson, P.C. Predicting the extent of lakeshore development using GIS datasets. Lake Reserv. Manag. 2015, 31, 169–179. [Google Scholar] [CrossRef]
- Dondajewska, R.; Gołdyn, R.; Kowalczewska-Madura, K.; Kozak, A.; Romanowicz-Brzozowska, W.; Rosińska, J.; Budzyńska, A.; Podsiadłowski, S. Hypertrophic Lakes and the Results of Their Restoration in Western Poland. In Polish River Basins and Lakes—Part II. The Handbook of Environmental Chemistry; Korzeniewska, E., Harnisz, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 87. [Google Scholar] [CrossRef]
- Wagner, T.; Erickson, L. Sustainable Management of Eutrophic Lakes and Reservoirs. J. Environ. Prot. 2017, 8, 436–463. [Google Scholar] [CrossRef]
- Bormans, M.; Maršálek, B.; Jančula, D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 407–422. [Google Scholar] [CrossRef]
- Xu, R.; Cai, Y.; Wang, X.; Li, C.; Liu, Q.; Yang, Z. Agricultural nitrogen flow in a reservoir watershed and its implications for water pollution mitigation. J. Clean. Prod. 2020, 267. [Google Scholar] [CrossRef]
- Matysik, M.; Absalon, D.; Habel, M.; Maerker, M. Surface Water Quality Analysis Using CORINE Data: An Application to Assess Reservoirs in Poland. Remote Sens. 2020, 12, 979. [Google Scholar] [CrossRef]
- Osuch, E.; Osuch, A.; Podsiadłowski, S.; Piechnik, L.; Chwirot, D. Project of coagulant dispenser in pulverization aerator with wind drive. J. Ecol. Eng. 2017, 18, 192–198. [Google Scholar] [CrossRef]
- Szarek-Gwiazda, E.; Mazurkiewicz-Boroń, G. A comparison between the water quality of the main tributaries to three submontane dam reservoirs and the sediment quality in those reservoirs. Oceanol. Hydrobiol. Stud. 2010, 39. [Google Scholar] [CrossRef]
- Statistics Poland. Environment 2019. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2019,1,20.html (accessed on 27 June 2020).
- Chen, S.; Little, J.C.; Carey, C.C.; McClure, R.P.; Lofton, M.E.; Lei, C. Three-dimensional effects of artificial mixing in a shallow drinkingwater reservoir. Water Resour. Res. 2018, 54, 425–441. [Google Scholar] [CrossRef]
- Wiech, A.K.; Marciniewicz-Mykieta, M.; i Toczko, B. Stan Środowiska w Polsce. Raport 2018. Warszawa: Główny Inspektorat Ochrony Środowiska/The State of the Environment in Poland; 2018 Report; Chief Inspectorate of Environmental Protection: Warsaw, Poland, 2018. [Google Scholar]
- Panek, P.; Ciećko, P. Zanieczyszczenia wód w Polsce—Stan śródlądowych wód powierzchniowych i podziemnych. Monografie Komitetu Inżynierii Środowiska. In Zanieczyszczenia Wód w Polsce. Stan, Przyczyny, Skutki; Monografie Nr 164; Gromiec, M., Pawłowski, L., Polskiej Akademii Nauk, Komitet Inżynierii Środowiska PAN, Eds.; Wydawnictwo Polskiej Akademii Nauk: Lublin, Poland, 2019; p. 107. ISBN 578-83-63714-63-5. [Google Scholar]
- Mendiondo, E.M. Limnologia by J G Tundisi and T Matsumura Tundisi. Braz. J. Biol. 2009, 69, 229. [Google Scholar] [CrossRef][Green Version]
- Song, H.; Li, X.; Lu, X.; Inamori, Y. Investigation of microcystin removal from eutrophic surface water by aquatic vegetable bed. Ecol. Eng. 2009, 35, 1589–1598. [Google Scholar] [CrossRef]
- Chief Inspectorate of Environmental Protection. Available online: http://www.gios.gov.pl/pl/ (accessed on 27 June 2020).
