Effect of Natural Organic Matter on the Ozonation Mechanism of Trimethoprim in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Ozonation Experiments Setup
2.3. TMP Ozonation Study
2.3.1. Removal of TMP in O3 Solution
2.3.2. Ozonation of TMP in O3 Contactor
2.4. Analytical Methods
3. Results and Discussion
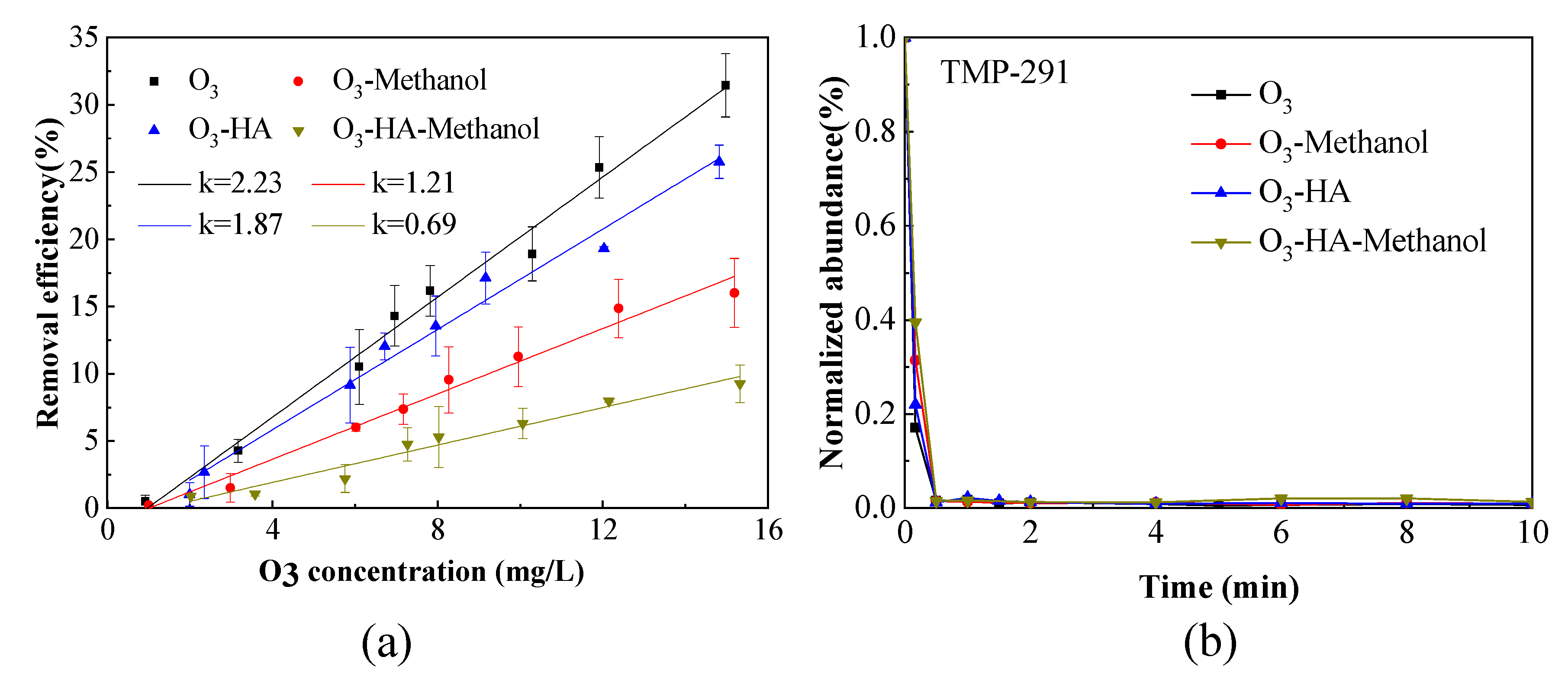
3.1. Removal Efficiency of TMP by Ozonation
3.1.1. Removal Efficiency of TMP in O3 Saturated Water
3.1.2. Removal Efficiency of TMP in O3 Contactor
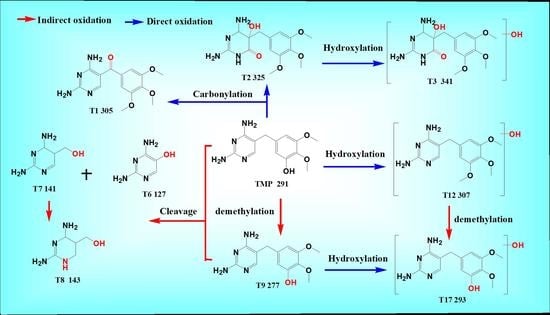
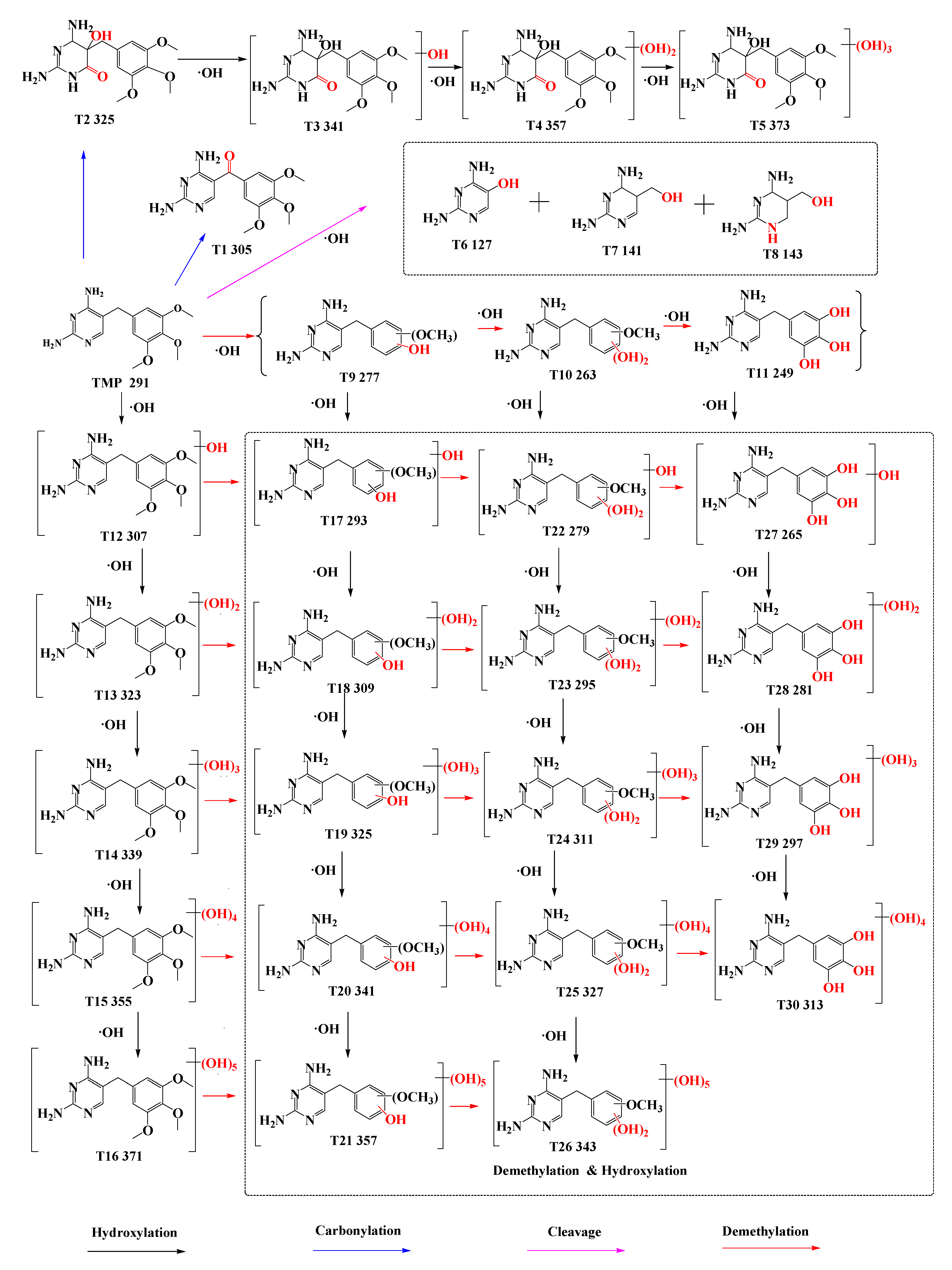
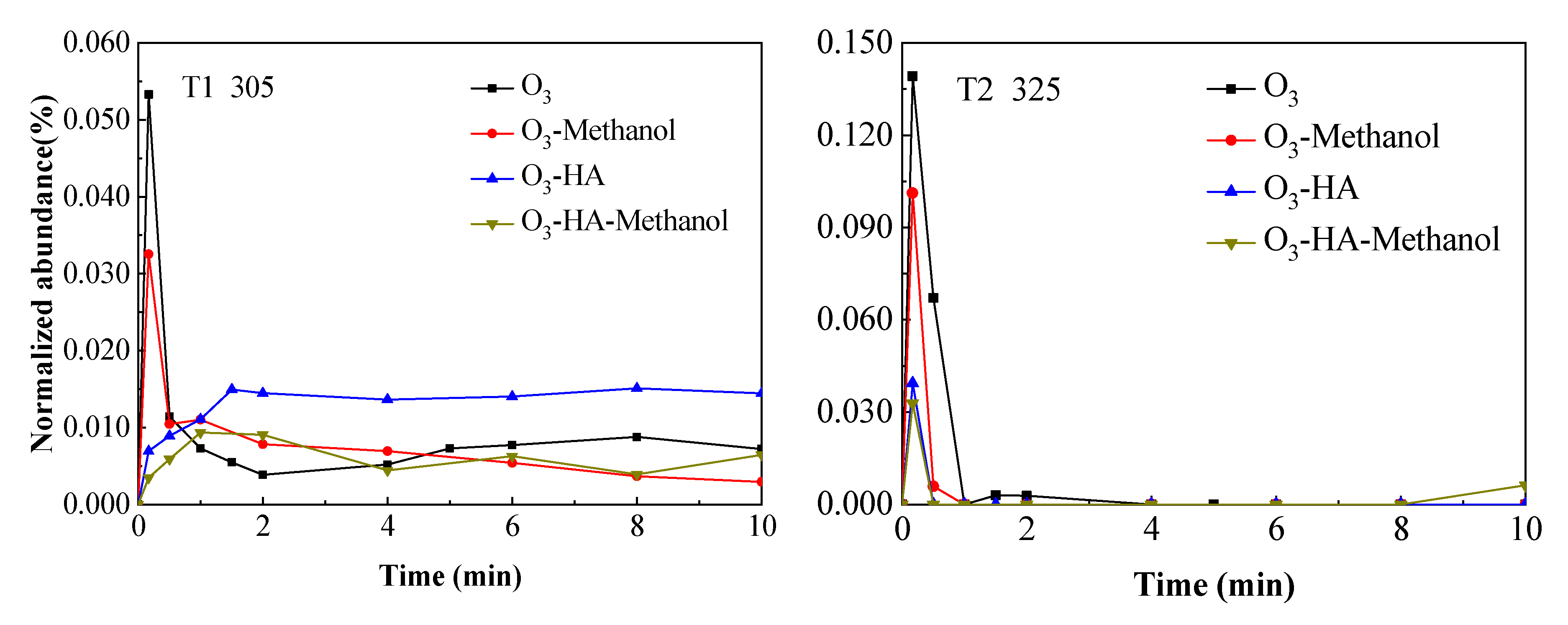
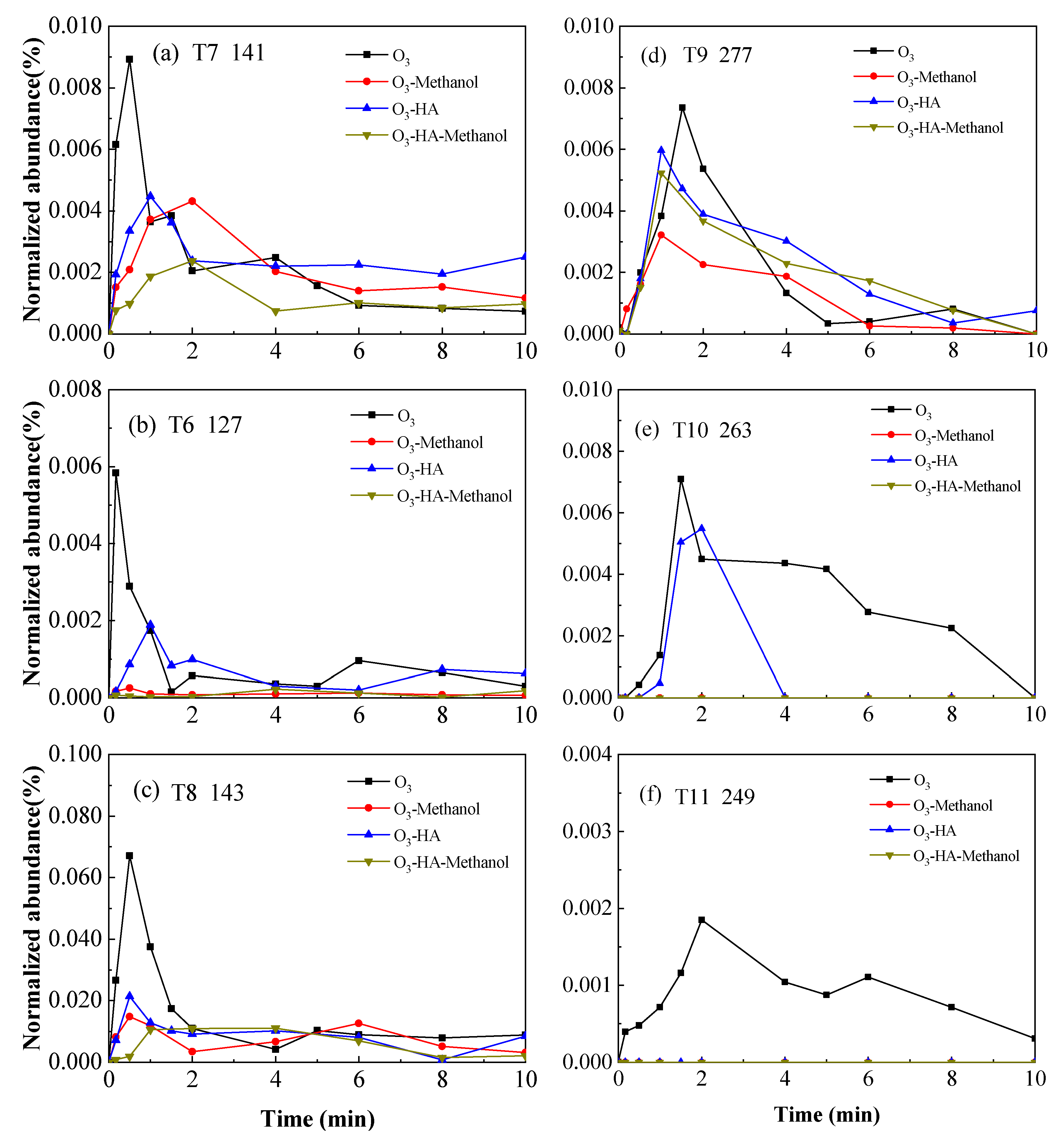
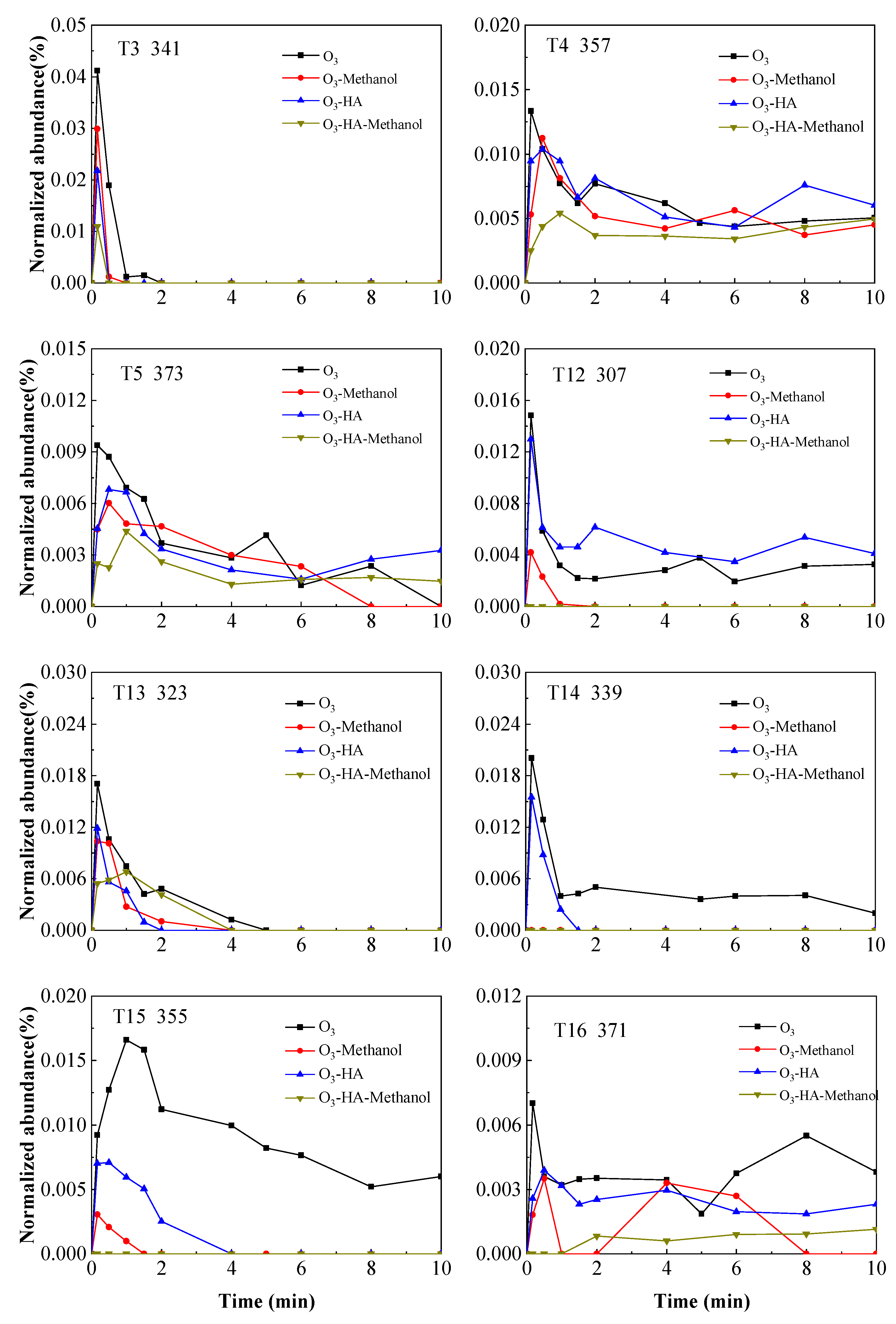
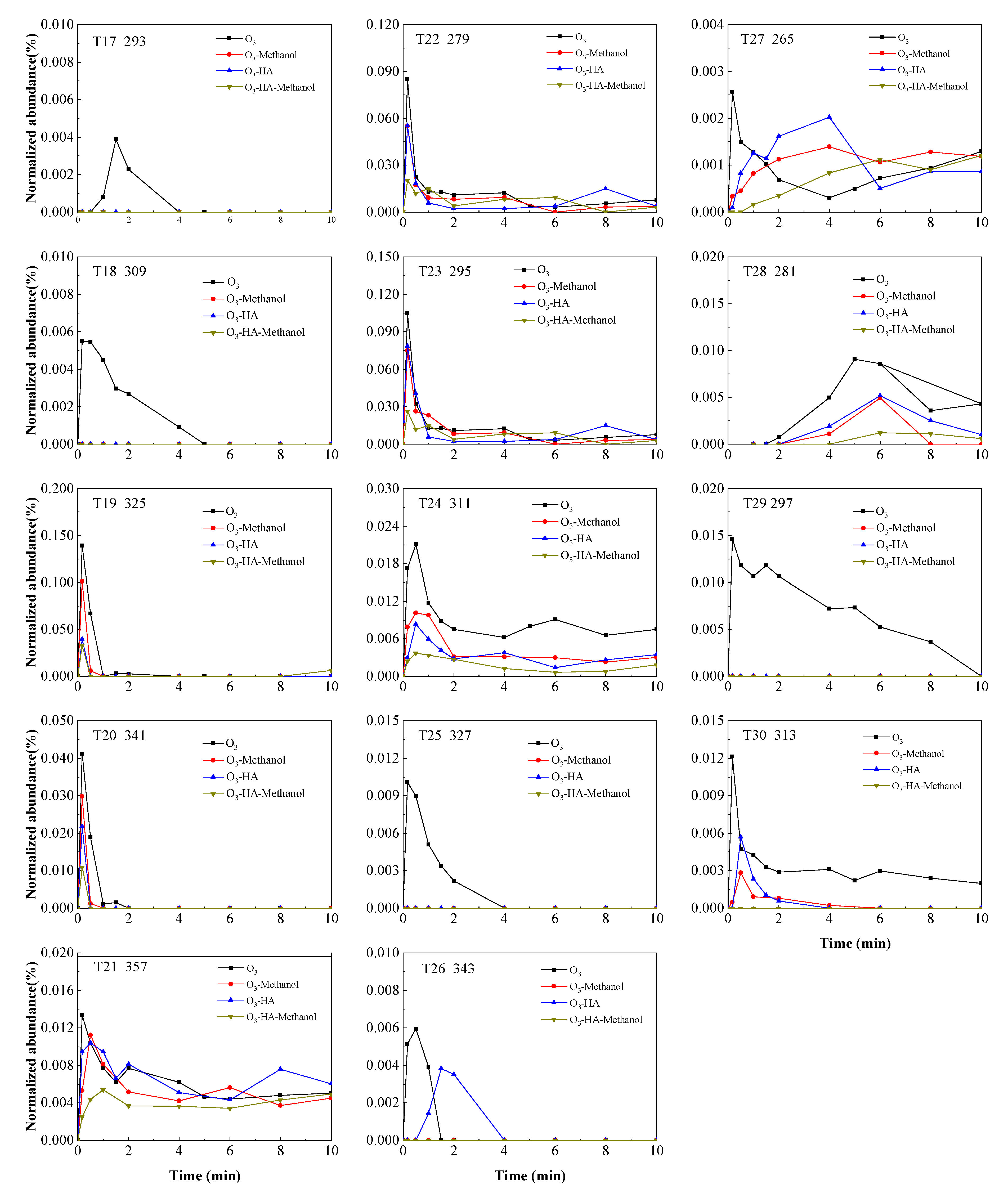
3.2. Degradation Pathway of TMP
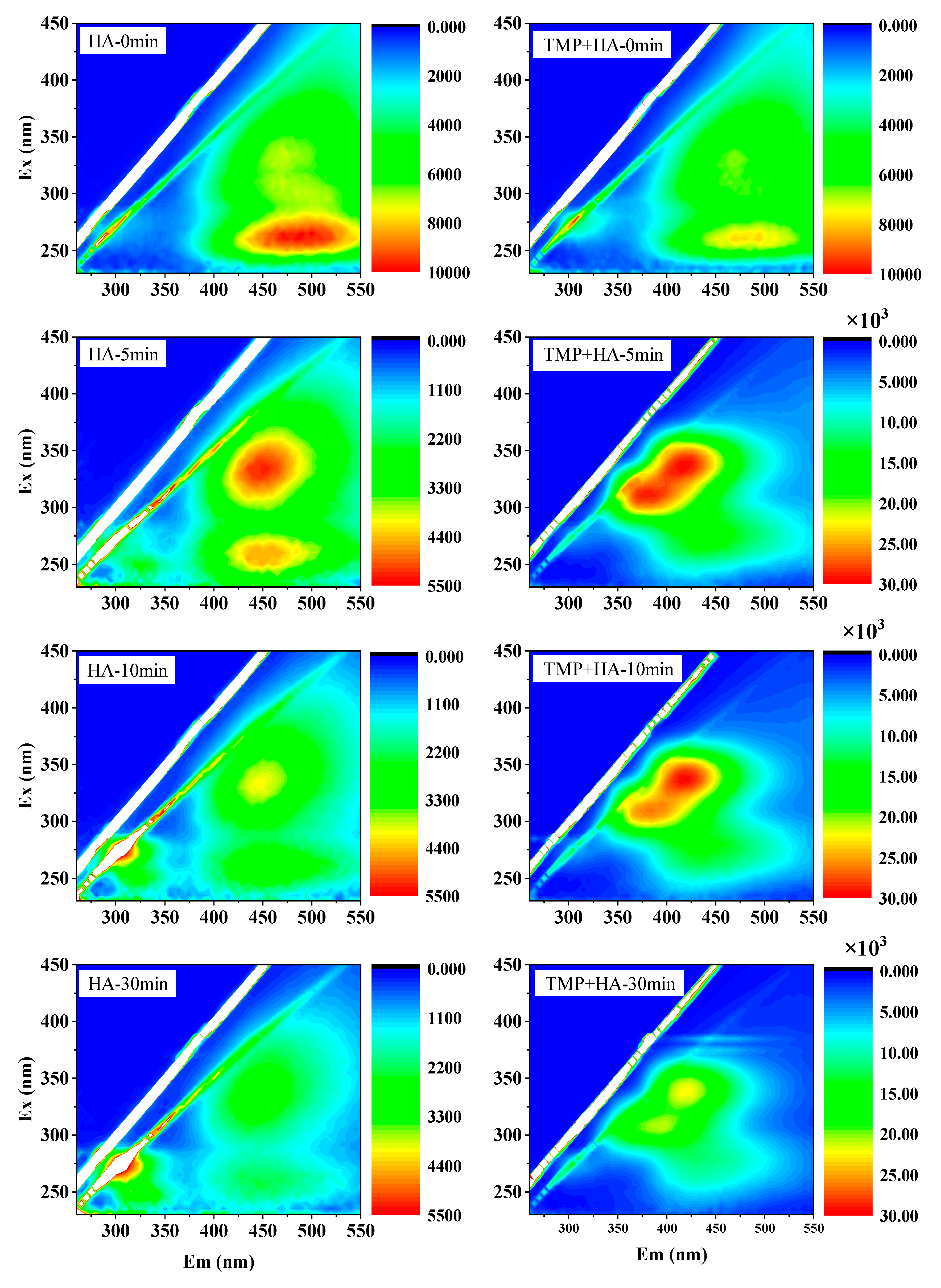
3.3. EEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Padhye, L.P.; Yao, H.; Kung’u, F.T.; Huang, C.H. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2005, 77, 3807–3838. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Trujillano, R.; Vicente, M.A.; Rives, V.; Korili, S.A.; Gil, A. Photocatalytic degradation of trimethoprim on doped Ti-pillared montmorillonite. Appl. Clay Sci. 2019, 167, 43–49. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Liu, X.; Zeng, H.; Zhang, H.; Crittenden, J. Efficient sulfadiazine degradation via in-situ epitaxial grow of Graphitic Carbon Nitride (g-C3N4) on carbon dots heterostructures under visible light