Removal of Diclofenac in Effluent of Sewage Treatment Plant by Photocatalytic Oxidation
Abstract
1. Introduction
2. Material and Methods
2.1. Material and Reagents
2.2. Photocatalytic Degradation Experiment
2.3. Analytical Methods
3. Results and Discussion
3.1. Photocatalytic Oxidation of DCF in Secondary Effluent
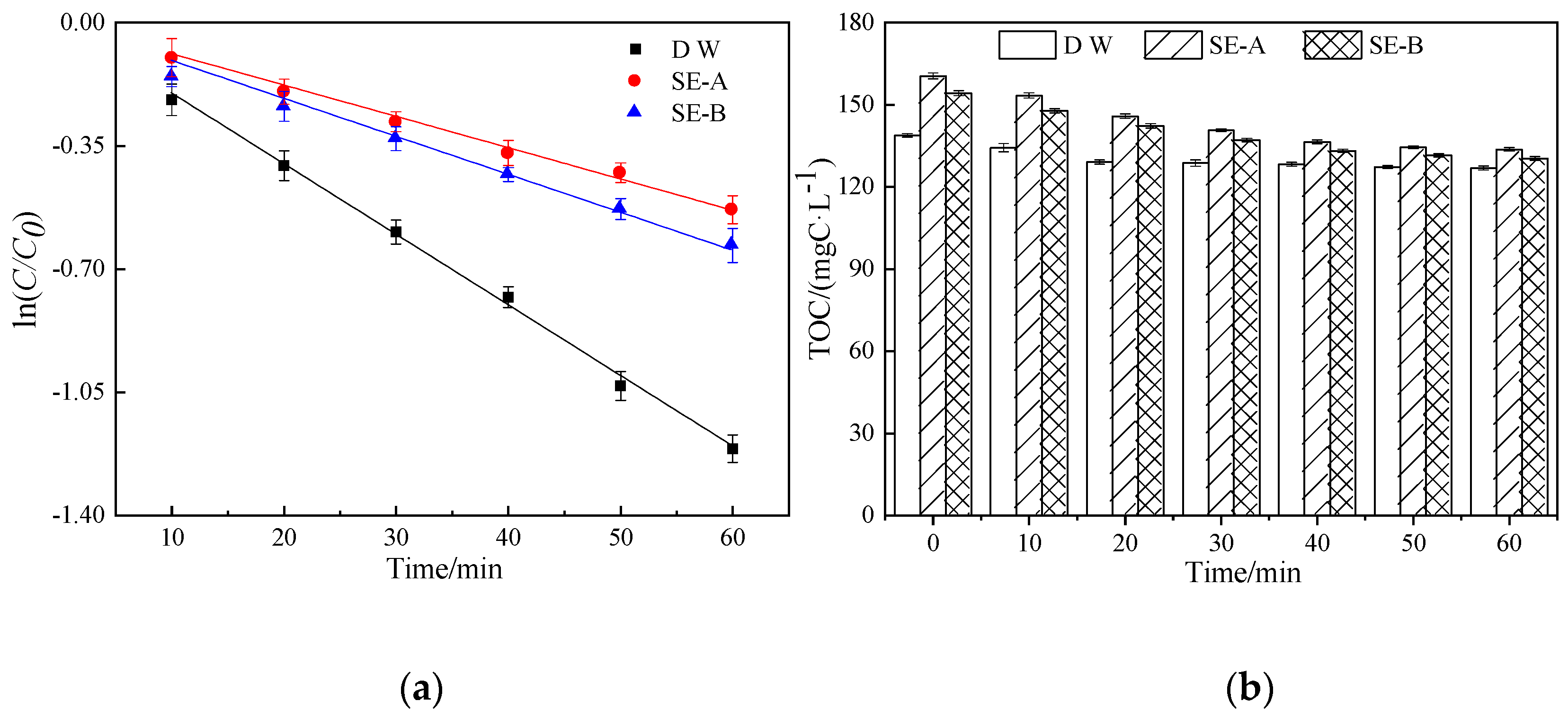
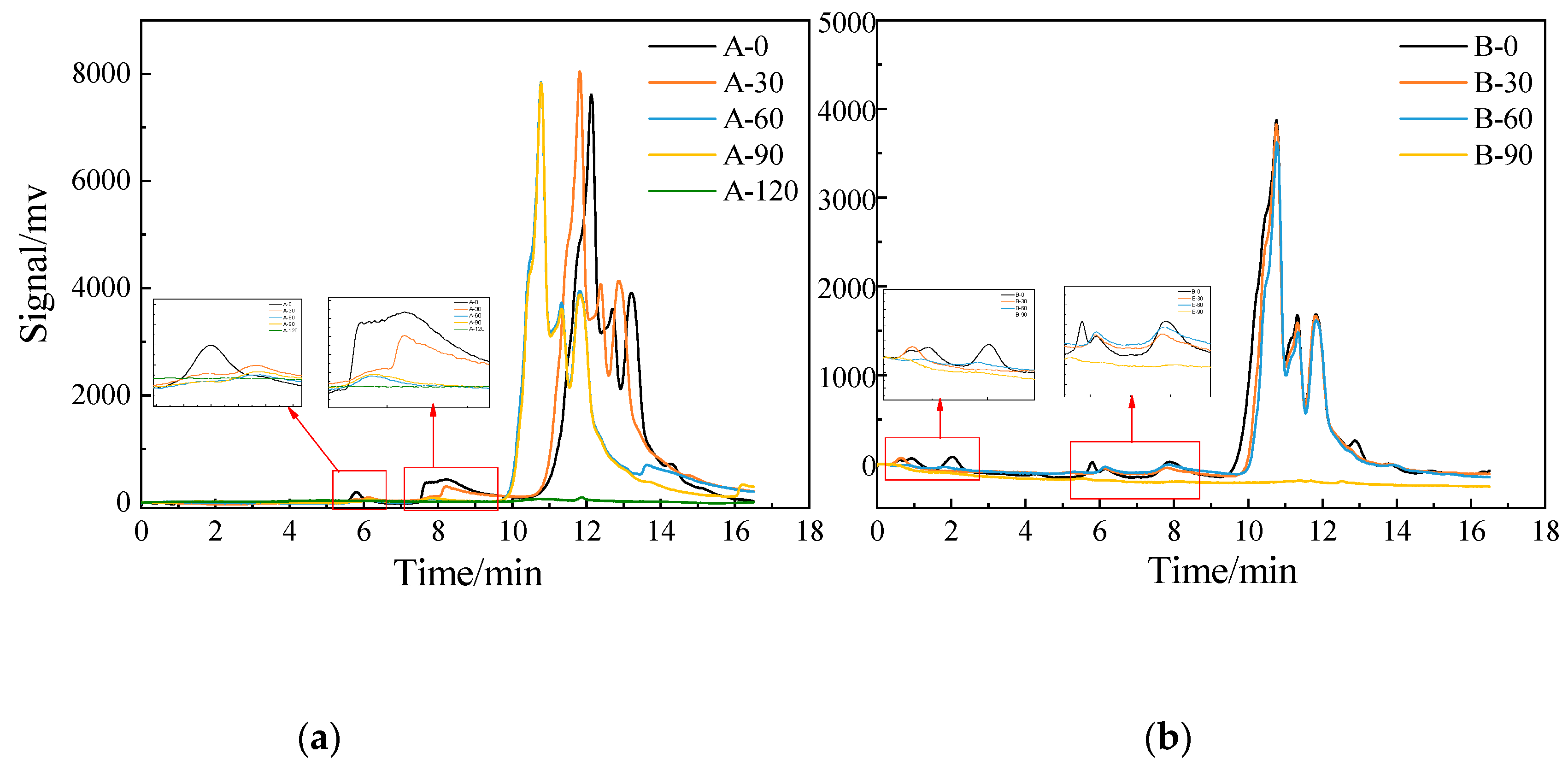
3.1.1. Degradation of DCF in SE-A and SE-B
3.1.2. Effects of DOM, Anion, and Cation
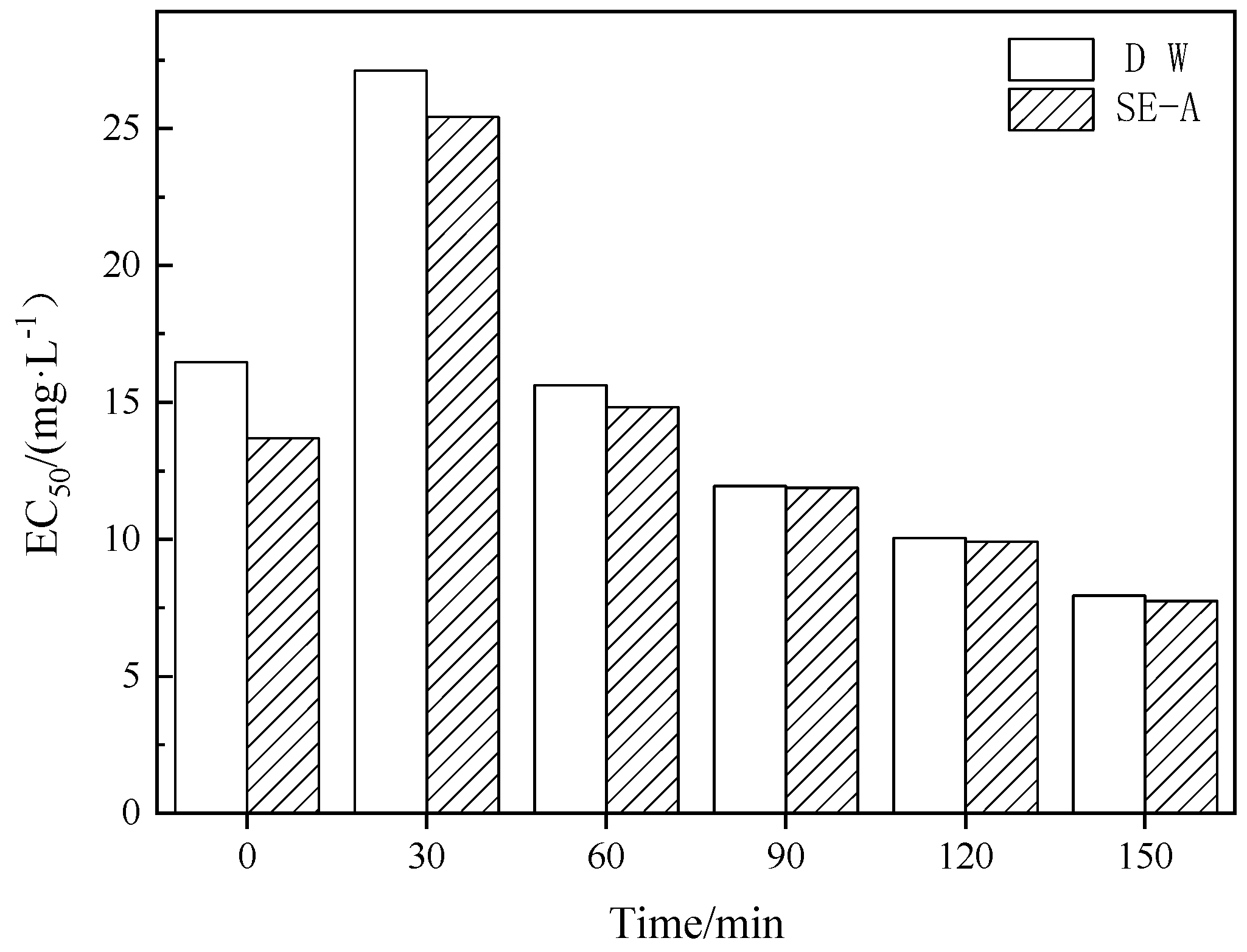
3.1.3. Biological Toxicities of DCF
3.2. Characteristics of DOM During Photocatalytic Degradation
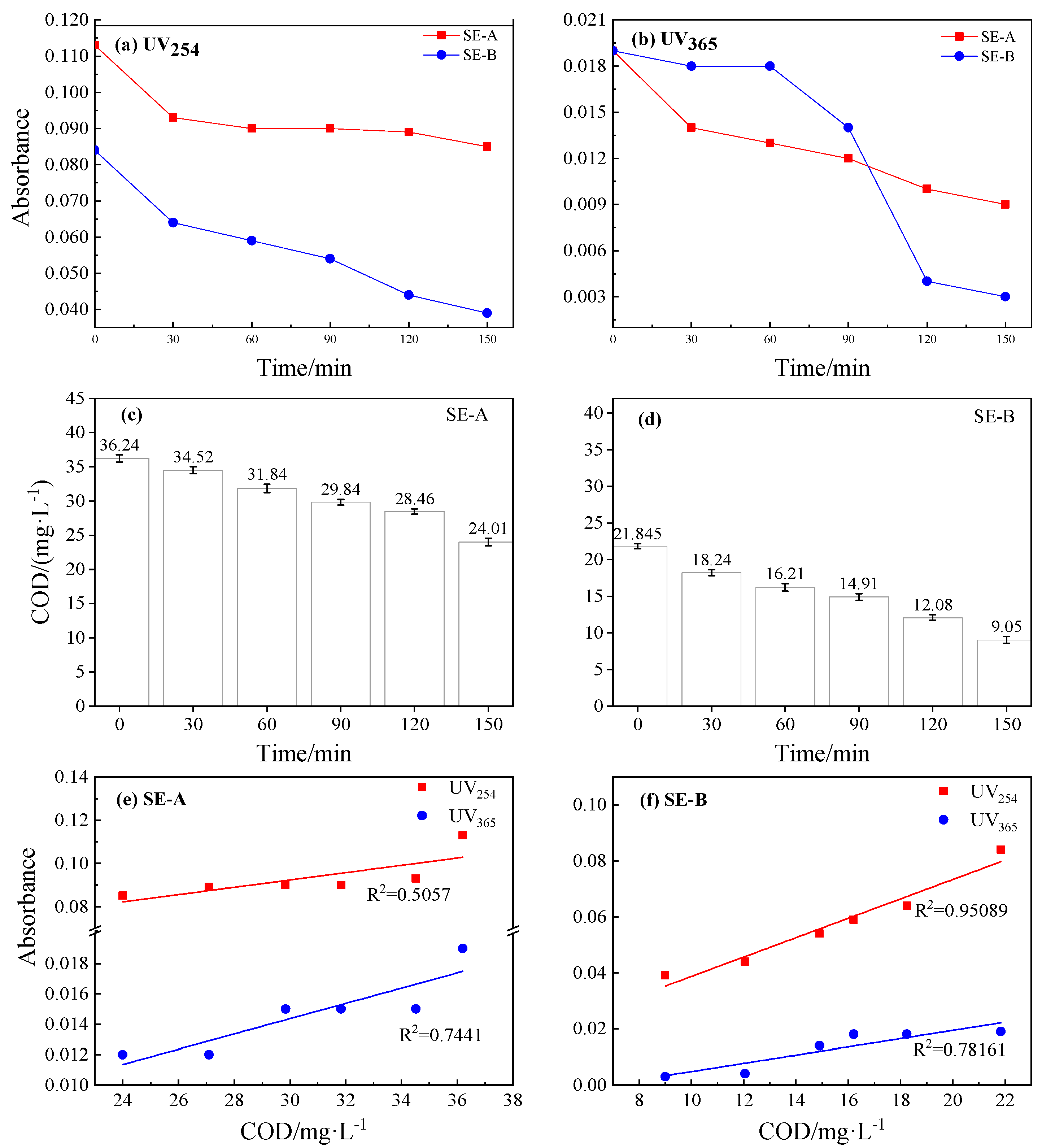
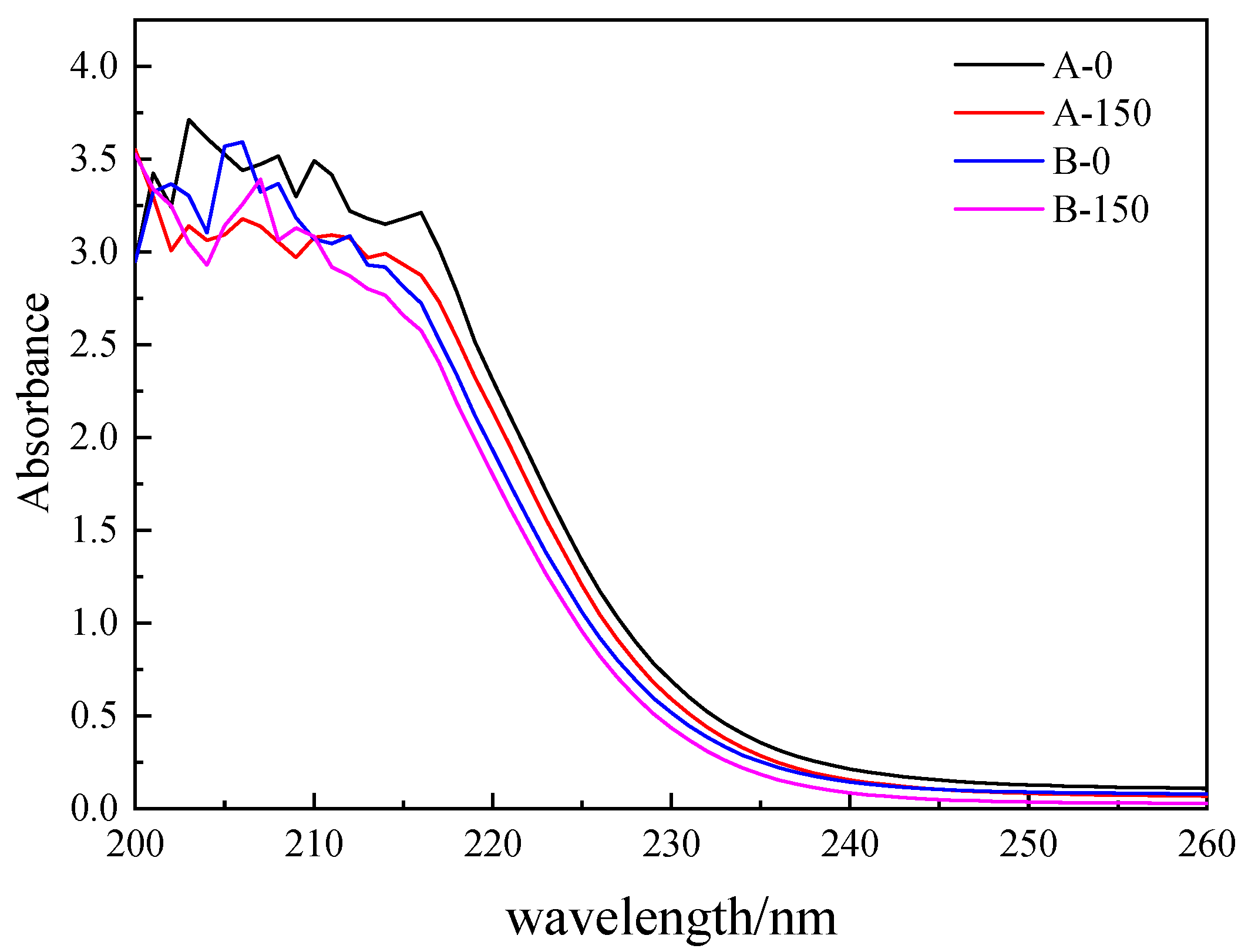
3.2.1. COD, UV254 and UV365
3.2.2. Three-Dimensional Fluorescence of DOM
3.2.3. AMW of DOM
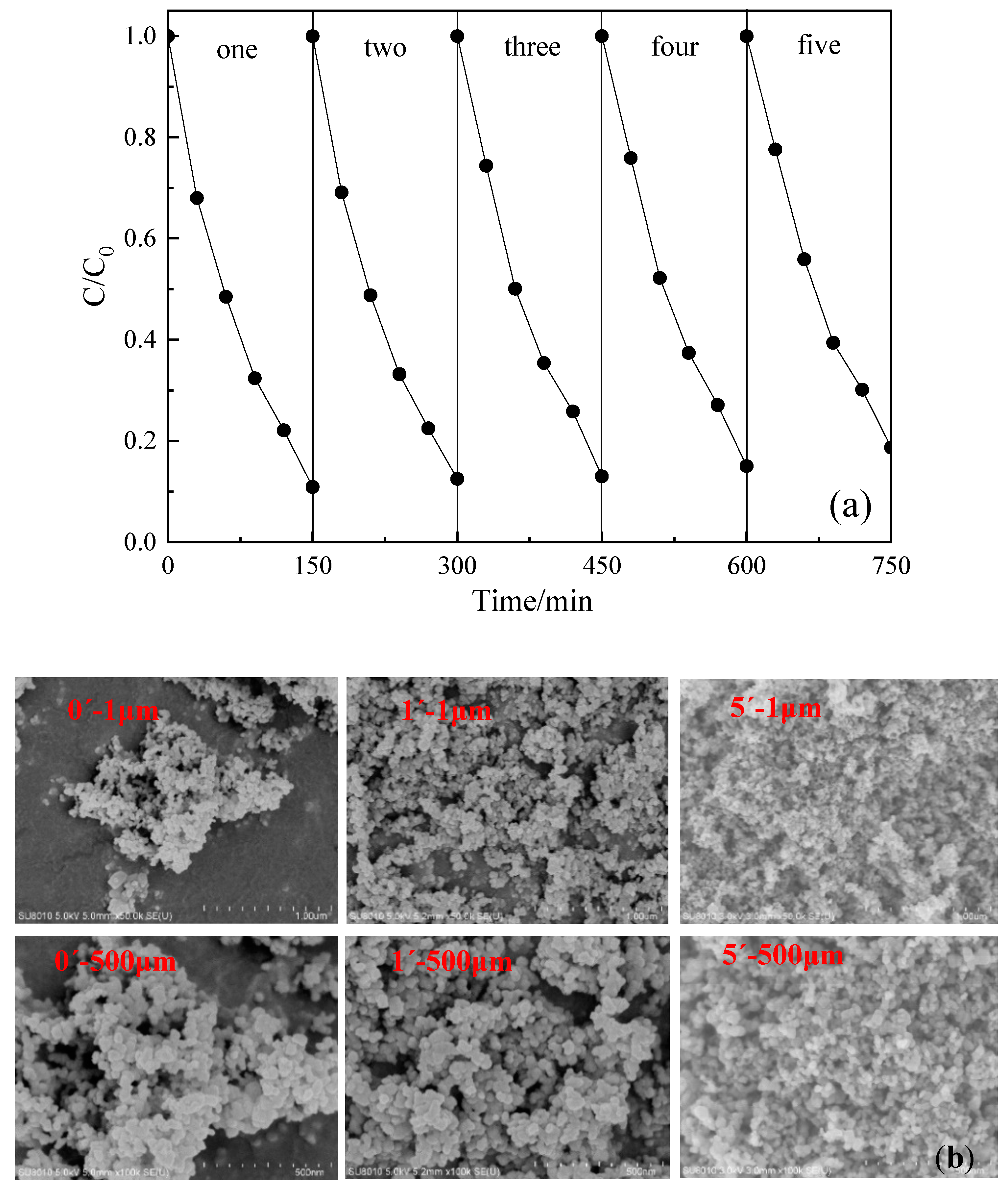
3.3. Repeatability of Photocatalyst
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daughton, C.G. Pharmaceuticals and Personal Care Products in the Environment: Overarching Issues and Overview; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Deo, R.; Halden, R. Pharmaceuticals in the Built and Natural Water Environment of the United States. Water 2013, 5, 1346–1365. [Google Scholar] [CrossRef]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ying, G.G.; Zhao, J.L.; Yang, X.B.; Chen, F.; Tao, R.; Liu, S.; Zhou, L.J. Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of north China. Sci. Total Environ. 2010, 408, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, J.; Zhou, J.; Xi, Q.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Fabricating I doped TiO2 photoelectrode for the degradation of diclofenac: Performance and mechanism study. Chem. Eng. J. 2019, 369, 968–978. [Google Scholar] [CrossRef]