- Environment 2019—The European Environment Agency and the European Commission. The Report on European Bathing Water Quality in 2018. Available online: https://ec.europa.eu/environment/water/water-bathing/index_en.html (accessed on 27 June 2020).
- Mazur, R. Lakes Restoration: Analysis of terminology incorrectly used in the scientific literature. Acta Sci. Pol. Form. Circumiectus 2019, 18, 135–146. [Google Scholar] [CrossRef]
- Touchette, B.W.; Edwards, C.T.; Alexander, J. A Comparison of Cyanotoxin Release Following Bloom Treatment with Copper Sulfate or Sodium Carbonate Peroxyhdrate. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 314–315. [Google Scholar]
- Kuster, A.C.; Kuster, A.T.; Huser, B.J. A comparison of aluminum dosing methods for reducing sediment phosphorus release in lakes. J. Environ. Manag. 2020, 261, 110195. [Google Scholar] [CrossRef]
- EPA—United States Environmental Protection Agency. 2018 Final Aquatic Life Criteria for Aluminum in Freshwater. Available online: https://www.epa.gov/wqc/2018-final-aquatic-life-criteria-aluminum-freshwater (accessed on 27 June 2020).
- Huser, B.J. Aluminum application to restore water quality in eutrophic lakes: Maximizing binding efficiency between aluminum and phosphorus. Lake Reserv. Manag. 2017, 33, 143–151. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Dong, X.; Liu, E. Environmental changes in Chaohu Lake (southeast, China) since the mid 20th century: The interactive impacts of nutrients, hydrology and climate. Limnol. Ecol. Manag. Inland Waters 2013, 43, 10–17. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Hfuisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef]
- Podsiadłowski, S.; Osuch, E.; Przybył, J.; Osuch, A.; Buchwald, T. Pulverizing aerator in the process of lake restotation. Ecol. Eng. 2018, 121, 99–103. [Google Scholar] [CrossRef]
- Osuch, E.; Osuch, A.; Rybacki, P.; Przybylak, A. Analysis of the Theoretical Performance of the Wind-Driven Pulverizing Aerator in the Conditions of Góreckie Lake—MaximumWind Speed Method. Energies 2020, 13, 502. [Google Scholar] [CrossRef]
- Łopata, M.; Augustyniak, R.; Grochowska, J.; Parszuto, K.; Tandyrak, R. Selected Aspects of Lake Restorations in Poland. In Polish River Basins and Lakes—Part II. The Handbook of Environmental Chemistry; Korzeniewska, E., Harnisz, M., Eds.; Springer: Cham, Switzerland, 2020; Volume 87. [Google Scholar] [CrossRef]
- Cooke, G.D.; Welch, E.B.; Peterson, S.A.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; Cooke, G.D., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Lewis, M.A.; Weber, D.E.; Stanley, R.S.; Moore, J.C. Dredging impact on an urbanized Florida bayou: Effects on benthos and algal-periphyton. Environ. Pollut. 2001, 115, 161–171. [Google Scholar] [CrossRef]
- Dróżdż, D.; Malińska, K.; Mazurkiewicz, J.; Kacprzak, M.; Mrowiec, M.; Szczypiór, A.; Postawa, P.; Stachowiak, T. Fish pond sediments from aquaculture production—Current practices and potentials for nutrient recovery. Int. Agrophys. 2020, 34, 33–41. [Google Scholar] [CrossRef]
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef]
- Kozák, P.; Duris, Z.; Petrusek, A.; Buřič, M.; Horká, I.; Kouba, A.; Kozubíková-Balcarová, E.; Policar, T. Crayfish Biology and Culture; University of South Bohemia in České Budějovice, Faculty of Fisheries and Protection of Waters: Vodňany, Czech Republic, 2015. [Google Scholar]