irradiation: Synthesis, mechanisms and toxicity evaluation. J. Colloid Interface Sci. 2020, 561, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.; Araujo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Challis, J.K.; Carlson, J.C.; Friesen, K.J.; Hanson, M.L.; Wong, C.S. Aquatic photochemistry of the sulfonamide antibiotic sulfapyridine. J. Photochem. Photobiol. A Chem. 2013, 262, 14–21. [Google Scholar] [CrossRef]
- Rong, S.-P.; Sun, Y.-B.; Zhao, Z.-H. Degradation of sulfadiazine antibiotics by water falling film dielectric barrier discharge. Chin. Chem. Lett. 2014, 25, 187–192. [Google Scholar] [CrossRef]
- Garoma, T.; Umamaheshwar, S.K.; Mumper, A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.H.; Xiong, X.L.; Li, Z.G.; Jin, X.H.; Wu, Y.N. Study of the physicochemical properties of trimethoprim with beta-cyclodextrin in solution. J. Pharm. Biomed. 2005, 38, 370–374. [Google Scholar] [CrossRef]
- Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef] [PubMed]
- de Paula, F.C.; de Pietro, A.C.; Cass, Q.B. Simultaneous quantification of sulfamethoxazole and trimethoprim in whole egg samples by column-switching high-performance liquid chromatography using restricted access media column for on-line sample clean-up. J. Chromatogr. A 2008, 1189, 221–226. [Google Scholar] [CrossRef]