- Wang, J.; Lou, Y.; Zhuang, X.; Song, S.; Liu, W.; Xu, C. Magnetic Pr6O11/SiO2@Fe3O4 particles as the heterogeneous catalyst for the catalytic ozonation of acetochlor: Performance and aquatic toxicity. Sep. Purif. Technol. 2018, 197, 63–69. [Google Scholar] [CrossRef]
- Taggart, M.A.; Cuthbert, R.; Das, D.; Sashikumar, C.; Pain, D.J.; Green, R.E.; Feltrer, Y.; Shultz, S.; Cunningham, A.A.; Meharg, A.A. Diclofenac disposition in Indian cow and goat with reference to Gyps vulture population declines. Environ. Pollut 2007, 147, 60–65. [Google Scholar] [CrossRef]
- Balbi, T.; Montagna, M.; Fabbri, R.; Carbone, C.; Franzellitti, S.; Fabbri, E.; Canesi, L. Diclofenac affects early embryo development in the marine bivalve Mytilus galloprovincialis. Sci. Total Environ. 2018, 642, 601–609. [Google Scholar] [CrossRef]
- Guo, R.; Xia, X.; Zhang, X.; Li, B.; Zhang, H.; Cheng, X.; Xie, M.; Cheng, Q. Construction of Ag3PO4/TiO2 nano-tube arrays photoelectrode and its enhanced visible light driven photocatalytic decomposition of diclofenac. Sep. Purif. Technol. 2018, 200, 44–50. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Q.; Fu, Y.; Peng, B.; Zhou, G. Kinetics and mechanism of diclofenac removal using ferrate(VI): Roles of Fe(3+), Fe(2+), and Mn(2). Environ. Sci. Pollut. Res. Int. 2018, 25, 22998–23008. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Topics Curr. Chem. 2019, 378, 7. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic Degradation of Azo Dye Reactive Violet 5 on Fe-Doped Titania Catalysts under Visible Light Irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef]