- Nazir, R.; Rehman, S.; Nisa, N.; ali Baba, U. Chapter 7—Exploring Bacterial Diversity: From Cell to Sequence; Bandh, S.A., Shafi, S., Shameem, N., Eds.; Freshwater Microbiology, Academic Press: London, UK, 2019; pp. 263–306. [Google Scholar] [CrossRef]
- Ni, Z.; Wu, X.; Li, L.; Lv, Z.; Zhang, Z.; Hao, A.; Iseri, Y.; Kuba, T.; Zhang, X.; Wu, W.; et al. Pollution control and in situ bioremediation for lake aquaculture using an ecological dam. J. Clean. Prod. 2017, 172, 2256–2265. [Google Scholar] [CrossRef]
- Shan, M.; Wang, Y.; Xue, S. Study on bioremediation of eutrophic lake. J. Environ. Sci. 2009, 21, S16–S18. [Google Scholar] [CrossRef]
- Pandey, A.; Negi, S.; Soccol, C. Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hamilton, D.P.; Salmaso, N.; Paerl, H.W. Mitigating harmful cyanobacterial blooms: Strategies for control of nitrogen and phosphorus loads. Aquat. Ecol. 2016, 50, 351–366. [Google Scholar] [CrossRef]
- Dondajewska, R.; Kozak, A.; Rosińska, J.; Gołdyn, R. Water quality and phytoplankton structure changes under the influence of effective microorganisms (EM) and barley straw–Lake restoration case study. Sci. Total Environ. 2019, 660, 1355–1366. [Google Scholar] [CrossRef]
- Wolna-Maruwka, A.; Jakubus, M.; Jordanowska, J. ECO TABS™ preparation action in stabilization of sewage sludge part ii: Assessment of microbiological properties of sludge. Proc. Ecopole 2016, 10, 367–378. [Google Scholar] [CrossRef]
- Sitarek, M.; Napiórkowska-Krzebietke, A.; Mazur, R.; Czarnecki, B.; Pyka, J.P.; Stawecki, K.; Olech, M.; Sołtysik, S.; Kapusta, A. Application of Effective Microorganisms Technology as a lake restoration tool-A case study of Muchawka Reservoir. J. Elem. 2017, 22, 529–543. [Google Scholar] [CrossRef]
- Mazur, R. The Application of Microbiological Biopreparations in the Process of Water. Remediation of the Dam Reservoir in Głuchów. Acta Scientiarum Polonorum. Form. Circumiectus 2020, 19, 81–95. [Google Scholar] [CrossRef]
- Mazur, R.; Siatarek, M. Microbiological bioremediation of the Kamienna Gora dam reservoir. Acta Sci. Polonorum. Form. Circumiectus 2020, 19, 47–59. [Google Scholar] [CrossRef]
- Yin, C.; Lan, Z. The nutrient retention by ecotone wetlands and their modification for Baiyangdian Lake restoration. Water Sci. Technol. 1995, 32, 159–167. [Google Scholar] [CrossRef]
- Holland, M. (Ed.) Ecotones: The Role of Landscape Boundaries in the Management and Restoration of Changing Environments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- European Horizon 2020 Project. Available online: https://organic-plus.net/ (accessed on 27 June 2020).
- Trzepla, M.; Heliasz, Z.; Chybiorz, R.; Lewandowski, J.; Bojakowska, I.; Lis, J.; Pasieczna, A.; Wołkowicz, S.; Bujakowska, K.; Hrybowicz, G.; et al. Objaśnienia do Mapy Geośrodowiskowej Polski 1:50 000, Arkusz Żarki; PIG: Warszawa, Poland, 2004; p. 879. [Google Scholar]
- Regulation of the Minister of Marine Economy and Inland Navigation: Journal of Laws 2019 Pos. 2149. Regulation on the Classification of Ecological Status, Ecological Potential, Chemical Status and the Method of Classifying the Status of Surface Water Bodies as well as Environmental Quality Standards for Priority Substances. Available online: http://www.fao.org/faolex/results/details/en/c/LEX-FAOC192330/ (accessed on 27 June 2020).