- Li, R.; Huang, J.; Cai, M.; Huang, J.; Xie, Z.; Zhang, Q.; Liu, Y.; Liu, H.; Lv, W.; Liu, G. Activation of peroxymonosulfate by Fe doped g-C3N4/graphene under visible light irradiation for Trimethoprim degradation. J. Hazard Mater. 2020, 384, 121435. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, W.; Fan, Y.; Shi, Y.; Kong, D.; Lu, J. Degradation of trimethoprim by thermo-activated persulfate oxidation: Reaction kinetics and transformation mechanisms. Chem. Eng. J. 2016, 286, 16–24. [Google Scholar] [CrossRef]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II) untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef]
- Thiebault, T. Sulfamethoxazole/Trimethoprim ratio as a new marker in raw wastewaters: A critical review. Sci. Total Environ. 2020, 715, 136916. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Degradation of trimethoprim antibiotic by UVA photoelectro-Fenton process mediated by Fe (III)–carboxylate complexes. Appl. Catal. B Environ. 2015, 162, 34–44. [Google Scholar] [CrossRef]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I. Individual and simultaneous degradation of antibiotics sulfamethoxazole and trimethoprim by UV and solar radiation in aqueous solution using bentonite and vermiculite as photocatalysts. Appl. Clay Sci. 2017. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Liu, X.; Zhang, Q.; Liu, R.; Wang, Z.; Shi, X.; Quan, J.; Shen, X.; Zhang, F. Removal of sulfamethoxazole and trimethoprim from reclaimed water and the biodegradation mechanism. Front. Environ. Sci. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Deng, S.; Yu, G.; Fan, Q. Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res. 2010, 44, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Angela, L.; Batt, S.K.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W.; Joss, A. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Richard, H.; Lindberg, U.; Olofsson, P.; Rendahl, M.; Johansson, I.; Tysklind, M.; Andersson, B.A.V. Behavior of Fluoroquinolones and Trimethoprim during Mechanical, Chemical, and Active Sludge Treatment of Sewage Water and Digestion of Sludge. Environ. Sci. Technol. 2006, 40, 1042–1048. [Google Scholar]
- Sui, Q.; Huang, J.; Deng, S.; Chen, W.; Yu, G. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ. Sci. Technol. 2011, 45, 3341–3348. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Borges, W.; Chen, H.; Hsiao, B.S. Remediation of UO22+ from Water by Nitro-Oxidized Carboxycellulose Nanofibers: Performance and Mechanism. In Contaminants in Our Water: Identification and Remediation Methods; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 269–283. [Google Scholar]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, Z.; Greaves, J.; Cooper, W.J.; Song, W. Trimethoprim: Kinetic and mechanistic considerations in photochemical environmental fate and AOP treatment. Water Res. 2012, 46, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Hapeshi, E.; Osorio, V.; Perez, S.; Petrovic, M.; Zapata, A.; Malato, S.; Barceló, D.; Fatta-Kassinos, D. Solar photocatalytic treatment of trimethoprim in four environmental matrices at a pilot scale: Transformation products and ecotoxicity evaluation. Sci. Total Environ. 