- Xia, D.; Lo, I.M.C. Synthesis of magnetically separable Bi2O4/Fe3O4 hybrid nanocomposites with enhanced photocatalytic removal of ibuprofen under visible light irradiation. Water Res. 2016, 100, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic titanium dioxide based nanomaterials: Synthesis, characteristics, and photocatalytic application in pollutant degradation. J. Mater. Chem. A 2015, 3, 17511–17524. [Google Scholar] [CrossRef]
- Castro, J.; Paz, S.; Mena, N.; Urresta, J.; Machuca-Martinez, F. Evaluation of heterogeneous catalytic ozonation process for diclofenac degradation in solutions synthetically prepared. Environ. Sci. Pollut. Res. Int. 2019, 26, 4488–4497. [Google Scholar] [CrossRef]
- Deng, Y.; Ezyske, C.M. Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water Res. 2011, 45, 6189–6194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Khan, M.; Fang, L.; Lo, I.M.C. Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. J. Hazard. Mater. 2019, 370, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Arlos, M.J.; Hatat-Fraile, M.M.; Liang, R.; Bragg, L.M.; Zhou, N.Y.; Andrews, S.A.; Servos, M.R. Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points. Water Res. 2016, 101, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Hu, J. Decomposition of sulfamethoxazole and trimethoprim by continuous UVA/LED/TiO2 photocatalysis: Decomposition pathways, residual antibacterial activity and toxicity. J. Hazard. Mater. 2017, 323, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photoch. Photobio. C 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Carp, O. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Žunič, V.; Škapin, S.D.; Maček-Kržmanc, M.; Bračko, I.; Sever Škapin, A.; Suvorov, D. Influence of the triblock copolymer P123 and phosphorous on the physico-chemical properties of TiO2. Appl. Catal A-Gen. 2011, 397, 241–249. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem. Eng. J. 2010, 161, 53–59. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel Magnetic Carbon Nanotube-TiO2 Composites for Solar Light Photocatalytic Degradation of Pharmaceuticals in the Presence of Natural Organic Matter. J. Water Process. Eng. 2019, 31. [Google Scholar] [CrossRef]
- Salaeh, S.; Juretic Perisic, D.; Biosic, M.; Kusic, H.; Babic, S.; Lavrencic Stangar, U.; Dionysiou, D.D.; Loncaric Bozic, A. Diclofenac removal by simulated solar assisted photocatalysis using TiO2-based zeolite catalyst; mechanisms, pathways and environmental aspects. Chem. Eng. J. 2016, 304, 289–302. [Google Scholar] [CrossRef]
- Sahu, S.P.; Qanbarzadeh, M.; Ateia, M.; Torkzadeh, H.; Maroli, A.S.; Cates, E.L. Rapid Degradation and Mineralization of Perfluorooctanoic Acid by a New Petitjeanite Bi3O(OH)(PO4)2 Microparticle Ultraviolet Photocatalyst. Environ. Sci. Tech. Lett. 2018, 5, 533–538. [Google Scholar] [CrossRef]
- Murgolo, S.; Moreira, I.S.; Piccirillo, C.; Castro, P.M.L.; Ventrella, G.; Cocozza, C.; Mascolo, G. Photocatalytic Degradation of Diclofenac by Hydroxyapatite-TiO2 Composite Material: Identification of Transformation Products and Assessment of Toxicity. Materials 2018, 11, 1779. [Google Scholar] [CrossRef]
- Kowalska, K.; Maniakova, G.; Carotenuto, M.; Sacco, O.; Vaiano, V.; Lofrano, G.; Rizzo, L. Removal of carbamazepine, diclofenac and trimethoprim by solar driven advanced oxidation processes in a compound triangular collector based reactor: A comparison between homogeneous and heterogeneous processes. Chemosphere 2020, 238, 124665. [Google Scholar] [CrossRef]