- Araújo, F.; Becker, V.; Attayde, J.L. Shallow lake restoration and water quality management by the combined effects of polyaluminium chloride addition and benthivorous fish removal: A field mesocosm experiment. Hydrobiologia 2016, 778, 243–252. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Liu, Z. Lake restoration and management in a climate change perspective: An introduction. Water 2017, 9, 122. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Zhong, P.; Zhang, X.; Ning, J.; Larsen, S.E.; Chen, D.; Yiming, G.; He, H.; Jeppesen, E. Successful restoration of a tropical shallow eutrophic lake: Strong bottom-up but weak top-down effects recorded. Water Res. 2018, 146, 88–97. [Google Scholar] [CrossRef]
- OECD. Eutrophication of Waters: Monitoring, Assessments and Control; Organisation for Economic Co-Operation and Development: Paris, France, 1984. [Google Scholar] [CrossRef]
- Chróst, R. Bioremediation of microbial contaminated and eutrophicated water reservoirs: Myths and facts, for and against. Hydromicro 2017: Drobnoustroje—Osiągnięcia i Wyzwania, Proceedings of the IX Scientific Conference, Olsztyn, Poland, 4 April 2017. Available online: https://www.microbiology.pl/ix-ogolnopolska-konferencja-hydromikrobiologiczna-hydromicro-2017-drobnoustroje-osiagniecia-i-wyzwania/ (accessed on 27 June 2020).
- Osuch, E.; Osuch, A.; Podsiadłowski, S.; Rybacki, P.; Adamski, M.; Ratajczak, J. Assessment of the condition of samołęskie lake waters. J. Ecol. Eng. 2016, 17, 108–112. [Google Scholar] [CrossRef]
- Gołdyn, R.; Podsiadłowski, S.; Dondajewska-Pielka, R.; Kozak, A. The sustainable restoration of lakes—towards the challenges of the Water Framework Directive. Ecohydrol. Hydrobiol. 2014, 14. [Google Scholar] [CrossRef]
- Grochowska, J.; Augustyniak, R.; Łopata, M. How durable is the improvement of environmental conditions in a lake after the termination of restoration treatments. Ecol. Eng. 2017, 104, 23–29. [Google Scholar] [CrossRef]
- Palmieri, A.; Shah, F.; Annandale, G.W.; Dinar, A. Reservoir Conservation, Economic and Engineering Evaluation of Alternative Strategies for Managing Sedimentation in Storage Reservoirs: RESCON Approach; The RESCON Approach, World Bank: Washington, DC, USA, 2003; Volume I. [Google Scholar]
- De Oliveira, A.J.F.C.; De França, P.T.R.; Pinto, A.B. Antimicrobial resistance of heterotrophic marine bacteria isolated from seawater and sands of recreational beaches with different organic pollution levels in southeastern Brazil: Evidences of resistance dissemination. Environ. Monit. Assess. 2010, 169, 375–384. [Google Scholar] [CrossRef]
- Fiałkowska, E.; Fyda, J.; Pajdak-Stós, A.; Więckowski, K. Osad Czynny. Biologia i Analiza Mikroskopowa/Activated Sludge. Biology and Microscopic Analysis; Wydawnictwo Seidel-Przywecki: Piaseczno, Poland, 2010. [Google Scholar]
- Alobaidy, A.H.M.J.; Abid, H.S.; Maulood, B.K. Application of water quality index for assessment of Dokan lake ecosystem, Kurdistan region, Iraq. J. Water Resour. Prot. 2010, 792–798. [Google Scholar] [CrossRef]
- Watts, C.J. The effect of organic matter on sedimentary phosphorus release in an Australian reservoir. Hydrobiologia 2000, 431, 13–25. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Meng, X.; Tang, W.; Shan, B. Phosphorus transformations at the sediment–water interface in shallow freshwater ecosystems caused by decomposition of plant debris. Chemosphere 2018, 201, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.; Haghseresht, F.; Cloete, T.E. The efect of pH and anoxia on the performance of Phoslock®, a phosphorus binding clay. Harmful Algae 2008, 7, 545–550. [Google Scholar] [CrossRef]