2012, 430, 167–173. [Google Scholar] [CrossRef]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A study of TiO2 nanocrystal growth and environmental remediation capability of TiO2/CNC nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [PubMed]
- Oneby, M.A.; Bromley, C.O.; Borchardt, J.H.; Harrison, D.S. Ozone Treatment of Secondary Effluent at U.S. Municipal Wastewater Treatment Plants. Ozone Sci. Eng. 2010, 32, 43–55. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Khan, N.A.; Ahmed, S.; Dhingra, A.; Singh, C.P.; Khan, S.U.; Mohammadi, A.A.; Changani, F.; Yousefi, M.; Alam, S.; et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020, 269, 122411. [Google Scholar] [CrossRef]
- Wang, H.; Mustafa, M.; Yu, G.; Ostman, M.; Cheng, Y.; Wang, Y.; Tysklind, M. Oxidation of emerging biocides and antibiotics in wastewater by ozonation and the electro-peroxone process. Chemosphere 2019, 235, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Staehelln, J.; Holgné, J. Decomposition of Ozone in Water Rate of Initiation by Hydroxide Ions and Hydrogen Peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Yargeau, V.; Leclair, C. Impact of Operating Conditions on Decomposition of Antibiotics during Ozonation: A Review. Ozone Sci. Eng. 2008, 30, 175–188. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part, I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U.; Kaiser, H.-P. Hydroxyl Radical/Ozone Ratios during Ozonation Processes. II. The Effect of Temperature, pH, Alkalinity, and DOM Properties. Ozone Sci. Eng. 2000, 22, 123–150. [Google Scholar] [CrossRef]