- Bernabeu, A.; Vercher, R.F.; Santos-Juanes, L.; Simón, P.J.; Lardín, C.; Martínez, M.A.; Vicente, J.A.; González, R.; Llosá, C.; Arques, A.; et al. Solar photocatalysis as a tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Tang, T.; Lu, G.; Wang, W.; Wang, R.; Huang, K.; Qiu, Z.; Tao, X.; Dang, Z. Photocatalytic removal of organic phosphate esters by TiO2: Effect of inorganic ions and humic acid. Chemosphere 2018, 206, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, J.; Li, J.; He, J. Kinetics and mechanism of Horner–Wadsworth–Emmons reaction of weakly acidic phosphonate in solid–liquid phase-transfer catalysis system. Catal. Commun. 2013, 36, 98–103. [Google Scholar] [CrossRef]
- Pereira, J.H.; Reis, A.C.; Queiros, D.; Nunes, O.C.; Borges, M.T.; Vilar, V.J.; Boaventura, R.A. Insights into solar TiO2-assisted photocatalytic oxidation of two antibiotics employed in aquatic animal production, oxolinic acid and oxytetracycline. Sci. Total Environ. 2013, 463–464, 274–283. [Google Scholar] [CrossRef]
- Sheng, H.; Li, Q.; Ma, W.; Ji, H.; Chen, C.; Zhao, J. Photocatalytic degradation of organic pollutants on surface anionized TiO2: Common effect of anions for high hole-availability by water. Appl. Catal. B-Environ. 2013, 138–139, 212–218. [Google Scholar] [CrossRef]
- Rao, Y.; Han, F.; Chen, Q.; Wang, D.; Xue, D.; Wang, H.; Pu, S. Efficient degradation of diclofenac by LaFeO3-Catalyzed peroxymonosulfate oxidation---kinetics and toxicity assessment. Chemosphere 2019, 218, 299–307. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernández-Alba, A.R. Application of Photo-Fenton as a Tertiary Treatment of Emerging Contaminants in Municipal Wastewater. Environ. Sci. Technol. 