- Liu, C.; Du, Y.; Yin, H.; Fan, C.; Chen, K.; Zhong, J.; Gu, X. Exchanges of nitrogen and phosphorus across the sediment-water interface influenced by the external suspended particulate matter and the residual matter after dredging. Environ. Pollut. 2019, 246, 207–216. [Google Scholar] [CrossRef]
- Neidhardt, H.; Achten, F.; Kern, S.; Schwientek, M.; Oelmann, Y. Phosphorus pool composition in soils and sediments of transitional ecotones under the influence of agriculture. J. Environ. Qual. 2019, 48, 1325–1335. [Google Scholar] [CrossRef]
- Gizińska-Górna, M.; Czekała, W.; Jóźwiakowski, K.; Lewicki, A.; Dach, J.; Marzec, M.; Pytka, A.; Janczak, D.; Kowalczyk-Juśko, A.; Listosz, A. The possibility of using plants from hybrid constructed wetland wastewater treatment plant for energy purposes. Ecol. Eng. 2016, 95, 534–541. [Google Scholar] [CrossRef]
- De Backer, S.; Teissier, S.; Triest, L. Identification of Total Phosphate, Submerged Vegetation Cover and Zooplankton Size Thresholds for Success of Biomanipulation in Peri-Urban Eutrophic Ponds. Hydrobiologia 2014, 737, 281–296. [Google Scholar] [CrossRef]
- Marzec, M.; Gizińska-Górna, M.; Jóźwiakowski, K.; Pytka-Woszczyło, A.; Kowalczyk-Juśko, A.; Gajewska, M. The efficiency and reliability of pollutant removal in a hybrid constructed wetland with giant miscanthus and Jerusalem artichoke in Poland. Ecol. Eng. 2019, 127, 23–35. [Google Scholar] [CrossRef]
- Marzec, M.; Jóźwiakowski, K.; Dębska, A.; Gizińska-Górna, M.; Pytka-Woszczyło, A.; Kowalczyk-Juśko, A.; Listosz, A. The efficiency and reliability of pollutant removal in a hybrid constructed wetland with common reed, manna grass, and Virginia mallow. Water 2018, 10, 1445. [Google Scholar] [CrossRef]
- Gajewska, M.; Jóźwiakowski, K.; Ghrabi, A.; Masi, F. Impact of influent wastewater quality on nitrogen removal rates in multistage treatment wetlands. Environ. Sci. Pollut. Res. 2015, 22, 12840–12848. [Google Scholar] [CrossRef] [PubMed]
- Mazur, R.; Głodkowska, A.; Kujawiak, S.; Mazurkiewicz, J.; Górski, K.; Czekała, W. Reed bed system as alternative solution for badly operating lagoon in wastewater treatment plant. Ośrodek Informacji “Technika instalacyjna w budownictwie”. Instal 2013, 4, 49–57. [Google Scholar]
- Angeler, D.G.; Allen, C.R.; Birgé, H.E.; Drakare, S.; McKie, B.G.; Johnson, R.K. Assessing and managing freshwater ecosystems vulnerable to environmental change. Ambio 2014, 43, 113–125. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]



| Name of Water Reservoir | Surface Area (ha) | Volume (m3) | Depth (m) (average–max) | Lake Types |
|---|---|---|---|---|
| Słoneczko Reservoir | 6.5 | 68,685 | 1.3–2.2 | polymictic |
| The Measurement Campaigns in Years: 2016–2018 | |||||
|---|---|---|---|---|---|
| Parameters | Measurement Points | May | July | October | November |
| Ntot, Ptot. (mg·dm−3) | P1 | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. |
| P2 | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | |
| P3 | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | |
| P4 | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | |
| P5 | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | |
| P6 | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | 5 samp. × 6 rep. | |
| COD, BOD5 (mg O2·dm−3) TSS (mg·dm−3) | P1 | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. |
| P2 | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | |
| P3 | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | |
| P4 | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | |
| P5 | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | |
| P6 | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | 5 samp. × 4 rep. | |
| Sediment depth, transparency (cm) | P1 | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. |
| P2 | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | |
| P3 | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | |
| P4 | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | |
| P5 | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | |
| P6 | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | 5 meas. × 2 rep. | |
| pH, (-) O2 (mg·dm−3) | P1 | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. |
| P2 | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | |
| P3 | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | |
| P4 | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | |
| P5 | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | |
| P6 | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | 5 meas. × 4 rep. | |
| Indication | Unit | Research Method | The Limit of Quantification | Uncertainty Expressed as Precision |
|---|---|---|---|---|
| BOD5 | mgO2·dm−3 | PN-EN ISO 5815-1:2019-12 PN-EN ISO 934-2+A1:2012 | ±4.5 | 11% |
| COD–Cr | mgO2·dm−3 | ISO 15705 | ±8.5 | 9% |
| Total nitrogen | mgN·dm−3 | EN-ISO 11905/1 ISO 7890-1 | ±0.5 | 1.3% |
| Total phosphorus | mgP·dm−3 | EN-ISO 6878 | ±0.5 | 1.2% |
| Total suspended solids | mg·dm−3 | ISO 11923: 1997(R2019) | ±1.5 | 2% |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Df | Sum Sq | Mean Sq | F Value | Pr (>F) | ||
| Sediments | Months | 11 | 114,811.1 | 10,437.4 | 167.7 | 0.00 |
| Residuals | 44,064.9 | 708 | 62.2 | |||
| BOD5 | Groups | 11 | 85,784.7 | 7798.6 | 4420.81 | 0.00 |
| Residuals | 2519.1 | 1428 | 1.8 | |||
| COD | Groups | 11 | 112,290 | 10,208 | 1443.8 | 0.00 |
| Residuals | 10,097 | 1428 | 7 | |||
| Water transparence | Groups | 11 | 2,477,432 | 225,221 | 1118.57 | 0.00 |
| Residuals | 142554 | 708 | 201 | |||
| O2 | Groups | 11 | 850.3 | 77.3 | 388.1 | 0.00 |
| Residuals | 284.4 | 1428 | 0.2 | |||
| TN | Groups | 11 | 53.6275 | 4.875225 | 2200.6 | 0.00 |
| Residuals | 4.75877 | 2148 | 0.002215 | |||
| TP | Groups | 11 | 0.948310 | 0.08621 | 3039.9 | 0.00 |
| Residuals | 0.060917 | 2148 | 2.84 ×10−5 | |||
| TSS | Groups | 11 | 61,692.6 | 5608.4 | 1418.7 | 0.00 |
| Residuals | 5645 | 1428 | 4 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurkiewicz, J.; Mazur, A.; Mazur, R.; Chmielowski, K.; Czekała, W.; Janczak, D. The Process of Microbiological Remediation of the Polluted Słoneczko Reservoir in Poland: For Reduction of Water Pollution and Nutrients Management. Water 2020, 12, 3002. https://doi.org/10.3390/w12113002
Mazurkiewicz J, Mazur A, Mazur R, Chmielowski K, Czekała W, Janczak D. The Process of Microbiological Remediation of the Polluted Słoneczko Reservoir in Poland: For Reduction of Water Pollution and Nutrients Management. Water. 2020; 12(11):3002. https://doi.org/10.3390/w12113002
Chicago/Turabian StyleMazurkiewicz, Jakub, Agata Mazur, Robert Mazur, Krzysztof Chmielowski, Wojciech Czekała, and Damian Janczak. 2020. "The Process of Microbiological Remediation of the Polluted Słoneczko Reservoir in Poland: For Reduction of Water Pollution and Nutrients Management" Water 12, no. 11: 3002. https://doi.org/10.3390/w12113002
APA StyleMazurkiewicz, J., Mazur, A., Mazur, R., Chmielowski, K., Czekała, W., & Janczak, D. (2020). The Process of Microbiological Remediation of the Polluted Słoneczko Reservoir in Poland: For Reduction of Water Pollution and Nutrients Management. Water, 12(11), 3002. https://doi.org/10.3390/w12113002