- Haag, W.R.; Yao, C.C.D. Rate Constants for Reaction of Hydroxyl Radicals with Several Drinking Water Contaminants. Environ. Sci. Technol. 1992, 26, 1005–1013. [Google Scholar] [CrossRef]
- Michael, C.D.; Buffle, M.; Gunten, U.V. Oxidation of Antibacterial Molecules by Aqueous Ozone: Moiety-Specific Reaction Kinetics and Application to Ozone-Based Wastewater Treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar]
- Ling, W.; Ben, W.; Xu, K.; Zhang, Y.; Yang, M.; Qiang, Z. Ozonation of norfloxacin and levofloxacin in water: Specific reaction rate constants and defluorination reaction. Chemosphere 2018, 195, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Huang, J.; Wang, B.; Cao, Q.; Deng, S.; Yu, G. Ozonation of trimethoprim in aqueous solution: Identification of reaction products and their toxicity. Water Res. 2013, 47, 2863–2872. [Google Scholar] [CrossRef]
- Kima, H.; Yooa, H.; Honga, S.; Leea, S.; Parkc, B.; Parkd, H.; Leee, C.; Leef, J. Effects of inorganic oxidants on kinetics and mechanisms of WO3-mediated photocatalytic degradation. Appl. Catal. B Environ. 2015, 163, 515–523. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Audenaert, W.T.M.; Vandierendonck, D.; Van Hulle, S.W.H.; Nopens, I. Comparison of ozone and HO induced conversion of effluent organic matter (EfOM) using ozonation and UVH2O2 treatment. Water Res. 2013, 47, 2387–2398. [Google Scholar] [CrossRef]