2010, 44, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Calin, M.; Yanez-Gascon, M.J.; Garrido, I.; Perez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six endocrine disruptor chemicals in wastewater using ZnO at pilot plant scale under natural sunlight. Environ. Sci. Pollut. Res. Int. 2018, 25, 34995–35007. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J. Photoch. Photobio. A 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Becerril, M.E.; Ramírez-García, J.J.; Cavazos, N.; Serrano, A.R. Determination of the Kinetic Behavior of Diclofenac in Aqueous Solution by UV Light Radiation. Water Air Soil Poll. 2019, 230. [Google Scholar] [CrossRef]
- Uyguner-Demirel, C.S.; Bekbolet, M. Significance of analytical parameters for the understanding of natural organic matter in relation to photocatalytic oxidation. Chemosphere 2011, 84, 1009–1031. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Qiu, Z.; Lin, K. Clofibric acid degradation in UV254/H2O2 process: Effect of temperature. J. Hazard. Mater. 2010, 176, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Massa, L.; Sperlich, A.; Gnirss, R.; Jekel, M. UV254 absorbance as real-time monitoring and control parameter for micropollutant removal in advanced wastewater treatment with powdered activated carbon. Water Res. 2016, 94, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Kang, J.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E. Photolysis and UV/H2O2 of diclofenac, sulfamethoxazole, carbamazepine, and trimethoprim: Identification of their major degradation products by ESI–LC–MS and assessment of the toxicity of reaction mixtures. Process. Saf. Environ. 2017, 112, 222–234. [Google Scholar] [CrossRef]