- Quaranta, M.L.; Mendes, M.D.; MacKay, A.A. Similarities in effluent organic matter characteristics from Connecticut wastewater treatment plants. Water Res. 2012, 46, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, Y. Impact of Water Quality on the Formation of Bromate and Formaldehyde during Water Ozonation. J. Environ. Health Sci. 2007, 33, 441–450. [Google Scholar] [CrossRef]
- Conde-Cida, M.; Fernández-Calviñoa, D.; Nóvoa-Muñoza, J.C.; Arias-Estéveza, M.; Díaz-Raviñab, M.; Núñez-Delgadoc, A.; Fernández-Sanjurjoc, M.J.; Álvarez-Rodríguezc, E. Degradation of sulfadiazine, sulfachloropyridazine and sulfamethazine in aqueous media. J. Environ. Manag. 2018, 228, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–569. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Z.; Wang, D. Ozone direct oxidation kinetics of Cationic Red X-GRL in aqueous solution. J. Hazard Mater. 2006, 137, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Deng, J.; Wang, J.; Li, W. Highly efficient removal of trimethoprim based on peroxymonosulfate activation by carbonized resin with Co doping: Performance, mechanism and degradation pathway. Chem. Eng. J. 2019, 356, 717–726. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Q.; Wang, J. Degradation of trimethoprim by gamma irradiation in the presence of persulfate. Radiat. Phys. Chem. 2016, 127, 85–91. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M.; Ookawara, S.; Ohno, T. Photocatalytic degradation of trimethoprim using S-TiO2 and Ru/WO3/ZrO2 immobilized on reusable fixed plates. J. Water Process Eng. 2020, 33, 101023. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Characterization of atenolol transformation products in ozonation by using rapid resolution high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. Microchem. J. 2011, 99, 312–326. [Google Scholar] [CrossRef]
- Sirtori, C.; Aguera, A.; Gernjak, W.; Malato, S. Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways. Water Res. 2010, 44, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Zhou, B.; Yuan, R.; Wang, F.; Chen, H. Effect of Natural Organic Matter on the Ozonation Mechanism of Trimethoprim in Water. Water 2020, 12, 2935. https://doi.org/10.3390/w12102935
Zhang N, Zhou B, Yuan R, Wang F, Chen H. Effect of Natural Organic Matter on the Ozonation Mechanism of Trimethoprim in Water. Water. 2020; 12(10):2935. https://doi.org/10.3390/w12102935
Chicago/Turabian StyleZhang, Ning, Beihai Zhou, Rongfang Yuan, Fei Wang, and Huilun Chen. 2020. "Effect of Natural Organic Matter on the Ozonation Mechanism of Trimethoprim in Water" Water 12, no. 10: 2935. https://doi.org/10.3390/w12102935
APA StyleZhang, N., Zhou, B., Yuan, R., Wang, F., & Chen, H. (2020). Effect of Natural Organic Matter on the Ozonation Mechanism of Trimethoprim in Water. Water, 12(10), 2935. https://doi.org/10.3390/w12102935