- Shimabuku, K.K.; Kennedy, A.M.; Mulhern, R.E.; Summers, R.S. Evaluating Activated Carbon Adsorption of Dissolved Organic Matter and Micropollutants Using Fluorescence Spectroscopy. Environ. Sci. Technol. 2017, 51, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Guerard, J.J.; Miller, P.L.; Trouts, T.D.; Chin, Y.P. The role of fulvic acid composition in the photosensitized degradation of aquatic contaminants. Aquatic Ences 2009, 71, 160–169. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Shang, E.; Crittenden, J.C. Influence of dissolved organic matter on photogenerated reactive oxygen species and metal-oxide nanoparticle toxicity. Water Res. 2016, 98, 9–18. [Google Scholar] [CrossRef]
- Chow, C.W.K.; Fabris, R.; Leeuwen, J.V.; Wang, D.; Drikas, M. Assessing Natural Organic Matter Treatability Using High Performance Size Exclusion Chromatography. Environ. Sci. Technol. 2008, 42, 6683–6689. [Google Scholar] [CrossRef]
- Lewis, R.; van Leeuwen, J.A.; Smernik, R.J.; Chow, C.W.K.; Everson, A.; Nothrop, S.C.; Beecham, S. Changes in the organic character of post-coagulated Pinus radiata sulfite pulp mill wastewater under aerated stabilization basin treatment—A laboratory scale study. Chem. Eng. J. 2011, 175, 160–168. [Google Scholar] [CrossRef]
- Plant, E.L.; Smernik, R.J.; van Leeuwen, J.; Greenwood, P.; Macdonald, L.M. Changes in the nature of dissolved organics during pulp and paper mill wastewater treatment: A multivariate statistical study combining data from three analytical techniques. Environ. Sci. Pollut. Res. Int. 2014, 21, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
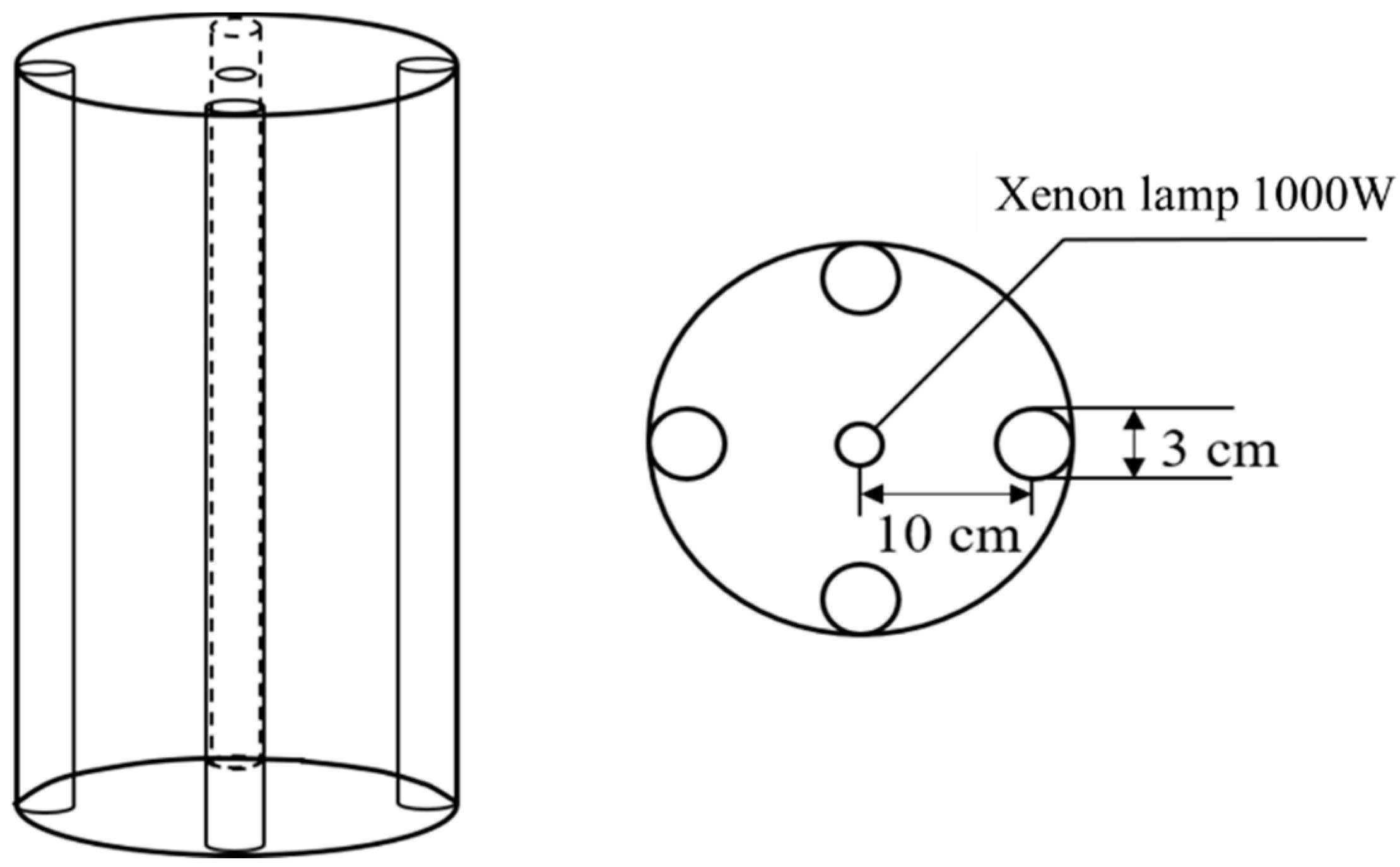



| Indicators | SE-A | SE-B | Level A Atandard of GB 18918-2002 |
|---|---|---|---|
| pH (-) | 8.03 | 7.51 | 6–9 |
| COD (mg/L) | 36 | 21 | 50 |
| TN (mg/L) | 24.33 | 16.24 | 15 |
| TP (mg/L) | 0.58 | 0.38 | 0.5 |
| NH3-N (mg/L) | 4.8 | 3.2 | 5(8) |
| Secondary Effluent | Peak Name | Residence Time (min) | Content (mg/L) | Content (mol) |
|---|---|---|---|---|
| SE-A | Cl− | 8.098 | 77.8068 | 2.192 |
| NO3− | 14.757 | 29.3798 | 0.474 | |
| SO42− | 23.998 | 47.2421 | 0.492 | |
| SE-B | Cl− | 7.940 | 61.6665 | 1.737 |
| NO3− | 14.032 | 12.0239 | 0.194 | |
| SO42− | 23.782 | 24.1031 | 0.251 |
| Peak Time/Min | 0.5–2.2 | 5.7–6.2 | 7.7–8.5 | 10.5–14 |
|---|---|---|---|---|
| Molecular Weight | 1,180,000–4,530,000 | 50,280–74,627 | 8175–15,378 | 106–1684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; Zhang, N.; Gao, L.; Yuan, R.; Chen, H.; Hou, X.; Hou, J.; Wang, F.; Zhou, B. Removal of Diclofenac in Effluent of Sewage Treatment Plant by Photocatalytic Oxidation. Water 2020, 12, 2902. https://doi.org/10.3390/w12102902
Ma N, Zhang N, Gao L, Yuan R, Chen H, Hou X, Hou J, Wang F, Zhou B. Removal of Diclofenac in Effluent of Sewage Treatment Plant by Photocatalytic Oxidation. Water. 2020; 12(10):2902. https://doi.org/10.3390/w12102902
Chicago/Turabian StyleMa, Nan, Nannan Zhang, Ling Gao, Rongfang Yuan, Huilun Chen, Xuerui Hou, Jiawei Hou, Fei Wang, and Beihai Zhou. 2020. "Removal of Diclofenac in Effluent of Sewage Treatment Plant by Photocatalytic Oxidation" Water 12, no. 10: 2902. https://doi.org/10.3390/w12102902
APA StyleMa, N., Zhang, N., Gao, L., Yuan, R., Chen, H., Hou, X., Hou, J., Wang, F., & Zhou, B. (2020). Removal of Diclofenac in Effluent of Sewage Treatment Plant by Photocatalytic Oxidation. Water, 12(10), 2902. https://doi.org/10.3390/w12102902





